Abstract
Accommodation has long been suspected to be involved in the development of myopia because near work, particularly reading, is known to be a risk factor. In this study, we measured several dynamic characteristics of accommodative behavior during extended periods of reading under close-to-natural conditions in 20 young emmetropic and stable myopic subjects. Accommodative responses, errors, and variability (including power spectrum analysis) were analyzed and related to accommodative demand and subject refractive error. All accommodative behaviors showed large inter-subject variability at all of the reading demands. Accommodative lags and variability significantly increased with closer demands for all subjects (ANOVA, p < 0.05). Myopes had significantly greater variability in their accommodation responses compared to emmetropes (ANOVA, p < 0.05) and had larger accommodative lags at further reading distances (unpaired t test p < 0.05). Power spectrum analysis showed a significant increase in the power of accommodative microfluctuations with closer demands (ANOVA, p < 0.05) and with increasing myopia at the closest reading demand (ANOVA, p < 0.01). The difference in the stability of the accommodative behavior between individuals with different refractive states suggests a possible relationship between variability in accommodation and the development of myopia.
Keywords: Accommodation, Myopia, Reading, Accommodative microfluctuations
1. Introduction
A correlation between myopia and education (e.g., Al-Bdour, Odat, & Tahat, 2001; Au Eong, Tay, & Lim, 1993; Hepsen, Evereklioglu, & Bayramlar, 2001; Richler & Bear, 1980; Young et al., 1969) is well known and has led to speculation that the amount of reading a child does may be a risk factor for the development of myopia. Additional research has suggested that accommodation is the link between near work and myopia (reviewed by Rosenfield, 1998; Rosenfield & Gilmartin, 1998b) but there is little direct evidence for or against this assertion. Several aspects of accommodation have been suggested as possible risk factors; decreased accommodative tonus (McBrien & Millodot, 1987; Rosenfield & Gilmartin, 1987), decreased accommodative amplitude (McBrien & Millodot, 1986), increased accommodative lags (Abbott, Schmid, & Strang, 1998; Gwiazda, Thorn, Bauer, & Held, 1993; Gwiazda, Thorn, & Held, 2005; Nakatsuka, Hasebe, Nonaka, & Ohtsuki, 2005), increased accommodative adaptation (Chen, Schmid, Brown, & Edwards, 2005; Culhane & Winn, 1999; Gilmartin & Bullimore, 1991; Gwiazda, Bauer, Thorn, & Held, 1995b; McBrien & Millodot, 1988; Strang, Winn, & Gilmartin, 1994), and increased accommodative fluctuations (Day, Strang, Seidel, Gray, & Mallen, 2006; Seidel, Gray, & Heron, 2003) have all been shown in myopes, although it is unclear how such risk factors might induce myopia.
Hyperopic retinal defocus resulting from a large accommodative lag during periods of extended near work might result in compensatory axial elongation of the eye and myopia (Abbott et al., 1998; Gwiazda, Bauer, Thorn, & Held, 1995a; Gwiazda et al., 1993; Gwiazda et al., 2005; Nakatsuka et al., 2005). Animal studies have shown that imposing hyperopic defocus with negative lenses induces a compensatory increase in eye growth and myopia (Graham & Judge, 1999; Hung, Crawford, & Smith, 1995; Schaeffel, Glasser, & Howland, 1988; Whatham & Judge, 2001). These studies, together with compensatory axial hyperopia seen in response to positive lenses (Schaeffel et al., 1988; Smith, Hung, & Harwerth, 1994; Whatham & Judge, 2001), provide strong evidence that eye growth and refractive development are visually guided (see Wallman & Winawer, 2004 for a recent review).
Variability in the accommodative response to near targets over sustained periods of time as in reading has not been described, but may be related to myopia in a number of ways: (1) fluctuations in retinal defocus associated with accommodative variability may be a risk factor for the development of myopia (Plainis, Ginis, & Pallikaris, 2005). Even if the mean accommodative responses show little lag, increased accommodative variability that increases hyperopic retinal defocus during near viewing could be integrated over time and lead to myopic shifts (Wallman & Winawer, 2004). (2) Accommodative variability may reflect the sensitivity of accommodation to hyperopic blur, and low blur sensitivity may be a risk factor for the development of myopia. Myopes have been reported to have reduced sensitivity to retinal blur (Jiang, 1997; Rosenfield & Abraham-Cohen, 1999), which would result in larger accommodative lags because a larger amount of hyperopic blur would be required to elicit an accommodative response and this could cause accommodation to oscillate during near viewing over a wider-than-normal range (Day et al., 2006; Seidel et al., 2003). (3) The elongation of the myopic eye may affect the shape of the anterior segment, which could theoretically increase variability in the accommodative response. Mutti, Jones, Moeschberger, and Zadnik (2000) hypothesized that increased mechanical tension on the ciliary body in the elongated eyes of myopes creates greater hyperopic lags (termed “pseudo-cycloplegia”), and accommodation has been suggested to affect eye shape as well (Walker & Mutti, 2002).
Studies of accommodation in humans are typically performed during brief near tasks under tightly controlled environments in order to understand the stimuli driving accommodation and the dynamics of the accommodative response (e.g., Alpern & David, 1958; Campbell & Westheimer, 1960; Fincham, 1951; Heath, 1956; Kruger, 1980; Kruger & Pola, 1989; Phillips & Stark, 1977). However, very little is known about accommodative behavior during sustained periods of reading in natural visual environments. In this study we concentrate our attention on the variability of the accommodative response. Understanding how individuals vary in their accommodative behavior and their ability to sustain focus on the plane of text while reading is important for understanding any relationship between accommodation, near work, and the development of myopia (Schaeffel, Weiss, & Seidel, 1999). The aim of this study is to identify and describe several aspects of accommodative behavior over sustained periods of reading in a close-to-natural setting and to determine whether differences in emmetropes and myopes exist. Preliminary reports of this study have been presented in abstract form (Harb, Thorn, & Troilo, 2003; Harb, Thorn, & Troilo, 2004).
2. Methods
2.1. Subjects
All research performed in this study adhered to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of The New England College of Optometry. All subjects who participated in this study also provided written informed consent. Accommodation during reading was examined in 20 subjects (optometry students aged 22-28 years) with normal binocular vision, stable spherical equivalent refractive errors ranging from +0.50 to -7.0 D (11 myopes and 9 emmetropes), and less than -0.75 D of astigmatism. Emmetropia was defined as +0.50 to -0.50 D. Stability of refractive error was self-reported by the subjects and defined as a change of ±0.50 D over the last two years. During testing myopic subjects were fully corrected with their contact lens corrections.
2.2. Reading task
Subjects read an excerpt from a novel (black letters on a white background—Times New Roman font) displayed on a computer monitor at three different distances (66.6, 40, and 28.6 cm) corresponding to accommodative demands of 1.5, 2.5, and 3.5 D. The accommodative response to a visual display terminal does not differ from printed text (Sorkin, Reich, & Pizzimenti, 2003), therefore, a computer monitor was used to allow for greater control over stimulus presentation. The actual size of a text window placed on the visual display terminal was modified to allow for a conserved angular subtense of 9.5° horizontal × 4.5° vertical at all reading distances. Letter height was also maintained at an angular subtense of 0.36° for all distances. On average the luminance of the visual display terminal was 14.3 c/m2 during the reading task. Subjects were instructed to read silently for 10 min. The subjects were able to control their own reading pace by scrolling within the text window. Subjects were instructed to read as they would normally and were allowed to look away from the text at their discretion while maintaining their head in the chin-rest. The number of fixation breaks was recorded. Although the subjects were not tested for reading speed or comprehension of the reading material, appropriate saccadic eye movements consistent with reading were observed by the examiner (E.H.) throughout the task. Reading tasks at all three working distances were performed in a random order within a single day.
2.3. Measurement of accommodation and eye position
Refractive state, pupil diameter, and eye position were measured binocularly (only one eye used for accommodation analysis) at a rate of 25 Hz using an eccentric infrared photorefractor (PowerRefractor-MultiChannel Systems; Reutlingen, Germany). For continuous recording, the PowerRefractor measures refractive state in the vertical meridian. The PowerRefractor was positioned 1 M away from the subject, 5°-8° above the text window, and was directed between the subject’s eyes. The infrared light source has been established for safe use with adults and children (Choi et al., 2000).
