Abstract
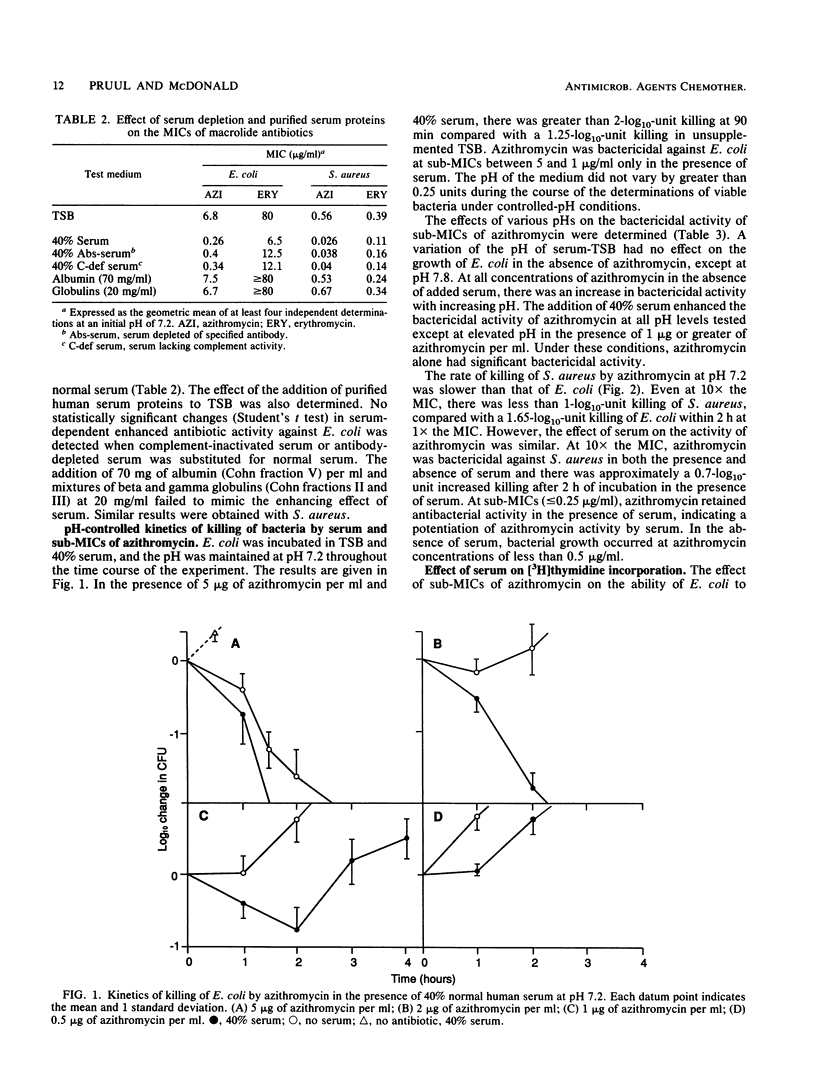
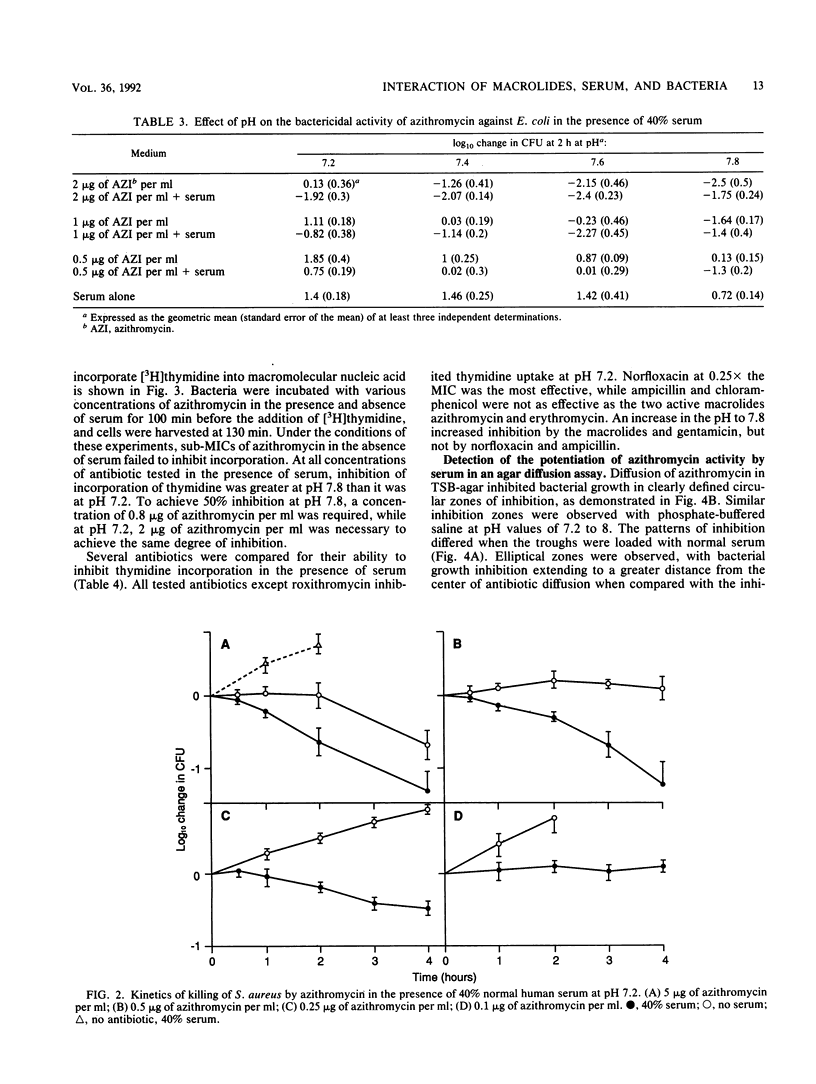
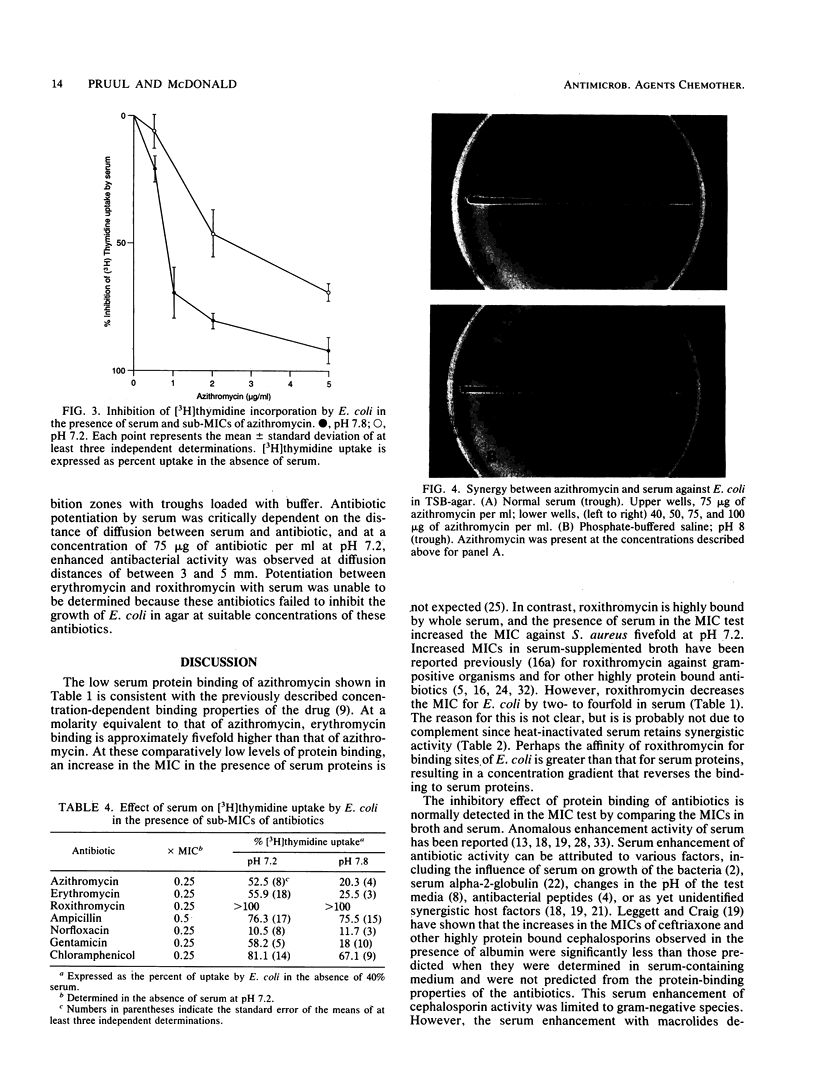
The interaction of azithromycin with normal human serum was examined in relation to serum protein binding, MIC, and kinetics of killing of bacteria. While the binding of azithromycin to serum proteins is low (8.5% at a concentration of 0.01 mM in 95% serum), the presence of 40% serum during the MIC test decreased MICs by 26-fold for serum-resistant Escherichia coli and 15-fold for Staphylococcus aureus. Erythromycin had a similar but lesser effect, while roxithromycin was less active against S. aureus in the presence of serum. The rate of killing of E. coli and S. aureus by azithromycin was increased in the presence of serum. The enhancement of antibiotic activity by serum was pH independent, and heat inactivation and preabsorption with homologous bacteria failed to inhibit enhancement by serum. The macromolecular incorporation of [3H]thymidine by E. coli continuously exposed to 2 micrograms of azithromycin per ml (0.25x the MIC) and 40% serum was decreased by 80% at pH 7.8 and by 48% at pH 7.2, while azithromycin alone failed to inhibit incorporation. Inhibition of nucleic acid biosynthesis at pH 7.2 in the presence of serum was also detected with sub-MICs of erythromycin, norfloxacin, and gentamicin but not roxithromycin. A diffusible serum factor was shown to interact with azithromycin to inhibit the growth of E. coli in an agar diffusion assay to detect antibiotic-serum synergy.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry A. L., Jones R. N., Thornsberry C. In vitro activities of azithromycin (CP 62,993), clarithromycin (A-56268; TE-031), erythromycin, roxithromycin, and clindamycin. Antimicrob Agents Chemother. 1988 May;32(5):752–754. doi: 10.1128/aac.32.5.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. R., Williams P. Influence of substrate limitation and growth phase on sensitivity to antimicrobial agents. J Antimicrob Chemother. 1985 Jan;15 (Suppl A):7–14. doi: 10.1093/jac/15.suppl_a.7. [DOI] [PubMed] [Google Scholar]
- Bryan C. S., Marney S. R., Jr, Alford R. H., Bryant R. E. Gram-negative bacillary endocarditis. Interpretation of the serum bactericial test. Am J Med. 1975 Feb;58(2):209–215. doi: 10.1016/0002-9343(75)90571-9. [DOI] [PubMed] [Google Scholar]
