Abstract
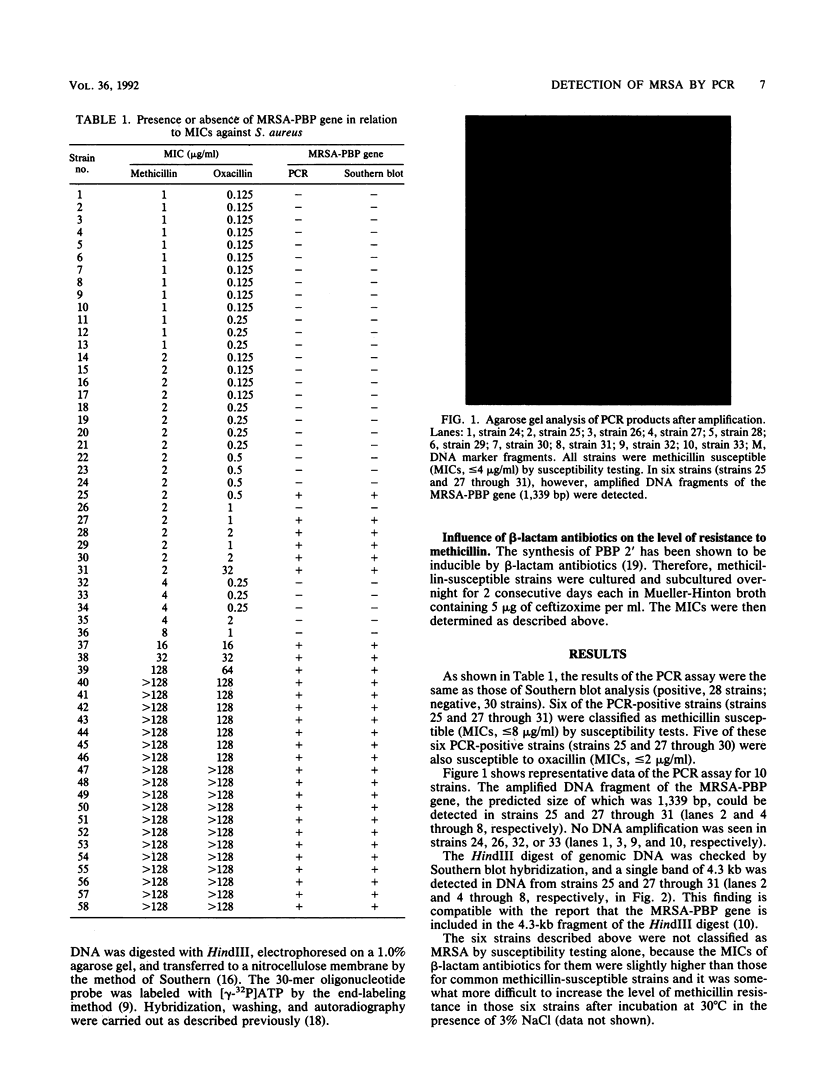
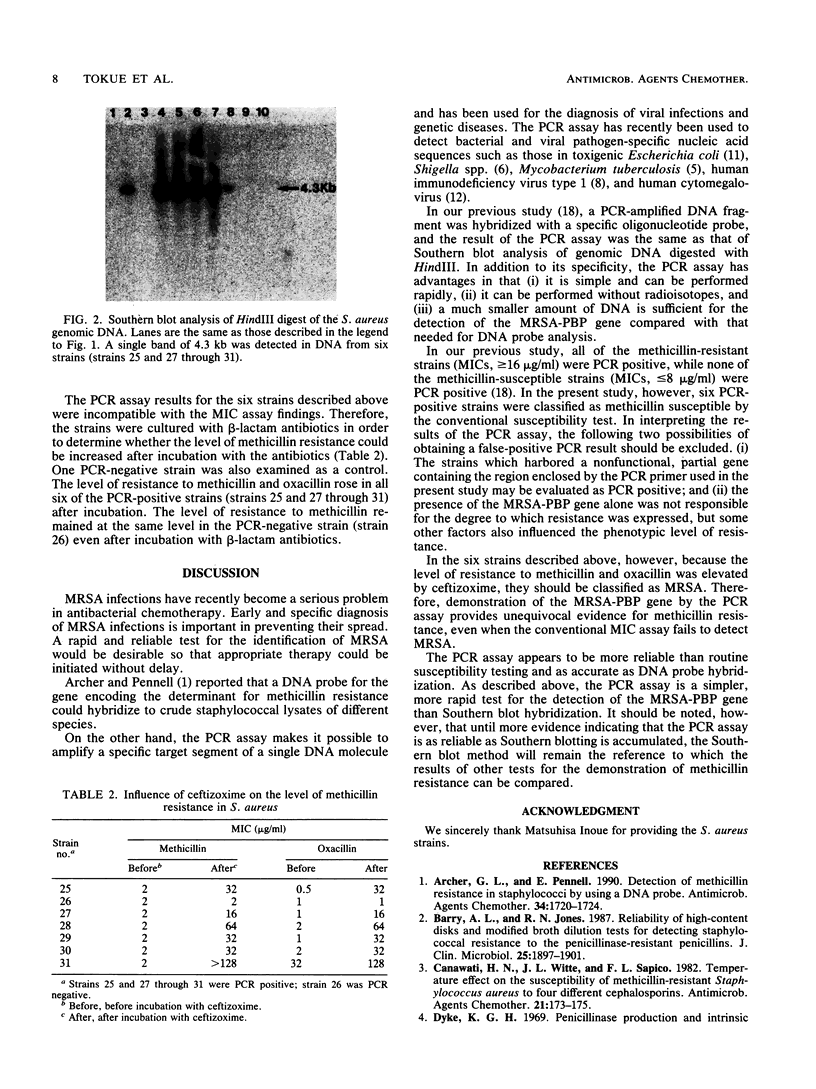
The presence or absence of a methicillin resistance gene in 58 clinical isolates of Staphylococcus aureus was examined by the polymerase chain reaction (PCR) and Southern blot analyses. The results were analyzed in relation to those of the MIC assay of methicillin and oxacillin. PCR assay results were identical to those of Southern blot analysis of genomic DNA digested with HindIII (positive, 28 strains; negative, 30 strains). Among the 28 PCR-positive strains, 6 strains showed methicillin susceptibility by the conventional susceptibility test (MICs, less than or equal to 8 micrograms/ml). Culturing of the six strains with ceftizoxime led to an increase in the phenotypic level of resistance to methicillin and oxacillin, indicating that these strains should be classified as methicillin-resistant S. aureus (MRSA). The PCR assay was found to be a sensitive and reliable procedure for the rapid diagnosis of MRSA infection, even in cases in which the conventional MIC assay failed to detect MRSA.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer G. L., Pennell E. Detection of methicillin resistance in staphylococci by using a DNA probe. Antimicrob Agents Chemother. 1990 Sep;34(9):1720–1724. doi: 10.1128/aac.34.9.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A. L., Jones R. N. Reliability of high-content disks and modified broth dilution tests for detecting staphylococcal resistance to the penicillinase-resistant penicillins. J Clin Microbiol. 1987 Oct;25(10):1897–1901. doi: 10.1128/jcm.25.10.1897-1901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canawati H. N., Witte J. L., Sapico F. L. Temperature effect on the susceptibility of methicillin-resistant Staphylococcus aureus to four different cephalosporins. Antimicrob Agents Chemother. 1982 Jan;21(1):173–175. doi: 10.1128/aac.21.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyke K. G. Penicillinase production and intrinsic resistance to penicillins in methicillin-resistant cultures of Staphylococcus aureus. J Med Microbiol. 1969 Aug;2(3):261–278. doi: 10.1099/00222615-2-3-261. [DOI] [PubMed] [Google Scholar]