The pupils of all subjects were dilated with two drops of 2.5% phenylephrine 45 min prior to measurements to prevent significant changes in pupil size. Although this is not considered a natural state for reading, subject’s pupils were dilated to reduce fluctuations in pupil size that could affect the performance of the optometer. The PowerRefractor has been shown to have no systematic change in refraction measures with varying pupil sizes (Choi et al., 2000) within a narrow range of relatively large pupil sizes (approximately 4-7 mm), but the accommodative demands in our set-up often resulted in pupil constriction outside of this reliable range. Phenylephrine has been reported to result in a small reduction in accommodative amplitude (11%) (Culhane, Winn, & Gilmartin, 1999; Gimpel, Doughty, & Lyle, 1994), but others found no interference with the accommodative response (Ciuffreda, 1998), including the dynamics of the response (Ostrin & Glasser, 2004). If present, the small decrease in the amplitude of accommodation associated with phenylephrine administration in young subjects with large amplitudes of accommodation (Hofstetter’s minimum amplitude=9.5 D for a 22-year-old and 8.0 D for a 28-year-old, (Hofstetter, Griffin, Berman, & Everson, 2000)) would not affect accommodative responses at normal reading distances. Although not specifically measured, it is plausible that the increased pupil sizes will increase the influence of aberrations on the accommodation measurements made by the PowerRefractor. Because of the arrangement of the LEDs, the PowerRefractor is less affected by asymmetric aberrations compared to photorefractors with a single source design (Roorda, Campbell, & Bobier, 1995). The effect of spherical aberrations, however, will theoretically be averaged into the readings and bias the measured refractions by small (<0.2 D) amounts. Although the effects of spherical aberrations on the operation of the PowerRefractor has not been empirically tested at this time, it has been shown in autorefractors that positive spherical aberration biases toward myopia and negative spherical aberration biases toward hyperopia (Hazel, Cox, & Strang, 2003; and reviewed by Charman, 2005).
2.4. Calibration of the PowerRefractor
The PowerRefractor was calibrated with a separate group of 21 subjects (21-28 years of age) with normal binocular vision and refractive errors ranging from +1.00 to -5.00 D (spherical equivalents). This range is within the limits of the PowerRefractor (±5 D) as described by Choi et al. (2000). Subjects were cyclopleged with two drops of 1.0% cyclopentolate 45 min prior to calibration measurements to control for pupil size and accommodation. Some subjects (n=12) were measured both while uncorrected and while wearing their contact lens corrections in order to increase the range of refractions measured.
After cycloplegia, retinoscopy and subjective refraction measures were performed by two separate experienced examiners (E.H. and F.T.) and were significantly correlated (R= 0.94, p < 0.01). In the same subjects, PowerRefractor measures were made in the vertical meridian while the subject looked at a high contrast 20/100 Snellen E presented at 7.5 M for 15-20 s. To directly compare the PowerRefractor measures to the retinoscopy measures, the refractive power of the vertical meridian was calculated from the overall sphero-cylindrical retinoscopy measures (J90 =Jsph sin2(θcyl-90°)). The PowerRefractor’s infrared light source illuminates the fundus and the brightness of the reflex (pixel intensity slope) across the vertical meridian of the pupil is converted into refractive state using a built-in calibration function (Seidemann, Schaeffel, Guirao, LopezGil, & Artal, 2002). We found that this function over-estimated the levels of myopia and hyperopia when compared to our cycloplegic retinoscopy (see Fig. 1). Using the cycloplegic retinoscopy measures as a standard, a new calibration function was determined by converting PowerRefractor measures back into pixel intensity slopes and then re-converted to refractions using our calibration function (refraction=-0.88 + 1.53 (pixel intensity slope)).
Fig. 1.
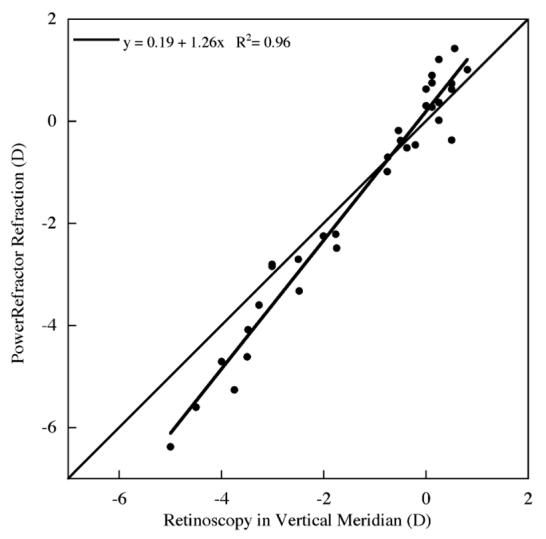
The PowerRefractor’s built-in calibration function over-estimated the amount of myopia and hyperopia compared to cycloplegic retinoscopy.
2.5. Filtering of the accommodative response
The accommodative response for each subject at each reading distance was measured continuously for 10 min. The response was filtered offline to remove measurement artifacts due to blinks and fixation breaks outside of the text window. To remove the artifacts associated with blinks without erroneously removing any true accommodation, we filtered out refractive changes that were too fast to be considered physiological (>10 D/s) (Adrian Glasser, personal communication; Ciuffreda & Kruger, 1988). These data were removed from the accommodative response by an iterative algorithm that located differences greater than 10 D/s between two data points and then removed the point that was furthest away from the mean accommodative response. Fig. 2 shows 60 s of accommodation for an individual before and after filtering.
Fig. 2.
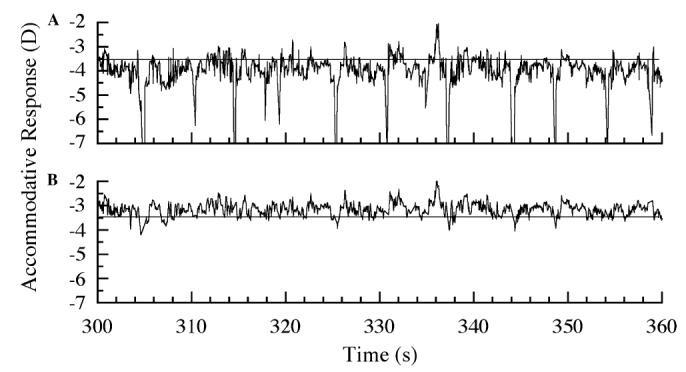
(A) Example of an accommodative response during 1 min of reading (horizontal line indicates the accommodative demand). The large myopic deflections are artifacts from blinks. (B) The same accommodative response after data corresponding to blinks and gaze breaks outside the text window were removed.
2.6. Analysis of the accommodative response
Several aspects of the accommodative response were identified and analyzed. The mean error in the accommodative response relative to the target demand (i.e., lead or lag) was determined for each subject in 10 one-minute periods for each reading distance. Individual stimulus-response functions were calculated using the three accommodative demands. The variability of accommodation response (determined from the standard deviation of the response) was determined for these periods and an analysis of the power spectrum of the accommodative response was also performed.
2.6.1. Power spectrum analysis
Fourier analysis of the accommodative data from each minute of reading was used to produce power spectra of each subject’s accommodative response during reading. Prior to the fast Fourier transform the DC component was removed by generating a running average in 0.12 s increments (low pass filter). A Gaussian function was then applied to the first and last 7 s of data to remove high amplitude edge effects. The 10 one-minute fast Fourier transforms were then averaged to generate a single power spectrum for each subject and the average power of low (LFC: 0.1-0.6 Hz), middle (MFC: 0.61-0.99 Hz), and high (HFC: 1.0-2.3 Hz) frequency components, which have been previously identified (Campbell, Robson, & Westheimer, 1959; Kotulak & Schor, 1986; Seidel et al., 2003; Winn, Pugh, Gilmartin, & Owens, 1990), was determined (see Fig. 3 for an example of an individual power spectrum).
Fig. 3.