- Carroll S. F., Martinez R. J. Antibacterial peptide from normal rat serum. 1. Isolation from whole serum, activity, and microbicidal spectrum. Biochemistry. 1981 Oct 13;20(21):5973–5981. doi: 10.1021/bi00524a008. [DOI] [PubMed] [Google Scholar]
- Craig W. A., Kunin C. M. Significance of serum protein and tissue binding of antimicrobial agents. Annu Rev Med. 1976;27:287–300. doi: 10.1146/annurev.me.27.020176.001443. [DOI] [PubMed] [Google Scholar]
- Dutcher B. S., Reynard A. M., Beck M. E., Cunningham R. K. Potentiation of antibiotic bactericidal activity by normal human serum. Antimicrob Agents Chemother. 1978 May;13(5):820–826. doi: 10.1128/aac.13.5.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds G., Shepard R. M., Johnson R. B. The pharmacokinetics of azithromycin in human serum and tissues. J Antimicrob Chemother. 1990 Jan;25 (Suppl A):73–82. doi: 10.1093/jac/25.suppl_a.73. [DOI] [PubMed] [Google Scholar]
- Girard A. E., Girard D., English A. R., Gootz T. D., Cimochowski C. R., Faiella J. A., Haskell S. L., Retsema J. A. Pharmacokinetic and in vivo studies with azithromycin (CP-62,993), a new macrolide with an extended half-life and excellent tissue distribution. Antimicrob Agents Chemother. 1987 Dec;31(12):1948–1954. doi: 10.1128/aac.31.12.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Bell A. Antibiotic uptake into gram-negative bacteria. Eur J Clin Microbiol Infect Dis. 1988 Dec;7(6):713–720. doi: 10.1007/BF01975036. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Wong P. G. Compounds which increase the permeability of the Pseudomonas aeruginosa outer membrane. Antimicrob Agents Chemother. 1984 Jul;26(1):48–52. doi: 10.1128/aac.26.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy D. J., Swanson R. N., Rode R. A., Marsh K., Shipkowitz N. L., Clement J. J. Enhancement of the in vitro and in vivo activities of clarithromycin against Haemophilus influenzae by 14-hydroxy-clarithromycin, its major metabolite in humans. Antimicrob Agents Chemother. 1990 Jul;34(7):1407–1413. doi: 10.1128/aac.34.7.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirashima A., Childs G., Inouye M. Differential inhibitory effects of antibiotics on the biosynthesis of envelope proteins of Escherichia coli. J Mol Biol. 1973 Sep 15;79(2):373–389. doi: 10.1016/0022-2836(73)90012-0. [DOI] [PubMed] [Google Scholar]
