Abstract
Mesenchymal stem cells (MSCs) in bone marrow (BM) regulate the differentiation and proliferation of adjacent hematopoietic precursor cells and contribute to the regeneration of mesenchymal tissues, including bone, cartilage, fat and connective tissue. BM is an important site for the pathogenesis of human cytomegalovirus (HCMV) where the virus establishes latency in hematopoietic progenitors and can transmit after reactivation to neighboring cells. Here we demonstrate that BM-MSCs are permissive to productive HCMV infection, and that HCMV alters the function of MSCs: i) by changing the repertoire of cell surface molecules in BM-MSCs, HCMV modifies the pattern of interaction between BM-MSCs and hematopoietic cells; ii) HCMV infection of BM-MSCs undergoing adipogenic or osteogenic differentiation impaired the process of differentiation. Our results suggest that by altering BM-MSC biology, HCMV may contribute to the development of various diseases.
Keywords: HCMV, virus-host interaction, bone marrow, mesenchymal stem cells, hematopoietic stem cells, hematopoiesis, cell differentiation, osteogenesis, adipogenesis, cell adhesion
Introduction
Bone marrow (BM) is a complex organ comprised of hematopoietic stem cells, their progenitors and stromal cells. In vitro, BM stromal cells are a heterogeneous population containing predominantly fibroblasts with small subsets of adipocytes, osteocytes, reticulocytes and endothelial cells. The stromal cells, which are most fibroblastoid cells are differentiated from mesenchymal stem cells (MSCs) that are also referred in some reports as marrow stromal precursor cells or stromal stem cells (Bruder et al., 1997; Majumdar et al., 1998; Owen and Friedenstein, 1988; Pittenger et al., 1999; Reyes et al., 2001). MSCs contribute to the regeneration of bone, cartilage and other mesenchymal tissues, and are present in many tissues but are most prevalent in BM. A homogeneous population of MSCs can proliferate in vitro as uncommitted cells capable of self-renewal, and can be induced to differentiate along multiple mesenchymal cell lineages including osteoblasts, adipocytes, chondrocytes, tendocytes, myofibroblasts, as well as endothelial-like and epithelial-like cells (Bruder et al., 1997; Jaiswal et al., 1997; Majumdar et al., 1998; Oswald et al., 2004; Pittenger et al., 1999; Reyes et al., 2001). The significance of BM-derived MSCs (BM-MSCs) as an important cellular and functional component of BM was demonstrated by their ability to support hematopoiesis and modulate differentiation of hematopoietic primitive stem cells in long-term cultures via expression of numerous cell adhesion molecules that are important for cell-to-cell and cell-to-matrix interactions, homing, mobilization and trafficking, and also by providing cytokines and growth factors (Cheng et al., 2000; Gronthos et al., 2003; Ishii et al., 2005; Majumdar et al., 1998; Majumdar et al., 2003; Owen and Friedenstein, 1988; Reyes et al., 2001).
Human cytomegalovirus (HCMV), a member of the beta-herpesvirus family, is a widespread ubiquitous pathogen (Mocarski, 2001). HCMV is a leading cause of congenital birth defects, as well as the major cause of a variety of life-threatening diseases in immunocompromised individuals such as organ and BM transplant and AIDS patients.
HCMV naturally infects a wide variety of cell types including fibroblasts, endothelial, epithelial, neuronal and smooth muscle cells, monocyte/macrophages, granulocytes and BM cells (Mocarski, 2001; Plachter et al., 1996). Following primary exposure, HCMV establishes a lifelong latency in the host. Although cellular reservoir(s) for latent HCMV and its persistency in the host have not been well characterized, latent virus has been found in hematopoietic myeloid lineage progenitor cells (Hahn et al., 1998; Kondo et al., 1996; Minton et al., 1994; Taylor-Wiedeman et al., 1991). Reactivation of the latent virus in BM-derived progenitor cells has been demonstrated in vitro, and CD34+ precursor cells were recognized as a more likely primary reservoir for latent HCMV within BM (Goodrum et al., 2004; Goodrum et al., 2002; Zhuravskaya et al., 1997). In addition, CD34+ cells purified from blood of healthy HCMV carriers were demonstrated to contain latent HCMV (Reeves et al., 2005). Therefore, current models suggest that BM hematopoietic progenitors serve as a reservoir for HCMV and may, after reactivation, transmit the viral genome to other cells including peripheral blood mononuclear cells (PBMCs) and cells of the stroma (Michelson et al., 2001; Streblow and Nelson, 2003). Because HCMV infection can cause latency, abortive or productive infection depending on cell type, it is important to study HCMV infection of distinct homogeneous cell populations. However, the pathogenic role of virus infection of various BM-derived specific cell populations remains poorly characterized.
Experiments in long-term BM cultures have indicated that mixed populations of BM stromal cells (BMSCs) are targets of productive HCMV infection, and that HCMV actively replicates in BMSCs (Apperley et al., 1989; Simmons et al., 1990; Taichman et al., 1997). Most of the studies on HCMV infection in BMSCs, except one with myofibroblasts (Michelson et al., 2001), were carried out using mixed populations of phenotypically and functionally heterogeneous BMSCs. The understanding of the cell types infected with HCMV and the consequences of the infection of distinct types of BM cells are of critical importance to understanding the pathobiology of HCMV infection.
BM-MSCs are unique multifunctional cells that not only regulate the differentiation and proliferation of hematopoietic precursor cells but also contribute to the regeneration of various mesenchymal tissues, including bone and fat tissue. Considering the significance of BM-MSCs, we focused our study on BM-MSCs, their multiple functions and how these functions are affected by HCMV infection. Here we demonstrate that BM-MSCs are permissive to productive HCMV infection, and that HCMV significantly alters the function of MSCs. Following infection, the repertoire of cell surface molecules in MSCs showed definite and differential changes that led to increased adherence of hematopoietic cells to infected MSCs, affecting the pattern of interaction between MSCs and hematopoietic cells. We also demonstrate that BM-MSCs induced into either adipogenesis or osteogenesis are permissive to HCMV infection at different phases of differentiation, and HCMV infection of MSCs undergoing adipogenic or osteogenic differentiation impaired the process of differentiation by blocking the progression of cells from the undifferentiated state to terminally differentiated osteoblasts and adipocytes. The results demonstrate the complexity of interaction between HCMV and BM and raise the possibility that MSCs may play a role in the development of HCMV-associated pathology.
Results
Susceptibility of MSCs to HCMV infection
It was demonstrated that HCMV actively replicates in mixed BM stromal cells whereas it remains latent in BM hematopoietic cells (Hahn et al., 1998; Simmons et al., 1990; Taichman et al., 1997; Zhuravskaya et al., 1997). We wanted to investigate whether HCMV can infect a homogenous population of BM-derived MSCs. To be able to monitor productive infection of MSCs by HCMV we used two recombinant HCMV strains expressing Green Fluorescent Protein (GFP). HCMV genome of laboratory AD169 and clinical TB40 HCMV strains were cloned as BAC plasmid in E. coli by homologous recombination using a pUSF-3 marker cassette containing GFP under control of an SV40 promoter and a chloramphenicol resistance gene as described (Marchini et al., 2001). To test MSCs’ susceptibility for HCMV infection, the cells were infected with TB40 BAC HCMV containing the GFP reporter gene at MOI of 1 PFU/cell. After 3 days, plaques of GFP expressing cells appeared in the monolayer of MSCs, and at day 10 over 95% of the cells showed GFP expression (Fig. 1A sup.), indicating the virus had established a fully permissive infection in MSCs.
To exclude the possibility that latent virus can survive in primary BM-MSCs during preparation and expansion in vitro, and because there was no record of HCMV testing of the BM donor, the presence of viral DNA in MSCs was tested by PCR amplification using primers for UL56 and β-actin (as a control) genes. No viral sequences were detected in lysates of uninfected MSCs, whereas the infected MSCs were highly positive for UL56 (Fig. 1B sup.).