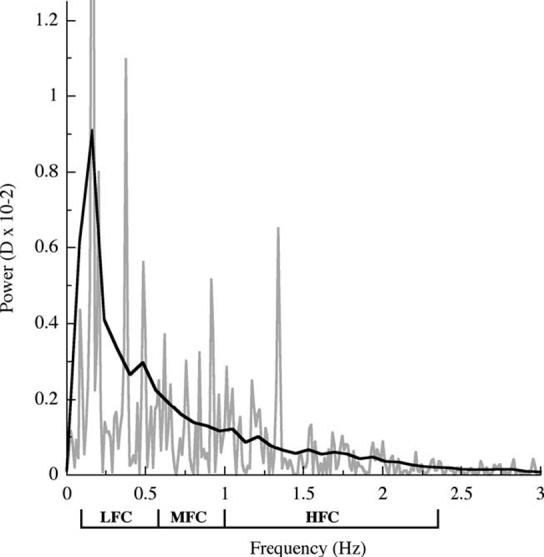
Example of a power spectrum derived from fast Fourier transforms for an individual (gray line) and a weighted mean curve fit was applied (black line). The mean power of three frequency components; LFC (0.1-0.60 Hz), MFC (0.61-0.99 Hz), and HFC (1.0-2.3 Hz) were calculated for each individual by the area under the curve for the corresponding frequency ranges and were normalized to the component frequency bandwidth.
2.7. Statistical analysis
Repeated measures ANOVAs were used to determine if the various accommodative measures changed over time or with different reading demands. Additionally, correlations were used to determine whether relationships existed between any of the accommodative measures and subject refractive state. Unpaired t tests were used to compare accommodative measures between the myopic and emmetropic groups. Statistical analysis was performed using Statview (Abacus Concepts, Inc., Berkeley, CA), and signal filtering, power spectrum analysis, and graphical analysis was performed using KaleidaGraph (Synergy Software, Reading, PA).
3. Results
3.1. Accommodative responses
The average accommodative error (the difference between the mean accommodative response and the target demand) observed at each of the reading distances was an accommodative lag. There was considerable inter-subject variability in the lag of accommodation at all reading distances (Fig. 4). The mean lag of accommodation for all subjects over the 10 min reading period significantly increased with closer reading distances (Fig. 4D, mean±SE, 0.69±0.08 D at 1.5 D; 0.88±0.10 D at 2.5 D; and 0.99±0.14 D at 3.5 D; repeated measures ANOVA p < 0.05). On average, the accommodative errors were greatest during the first 2-3 min of reading and then reduced (by approximately 0.2 D at the 2.5 and 3.5 D demands) and remained stable throughout the remainder of the reading period at all distances tested. One individual demonstrated a substantial lead of accommodation at the 1.5 and 2.5 D reading demand. This subject was later diagnosed with an accommodative excess and was not included in the group analysis because of failure to meet the subject inclusion criteria of normal binocular vision.
Fig. 4.
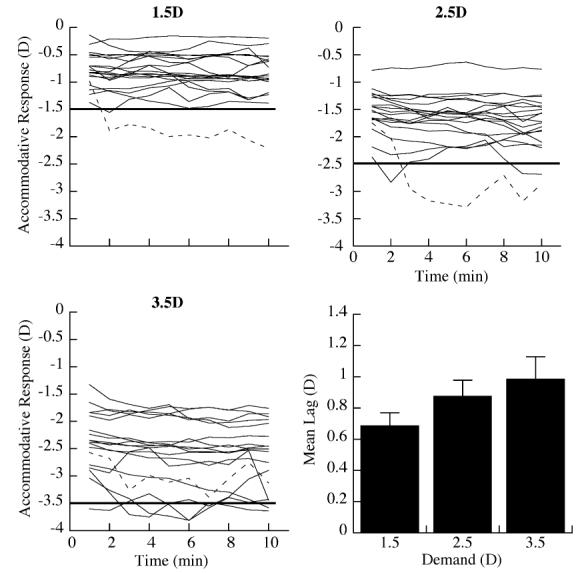
The average accommodative response during the reading period differed substantially between subjects at the three accommodative demands. Individual accommodative responses are shown as solid black lines. The dashed line indicates the response of a subject who demonstrated a lead of accommodation and was diagnosed with an accommodative excess. The straight horizontal line denotes the accommodative demand at each reading distance. The mean lag of accommodation (means±SE: 0.69±0.08 D at 1.5 D demand, 0.88±0.10 D at 2.5 D demand, and 0.99±0.14 D at 3.5 D demand, ANOVA p<0.01) for the group at each reading distance.
In general, the lag of accommodation was not significantly correlated with the subjects’ refractive state (R2=0.05, p=0.39 at 2.5 D; R2=0.02, p=0.59 at 3.5 D), although at the 1.5 D demand the lag of accommodation tended to be greater in myopes (R2=0.17, p=0.08) and the mean lag of all myopes was greater than for emmetropes (mean±SE myopes vs. emmetropes, 0.84±0.08 D vs. 0.56±0.09 D, unpaired t test p < 0.05).
There was substantial inter-subject variability in the slopes of the accommodative stimulus-response functions (determined from the slopes of linear regressions) (range: 0.41-1.36, mean ±SE, 0.85±0.06). There was no significant difference between accommodative stimulus-response slope between myopes and emmetropes (mean±SE myopes vs. emmetropes, 0.92±0.09 vs. 0.77±0.07; unpaired t test p=0.28).
3.2. Variability in accommodation
The variability of the filtered accommodative response for each subject, measured as the mean standard deviation of the response, differed substantially among the subjects and, on average, at each of the reading demands (Fig. 5). Examination of each minute shows that, on average, the mean variability did not change significantly over the 10 min reading period at any of the reading demands (ANOVA). The mean standard deviation of the accommodative response increased significantly with closer reading demands (Fig. 5D, mean±SE, 0.15±0.02 D at 1.5D; 0.20D±0.02 D at 2.5 D; 0.30±0.04 D at3.5 D; ANOVA p< 0.01). The variability of the accommodative response was proportional to the amplitude of the response (Fig. 6, R2=0.38, p<0.01).
Fig. 5.
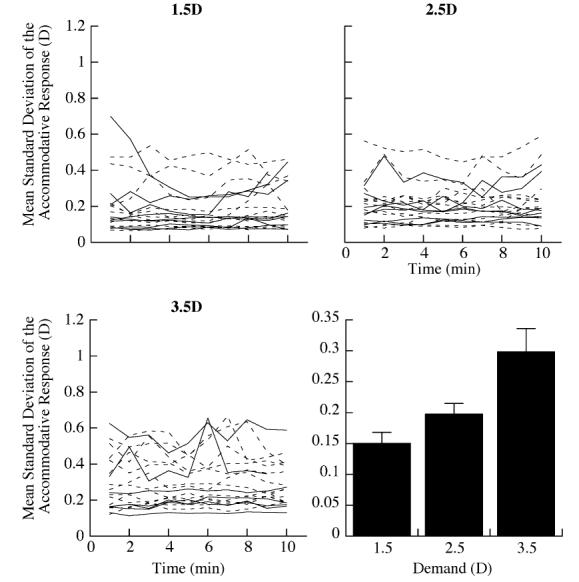
Variability of the accommodative response (measured as mean standard deviation (SD)) per minute of reading differed substantially at each of the accommodative demands. Individual data (dashed lines (myopes) and solid lines (emmetropes)). The mean variability for the entire group significantly increased at the closer reading distances ((mean±SE) 0.15±0.02 at 1.5 D demand, 0.20±0.02 at 2.5 D demand, 0.30±0.04 at 3.5 D demand).
Fig. 6.
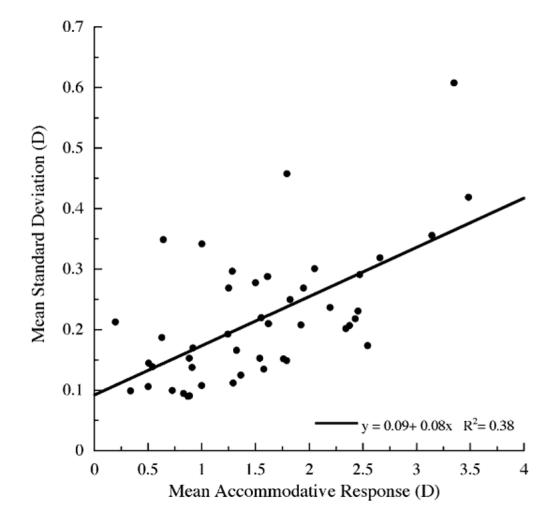
Linear regression demonstrates a proportional relationship (p < 0.01) between the mean accommodative responses and mean standard deviation of the response averaged over the entire reading period for all subjects and all three accommodative demands.