- Iida K., Koike M. Cell wall alterations of gram-negative bacteria by aminoglycoside antibiotics. Antimicrob Agents Chemother. 1974 Jan;5(1):95–97. doi: 10.1128/aac.5.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Barry A. L. Antimicrobial activity of ceftriaxone, cefotaxime, desacetylcefotaxime, and cefotaxime-desacetylcefotaxime in the presence of human serum. Antimicrob Agents Chemother. 1987 May;31(5):818–820. doi: 10.1128/aac.31.5.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Barry A. L. Unpredictable influence of human serum on antimicrobial activity of erythromycin and three oxime ether macrolides. Eur J Clin Microbiol. 1987 Feb;6(1):81–82. doi: 10.1007/BF02097206. [DOI] [PubMed] [Google Scholar]
- Klainer A. S., Russell R. R. Effect of the inhibition of protein synthesis on the Escherichia coli cell envelope. Antimicrob Agents Chemother. 1974 Aug;6(2):216–224. doi: 10.1128/aac.6.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang S. D., Cameron G. L., Mullins P. R. Anomalous effect of serum on the antimicrobial activity of cefoperazone. Drugs. 1981;22 (Suppl 1):52–59. doi: 10.2165/00003495-198100221-00012. [DOI] [PubMed] [Google Scholar]
- Leggett J. E., Craig W. A. Enhancing effect of serum ultrafiltrate on the activity of cephalosporins against gram-negative bacilli. Antimicrob Agents Chemother. 1989 Jan;33(1):35–40. doi: 10.1128/aac.33.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marca G., Veronese M., Petrescu D. Enhancement of the bacteriostatic and bactericidal activities of chloramphenicol and thiamphenicol by normal serum in vitro. Chemotherapy. 1973;18(2):91–98. doi: 10.1159/000221250. [DOI] [PubMed] [Google Scholar]
- Mett H., Gyr K., Zak O., Vosbeck K. Duodeno-pancreatic secretions enhance bactericidal activity of antimicrobial drugs. Antimicrob Agents Chemother. 1984 Jul;26(1):35–38. doi: 10.1128/aac.26.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moellering R. C., Jr, Eliopoulos G. M. Activity of cefotaxime against enterococci. Diagn Microbiol Infect Dis. 1984 Jun;2(3 Suppl):85S–90S. [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl T. M., Pfaller M. A., Houston A., Wenzel R. P. Effect of serum on the in vitro activities of 11 broad-spectrum antibiotics. Antimicrob Agents Chemother. 1990 Nov;34(11):2234–2239. doi: 10.1128/aac.34.11.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson L. R., Gerding D. N. Influence of protein binding of antibiotics on serum pharmacokinetics and extravascular penetration: clinically useful concepts. Rev Infect Dis. 1980 May-Jun;2(3):340–348. doi: 10.1093/clinids/2.3.340. [DOI] [PubMed] [Google Scholar]