To more carefully evaluate the virus growth in MSCs, the growth properties of laboratory AD169 and clinical TB40 HCMV strains were compared in single-step (MOI = 5 PFU/cell) and multiple-step (MOI = 0.1 PFU/cell) growth curve experiments on both MSC and HFF cells (Fig. 1). There were no differences observed in virus replication between MSC and HFF cells at low MOI. Both AD169 and TB40E strains showed similar growth kinetics and peak titers. Interestingly, at high multiplicity (5 PFU/cell), the growth rate of laboratory AD169 HCMV strain on MSCs was significantly lower than that of clinical TB40E HCMV strain, which produced virus at levels comparable to those on HFFs (Fig. 1). The yield of AD169 HCMV on MSCs at MOI of 5 PFU/cell was consistently 10-fold less than that on HFFs. It appeared that at high MOI, cells infected with AD169 HCMV quickly became abrogated and died within 4 to 6 days post infection, whereas clinical TB40 strain was able to retain MSCs from dying for prolonged time (8 days), therefore, yielding higher viral titers.
Fig. 1. Characterization of HCMV infection in MSCs.
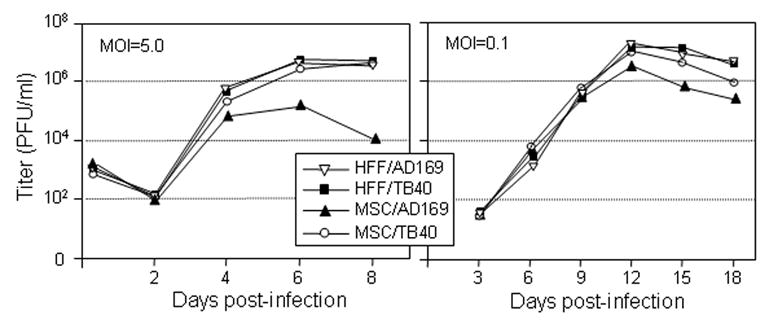
Growth kinetics of laboratory AD169 and clinical TB40 HCMV strains were compared in MSC and HFF cells. The cells were infected with virus at a high MOI of 5 PFU/cell (single-step growth curves, left panel) or at a low MOI of 0.1 PFU/cell (multistep growth curves, right pane). Cell-free and cell-associated virus was collected at indicated times and the virus titer was determined. Shown data is from one representative experiment out of three independent experiments.
HCMV infection alters expression of cell surface markers in BM-MSCs
BM-MSCs express numerous cell surface molecules important for cell-to-cell and cell-to-matrix interactions that have been demonstrated to play a central role in support of optimal conditions for hematopoiesis within BM, as well as cell migration, recognition, trafficking, growth and survival (Gronthos et al., 2003; Majumdar et al., 2003). Therefore, it was of interest to investigate the effect of HCMV infection on the expression of cell surface molecules that are relevant for MSC functions in BM. Because HFFs are one of the terminally differentiated cell types along mesenchymal lineage, and there is a high similarity in expression patterns of many CD markers between mesenchymal stem cells and fibroblasts (Ishii et al., 2005), we also extended this analysis to HFFs.
MSC and HFF cells were left uninfected or HCMV-infected at MOI of 3–5 PFU/cell for 72 hr resulting in about 95% of the cells expressing GFP. Expression levels of cell surface CD29 (integrin-beta1), CD44 (receptor for osteopontin and hyaluronic acid/fibronectin), CD54 (ICAM-1), CD58 (LFA-3), CD73 (ecto-5’-nucleotidase), CD90 (Thy-1), CD105 (endoglin), CD106 (VCAM-1), MHC class I (HLA-ABC), and MHC class II (HLA-DR) were evaluated in the GFP-positive cells by flow cytometry (Fig. 2 and data not shown). Although overall expression profiles of cell surface molecules in uninfected MSC and HFF cells were generally similar, certain differences in the expression level of CD markers was also observed. For instance, CD44, CD73, and CD105 markers consistently showed 2 to 3 times higher levels of expression in MSCs than in HFFs. In contrast, the expression level of ICAM-1 (CD54) adhesion molecule in uninfected MSCs was only weakly detectable (1.5 times over isotype control), whereas HFFs demonstrated moderate expression of this CD marker. In addition, MSCs were weakly positive for VCAM-1 (CD106) expression, whereas HFFs were negative (data not shown).
Fig. 2. Modulation of cell surface molecule expression in MSC and HFF cells infected with HCMV.
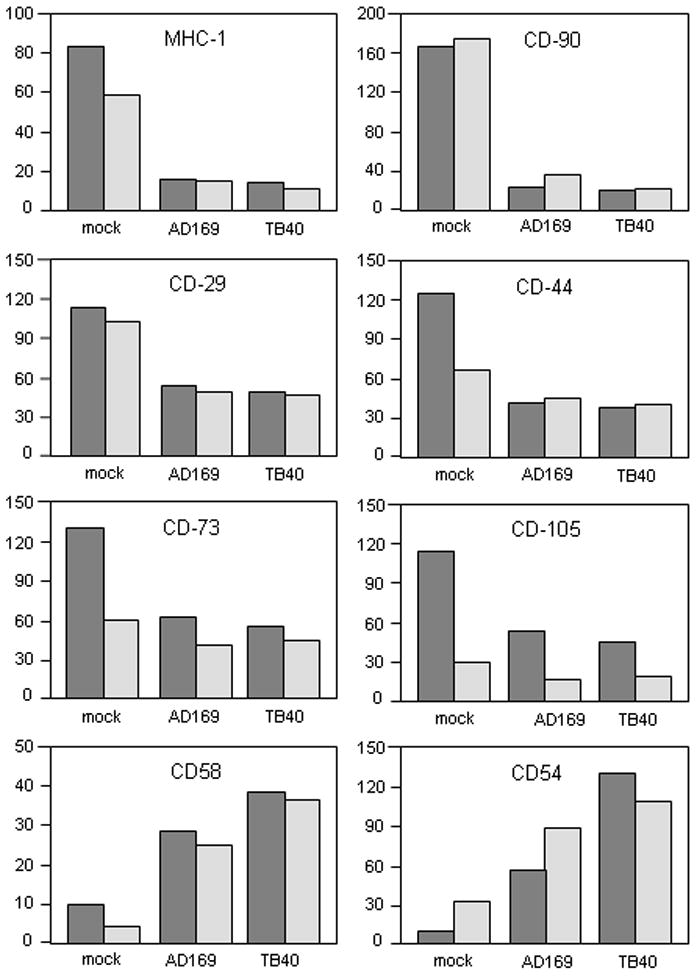
Analysis of the expression of cell surface molecules on MSCs (dark shaded bars) and HFFs (light shaded bars) was performed with the use of flow cytometry. Uninfected or HCMV-infected (3 days post infection) cells were collected and incubated with the following conjugated monoclonal antibodies: CD29-PE, CD44-PE, CD73-PE, CD90-PE, CD105-FITC and HLA-ABC-PE; or with CD54 or CD58 antibodies and subsequently with FITC-conjugated secondary antibody. Nonspecific fluorescence was determined by incubation of the cells with isotype-matched mouse monoclonal antibodies. Antibody-treated cells were subjected to flow cytometry. Data are expressed as relative fluorescence intensity (RFI) as a percentage of the baseline value (isotype control). Shown data is from one representative experiment out of three independent experiments.