Variability in an individuals’ accommodative response was related to accommodative demand and to refractive state. Accommodative variability was proportional to the degree of myopia at each of the three reading demands (Fig. 7, R2=0.49, p<0.01 at 1.5 D; R2=0.31, p<0.05 at 2.5 D; and R2=0.34, p<0.05 at 3.5 D). Furthermore, myopes D showed greater accommodative variability than emmetropes especially at the closest reading demand (mean±SE myopes vs. emmetropes, 0.34±0.04 vs. 0.21±0.02; unpaired t test p <0.05).
Fig. 7.
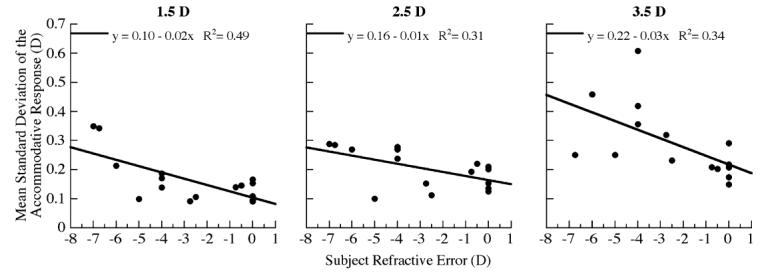
Linear regression analysis demonstrating the relationship between subject refractive error and mean standard deviation of the accommodative response at each of the reading demands (correlations p < 0.01 at 1.5 D, p < 0.05 at 2.5 D and 3.5 D).
3.2.1. Microfluctuations
The mean power (D×10-2) of the three frequency components of accommodative microfluctuations increased significantly with closer reading distances (Fig. 8, repeated measures ANOVA; LFC, p=0.01; MFC, p<0.05; HFC, p=0.05). The mean power of the microfluctuations for all frequency components significantly increased with increasing myopia at the 3.5 D demand (Fig. 9; LFC, R2=0.43, p<0.05; MFC, R2=0.48, p<0.05; HFC, R2=0.50, p<0.01), but not at the 1.5 and 2.5 D demands.
Fig. 8.
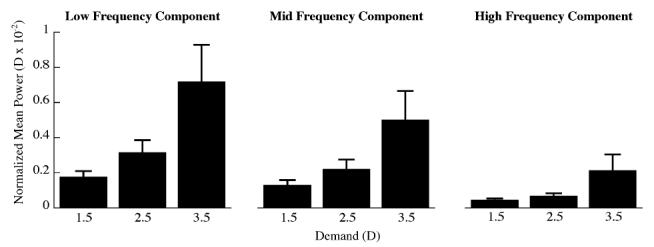
Mean (±SE) power of each of the frequency components calculated from the Fourier derived power spectra for each subject at each demand (repeated measures ANOVA, LFC p= 0.01; MFC p < 0.05; HFC p =0.05). The mean power of each frequency component has been normalized to the frequency bandwidth.
Fig. 9.
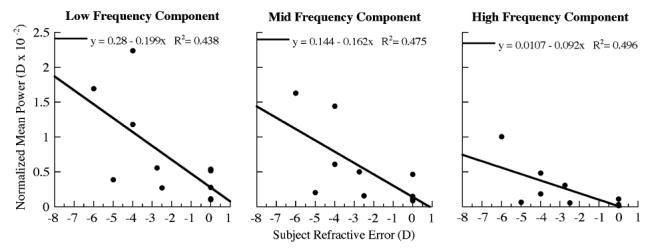
Linear regression showing a proportional relationship between subjects refractive error and the mean power of accommodative microfluctuations, for all three frequency components, at the 3.5 D reading demand (correlation LFC and MFC p < 0.05, HFC p < 0.01). The power of each frequency component was normalized to the component frequency bandwidth.
3.2.2. PowerRefractor control experiments
Several possible sources of variability in the PowerRefractor measures were considered in a series of control experiments. Because we were concerned that the PowerRefractor readings might increase in variability at closer reading distances because of increased variability with steeper pixel intensity slopes, an analysis of uncorrected myopes (range: -0.25 to -5.0 D, n=7) was performed. In this experiment, the subjects were instructed to look at a distant target (high contrast 20/100 Snellen letter presented at 6 m) while measures were made continuously for approximately 15 s. A small but significant increase in the variability (standard deviation) of the measures with increasing myopia was found (y=0.07-0.03x; R2=0.59; p<0.05), but it was too small to account for the increase in variability of accommodation seen at closer reading demands. To determine how much variability in the accommodative response might be due to measurement noise from eye movements during reading we performed an analysis on a sample of cyclopleged subjects (n=23) to compare the difference in the variability of measures made while fixating on a near target (isolated letter) and while reading at two near distances (28.6 and 66.6 cm). Since the subjects were unable to accommodate, their right eye was fully corrected with an appropriate trial lens for each of the near targets while an infrared filter was used over their left eye that allowed the PowerRefractor to measure fluctuations in refractions associated with changing eye position. The difference in the standard deviation of the response between fixating and reading conditions was not significant at the 1.5 D demand (mean±SE looking vs. reading, 0.17±0.03 vs. 0.23±0.03, paired t test p=0.53). At the 3.5 D demand, the variability in the response was significantly less during reading compared to fixating (mean±SE looking vs. reading 0.32±0.04 vs. 0.18±0.04, paired t test p<0.01), suggesting that eye movements during reading are not a source of increased variability.
3.3. Fixation breaks
The number of fixation breaks over the reading period was analyzed for each subject. While there were large differences between individuals, on average there was a significant increase in the number of fixation breaks at the 3.5 D demand (Fig. 10, mean±SE, 13.07±5.46 at 1.5 D; 12.86±6.13 at 2.5 D; 32.00±11.20 at 3.5 D; repeated measures ANOVA, p<0.05). Myopes had signiffcantly fewer fixation breaks than emmetropes at the 2.5 and 3.5 D reading demands (Fig. 10, mean±SE, myopes, 1.00±0.63; emmetropes, 28.50±11.92 at 2.5 D, ANOVA p<0.05; myopes, 1.83±1.25; emmetropes, 46.33±19.05 at 3.5 D, ANOVA p<0.05).
Fig. 10.
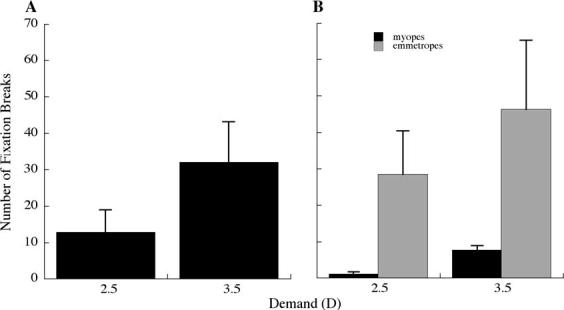
(A) The number of fixation breaks away from the text windows was significantly greater at the closer reading demand (repeated measures ANOVA: p < 0.05). (B) Myopes had significantly fewer fixation breaks at the 2.5 D and 3.5 D demands compared to emmetropes (ANOVA: p < 0.05).
4. Discussion
Accommodative behavior associated with the type of near work commonly seen in school-aged children has been suspected to be a factor in the development of myopia. Several investigators (Abbott et al., 1998; Gwiazda et al., 1995a, 1993; Jiang, 1997) have specifically hypothesized that large lags in accommodation degrade retinal image quality by producing hyperopic retinal defocus, and may affect eye growth and refraction in much the same way that negative lens-rearing affects eye growth and refraction in animal models (Graham & Judge, 1999; Hung et al., 1995; Schaeffel et al., 1988; Whatham & Judge, 2001). Reading is a sustained near work task that uses unique visual stimulus characteristics and requires extraordinarily long periods of visual attention, serial saccades, and accommodation to a near target. Accommodative errors, and the associated retinal defocus during sustained periods of reading might make a child’s eye more vulnerable to the effects of hyperopic defocus. In this paper, we examine several aspects of accommodative behavior that may be additional risk factors for myopia development.
