Abstract
Sperm of many animals must complete an exocytotic event, the acrosome reaction, in order to fuse with eggs. In mammals, acrosome reactions are triggered during sperm contact with the egg extracellular matrix, or zona pellucida, by the matrix glycoprotein ZP3. Here, we show that ZP3 stimulates production of phosphatidylinositol-(3,4,5)-triphosphate in sperm membranes. Phosphatidylinositol-3-kinase antagonists that prevent the production of this phosphoinositide blocked acrosome reactions and fertilization in vitro, while generation of this phosphoinositide in the absence of ZP3 triggered acrosome reactions. Downstream effectors of phosphatidylinositol-(3,4,5)-triphosphate in sperm include the protein kinases, Akt and PKCζ. These studies outline a signal transduction pathway that plays an essential role in the early events of mammalian fertilization.
Keywords: fertilization; acrosome reaction; sperm; Akt; protein kinase C; phosphatidylinositol; phosphatidylinositol 3-kinase; phosphatidylinositol (3,4,5)-triphosphate
Introduction
Mammalian sperm contain a single secretory vesicle, or acrosome, in the apical region of the head. Exocytosis occurs during the acrosome reaction, resulting in the release of vesicular contents and in the display of a new membrane domain at the cell surface. One functional consequence of the acrosome reaction is that sperm first exhibit the ability to interact with and fuse with the egg plasma membrane. Acrosome reactions can be triggered during sperm contact with the egg extracellular matrix, or zona pellucida. The stimulatory activity was subsequently assigned to ZP3, a glycoprotein component of the zona pellucida (reviewed by Florman and Ducibella, 2006). Interest in the mechanism of ZP3 action was further enhanced with the recognition that a failure of this process may be associated with infertility syndromes in humans (Liu and Baker, 2000, 2003).
One element of ZP3 signal transduction is the hydrolysis of the membrane phosphoinositide, phosphatidylinositol-4,5-diphosphate (PI(4,5)P2), by phospholipase C (Roldan et al., 1994; Fukami et al., 2001, 2003), resulting in an elevation of cytosolic Ca2+ due to sustained influx through TRPC channels (O’Toole et al., 2000; Jungnickel et al., 2001) and, possibly, release from internal stores (Walensky and Snyder, 1995; De Blas et al., 2002). However, the role of other aspects of phosphoinositide signaling during ZP3 signaling has not yet been explored extensively.
Membrane phosphoinositides are also substrates for 1-phosphatidylinositol-3 (PI3) kinases. The phosphoinositide products which are phosphorylated at the D3 hydroxyl provide association sites for a number of protein domains, permitting the local formation of protein assemblies at the cytosolic face of membranes. These protein complexes are linked to the control cytoskeletal and membrane dynamics, chemotaxis and other signal transduction events (Vanhaesebroeck et al., 2001; Cantley, 2002; Weiner, 2002; DiNitto et al., 2003; Czech, 2003). PI3 kinase is also implicated in the control of exocytosis in a number of systems, including that of vesicles containing glucose transporters with plasma membranes of adipocytes and muscle (Bryant et al., 2002; Czech, 2003) and of vesicles containing TRPC5 with plasma membranes of cultured hippocampal neurons (Bezzerides et al., 2004), as well the exocytic release of glutamate from synaptosomes and hippocampal CA1 neurons (Cousin et al., 2003). Further, two observations hint at a role of D3-phosphoinositides in sperm exocytosis. First, sperm express a putative scaffold protein, enkurin, which may tether p85, the regulatory subunit of class 1A PI3 kinase, to a ZP3-activated TRPC cation channel (Sutton et al., 2004). Second, acrosome reactions can be triggered experimentally by factors of uncertain physiological relevance (Harper and Publicover, 2005) and, in some cases, these are blocked by PI3 kinase inhibitors (Feng et al., 1998, 2005; Fisher et al., 1998). However, the role of PI3 kinase or of D-3 phosphoinositides in ZP3 signaling has not been examined.
Here, we provide evidence that ZP3 stimulation leads to the accumulation of phosphatidylinositol-(3,4,5)-triphosphate (PI(3,4,5)P3) in sperm, resulting in the activation of the downstream effector protein kinase, Akt (protein kinase B). Additional data indicates a role of a second protein kinase, PKCζ. This signaling cascade is essential for the acrosome reaction.