The expression pattern of CD markers was dramatically changed upon infection (Fig. 2 and data not shown). After 72 hr, a 2- to 3-fold reduction of the expression level of CD29, CD44, CD73, CD105 surface proteins was observed in MSCs, comparing uninfected versus HCMV-infected cells. Even a more profound, up to 10 times, inhibition of expression of CD90 and MHC class I markers was seen in infected MSCs. Nevertheless, detectable levels of expression of CD90 and MHC class I molecules were observed 72 and up to 168 hr post infection (Fig. 2 and data not shown). Similar down-regulation of cell surface molecule expression was observed in infected HFFs with the exception of two molecules. Interestingly, in contrast to MSCs, the expression level of CD44 and CD73 surface proteins in HFFs showed little or no alteration in response to HCMV infection. Both MSC and HFF cells showed significant up-regulation of ICAM-1 (CD54) and LFA-3 (CD58) surface molecule expression upon HCMV infection.
HCMV infection affects interaction between BM-MSCs and hematopoietic cells
In light of such profound HCMV-related modulation of cell surface molecule expression on BM-MSCs, we next investigated functional consequences of this modulation for specific cell-to-cell interactions within BM. A whole cell adhesion assay between BM-MSCs and various hematopoietic transformed cell lines was used as a model. Each of the cell lines we used represents a different stage in hematopoiesis toward monocytes according to a review by Harris (Harris, 1996): KG1a (early pluripotent CD34+ hematopoietic stem cells), K562 (late pluripotent hematopoietic stem cells), HL-60 (monocyte or granulocyte progenitor-like), U937 (promonocytic), and THP-1 (monocytic) cells. In addition, Jurkat, Molt-4 and Raji cells, representing mature T and B lymphoblastic cell lineages, respectively, were included in the analysis.
Results of cell adhesion experiments between hematopoietic cell lines and non-infected monolayers of MSCs (Fig. 3A) were quite similar to what has been reported (Majumdar et al., 2003). We also observed preferential binding of mature hematopoietic cell lines and T lymphoblastic cells to MSCs. Cell lines representing early stages of hematopoietic differentiation adhered to MSC monolayers at significantly lower rates. Following infection for 96 hr with TB40 HCMV strain at MOI of 3–5, MSCs showed a visible increase in adherence of all studied hematopoietic cell lines compared to uninfected cells. However, the most dramatic changes in binding affinity were observed for the promonocytic cell line U937, early pluripotent CD34+ stem-like cell line KG1a, and late pluripotent hematopoietic stem-like cell line K562 (Fig. 3A). Adherence of these cells to HCMV-infected MSCs versus the uninfected cells increased five, three and two times, respectively.
Fig. 3. Effect of HCMV infection on interaction between MSCs and hematopoietic cells.
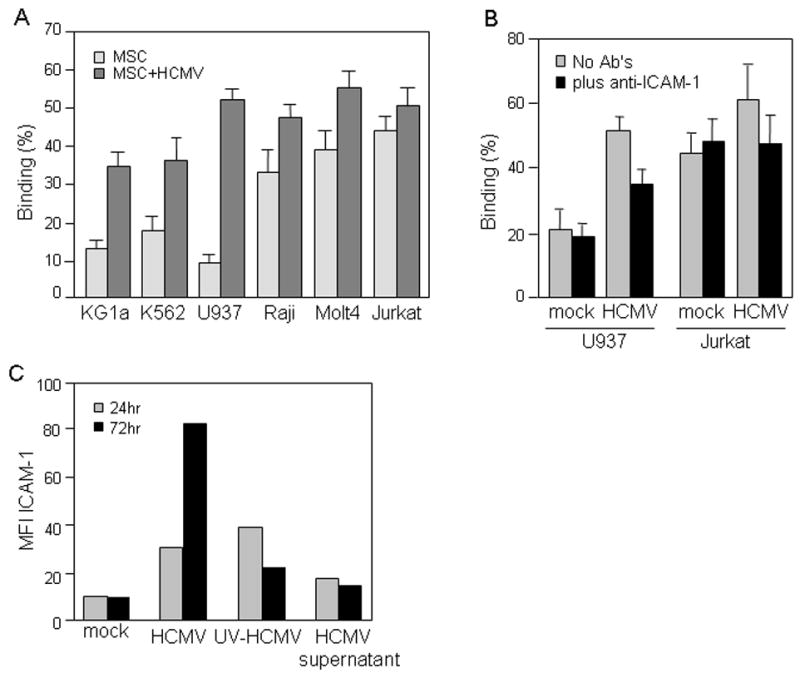
(A and B) Adherent MSCs were seeded in 96-well plates, left uninfected or infected with TB40 HCMV at MOI of 3–5 PFU/cell for 72 hr, and treated with blocking ICAM antibody (B) or left untreated (A). Suspension cell lines shown on the figure were labeled with PKH26 red fluorescent dye and allowed to adhere to MSCs for 1 hr at 37°C at a ratio of 5–10 suspension/monolayer cells. Unbound cells were washed out and the fluorescence of cells retained in the wells was measured. Results are expressed as the percentage of adherent cells. The adhesion assay was performed in quadruplicate format for each target cell line. (C) MSCs were left uninfected (mock), infected with TB40 HCMV at MOI of 3–5 PFU/cell or treated with either UV-inactivated HCMV virions (UV-HCMV) or HCMV-free supernatants (HCMV supernatant); and ICAM-1 expression was analyzed by flow cytometry at 24 and 72 hr post infection. Data are expressed as RFI as a percentage of the baseline value (isotype control). Shown data is from one representative experiment out of three independent experiments.
A significant decrease of binding of U937 and Jurkat cells to infected MSCs occurred in the presence of blocking ICAM-1 antibodies (Fig. 3B), demonstrating the impact of up-regulation of ICAM-1 expression on cell adherence. Next we investigated whether the observed up-regulation of ICAM-1 expression (Fig. 2) is a direct effect of virus infection or a bystander effect mediated by cytokines produced by CMV-infected cells. The level of ICAM-1 expression was moderately upregulated after 24 hr of treatment of MSCs with either intact or UV inactivated HCMV virions (Fig. 3C). HCMV-free supernatants only slightly modulated ICAM-1 expression. However, at 72 hr only direct HCMV infection led to strong up-regulation of the level of cell surface ICAM-1 expression on MSC.
HCMV infects MSCs differentiating into adipogenic or osteogenic lineage
Since MSCs are pluripotent cells capable of differentiation along mesenchymal cell lineage, we next investigated the effect of HCMV infection on MSC differentiation into adipocytes and osteocytes.
For adipogenic differentiation MSCs were cultured in differentiation medium for 28 days with three cycles of induction/maintenance as described in Materials and Methods. Subsets of the differentiating cells were infected at the beginning of each induction cycle (days 0, 7, 14 and 21) with TB40 BAC-GFP HCMV at MOI of 3–5 PFU/cell. Progression of HCMV infection in differentiating cells was monitored by visualization of GFP expression. HCMV titers in differentiating cell cultures infected at various time points were also determined at day 14 post infection.
At the end of adipogenesis (day 28) more than 90% of uninfected MSCs differentiated into adipocytes as revealed by observation of changes in the cell morphology and formation of lipid vacuoles visualized with Oil-red-O staining (Fig. 4A and B). In contrast, HFFs retained their fibroblastic shape and did not show any changes in cell morphology under adipogenic differentiation conditions (not shown). MSCs undergoing differentiation into adipocytes were permissive to HCMV infection at any stage of the induction (Fig. 4C and D). Cell cultures infected at early stages (0 and 7 days, data not shown) developed full cytopathic effects (CPE) at day 28, and the virus titer obtained from cell cultures at day 14 post infection was comparable to that on HFF cells (107 PFU/ml, data not shown). Cell cultures infected with the virus at either day 14 or day 21 of induction were also susceptible to HCMV infection (Fig. 4C and D). Examination by light microscopy showed that development of HCMV infection in these cultures was gradually accompanied by the release of fat vacuoles from the cells into culture medium, and by the partial change of cell morphology from lipid vacuole containing adipocyte-like phenotype to spindle shaped MSC-like phenotype. Moreover, the progression of virus infection negatively correlated with the size of accumulated fats in adipocytes. However, the virus yield finally generated from these cultures at day 14 post infection was still relatively high (titer range between 105 to 106 PFU/ml; data not shown). There was no difference observed in virus replication in HFFs under conditions of adipogenic induction compared to regular culture conditions (data not shown).