- Eisenach K. D., Cave M. D., Bates J. H., Crawford J. T. Polymerase chain reaction amplification of a repetitive DNA sequence specific for Mycobacterium tuberculosis. J Infect Dis. 1990 May;161(5):977–981. doi: 10.1093/infdis/161.5.977. [DOI] [PubMed] [Google Scholar]
- Frankel G., Riley L., Giron J. A., Valmassoi J., Friedmann A., Strockbine N., Falkow S., Schoolnik G. K. Detection of Shigella in feces using DNA amplification. J Infect Dis. 1990 Jun;161(6):1252–1256. doi: 10.1093/infdis/161.6.1252. [DOI] [PubMed] [Google Scholar]
- Kwok S., Mack D. H., Mullis K. B., Poiesz B., Ehrlich G., Blair D., Friedman-Kien A., Sninsky J. J. Identification of human immunodeficiency virus sequences by using in vitro enzymatic amplification and oligomer cleavage detection. J Virol. 1987 May;61(5):1690–1694. doi: 10.1128/jvi.61.5.1690-1694.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuhashi M., Song M. D., Ishino F., Wachi M., Doi M., Inoue M., Ubukata K., Yamashita N., Konno M. Molecular cloning of the gene of a penicillin-binding protein supposed to cause high resistance to beta-lactam antibiotics in Staphylococcus aureus. J Bacteriol. 1986 Sep;167(3):975–980. doi: 10.1128/jb.167.3.975-980.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive D. M. Detection of enterotoxigenic Escherichia coli after polymerase chain reaction amplification with a thermostable DNA polymerase. J Clin Microbiol. 1989 Feb;27(2):261–265. doi: 10.1128/jcm.27.2.261-265.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive D. M., al Mufti S., Simsek M., Fayez H., al Nakib W. Direct detection of human cytomegalovirus in urine specimens from renal transplant patients following polymerase chain reaction amplification. J Med Virol. 1989 Dec;29(4):232–237. doi: 10.1002/jmv.1890290403. [DOI] [PubMed] [Google Scholar]