Materials and Methods
Reagents
Phospholipids were obtained from Eschelon Biosciences (Salt Lake City UT; PI(3)P) or from Avanti Polar Lipids (Alabaster AL; all other phospholipids). [32P]-PO4-2 (carrier-free, 10 mCi/ml) was from Perkin Elmer (Wellesley MA). Antibodies were from: Alexafluor-546 goat anti-rabbit IgG, Molecular Probes (Eugene OR); antibody against Akt [pT308], Biosource International (Camarillo CA); and all others, Santa Cruz Biotechnology, Inc. (Santa Cruz CA). Protein kinase inhibitors were obtained from: Akt inhibitors IV and V, EMD Biosciences-Calbiochem (La Jolla CA); Akt inhibitors SH-5 and SH-6, Alexis Corp. (Lausen CH); myristolated and non-myristolated pseudosubstrate PKC isoform inhibitors, EMD Biosciences-Calbiochem; and myristolated scrambled peptide control for protein kinase Cζ inhibitor (myr-SIYRRGARRWRKL), Quality Controlled Biochemicals (Hopkinton MA). 740 Y-P (RQIKIWFQNRRMKWKKSDGG[pY]MDMS) was from Tocris Biosciences (Ellisville, MO); PTEN inhibitors bpV(OHpic), bpV(bipy), bpV(phen) and bpV(pic) from EMD Biosciences-Calbiochem; and all other chemicals and reagents were obtained from Sigma (St Louis MO).
Preparation of sperm
Sperm are released from caudae epididymides of 12-16 week old Swiss albino mice (CD-1, Charles River Lab., Wilmington MA) into a modified Whitten’s medium (mW), containing (mM): NaCl (100), KCl (4.7), KH2PO4 (1.2), MgSO4 (1.2), glucose (5.56), Na+-pyruvate- (1), Na+-lactate (3.2), Ca2+-lactate (1.6), Hepes (20) pH 7.4. Sperm were washed by sedimentation (700xg, 10 min, room temperature), resuspended to a final concentration of 106 sperm/ml in either a non-capacitating (mW) or capacitating medium (mWab: mW, 10 mg/ml bovine serum albumin, 20 mM NaHCO3), and incubated (37°C in air) for 1 hr. This medium supports sperm capacitation and fertilization (Eppig, 1999).
For in vitro fertilization experiments capacitated sperm (104/ml) were incubated with ovulated eggs for 4 hrs in mWab medium. These conditions are designed so that fertilization rate is sensitive to changes in sperm fecundity, although absolute fertilization levels are not optimal (Amann and Hammerstedt, 2002). Criteria for fertilization included either a decondensing sperm head or two pronuclei within Hoechst 33342-stained eggs. Acrosome reaction assays were carried out by incubating capacitated sperm (106/ml) for 20 min in mWab containing ZPse (5 ZP/ul, ∼20 ug/ml), purified zona pellucida glycoproteins from an equivalent concentration of zonae pellucidae (ZP1, ZP2-10 ug/ml; ZP3, 5 ug/ml), or control proteins (20 ug/ml)(O’Toole et al., 2000; Jungnickel et al., 2001). Experiments were typically carried out with ZPse and results confirmed with ZP3. ZP3 and ZPse produced similar effects and these data were frequently pooled for presentation. Acrosome reactions were determined in fixed sperm by Coomassie blue staining patterns (Thaler and Cardullo, 1995).
Zonae pellucidae were isolated from ovarian homogenates of CD-1 mice (5-6 weeks old) by density sedimentation and solubilized in 5 mM NaH2PO4 (pH 2.5; 60°C; 1 hr) as described previously (Jungnickel et al., 2001). When required, ZP1, ZP2 and ZP3 were isolated by HPCL and purity confirmed by SDS-PAGE.
Lipid analysis
Sperm (4×107/ml) were incubated for 1 hr in mW or mWab containing no KH2PO4 and supplemented with 150 μCi/ml [324 P]-PO4-2 in order to label ATP pools. Control experiments showed that sperm incubated in mWab under these conditions were capacitated (data not shown), as assessed in zona pellucida-evoked acrosome reaction assays (Ward and Storey, 1984).
Sperm lipids were extracted in CHCl3:CH3OH: HCl (500:1000:6, v/v), dried under N2, resuspended in CHCl3:CH3OH (2:1, v/v), and applied to silica-gel 60 plates (0.25 mm thickness; E. Merck, Darnstadt DE) that had been impregnated with potassium oxalate (1% w/v)/EGTA (4 mM) and activated (1 hr, 110°C). Thin layer chromatograms were developed in a CHCl3:CH3OH/NH3/H20 (100:70:14:25 v/v) solvent. PI(3)P, PI(3,4)P2, PI(3,5)P2, PI(4,5)P2, and PI(3,4,5)P3 standards were identified with I2 vapors. [32P]-Phospholipids were quantified with a Storm PhosphorImager (Molecular Dynamics, Sunnyvale CA). Data for each phospholipid species were normalized within each experiment to [32P] levels prior to treatment with ZP3 or other proteins and expressed as fold-increase.