4.1. Accommodative lags
Accommodative lags in young progressing myopes are larger than in emmetropes (Gwiazda et al., 1993; Gwiazda et al., 2005; He, Gwiazda, Thorn, Held, & Vera-Diaz, 2005; Nakatsuka et al., 2005), but adaptations within the accommodative system appear to increase the accommodative response so that in adults with stable myopia the mean lag is not different from that of adult emmetropes (Abbott et al., 1998; Nakatsuka, Hasebe, Nonaka, & Ohtsuki, 2003; Seidemann & Schaeffel, 2003). Our findings are consistent with this because there was no statistical difference in the accommodative lags between myopes and emmetropes. Although we find large differences in accommodative lags between subjects, the mean lag during extended periods of reading was similar to that shown in other studies during brief periods of accommodation (Gwiazda et al., 1993; McBrien & Millodot, 1986; Nakatsuka et al., 2003, 2005). We also observed that, after an initial period of 2-3 min, the lags were reduced slightly and then remained relatively constant over the remaining reading period. A reduction in the lag of accommodation within the first few minutes of reading has been previously observed and has been hypothesized to result from increased output of a slow, blur-driven, accommodative response mediated by the sympathetic nervous system (Rosenfield & Gilmartin, 1998a; Schor, Kotulak, & Tsuetaki, 1986). Such adaptation may increase the accuracy of the accommodative response during a sustained reading period and ultimately increase subjective clarity. Vera-Diaz, Gwiazda, Thorn, and Held (2004) also reported an increase in accommodative gain in myopes following viewing of a blurred stimulus that could not be cleared by accommodation. This adaptation was hypothesized to be a result of differences in the use of sensory blur cues between myopes and emmetropes.
We also found that, on average, the lag of accommodation increased significantly with closer reading distances, consistent with earlier reports (Charman, 1999; Gwiazda et al., 1993; Seidemann & Schaeffel, 2003). Interestingly, some of the subjects in our study showed particularly large lags of accommodation (>1.0 D), but this did not seem to compromise their ability to read, raising questions about how the subjects were able to read through substantial hyperopic defocus. We speculate that large accommodative lags may be tolerated during reading in certain individuals for several possible reasons. (1) High contrast and low spatial frequency content (near the peak of the contrast sensitivity function) are typically found in text and does not appear to require particularly accurate accommodation to be visible (Tucker & Charman, 1987; Ward, 1987). Studies have also shown that reading speed and word recognition are not affected even when text contrast is reduced 30% (Legge, Kersten, & Burgess, 1987; van Nes & Jacobs, 1981). (2) Optical aberrations in the eye may also play an important role in a subject’s ability to read through significant defocus (for a recent review seeCharman, 2005). Increased aberrations induce a greater depth of focus that would impart greater perceived clarity to a defocused retinal image. (3) Adaptations to blur (Mon-Williams, Tresilian, Strang, Kochhar, & Wann, 1998; Rosenfield & Gilmartin, 2004; Webster, Georgeson, & Webster, 2002) may be greater in some individuals, resulting in a variety of accommodative lags, and could account for the ability to read through a significant lag of accommodation with perceived clarity.
4.2. Accommodative fluctuations
Although the accommodative lags of the myopic subjects were similar to those of the emmetropic subjects in our study, the variability of their accommodative responses was significantly greater as shown by several criteria. Our data show that accommodation during extended periods of reading is proportionally more variable in myopes in terms of both the standard deviation of the accommodative response and the power spectra of accommodative microfluctuations. Both measures vary greatly between individual subjects but, overall, show increases with increasing accommodative demand. Increases in accommodative microfluctuations have also been observed with increasing accommodative demands during brief periods of accommodation by others (Campbell et al., 1959; Ciuffreda & Kruger, 1988; Day et al., 2006; Kotulak & Schor, 1986; Plainis et al., 2005; Seidel et al., 2003) and could be a product of signal-dependent noise (Harris & Wolpert, 1998). Differences in the variability of the accommodative response with the subjects’ refractive state, however, suggest a more complex relationship. We hypothesize that increased depth of focus in myopes, possibly because of increased aberrations (Charman, 2005), may be responsible for a reduction in blur sensitivity (Rosenfield & Abraham-Cohen, 1999) and lead to increased accommodative variability (Day et al., 2006; Seidel et al., 2003). Myopes have been shown in numerous studies to have increased astigmatism and higher order aberrations compared to emmetropes (Cheng et al., 2000; Collins, Buehren, & Iskander, 2006; He et al., 2002, 2005; Kirwan, O’Keefe, & Soeldner, 2006; Marcos, Moreno-Barriuso, Llorente, Navarro, & Barbero, 2000; Simonet, Hamam, Brunette, & Campbell, 1999; Sun, Sun, & He, 2000, but see Llorente, Barbero, Cano, Dorronsoro, & Marcos, 2004), which may be the result of poor compensation of corneal aberrations by internal optics (Benito & Artal, 2004). In addition, changes in the eye’s aberrations have also been reported to occur during accommodation. These changes could affect the accuracy of the accommodative response during reading. In particular, positive spherical aberrations have been reported to become more negative during accommodation and would increase the demand needed to produce a clear retinal image (He, Burns, & Marcos, 2000; Plainis et al., 2005). Although this varies widely among studies (Charman, 2005; Cheng et al., 2004; He et al., 2000; Ninomiya et al., 2002; Vilupuru, Roorda, & Glasser, 2004), it has been reported to occur to a greater degree in myopes during accommodation and after sustained periods of reading (Buehren, Collins, & Carney, 2005; Collins et al., 2006; He et al., 2000). Finally, the microfluctuations of higher order aberrations have been shown to have similar frequency characteristics to accommodative microfluctuations (Zhu, Collins, & Iskander, 2004) and both may be related to the dynamics of accommodation during sustained reading.
Our data suggest that the increased variability of the accommodative response observed in myopes is specific to the operation of the accommodation controller, and possibly the accommodative plant, and not to the general increase in variability associated with increasing demands. In this study, both myopes and emmetropes show approximately the same average accommodative response for a given target. Therefore, accommodation in myopes appears to possess characteristics that result in greater fluctuations without changing the average accommodative response. Adult myopes are less sensitive than emmetropes to the defocus cues that drive accommodation (Rosenfield & Abraham-Cohen, 1999; Thorn, Cameron, Arnel, & Thorn, 1998), although myopic children may have similar blur detection thresholds to age-matched emmetropes (Schmid, Iskander, Li, Edwards, & Lew, 2002). The accommodative response of young myopes is reduced (lags are greater) when their myopia is progressing but improves to the level of emmetropes as myopic progression slows and stabilizes (Abbott et al., 1998; Gwiazda et al., 1995a; Nakatsuka et al., 2003) suggesting accommodative gain adjustments. Pairing adaptable system gain with decreased sensitivity will result in response instability in the accommodative controller, as in any servosystem. Finally, it is also plausible that the shape of the myopic eye affects the operation of the ciliary muscle and may affect the accommodative response (Mutti et al., 2000) by increasing the tension on the ciliary zonules and the accommodative effort for a given demand. In theory, the increased accommodative strain put upon the accommodative plant could also cause an increase in the variability of the response.
The increases in accommodative variability and lags, associated with closer reading demands may have important clinical implications. Individuals preferring closer reading distances may be more susceptible to myopia because both the fluctuations and lags are greater. The associated increases in hyperopic blur may signal increased eye growth (Bartmann & Schaeffel, 1994; Flitcroft, 1998; Goss & Wickham, 1995; Hung & Ciuffreda, 2000; Jiang & Morse, 1999). Myopic children have habitually closer working distances than emmetropic children (Haro, Poulain, & Drobe, 2000) and myopia progression is significantly greater in children with closer near working distances (Parssinen & Lyyra, 1993). Moreover, the Correction of Myopia Evaluation Trial (COMET) has recently reported that children wearing progressive addition lenses who had a lag of accommodation greater than 0.43 D and a closer than normal reading distance (<31.2cm) had a significantly greater treatment effect in the reduction of myopia compared to single vision lens wearers (a 0.44 D difference over three years) (Gwiazda, Hyman, & Norton, 2004). Interestingly, we also observed that myopes had significantly fewer fixation breaks than emmetropes at the closest reading distance, suggesting that breaks from reading at a close distance may have a protective effect. This is consistent with the fact that even very brief periods of myopic defocus have been shown to inhibit compensatory axial growth to sustained periods of hyperopic defocus in animal models (Zhu, Winawer, & Wallman, 2003).