Fig. 4. HCMV infection of MSCs differentiating into adipogenic or osteogenic lineage.
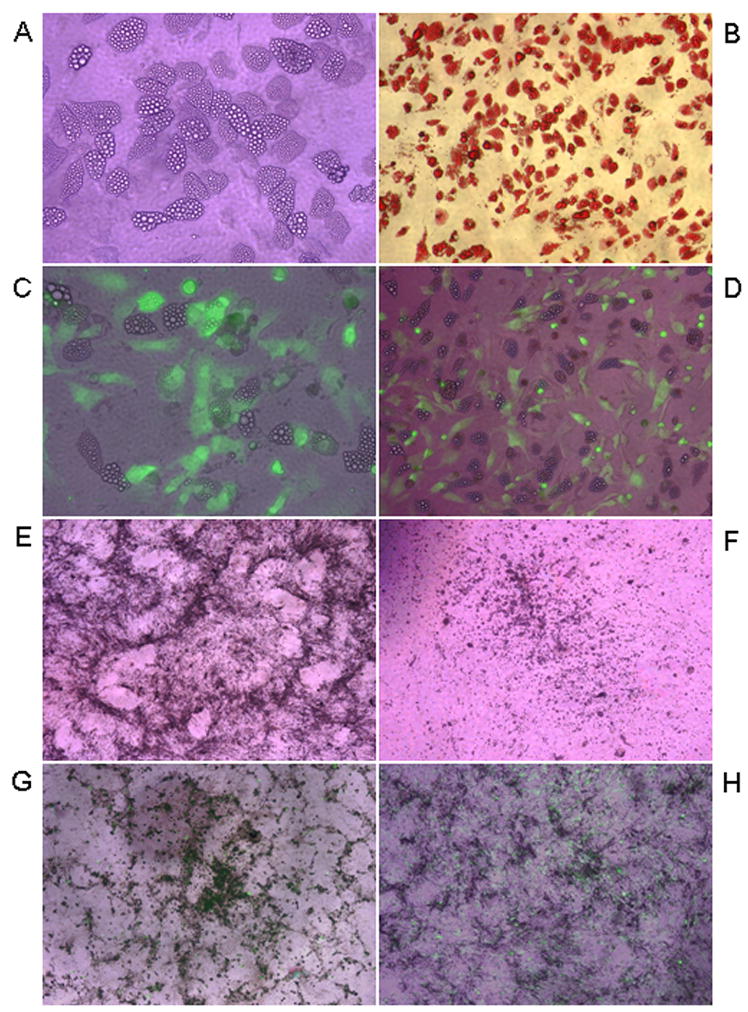
MSCs were incubated in either adipogenic (A–D) or osteogenic (E, G and H) differentiation media for 28 days, left uninfected or infected with TB40 BAC-GFP HCMV at MOI of 3–5 PFU/cell at days 0, 7, 14 and 21 of differentiation. HFFs (F) were also incubated in osteogenic differentiation media for 28 days. All cultures were analyzed at day 28 of the differentiation. Lipid vacuole accumulation in uninfected adipogenic MSC cultures was observed by regular light microscopy (A; X600, regular light microscopy) and was confirmed with Oil red O staining (B; X400, regular light microscopy). GFP expression was used to monitor productive HCMV infection in adipogenic cultures infected at day 14 (C; X600, merged regular and fluorescent light microscopy) or day 21 of differentiation (D; X400; merged regular and fluorescent light microscopy). MSCs (E) and HFFs (F), incubated in osteogenic differentiation media, were photographed to observe calcium deposition in the cells (X400; regular light microscopy). GFP expression was used to monitor productive HCMV infection in osteogenic cultures infected at day 7 (G) or day 21 of differentiation (H) (X400; merged regular and fluorescent light microscopy).
To further evaluate the effect of HCMV infection on adipogenesis we compared effects of active HCMV infection with that of UV-inactivated HCMV virions and virus-free supernatants on the differentiation potential of MSCs. As shown in Fig. 5A, there is an evident inhibition of adipocyte formation in the presence of virus-free supernatants and UV-inactivated virions. No loss in cell number was observed as determined with Giemsa staining (data not shown). However, in cultures treated with infectious virions, the effect of inhibition of adipocyte formation was more profound and was accompanied by loss of cells as a result of lytic infection.
Fig. 5. Effect of HCMV infection on adipogenesis and osteogenesis.
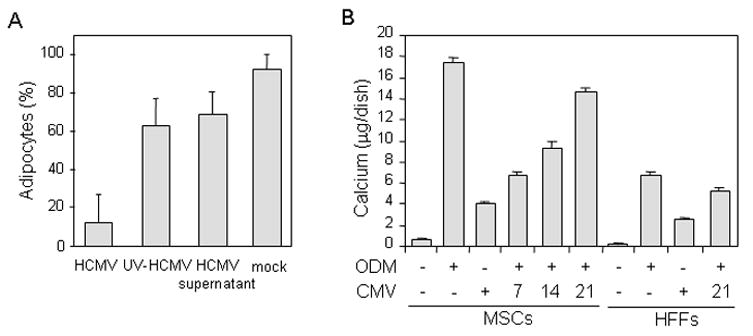
(A) MSCs were seeded in 12 well plates and left uninfected (mock), infected with TB40 HCMV at MOI of 3–5 PFU/cell or treated with either UV-inactivated HCMV virions (UV-HCMV) or HCMV-free supernatants (HCMV supernatant) for 1 day at the beginning of each cycle of induction/maintenance and then maintained in differentiation medium on the same schedule as described above in figure 4 legend for 2 cycles. After 14 days the adipogenic cultures were stained with Oil red O and adipocytes (cells containing lipid vacuoles) were counted. The number of adipocytes is shown as a percentage of the total number of all cells. Data represents results of 5 counting’s in each out of three wells. (B) MSCs and HFFs were cultured in standard or osteogenic differentiation media (ODM) for 28 days. Cells were left uninfected or infected with TB40 BAC-GFP HCMV at indicated days (7, 14 and 21) of osteogenic differentiation. Calcium deposition in the cells was determined at day 28. Total calcium (μg/dish) was calculated from standard solutions. Assays were done in triplicate at each time point.
To induce osteogenesis, MSCs were cultured in osteogenic differentiation medium for 28 days and were infected with HCMV at either day 0, 7, 14 or 21 of induction. For comparison, HFFs were also cultured in osteogenic medium and infected with virus on the same schedule as MSCs. At day 28 of differentiation MSCs showed changes in cell morphology, from spindle shaped to cuboidal shaped, and a specific formation of nodular aggregates and calcium mineralization (black granular deposits) were evident (Fig. 4E). HFFs only modestly responded to osteogenic medium by calcium deposition (Fig. 4F). Mineralization of the extracellular matrix was apparent beginning at days 5 to 7 and was confirmed by calcium accumulation assay (Fig. 5B). Osteogenesis-induced MSCs were permissive for virus infection at all times tested (Fig. 4G and H, and data not shown). In MSCs infected with HCMV at days 0 or 7 of osteogenic induction (Fig. 4G, and data not shown), full CPE developed by day 28. In MSCs infected with HCMV at days 14 or 21 of osteogenic induction (Fig. 4H, and data not shown), specific formation of nodular aggregates and calcium mineralization were significantly impaired. To quantify effects of HCMV infection on osteogenic differentiation, mineral deposition in cell cultures was measured at the end of osteogenic differentiation (day 28; Fig. 5B). While MSCs grown in osteogenic differentiation medium without infection showed significant calcium deposition, when HCMV was added into differentiating cell cultures, calcium mineralization was inhibited in a time-dependent manner (Figs. 4G and H, and5B). It appeared that cells infected at an early stage of differentiation demonstrated a lower rate of mineralization than those infected at later times (Figs. 4G and H, and 5B). Interestingly, while the cells grown in control medium failed to deposit any detectable calcium throughout the culture period, HCMV infection alone was able to induce definite levels of mineralization in MSCs without osteogenic induction. Low calcium accumulation in response to virus infection was also observed in HFFs. Osteogenic differentiating cells infected at day 0 of differentiation yielded 106 PFU/ml at day 14 post infection, whereas virus titer was substantially reduced (104 PFU/ml; day 14 post infection) in differentiating MSCs infected at day 21 of differentiation. We also observed slight reduction (10 fold) in virus titer in HFFs under conditions of ostegenic induction compared to regular culture conditions (data not shown).