- Pruul H., McDonald P. J. Damage to bacteria by antibiotics in vitro and its relevance to antimicrobial chemotherapy: a historical perspective. J Antimicrob Chemother. 1988 Jun;21(6):695–698. doi: 10.1093/jac/21.6.695. [DOI] [PubMed] [Google Scholar]
- Pruul H., Reynolds B. L. Interaction of complement and polymyxin with gram-negative bacteria. Infect Immun. 1972 Nov;6(5):709–717. doi: 10.1128/iai.6.5.709-717.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer L. G., Stratton C. W., Reller L. B. Minimum inhibitory and bactericidal concentrations of 44 antimicrobial agents against three standard control strains in broth with and without human serum. Antimicrob Agents Chemother. 1981 Jun;19(6):1050–1055. doi: 10.1128/aac.19.6.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retsema J. A., Girard A. E., Girard D., Milisen W. B. Relationship of high tissue concentrations of azithromycin to bactericidal activity and efficacy in vivo. J Antimicrob Chemother. 1990 Jan;25 (Suppl A):83–89. doi: 10.1093/jac/25.suppl_a.83. [DOI] [PubMed] [Google Scholar]
- Retsema J., Girard A., Schelkly W., Manousos M., Anderson M., Bright G., Borovoy R., Brennan L., Mason R. Spectrum and mode of action of azithromycin (CP-62,993), a new 15-membered-ring macrolide with improved potency against gram-negative organisms. Antimicrob Agents Chemother. 1987 Dec;31(12):1939–1947. doi: 10.1128/aac.31.12.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B. L., Pruul H. Sensitization of complement-resistant smooth gram-negative bacterial strains. Infect Immun. 1971 Mar;3(3):365–372. doi: 10.1128/iai.3.3.365-372.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton C. W., Reller L. B. Serum dilution test for bactericidal activity. I. Selection of a physiologic diluent. J Infect Dis. 1977 Aug;136(2):187–195. doi: 10.1093/infdis/136.2.187. [DOI] [PubMed] [Google Scholar]
- Sullam P. M., Drake T. A., Täuber M. G., Hackbarth C. J., Sande M. A. Influence of the developmental state of valvular lesions on the antimicrobial activity of cefotaxime in experimental enterococcal infections. Antimicrob Agents Chemother. 1985 Mar;27(3):320–323. doi: 10.1128/aac.27.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]