5. Conclusion
In this study, we show that in adult subjects with stable myopia, the average accommodative response amplitude during an extended period of reading is virtually identical to that of adult emmetropes. We find, however, that there is more variability in myopes, which is consistent with the hypothesis that myopes are less sensitive to defocus. While the small fluctuations in the accommodative response may be individually too small to stimulate eye growth, temporal integration of the fluctuations over periods of sustained reading may be sufficient to produce a blur signal that may lead to myopia. Investigation in individuals with progressing myopia, particularly children, is necessary to determine whether these differences are risk factors for the development of myopia.
Footnotes
Supported by NIH R01 EY11228 (D.T.) and T35 EY07149 (E.H.).
References
- Abbott ML, Schmid KL, Strang NC. Differences in the accommodation stimulus-response curves of adult myopes and emmetropes. Ophthalmic and Physiological Optics. 1998;18(1):13–20. [PubMed] [Google Scholar]
- Al-Bdour MD, Odat TA, Tahat AA. Myopia and level of education. European Journal of Ophthalmology. 2001;11(1):1–5. doi: 10.1177/112067210101100101. [DOI] [PubMed] [Google Scholar]
- Alpern M, David H. Effects of illuminance quantity on accommodation of the eyes. Industrial Medicine and Surgery. 1958;27:551–555. [PubMed] [Google Scholar]
- Au Eong KG, Tay TH, Lim MK. Education and myopia in 110,236 young Singaporean males. Singapore Medical Journal. 1993;34:489–492. [PubMed] [Google Scholar]
- Bartmann M, Schaeffel F. A simple mechanism for emmetropization without cues from accommodation or colour. Vision Research. 1994;34(7):873–876. doi: 10.1016/0042-6989(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Benito A, Artal P. Compensation of corneal aberrations by the internal optics is better in hyperopic eyes. Investigative Ophthalmology and Visual Science. 2004 2004 ARVO E-abstract 1076. [Google Scholar]
- Buehren T, Collins M, Carney L. Near work induced wavefront aberrations in myopia. Vision Research. 2005;45:1297–1312. doi: 10.1016/j.visres.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Campbell FW, Robson JG, Westheimer G. Fluctuations of accommodation under steady state viewing conditions. Journal of Physiology. 1959;145:579–594. doi: 10.1113/jphysiol.1959.sp006164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell FW, Westheimer G. Dynamics of accommodation responses of the human eye. Journal of Physiology. 1960;151:285–295. doi: 10.1113/jphysiol.1960.sp006438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman WN. Near vision, lags of accommodation and myopia. Ophthalmic and Physiological Optics. 1999;19(2):126–133. doi: 10.1046/j.1475-1313.1999.00414.x. [DOI] [PubMed] [Google Scholar]
- Charman WN. Aberrations and myopia. Ophthalmic and Physiological Optics. 2005;25:285–301. doi: 10.1111/j.1475-1313.2005.00297.x. [DOI] [PubMed] [Google Scholar]
- Chen J, Schmid KL, Brown B, Edwards M. The effect of a β-adrenoceptor antagonist on accommodative adaptation in Hong Kong children. Current Eye Research. 2005;30:179–188. doi: 10.1080/02713680490908571. [DOI] [PubMed] [Google Scholar]
- Cheng H, Barnett J, Vilupuru A, Marsack J, Kasthurirangan S, Applegate R, Roorda A. A population study on changes in wave aberrations with accommodation. Journal of Vision. 2004;4:272–280. doi: 10.1167/4.4.3. [DOI] [PubMed] [Google Scholar]
- Cheng X, Thibos L, Hong X, Bradley A, Himebaugh N, Riley C, et al. Increased optical aberrations in myopia; Myopia 2000: Proceedings of the VIII International Conference on Myopia; Boston. 2000.pp. 122–126. [Google Scholar]
- Choi M, Weiss S, Schaeffel F, Seidmann A, Howland HC, Wilhelm B. Laboratory, clinical, and kindergarten test of a new eccentric infrared photorefractor (PowerRefractor) Optometry and Vision Science. 2000;77(10):537–548. doi: 10.1097/00006324-200010000-00008. [DOI] [PubMed] [Google Scholar]
- Ciuffreda KJ. Borish’s Clinical Refraction. third ed. W.B. Saunders; Philadelphia: 1998. Accommodation, the pupil, and presbyopia; pp. 78–120. [Google Scholar]
- Ciuffreda KJ, Kruger PB. Dynamics of human voluntary accommodation. American Journal of Optometry and Physiological Optics. 1988;65(5):365–370. doi: 10.1097/00006324-198805000-00010. [DOI] [PubMed] [Google Scholar]
- Collins M, Buehren T, Iskander DR. Retinal image quality, reading and myopia. Vision Research. 2006;46(12):196–215. doi: 10.1016/j.visres.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Culhane HM, Winn B. Dynamic accommodation and myopia. Investigative Ophthalmology and Visual Science. 1999;40(9):1968–1974. [PubMed] [Google Scholar]
- Culhane HM, Winn B, Gilmartin B. Human dynamic closedloop accommodation augmented by sympathetic inhibition. Investigative Ophthalmology and Visual Science. 1999;40(6):1137–1143. [PubMed] [Google Scholar]
- Day M, Strang NC, Seidel D, Gray L, Mallen EAH. Refractive group differences in accommodation microfluctuations with changing accommodation stimulus. Ophthalmic and Physiological Optics. 2006;26:88–96. doi: 10.1111/j.1475-1313.2005.00347.x. [DOI] [PubMed] [Google Scholar]
- Fincham EF. The accommodation reflex and its stimulus. British Journal of Ophthalmology. 1951;35:381–393. doi: 10.1136/bjo.35.7.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flitcroft DI. A model of the contribution of oculomotor and optical factors to emmetropization and myopia. Vision Research. 1998;38(19):2869–2879. doi: 10.1016/s0042-6989(98)00087-x. [DOI] [PubMed] [Google Scholar]
- Gilmartin B, Bullimore MA. Adaptation of tonic accommodation to sustained visual tasks in emmetropia and late-onset myopia. Optometry and Vision Science. 1991;68(1):22–26. doi: 10.1097/00006324-199101000-00004. [DOI] [PubMed] [Google Scholar]
- Gimpel G, Doughty MJ, Lyle WM. Large sample study of the effects of phenylephrine 2.5% eye drops on the amplitude of accommodation in man. Ophthalmic and Physiological Optics. 1994;14(2):123–128. doi: 10.1111/j.1475-1313.1994.tb00099.x. [DOI] [PubMed] [Google Scholar]
- Goss D, Wickham MG. Retinal image-mediated ocular growth as a possible etiological factor in juvenile-onset myopia. Documenta Ophthalmologica. 1995;90:341–375. doi: 10.1007/BF01268122. [DOI] [PubMed] [Google Scholar]
- Graham B, Judge SJ. The effects of spectacle wear in infancy on eye growth and refractive error in the marmoset (Callithrix jacchus) Vision Research. 1999;39(2):189–206. doi: 10.1016/s0042-6989(98)00189-8. [DOI] [PubMed] [Google Scholar]
- Gwiazda J, Bauer J, Thorn F, Held R. A dynamic relationship between myopia and blur-driven accommodation in school-aged children. Vision Research. 1995;35(a)(9):1299–1304. doi: 10.1016/0042-6989(94)00238-h. [DOI] [PubMed] [Google Scholar]
- Gwiazda J, Bauer J, Thorn F, Held R. Shifts in tonic accommodation after near work are related to refractive errors in children. Ophthalmic and Physiological Optics. 1995;15(b)(2):93–97. [PubMed] [Google Scholar]
- Gwiazda J, Hyman L, Norton T, et al. Accommodation and related risk factors associated with myopia progression and their interaction with treatment in COMET children. Investigative Ophthalmology and Visual Science. 2004;45(7):2143–2151. doi: 10.1167/iovs.03-1306. [DOI] [PubMed] [Google Scholar]