Discussion
HCMV is a widespread herpes virus able to establish life-long latency in its host. Although HCMV infection is generally asymptomatic in healthy individuals, HCMV is associated with the development of several pathologies, including atherosclerosis, and HCMV recurrence from latency is a major risk factor in immunocompromised individuals such as bone marrow transplantation patients (Boeckh et al., 2003). The bone marrow (BM), and specifically BM hematopoietic myeloid progenitor cells, were demonstrated to harbor the latent virus (von Laer et al., 1995). Studies demonstrated that HCMV reactivation is a rather frequent event not only in immunosuppressed patients but also in healthy donors (Prosch et al., 1999). Importantly, it was demonstrated that amount of HCMV-reactive CD8+ T cells increases with age and can constitute up to one-quarter of the total CD8+ T cell population in elderly individuals (Khan et al., 2002), suggesting that the immune system is increasingly aware of the viral presence and constantly trying to eliminate the virus from the host. Frequent viral reactivation from BM myeloid progenitor cells should cause infection of other BM cells including stromal cells (Streblow and Nelson, 2003). Depending on cell type, HCMV infection can lead to productive or abortive infection, or latency. Therefore, to better elucidate HCMV pathogenesis it is important to study HCMV infection of specific homogenous cell populations.
Function of the bone marrow is complex and includes maintenance of hematopoietic and stromal progenitors. Recently identified BM-derived MSCs support proper hematopoiesis through cell-to-cell contact and by providing cytokines and other factors which affect hematopoietic progenitors (Gronthos et al., 2003; Majumdar et al., 2003; Verfaillie, 1998; Kronenwett et al., 2000). In addition, during injury and as a part of constant renewal of various tissues such as bone and cartilage, BM-MSCs are likely to serve as a major source for regeneration and renewal of the cells of mesenchymal lineage. Considering the importance of MSCs, we investigated whether HCMV can infect BM-derived purified MSCs and the mode and the impact of the virus infection on self renewal and differentiation potential of the MSCs as well as on their function in supporting hematopoiesis.
We first demonstrated that HCMV was able to establish productive infection in a homogenous population of BM-derived MSC cells (Figs. 1 and 1supplement). Both attenuated laboratory AD169 HCMV strain, which infects only HFFs, and clinical TB40 HCMV strain replicated equally well in MSC and HFF cells with similar growth kinetics at MOI of 0.1 PFU/cell (Fig. 1). However, at high MOI of 5 PFU/cell, the titer of AD169 HCMV strain on MSCs at days 6 and 8 post infection was consistently 10-fold less than that on HFFs. In contrast, the yield of clinical TB40 HCMV strain appeared to be similar on MSC and HFF cells regardless of MOI (Fig. 1). We also observed that at high MOI, cytopathic effects (CPE) induced by AD169 HCMV strain in MSCs were apparent within 4 to 6 days, whereas CPE produced by TB40 HCMV in MSCs appeared at day 8 post infection. HCMV developed several mechanisms to manipulate cell cycle progression and apoptosis of the infected cells (Mocarski, Jr., 2002). It was demonstrated that some of these HCMV-encoded anti-apoptotic proteins are mutated in laboratory strains and are dispensable for HCMV replication in HFFs in vitro (Skaletskaya et al., 2001). Therefore, our data suggest that MSCs are more sensitive to HCMV-induced cell killing, and that some viral genes, which are likely to be present in clinical and not in laboratory HCMV strains, may be required to prolong the viability of HCMV-infected cells allowing more efficient viral replication.
HCMV modulates many aspects of immune response through a variety of mechanisms including expression of several viral immunomodulatory proteins such as cmvIL-10, and dysregulation of expression of cellular cytokines and surface molecules in infected cells (Kotenko et al., 2000; Mocarski, Jr., 2002). It was shown that the expression level of adhesion molecules in a variety of cell types is increased upon HCMV infection and affect the adherence and activation of leukocytes to infected cells (Knight et al., 1999; Michelson et al., 2001; Rahbar and Soderberg-Naucler, 2005). In contrast, cell surface markers that are involved in immune surveillance are down-regulated by HCMV (Ahn et al., 1996; Leis et al., 2004). Because BM-MSCs express numerous cell surface molecules which can affect cell-to-cell interactions and modulate hematopoiesis, we next analyzed the effect of HCMV infection on the expression pattern of several cell adhesion molecules from integrin and immunoglobulin families, that are important for interaction of MSCs with cells of hematopoietic lineage (Majumdar et al., 2003). We observed that the expression level of ICAM-1 (CD54) and LFA-3 (CD58) were up-regulated in MSCs upon HCMV infection (Fig. 2). To test whether modulation of adhesion molecule expression has functional implications, we then tested the interaction between hematopoietic cell lines and HCMV-infected MSCs. We observed increased adherence of all hematopoietic cell lines we investigated (Fig. 3A). ICAM-1 strongly contributed to the increase in adherence of hematopoietic cells to HCMV-infected MSCs (Fig. 3B) and up-regulation of ICAM-1 expression required direct virus infection (Fig. 3C). Interestingly, while the cells of lymphoid origin (lymphoblastic Jurkat and Molt-4 T cells, and Raji B cells) showed higher binding affinity to uninfected MSCs then cells of the myeloid lineage, when MSCs were infected with HCMV, cells of myeloid origin (U937 and K562) as well as the CD34+ progenitor cell line KG1a exhibited the greatest increases in adherence (Fig. 3A). These HCMV-mediated changes in interaction between MSCs and hematopoietic cells may have various biological consequences such as dysregulation of hematopoiesis and myelosuppression which is known to occur during HCMV infections (Randolph-Habecker et al., 2002). Increased cell adherence can also promote the spread of HCMV infection between various cells of BM.
In contrast to ICAM-1 and LFA-3, molecules involved in immune recognition of infected cells such as MHC class I and II antigens were strongly down-regulated upon HCMV infection (Fig. 2) confirming the ability of HCMV to circumvent immune surveillance. Other CD markers such as CD29, CD44, CD73 and CD105 were also down-regulated in HCMV-infected MSCs demonstrating that HCMV exerts a profound effect on the expression of many cellular proteins. Importance of the modulation of the expression level of many cell surface molecules in MSCs and other cells for HCMV pathogenesis remains to be further investigated.