Immunofluorescence and Immunoblotting
Immunoblotting was performed as described previously (Sutton et al., 2004). In immunofluorescence studies excitation illumination was provided by the 75W Xe lamp of a PolyChrome II monochromator (TILL Photonics, Grafelfing DE) and directed towards the excitation port of an Axiovert 200 microscope (Zeiss, Thornwood NY) equipped with a 100x Plan-Neofluar 1.3 N.A. objective. Images were acquired using a C4742.80 digital camera (Hamamatsu Photonics, Bridgewater NJ) coupled to VS4-1845 image intensifier (Videoscope International, Dulles VA). Image acquisition and off-line processing was accomplished using OpenLab 5.0.1 (Improvision Inc., Lexington MA).
Immunfluorescence controls included use of pre-immune serum, (when available) and lots of non-immune rabbit Ig; parallel studies with primary antibodies against proteins that are not present in mouse sperm (p110β, TRPC4, PKCε); as well no-primary antibody controls. In addition, only antibodies that were selective and specific in immunoblot experiments were used for immunofluorescence localization. Akt activation as assessed from immunofluorescence signals using an activation-state anti-pT308-Akt as well as a pan-Akt antibody to monitor total Akt levels.
Results
Effects of PI3 kinase inhibitors on ZP3-evoked acrosome reactions
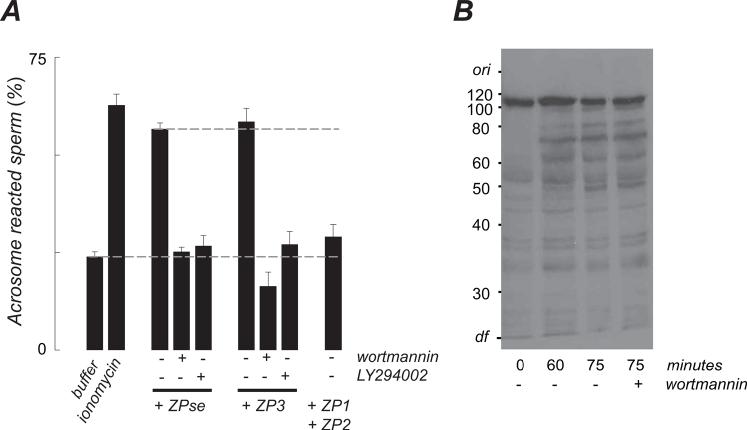
Capacitated sperm1 were stimulated with either a soluble extract of zonae pellucidae (ZPse) or with the purified zona pellucida glcyoproteins ZP1, ZP2, and ZP3. As reported previously, ZPse stimulated acrosome reactions and agonist activity was retained by ZP3 following purification but not by ZP1 or ZP2 (Bleil and Wassarman, 1983). Evoked exocytosis was suppressed by >90% when sperm were pretreated with either of two PI3 kinase inhibitors, 100 nM wortmannin or 10 μM 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002)(Fig. 1A). These agents act by different mechanisms and at doses in sperm that were similar to those required for inhibition of PI3 kinase in other cells, where IC50 values are in the low nanomolar and low micromolar range for wortmannin and LY294002, respectively (Walker et al., 2000). Taken together, these observations suggest a role of PI3 kinase in ZP3 signal transduction.
Figure 1.
Effects of PI3 kinase inhibitors on the zona pellucida-induced acrosome reaction and capacitation. (A) Acrosome reactions are triggered by ZPse (20 μg/ml) or by ZP3 (5 μg/ml) and these were inhibited by PI3 kinase antagonists (100 nM wortmannin, 10 uM LY294002). Dashed lines indicate acrosome reaction levels in sperm suspensions treated with buffer and with ZPse. The maximum exocytotic response of the preparation is evoked by the Ca2+-conducting ionophore (10 μM ionomycin). Data represents mean (± SD) of 3-5 separate experiments with >200 sperm assessed in each experiment. (B) Distribution of tyrosine phosphorylated proteins in sperm. Sperm were incubated 75 min in a capacitating medium. Wortmannin or solvent were added for the final 15 min.