- Gwiazda J, Thorn F, Bauer J, Held R. Myopic children show insufficient accommodative response to blur. Investigative Ophthalmology and Visual Science. 1993;34(3):690–694. [PubMed] [Google Scholar]
- Gwiazda J, Thorn F, Held R. Accommodation, accommodation convergence, and response AC/A ratios before and at the onset of myopia in children. Optometry and Vision Science. 2005;82(4):273–278. doi: 10.1097/01.opx.0000159363.07082.7d. [DOI] [PubMed] [Google Scholar]
- Harb EN, Thorn F, Troilo D. Accommodation during sustained near reading. Investigative Ophthalmology and Visual Science. 2003;12(44) E-Abstract 2732 (available at www.iovs.org)
- Harb E, Thorn F, Troilo D. Temporal characteristics of sustained accommodation in myopes and emmetropes. Optometry and Vision Science. 2004;81(12s):23. [Google Scholar]
- Haro C, Poulain I, Drobe B. Investigation of working distance in myopic and non-myopic children. Optometry and Vision Science. 2000;77(Suppl):189. [Google Scholar]
- Harris CM, Wolpert DM. Signal-dependent noise determines motor planning. Nature. 1998;394(6695):780–784. doi: 10.1038/29528. [DOI] [PubMed] [Google Scholar]
- Hazel C, Cox M, Strang NC. Wavefront aberration and its relationship to accommodative stimulus-response function in myopic subjects. Optometry and Vision Science. 2003;80(2):151–158. doi: 10.1097/00006324-200302000-00011. [DOI] [PubMed] [Google Scholar]
- He JC, Burns SA, Marcos S. Monochromatic aberrations in the accommodated human eye. Vision Research. 2000;40(1):41–48. doi: 10.1016/s0042-6989(99)00156-x. [DOI] [PubMed] [Google Scholar]
- He JC, Gwiazda J, Thorn F, Held R, Vera-Diaz F. The association of wavefront aberration and accommodative lag in myopes. Vision Research. 2005;45:285–290. doi: 10.1016/j.visres.2004.08.027. [DOI] [PubMed] [Google Scholar]
- He JC, Sun P, Held R, Thorn F, Sun XR, Gwiazda JE. Wavefront aberrations in eyes of emmetropic and moderately myopic school children and young adults. Vision Research. 2002;42(8):1063–1070. doi: 10.1016/s0042-6989(02)00035-4. [DOI] [PubMed] [Google Scholar]
- Heath GG. Components of accommodation. American Journal of Optometry and Archives of the American Academy of Optometry. 1956;33:569–579. doi: 10.1097/00006324-195611000-00001. [DOI] [PubMed] [Google Scholar]
- Hepsen IF, Evereklioglu C, Bayramlar H. The effect of reading and near-work on the development of myopia in emmetropic boys: A prospective, controlled, three-year follow-up study. Vision Research. 2001;41(19):2511–2520. doi: 10.1016/s0042-6989(01)00135-3. [DOI] [PubMed] [Google Scholar]
- Hofstetter H, Griffin J, Berman M, Everson R. Dictionary of visual science and related clinical terms. Butterworth Heinemann; Boston: 2000. [Google Scholar]
- Hung L-F, Crawford MLJ, Smith EL. Spectacle lenses alter eye growth and the refractive status of young monkeys. Nature Medicine. 1995;1(8):761–765. doi: 10.1038/nm0895-761. [DOI] [PubMed] [Google Scholar]
- Hung LF, Ciuffreda K. A unifying theory of refractive error development. Bulletin of Mathematical Biology. 2000;62:1087–1108. doi: 10.1006/bulm.2000.0199. [DOI] [PubMed] [Google Scholar]
- Jiang B-C. Integration of sensory components into the accommodation model reveals differences between emmetropia and late onset myopia. Investigative Ophthalmology and Visual Science. 1997;38(8):1511–1516. [PubMed] [Google Scholar]
- Jiang B, Morse SE. Oculomotor functions and late-onset myopia. Ophthalmic and Physiological Optics. 1999;19(2):165–172. doi: 10.1046/j.1475-1313.1999.00431.x. [DOI] [PubMed] [Google Scholar]
- Kirwan C, O’Keefe M, Soeldner H. Higher-order aberrations in children. American Journal of Ophthalmology. 2006;141:67–70. doi: 10.1016/j.ajo.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Kotulak JC, Schor CM. Temporal variations in accommodation during steady-state conditions. Journal of the Optical Society of America A. 1986;3:223–227. doi: 10.1364/josaa.3.000223. [DOI] [PubMed] [Google Scholar]
- Kruger PB. The effect of cognitive demand on accommodation. American Journal of Ophthalmic and Physiological Optics. 1980;57(7):440–445. doi: 10.1097/00006324-198007000-00006. [DOI] [PubMed] [Google Scholar]
- Kruger PB, Pola J. Accommodation to size and blur changing in counterphase. Optometry and Vision Science. 1989;66(7):455–458. doi: 10.1097/00006324-198907000-00008. [DOI] [PubMed] [Google Scholar]
- Legge GE, Kersten D, Burgess AE. Psychophysics of reading—V. The role of contrast in normal vision. Vision Research. 1987;27:1165–1177. doi: 10.1016/0042-6989(87)90028-9. [DOI] [PubMed] [Google Scholar]
- Llorente L, Barbero S, Cano D, Dorronsoro C, Marcos S. Myopic versus hyperopic eyes: Axial length, corneal shape and optical aberrations. Journal of Vision. 2004;4(4):288–298. doi: 10.1167/4.4.5. [DOI] [PubMed] [Google Scholar]
- Marcos S, Moreno-Barriuso E, Llorente L, Navarro R, Barbero S. Do myopic eyes suVer from larger amounts of aberrations; Myopia 2000: Proceedings of the VIII International Conference on Myopia; Boston. 2000.pp. 118–121. [Google Scholar]
- McBrien NA, Millodot M. Amplitude of accommodation and refractive error. Investigative Ophthalmology and Visual Science. 1986;27(7):1187–1190. [PubMed] [Google Scholar]
- McBrien NA, Millodot M. The relationship between tonic accommodation and refractive error. Investigative Ophthalmology and Visual Science. 1987;28(6):997–1004. [PubMed] [Google Scholar]
- McBrien NA, Millodot M. Differences in adaptation of tonic accommodation with refractive state. Investigative Ophthalmology and Visual Science. 1988;29(3):460–469. [PubMed] [Google Scholar]
- Mon-Williams M, Tresilian JR, Strang NC, Kochhar P, Wann JP. Improving vision: Neural compensation for optical defocus. Proceedings of the Royal Society of London. 1998;265:71–77. doi: 10.1098/rspb.1998.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutti DO, Jones L, Moeschberger ML, Zadnik K. AC/A ratio, age, and refractive error in children. Investigative Ophthalmology and Visual Science. 2000;41(9):2469–2478. [PubMed] [Google Scholar]
- Nakatsuka C, Hasebe S, Nonaka F, Ohtsuki H. Accommodative lag under habitual seeing conditions: Comparison between adult myopes and emmetropes. Japanese Journal of Ophthalmology. 2003;47:291–298. doi: 10.1016/s0021-5155(03)00013-3. [DOI] [PubMed] [Google Scholar]
- Nakatsuka C, Hasebe S, Nonaka F, Ohtsuki H. Accommodative lag under habitual seeing conditions: Comparison between myopic and emmetropic children. Japanese Journal of Ophthalmology. 2005;49:189–194. doi: 10.1007/s10384-004-0175-7. [DOI] [PubMed] [Google Scholar]
- Ninomiya S, Fujikado T, Kuroda T, Maeda N, Tano Y, Oshika T, Hirohara Y, Mihashi T. Changes of ocular aberration with accommodation. American Journal of Ophthalmology. 2002;134(6):924–926. doi: 10.1016/s0002-9394(02)01856-1. [DOI] [PubMed] [Google Scholar]
- Ostrin LA, Glasser A. The eVect of phenylephrine on pupil diameter and accommodation in rhesus monkeys. Investigative Ophthalmology and Visual Science. 2004;45(1):215–221. doi: 10.1167/iovs.03-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parssinen O, Lyyra AL. Myopia and myopic progression among school children—A three-year follow-up study. Investigative Ophthalmology and Visual Science. 1993;34(9):2794–2802. [PubMed] [Google Scholar]
- Phillips S, Stark L. Blur: A suffcient accommodative stimulus. Documenta Ophthalmologica. 1977;43(1):65–89. doi: 10.1007/BF01569293. [DOI] [PubMed] [Google Scholar]