Because MSCs have the capacity to differentiate along mesenchymal cell lineages and, therefore, contribute to the regeneration of bone, cartilage and other mesenchymal tissues, we also investigated the effect of HCMV infection on MSC differentiation into adipocytes and osteocytes. For the first time we demonstrated that cells induced into either adipogenesis or osteogenesis are permissive to HCMV infection at every step of differentiation progression from undifferentiated precursors to differentiated adipocytes or osteoblasts (Fig. 4). Although even highly differentiated cultures were permissive to HCMV infection, the virus yield (day 14 post infection) generated from differentiating cultures infected at day 21 of differentiation was reduced in comparison to the viral yield harvested from cultures infected at day 0 of differentiation, suggesting that differentiated cells were less supportive for productive viral replication. We also observed that HCMV infection of MSCs undergoing adipogenic or osteogenic differentiation impaired the process of differentiation. During adipogenesis HCMV infection was accompanied by the gradual release of fat vacuoles from the infected cells into culture medium, and by the partial change of cellular morphology form adiposite-like to MSC-like appearance (Fig. 4). During osteogenesis HCMV infection reduced calcium deposition in cells in a time-dependent manner: cell cultures infected at an early stage of osteogenic differentiation demonstrated a lower rate of mineralization than those infected at later times (Figs. 4 and 5).
In conclusion, our experiments demonstrate that BM-derived MSCs are permissive to productive HCMV infection, and that HCMV can significantly alter the function of MSCs. The following scenario can be proposed. After reactivation of HCMV from its latency in hematopoietic progenitor cells in BM, MSCs, residing in close vicinity, may represent the first easily accessible target for successful replication and dissemination of the virus. Therefore, frequently occurring HCMV reactivations may result in the depletion of uncommitted MSCs from BM and/or may lead to the substantial loss of MSC potential for renewal and ability to differentiate into specialized cells and tissues. This may have severe consequences. For example, HCMV-caused depletion and inability of MSCs to support osteoblast regeneration may contribute to the development of osteoporosis in elderly people. Similarly, HCMV-mediated deterioration of MSCs may result in impaired regeneration of cartilage tissues and, therefore, contribute to the pathogenesis of arthritis. The infection could also alter cytokine milieu in BM. These changes may affect hematopoiesis and change the properties and functions of immune progenitor cells. Therefore, frequent virus reactivations in BM cells and subsequent productive HCMV infection of MSCs may be an underlying cause of various pathological conditions.
Methods
Cells
MSCs at passage two were purchased from Cambrex Bio Science (Walkersville, MD) or established from normal iliac crest bone marrow aspirates obtained from two healthy volunteers as described (Potian et al., 2003). Based on the position outlined by the International Society for Cellular Therapy on the nomenclature of MSCs, we have designated the cells as MSCs (Horwitz et al., 2005). At passage 5, MSCs were verified by morphology, phenotype, and differentiation (Conget and Minguell, 1999). The cells were adherent to plastic, morphologically symmetrical compared with the asymmetry of fibroblasts, replicated by asymmetry, expressive of telomerase, and able to differentiate into several cell lineages. Based on these indicators, the MSCs used in this study were more than 99% pure. MSCs were cultured in MSC Growth Medium (MGM) consisting of a mixture (1:1) of Dulbecco's modified Eagle's medium DMEM (low glucose)/Ham’s F-12 plus 10% selected FCS (HyClone, Logan, UT). Pooled population of human foreskin fibroblasts (HFFs) obtained from several donors were propagated in DMEM supplemented with 10% FBS. In this study, MSCs and HFFs were used at passage 4 to 8 and 10 to 15, respectively. All transformed suspension cell lines except KG1a were grown in RPMI medium with 10% FBS. KG1a cells were grown in Iscove’ DMEM with 20% FBS.
HCMV strains and virus preparation
Two strains of HCMV were used in this study: laboratory strain AD169 (ATCC) and clinical strain TB40E (TB40) (C. Sinzger, Tübingen, Germany), which was preferably propagated on endothelial cells (ECs) and prepared from single round infected HFF cells. Restriction digestion of HCMV TB40E and AD169 BACs was performed and demonstrated that the pattern of DNA fragments of TB40E BAC is different than those of AD169 BAC (Fig. 1C sup.).
HCMV BAC-GFP viruses were engineered by introducing BAC vector (Marchini et al., 2001) into the viral genome of the wild-type AD169 and TB40 strains. In these constructs the US1-11 region was replaced with a pUSF-3 marker cassette containing GFP under control of an SV40 promoter and a chloramphenicol resistance gene as described (Marchini et al., 2001). The BAC viruses were reconstituted by electroporation of BAC DNA into fibroblasts, together with a plasmid expressing the viral pp71 protein to enhance the infectivity of viral DNA (Baldick, Jr. et al., 1997).
Infected cells and supernatants were harvested, thawed and frozen twice, and cleared of cell debris, resulting in HCMV-containing media. HCMV-free supernatants were generated by ultracentrifugation of HCMV-containing media at 55K x g for 1hr followed by the exposure of virus-free supernatants to 254 nm UV light (40 W) for 1 hr. The absence of infectious virions was confirmed by plaque assay on HFFs. Virions were purified by density gradient centrifugation through a D-sorbitol cushion at 55K x g for 1hr as described (Stinski, 1976). Virions were resuspended in DMEM with 2% FBS, and virus stocks were stored at −80°C. Virus titers were determined in duplicate by plaque assay on HFFs. For inactivation, virus stocks were exposed to 254 nm UV light (40 W) for 1 hr prior to infection.
Growth curves
2×105 MSC or HFF cells/well in a 12-well plate were infected with virus at a high multiplicity of infection (MOI) of 5 PFU/cell for single-step growth curves, or at a low MOI of 0.1 PFU/cell for multistep growth curves. At the times indicated in Fig. 1, infected cells and supernatants were harvested and stored at −80°C until the end of the experiment. The samples were thawed and frozen, cleared of cell debris, and the virus titer was determined by plaque assay on HFFs.
FACS analysis
Analyses of cell surface molecules on MSC and HFF cells were performed using flow cytometry. Cells recovered from flasks by trypsin-EDTA treatment were washed in flow cytometry buffer consisting of 2% BSA and 0.1% sodium azide in PBS. Aliquots (1×105 cells) were incubated with the following conjugated monoclonal antibodies: CD29-PE, CD44-PE, CD73-PE, CD90-PE, CD105-FITC, HLA-ABC-PE, HLA-DR-PE (PharMingen, San Diego, CA); or with CD54 (ICAM-1)-purified, CD58 (LFA-3)-purified, and CD106 (VCAM-1)-purified (R&D Systems, Minneapolis, MN), followed by incubation with secondary goat anti-mouse PE-conjugated antibodies where necessary. After incubation, cells were washed and resuspended in flow cytometry buffer containing 1% paraformaldehyde. Nonspecific fluorescence was determined by incubation of similar cell aliquots with isotype-matched mouse monoclonal antibodies (PharMingen), or with secondary antibody alone. Data were analyzed by collecting 10,000 events on a Becton- Dickinson Vantage instrument. The resultant values were normalized to the values obtained for the baseline (isotype control). Data were expressed as relative fluorescence intensity (RFI) as a percentage of this baseline value.
Cell Adhesion Assay
MSCs were seeded at 10,000 cells/well in 96-well plates. When cells reached confluence, they were infected at MOI of 3–5 PFU or left uninfected for 72 hr. For experiments with blocking antibody, MSCs were treated with 1 μg/well mouse anti-human ICAM-1 (CD54) monoclonal antibody (R&D Systems, Minneapolis, MN) for 1hr at 37°C. The number of uninfected and infected cells was counted on the day of experiment. Transformed suspension cell lines were labeled with the PKH26 Red Fluorescent Cell linker kit (Sigma Diagnostics, St. Louis, MO) according to the manufacturer’s instructions. Cell viability and labeling were both 100%, as determined by trypan blue exclusion and fluorescence microscopy, respectively. Wells of MSCs were washed with PBS three times and 100,000 labeled target cells in 100μl PBS were added into each well so that a ratio of 5–10 suspension/monolayer cells was achieved. For each target cell line, the adhesion assay was performed in quadruplicate format. After 1.5 hr incubation at 37°C, the wells were carefully washed 3 times with PBS. Fluorescence was read using Microplate Fluorescence Reader FL500 (Bio-Tek Instruments, Winooski, VT) at excitation of 535 nm and emission of 580 nm. To correlate the retained fluorescence with the number of bound cells in each well, a series of dilutions for each labeled target cell line was prepared and average fluorescence of quadruplicate wells was determined. Wells with only MSCs were used as background values. Results were expressed as the percentage of adherent cells.