- Reynolds P. E., Brown D. F. Penicillin-binding proteins of beta-lactam-resistant strains of Staphylococcus aureus. Effect of growth conditions. FEBS Lett. 1985 Nov 11;192(1):28–32. doi: 10.1016/0014-5793(85)80036-3. [DOI] [PubMed] [Google Scholar]
- Sabath L. D., Wallace S. J., Gerstein D. A. Suppression of intrinsic resistance to methicillin and other penicillins in Staphylococcus aureus. Antimicrob Agents Chemother. 1972 Nov;2(5):350–355. doi: 10.1128/aac.2.5.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M. D., Wachi M., Doi M., Ishino F., Matsuhashi M. Evolution of an inducible penicillin-target protein in methicillin-resistant Staphylococcus aureus by gene fusion. FEBS Lett. 1987 Aug 31;221(1):167–171. doi: 10.1016/0014-5793(87)80373-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thornsberry C., McDougal L. K. Successful use of broth microdilution in susceptibility tests for methicillin-resistant (heteroresistant) staphylococci. J Clin Microbiol. 1983 Nov;18(5):1084–1091. doi: 10.1128/jcm.18.5.1084-1091.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokue Y., Shoji S., Satoh K., Watanabe A., Motomiya M. Detection of methicillin-resistant Staphylococcus aureus (MRSA) using polymerase chain reaction amplification. Tohoku J Exp Med. 1991 Jan;163(1):31–37. doi: 10.1620/tjem.163.31. [DOI] [PubMed] [Google Scholar]
- Ubukata K., Nonoguchi R., Matsuhashi M., Konno M. Expression and inducibility in Staphylococcus aureus of the mecA gene, which encodes a methicillin-resistant S. aureus-specific penicillin-binding protein. J Bacteriol. 1989 May;171(5):2882–2885. doi: 10.1128/jb.171.5.2882-2885.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubukata K., Yamashita N., Konno M. Occurrence of a beta-lactam-inducible penicillin-binding protein in methicillin-resistant staphylococci. Antimicrob Agents Chemother. 1985 May;27(5):851–857. doi: 10.1128/aac.27.5.851. [DOI] [PMC free article] [PubMed] [Google Scholar]