- Plainis S, Ginis HS, Pallikaris A. The effect of ocular aberrations in steady-state errors of accommodative response. Journal of Vision. 2005;5(5):466–477. doi: 10.1167/5.5.7. [DOI] [PubMed] [Google Scholar]
- Richler A, Bear JC. Refraction, nearwork, and education. A population study in Newfoundland. Acta Ophthalmologica. 1980;58:468–478. doi: 10.1111/j.1755-3768.1980.tb05748.x. [DOI] [PubMed] [Google Scholar]
- Roorda A, Campbell MC, Bobier WR. Geometrical theory to predict eccentric photorefraction intensity profiles in the human eye. Journal of the Optical Society of America, A, Optics, Image Science, & Vision. 1995;12(8):1647–1656. doi: 10.1364/josaa.12.001647. [DOI] [PubMed] [Google Scholar]
- Rosenfield M. Accommodation and myopia. In: Rosenfield M, Gilmartin B, editors. Myopia and Nearwork. Butterworth Heinemann; Oxford: 1998. pp. 91–116. [Google Scholar]
- Rosenfield M, Abraham-Cohen JA. Blur sensitivity in myopes. Optometry and Vision Science. 1999;76(5):303–307. doi: 10.1097/00006324-199905000-00018. [DOI] [PubMed] [Google Scholar]
- Rosenfield M, Gilmartin B. Effect of a near-vision task on the response AC/A of a myopic population. Ophthalmic and Physiological Optics. 1987;7:225–233. [PubMed] [Google Scholar]
- Rosenfield M, Gilmartin B. Accommodative adaptation during sustained near-vision reduces accommodative error. Investigative Ophthalmology and Visual Science. 1998;39(a):S639. [Google Scholar]
- Rosenfield M, Gilmartin B. Myopia and nearwork: Causation or merely association. In: Rosenfield M, Gilmartin B, editors. Myopia and Nearwork. b. Butterworth Heinemann; Oxford: 1998. pp. 193–206. [Google Scholar]
- Rosenfield M, Gilmartin B. Blur adaptation in myopes. Optometry and Vision Science. 2004;81(9):657–662. doi: 10.1097/01.opx.0000144743.34976.da. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Glasser A, Howland HC. Accommodation, refractive error and eye growth in chickens. Vision Research. 1988;28(5):639–657. doi: 10.1016/0042-6989(88)90113-7. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Weiss S, Seidel J. How good is the match between the plane of the text and the plane of focus during reading. Ophthalmic and Physiological Optics. 1999;19(2):180–192. doi: 10.1046/j.1475-1313.1999.00430.x. [DOI] [PubMed] [Google Scholar]
- Schmid K, Iskander DR, Li R, Edwards M, Lew J. Blur detection thresholds in childhood myopia: single and dual target presentation. Vision Research. 2002;42:239–247. doi: 10.1016/s0042-6989(01)00277-2. [DOI] [PubMed] [Google Scholar]
- Schor CM, Kotulak JC, Tsuetaki T. Adaptation of tonic accommodation reduces accommodative lag and is masked in darkness. Investigative Ophthalmology and Visual Science. 1986;27(5):820–827. [PubMed] [Google Scholar]
- Seidel D, Gray L, Heron G. Retinotopic accommodation responses in myopia. Investigative Ophthalmology and Visual Science. 2003;44:1035–1041. doi: 10.1167/iovs.02-0264. [DOI] [PubMed] [Google Scholar]
- Seidemann A, Schaeffel F. An evaluation of the lag of accommodation using photorefraction. Vision Research. 2003;43(4):419–430. doi: 10.1016/s0042-6989(02)00571-0. [DOI] [PubMed] [Google Scholar]
- Seidemann A, Schaeffel F, Guirao A, Lopez-Gil N, Artal P. Peripheral refractive errors in myopia, emmetropic, and hyperopic young subjects. Journal of the Optical Society of America. 2002;19(12):2363–2373. doi: 10.1364/josaa.19.002363. [DOI] [PubMed] [Google Scholar]
- Simonet P, Hamam H, Brunette I, Campbell M. Influence of ametropia on the optical quality of the human eye. Investigative Ophthalmology and Visual Science. 1999;40(4):S448. [Google Scholar]
- Smith EL, III, Hung LF, Harwerth RS. Effects of optically induced blur on the refractive status of young monkeys. Vision Research. 1994;34(3):293–301. doi: 10.1016/0042-6989(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Sorkin R, Reich L, Pizzimenti J. Accommodative response to PRIO computer vision tester versus printed text. Optometry. 2003;74(12):782–786. [PubMed] [Google Scholar]
- Strang NC, Winn B, Gilmartin B. Repeatability of post task regression of accommodation in emmetropia and late-onset myopia. Ophthalmic and Physiological Optics. 1994;14:88–91. doi: 10.1111/j.1475-1313.1994.tb00565.x. [DOI] [PubMed] [Google Scholar]
- Sun P, Sun X, He JC. The change in wave-front aberrations of the eye with age for Chinese school children and young adults; Myopia 2000: Proceedings of the VIII International Conference on Myopia; Boston. 2000.pp. 132–135. [Google Scholar]
- Thorn F, Cameron L, Arnel J, Thorn S. Myopic adults see through defocus better than emmetropes; Proceedings of the 6th International Conference on Myopia; Tokyo: Springer-Verlag. 1998.pp. 368–374. [Google Scholar]
- Tucker J, Charman WN. EVect of target content at higher spatial frequencies on the accuracy of the accommodative response. Ophthalmic and Physiological Optics. 1987;7(2):137–142. doi: 10.1111/j.1475-1313.1987.tb01009.x. [DOI] [PubMed] [Google Scholar]
- van Nes FL, Jacobs JC. The effects of contrast on letter and word recognition. IPO Annual Progress Reports. 1981;16:72–80. [Google Scholar]
- Vera-Diaz FA, Gwiazda J, Thorn F, Held R. Increased accommodation following adaptation to image blur in myopes. Journal of Vision. 2004;4(12):1111–1119. doi: 10.1167/4.12.10. [DOI] [PubMed] [Google Scholar]
- Vilupuru A, Roorda A, Glasser A. Spatially variant changes in lens power during ocular accommodation in a rhesus monkey eye. Journal of Vision. 2004;4:299–309. doi: 10.1167/4.4.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T, Mutti DO. The effect of accommodation on ocular shape. Optometry and Vision Science. 2002;79(7):424–430. doi: 10.1097/00006324-200207000-00010. [DOI] [PubMed] [Google Scholar]
- Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43(4):447–468. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Ward PA. The effect of stimulus contrast on the accommodation response. Ophthalmic and Physiological Optics. 1987;7(1):9–15. [PubMed] [Google Scholar]
- Webster M, Georgeson M, Webster S. Neural adjustments to image blur. Nature Neuroscience. 2002;5(9):839–840. doi: 10.1038/nn906. [DOI] [PubMed] [Google Scholar]
- Whatham AR, Judge SJ. Compensatory changes in eye growth and refraction induced by daily wear of soft contact lenses in young marmosets. Vision Research. 2001;41(3):267–273. doi: 10.1016/s0042-6989(00)00250-9. [DOI] [PubMed] [Google Scholar]
- Winn B, Pugh JR, Gilmartin B, Owens H. The frequency characteristics of accommodative microfluctuations for central and peripheral zones of the human crystalline lens. Vision Research. 1990;30(7):1093–1099. doi: 10.1016/0042-6989(90)90117-4. [DOI] [PubMed] [Google Scholar]
- Young FA, Leary GA, Baldwin WR, West DC, Box RA, Harris E. The transmission of refractive errors within Eskimo families. American Journal of Optometry and Archives of American Academy of Optometry. 1969;46:676–685. doi: 10.1097/00006324-196909000-00005. [DOI] [PubMed] [Google Scholar]
- Zhu M, Collins M, Iskander DR. Microfluctuations of wavefront aberrations if the eye. Ophthalmic and Physiological Optics. 2004;24:562–571. doi: 10.1111/j.1475-1313.2004.00237.x. [DOI] [PubMed] [Google Scholar]
- Zhu X, Winawer J, Wallman J. The potency of myopic defocus in spectacle lens compensation. Investigative Ophthalmology and Visual Science. 2003;44:2818–2827. doi: 10.1167/iovs.02-0606. [DOI] [PubMed] [Google Scholar]