Adipogenic and osteogenic differentiation of MSCs
To induce osteogenic or adipogenic differentiation of MSCs, the protocols described by the manufacturer (Cambrex) were used. MSCs at passage 4 to 8 were seeded at 4×104 cells per well in a 24-well plate and cultured in MGM to confluence.
For induction of adipogenesis MSCs were stimulated with three cycles of induction/maintenance. Each cycle consisted of feeding MSCs with adipogenic differentiation medium supplemented with h-Insulin, dexamethasone, indomethacin, IBMX (3-isobuty-l-methyl-xanthine) for 3–4 days followed by 1–3 days of culturing in adipogenic maintenance medium (Cambrex). After 3 complete cycles of induction/maintenance, the cultures were maintained for 7 more days in adipogenic maintenance medium with medium replacement every 2–3 days. Adipogenic differentiation was visualized by staining with fresh Oil-red-O solution (Sigma). For control, MSCs were cultured in MGM followed by adipogenic maintenance medium on the same schedule. For comparison purposes the experiment in the same format was performed with HFF cells.
For induction of osteogenesis the growth medium was replaced with osteogenic differentiation medium (Cambrex) supplemented with 10 mM β-glycerophosphate, 100 nM dexamethasone, and 50 μg/ml ascorbic acid-2-phosphate. The induced cultures were maintained in osteogenic differentiation medium for 3 weeks and fed twice a week by completely replacing the medium with fresh differentiation medium. Untreated cultures of MSCs were maintained in MGM on the same schedule during this period of time. For comparison purposes, the experiment in the same format was performed with HFFs.
Virus infection in differentiating cells
At various times (days 0, 7, 14, 21) of differentiation process, subsets of the differentiating cells (3 wells of the cells per infection time) were infected with TB40 BAC-GFP HCMV at MOI of 3–5 PFU/cell in MGM. After 2 hr of incubating with virus, the cells were washed once with MGM and the differentiation medium was replaced. The cells were maintained in differentiation medium on the same schedule as described above. The total virus titer in differentiating cell cultures was determined at day 14 post infection by plaque assay on HFFs. To check if the differentiation medium may have an effect on virus replication and for comparison purposes, the experiment in the same format was performed with HFFs.
Calcium assay
Cell layers were rinsed twice with PBS and scraped off the dish in 0.5 N HCl. The calcium was extracted from the cell layers by shaking for 4 h at 4°C, and then centrifuging at 1,000g for 5 min. The resulting supernatant was used for quantitative calcium determination by calcium dye complex method according to the manufacturer’s instructions (Diagnostic Chemicals Limited, Charlottetown, PE, Canada). Absorbency of samples was determined at 575 nm. Total calcium was calculated from standard solutions prepared in parallel, and expressed as μg per dish. Assays were done in triplicate at each time point.
Supplementary Material
Acknowledgments
We thank Raymond Birge and Sukhwindi Singh for advice with microscopy and cell adhesion assay; Kelly E. Corcoran for assistance in experiments with MSCs. This study was supported in part by United States Public Health Services Grants RO1 AI051139 and AI057468 from the National Institute of Allergy and Infectious Diseases and by American Heart Association Grant AHA #0245131N to S.V.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Ahn K, Angulo A, Ghazal P, Peterson PA, Yang Y, Fruh K. Human cytomegalovirus inhibits antigen presentation by a sequential multistep process. Proc Natl Acad Sci USA. 1996;93 (20):10990–10995. doi: 10.1073/pnas.93.20.10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apperley JF, Dowding C, Hibbin J, Buiter J, Matutes E, Sissons PJ, Gordon M, Goldman JM. The effect of cytomegalovirus on hemopoiesis: in vitro evidence for selective infection of marrow stromal cells. Exp Hematol. 1989;17 (1):38–45. [PubMed] [Google Scholar]
- Baldick CJ, Jr, Marchini A, Patterson CE, Shenk T. Human cytomegalovirus tegument protein pp71 (ppUL82) enhances the infectivity of viral DNA and accelerates the infectious cycle. J Virol. 1997;71 (6):4400–4408. doi: 10.1128/jvi.71.6.4400-4408.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckh M, Nichols WG, Papanicolaou G, Rubin R, Wingard JR, Zaia J. Cytomegalovirus in hematopoietic stem cell transplant recipients: Current status, known challenges, and future strategies. Biol Blood Marrow Transplant. 2003;9 (9):543–558. doi: 10.1016/s1083-8791(03)00287-8. [DOI] [PubMed] [Google Scholar]
- Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64 (2):278–294. doi: 10.1002/(sici)1097-4644(199702)64:2<278::aid-jcb11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Cheng L, Qasba P, Vanguri P, Thiede MA. Human mesenchymal stem cells support megakaryocyte and pro-platelet formation from CD34(+) hematopoietic progenitor cells. J Cell Physiol. 2000;184 (1):58–69. doi: 10.1002/(SICI)1097-4652(200007)184:1<58::AID-JCP6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Conget PA, Minguell JJ. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J Cell Physiol. 1999;181:67–73. doi: 10.1002/(SICI)1097-4652(199910)181:1<67::AID-JCP7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Goodrum F, Jordan CT, Terhune SS, High K, Shenk T. Differential outcomes of human cytomegalovirus infection in primitive hematopoietic cell subpopulations. Blood. 2004;104 (3):687–695. doi: 10.1182/blood-2003-12-4344. [DOI] [PubMed] [Google Scholar]
- Goodrum FD, Jordan CT, High K, Shenk T. Human cytomegalovirus gene expression during infection of primary hematopoietic progenitor cells: a model for latency. Proc Natl Acad Sci USA. 2002;99 (25):16255–16260. doi: 10.1073/pnas.252630899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronthos S, Zannettino AC, Hay SJ, Shi S, Graves SE, Kortesidis A, Simmons PJ. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003;116 (Pt9):1827–1835. doi: 10.1242/jcs.00369. [DOI] [PubMed] [Google Scholar]
- Hahn G, Jores R, Mocarski ES. Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells. Proc Natl Acad Sci USA. 1998;95 (7):3937–3942. doi: 10.1073/pnas.95.7.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P. Human myeloid cell lines. In: Herzenberg LaBC., editor. The intergrated immune system. 5. IV. Blackwell Science; Cambridge, MA: 1996. pp. 173.1–173.16. [Google Scholar]
- Horwitz EM, Le BK, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, Keating A. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- Ishii M, Koike C, Igarashi A, Yamanaka K, Pan H, Higashi Y, Kawaguchi H, Sugiyama M, Kamata N, Iwata T, Matsubara T, Nakamura K, Kurihara H, Tsuji K, Kato Y. Molecular markers distinguish bone marrow mesenchymal stem cells from fibroblasts. Biochem Biophys Res Commun. 2005;332 (1):297–303. doi: 10.1016/j.bbrc.2005.04.118. [DOI] [PubMed] [Google Scholar]
- Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64 (2):295–312. [PubMed] [Google Scholar]
- Khan N, Shariff N, Cobbold M, Bruton R, Ainsworth JA, Sinclair AJ, Nayak L, Moss PA. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol. 2002;169 (4):1984–1992. doi: 10.4049/jimmunol.169.4.1984. [DOI] [PubMed] [Google Scholar]
- Knight DA, Waldman WJ, Sedmak DD. Cytomegalovirus-mediated modulation of adhesion molecule expression by human arterial and microvascular endothelial cells. Transplantation. 1999;68 (11):1814–1818. doi: 10.1097/00007890-199912150-00030. [DOI] [PubMed] [Google Scholar]
- Kotenko SV, Saccani S, Izotova LS, Mirochnitchenko OV, Pestka S. Human cytomegalovirus harbors its own unique IL-10 homolog (cmvIL-10) Proc Natl Acad Sci USA. 2000;97 (4):1695–1700. doi: 10.1073/pnas.97.4.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenwett R, Martin S, Haas R. The role of cytokines and adhesion molecules for mobilization of peripheral blood stem cells. Stem Cells. 2000;18:320–330. doi: 10.1634/stemcells.18-5-320. [DOI] [PubMed] [Google Scholar]
- Leis M, Marschall M, Stamminger T. Downregulation of the cellular adhesion molecule Thy-1 (CD90) by cytomegalovirus infection of human fibroblasts. J Gen Virol. 2004;85 (Pt7):1995–2000. doi: 10.1099/vir.0.79818-0. [DOI] [PubMed] [Google Scholar]
- Majumdar MK, Keane-Moore M, Buyaner D, Hardy WB, Moorman MA, McIntosh KR, Mosca JD. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci. 2003;10 (2):228–241. doi: 10.1007/BF02256058. [DOI] [PubMed] [Google Scholar]
- Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol. 1998;176 (1):57–66. doi: 10.1002/(SICI)1097-4652(199807)176:1<57::AID-JCP7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Marchini A, Liu H, Zhu H. Human cytomegalovirus with IE-2 (UL122) deleted fails to express early lytic genes. J Virol. 2001;75 (4):1870–1878. doi: 10.1128/JVI.75.4.1870-1878.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson S, Rohrlich P, Beisser P, Laurent L, Perret E, Prevost MC, Monchatre E, Duval M, Marolleau JP, Charbord P. Human cytomegalovirus infection of bone marrow myofibroblasts enhances myeloid progenitor adhesion and elicits viral transmission. Microbes Infect. 2001;3 (12):1005–1013. doi: 10.1016/s1286-4579(01)01464-2. [DOI] [PubMed] [Google Scholar]
- Kondo K, Xu J, Mocarski ES. Human cytomegalovirus latent gene expression in granulocyte-macrophage progenitors in culture and in seropositive individuals. Proc Natl Acad Sci USA. 1996;93:11137–11142. doi: 10.1073/pnas.93.20.11137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton EJ, Tysoe C, Sinclair JH, Sissons JG. Human cytomegalovirus infection of the monocyte/macrophage lineage in bone marrow. J Virol. 1994;68 (6):4017–4021. doi: 10.1128/jvi.68.6.4017-4021.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocarski ES., Jr Immunomodulation by cytomegaloviruses: manipulative strategies beyond evasion. Trends Microbiol. 2002;10 (7):332–339. doi: 10.1016/s0966-842x(02)02393-4. [DOI] [PubMed] [Google Scholar]
- Mocarski ES. Cytomegaloviruses and their replication. In: Knipe DM, editor. Virology. Vol. 4. Lippincott-Raven; Philadelphia, PA: 2001. pp. 2629–2673. [Google Scholar]
- Oswald J, Boxberger S, Jorgensen B, Feldmann S, Ehninger G, Bornhauser M, Werner C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22 (3):377–384. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284 (5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Plachter B, Sinzger C, Jahn G. Cell types involved in replication and distribution of human cytomegalovirus. Adv Virus Res. 1996;46:195–261. doi: 10.1016/s0065-3527(08)60073-1. [DOI] [PubMed] [Google Scholar]
- Potian JA, Aviv H, Ponzio NM, Harrison JS, Rameshwar P. Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol. 2003;171 (7):3426–3434. doi: 10.4049/jimmunol.171.7.3426. [DOI] [PubMed] [Google Scholar]
- Prosch S, Docke WD, Reinke P, Volk HD, Kruger DH. Human cytomegalovirus reactivation in bone-marrow-derived granulocyte/monocyte progenitor cells and mature monocytes. Intervirology. 1999;42 (5–6):308–313. doi: 10.1159/000053965. [DOI] [PubMed] [Google Scholar]
- Rahbar A, Soderberg-Naucler C. Human cytomegalovirus infection of endothelial cells triggers platelet adhesion and aggregation. J Virol. 2005;79 (4):2211–2220. doi: 10.1128/JVI.79.4.2211-2220.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph-Habecker J, Iwata M, Torok-Storb B. Cytomegalovirus mediated myelosuppression. J Clin Virol. 2002;25(Suppl 2):S51–S56. doi: 10.1016/s1386-6532(02)00092-6. [DOI] [PubMed] [Google Scholar]
- Reeves MB, MacAry PA, Lehner PJ, Sissons JG, Sinclair JH. Latency, chromatin remodeling, and reactivation of human cytomegalovirus in the dendritic cells of healthy carriers. Proc Natl Acad Sci USA. 2005;102:4140–4145. doi: 10.1073/pnas.0408994102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes M, Lund T, Lenvik T, Aguiar D, Koodie L, Verfaillie CM. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood. 2001;98 (9):2615–2625. doi: 10.1182/blood.v98.9.2615. [DOI] [PubMed] [Google Scholar]
- Simmons P, Kaushansky K, Torok-Storb B. Mechanisms of cytomegalovirus-mediated myelosuppression: perturbation of stromal cell function versus direct infection of myeloid cells. Proc Natl Acad Sci USA. 1990;87 (4):1386–1390. doi: 10.1073/pnas.87.4.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaletskaya A, Bartle LM, Chittenden T, McCormick AL, Mocarski ES, Goldmacher VS. A cytomegalovirus-encoded inhibitor of apoptosis that suppresses caspase-8 activation. Proc Natl Acad Sci USA. 2001;98 (14):7829–7834. doi: 10.1073/pnas.141108798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski MF. Human cytomegalovirus: glycoproteins associated with virions and dense bodies. J Virol. 1976;19 (2):594–609. doi: 10.1128/jvi.19.2.594-609.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streblow DN, Nelson JA. Models of HCMV latency and reactivation. Trends Microbiol. 2003;11 (7):293–295. doi: 10.1016/s0966-842x(03)00149-5. [DOI] [PubMed] [Google Scholar]
- Taichman RS, Nassiri MR, Reilly MJ, Ptak RG, Emerson SG, Drach JC. Infection and replication of human cytomegalovirus in bone marrow stromal cells: effects on the production of IL-6, MIP-1alpha, and TGF-beta1. Bone Marrow Transplant. 1997;19 (5):471–480. doi: 10.1038/sj.bmt.1700685. [DOI] [PubMed] [Google Scholar]
- Taylor-Wiedeman J, Sissons JG, Borysiewicz LK, Sinclair JH. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol. 1991;72 (Pt9):2059–2064. doi: 10.1099/0022-1317-72-9-2059. [DOI] [PubMed] [Google Scholar]
- Verfaillie CM. Adhesion receptors as regulators of the hematopoietic process. Blood. 1998;92:2609–2612. [PubMed] [Google Scholar]
- von Laer D, Meyer-Koenig U, Serr A, Finke J, Kanz L, Fauser AA, Neumann-Haefelin D, Brugger W, Hufert FT. Detection of cytomegalovirus DNA in CD34+ cells from blood and bone marrow. Blood. 1995;86 (11):4086–4090. [PubMed] [Google Scholar]
- Zhuravskaya T, Maciejewski JP, Netski DM, Bruening E, Mackintosh FR, St Jeor S. Spread of human cytomegalovirus (HCMV) after infection of human hematopoietic progenitor cells: model of HCMV latency. Blood. 1997;90 (6):2482–2491. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