The acrosome reaction is a critical step in fertilization and so if sperm PI3 kinase activation is essential for exocytosis then inhibition of this enzyme should reduce fertilization rates. To assess this, capacitated sperm were treated for 15 min with 100 nM wortmannin, which inhibits PI3 kinase irreversibly (Walker et al., 2000), and then sperm were incubated with eggs in wortmannin-free medium for 4 hrs. Fertilization by wortmannin-treated sperm was reduced by ∼75% relative to solvent-treated controls when experiments were carried out under conditions in which fertilization was dependent upon sperm concentration (eggs fertilized [%]: solvent control, 24/67 [35.8%]; 100 nM wortmannin, 6/70 [8.6%]). This was not due to an inhibition of egg PI3 kinase, as shown in experiments in which wortmannin was first pre-incubated in cell-free culture medium for 15 min and then added to eggs in the absence of sperm for an additional 15 min. These eggs were subsequently inseminated with sperm that had not been pre-incubated with wortmannin. No inhibition of fertilization was observed relative to wortmannin-free control inseminations (data not shown), consistent with the instability of wortmannin in culture medium (Holleran et al., 2003) and with reports that wortmannin inhibition of egg PI3 kinase does not block mouse fertilization in vitro (Mehlmann et al., 2001). Other control experiments showed that there was no apparent effect of wortmannin on sperm viability, as assessed by the fraction of motile cells or by the incidence of agonist-independent acrosome reactions (data not shown).
Since mammalian sperm must be capacitated in order to undergo ZP3-induced acrosome reactions (Ward and Storey, 1984), and as PI3 kinase activation has been implicated in aspects of capacitation (Nagdas et al., 2002; Nauc et al., 2004), then the effects of PI3 kinase inhibitors on sperm exocytosis might be an indirect consequence of a suppression of capacitation. Although sperm were routinely capacitated prior to use here, it remained possible that inhibition of sperm PI3 kinase reversed the capacitation state and placed sperm in a ZP3-refractory state. The enhanced tyrosine phosphorylation of sperm proteins that accompanies capacitation (Visconti et al., 1995; Florman and Ducibella, 2006) provided a means for testing this possibility. We found that addition of wortmannin to capacitated sperm had no detectable effects on this wave of protein tyrosine phosphorylation (Fig. 1B). This suggests that a capacitated state, once achieved, is not reversed by PI3 kinase inhibitors, and in turn suggests that sperm PI3 kinase plays a role in ZP3 signal transduction during fertilization.
Effects of ZP3 on sperm phosphoinositides
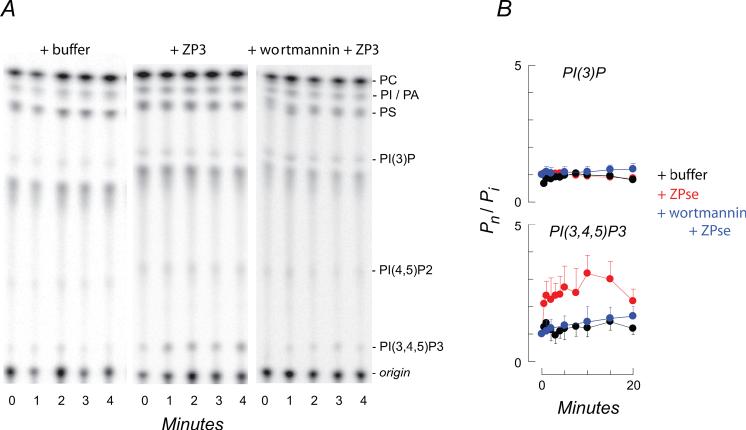
The major products of the wortmannin/LY294002-sensitive PI3 kinases are PI(3)P, PI(3,4)P2, and PI(3,4,5)P3. To detect these products sperm ATP pools were radiolabeled during incubation with 32PO4-2, phospholipids extracted following stimulation with ZP3 or ZPse, and phosphoinositides were resolved by thin layer chromatography (Fig. 2A).
Figure 2.
Zona pellucida agonists activate phosphoinositide signaling pathways in sperm. (A) Examples of the early effects of ZP3 on sperm phosphoinositides are shown. ZP3 (5 μg/ml) stimulated labeling of PI(3,4,5)P3 but not of PI(3)P. Enhanced labeling was inhibited by 100 nM wortmannin. Relative migration of sperm phosphoinositides and other phospholipids were identified using purified standards. Abbreviations: PC, phosphatidylcholine; PA, phosphatidic acid; phosphatidylinositides were defined previously. The spot migrating beneath PI(3)P could not be identified but did not change during treatment with ZPse or ZP3. (B) Time courses of the effects of ZP3 or of ZPse (5 and 20 μg/ml, respectively) on sperm phosphoinositides are shown. Data (mean ± SD, 7-10 experiments) were obtained from chromatograms of response time courses, similar to those in panel A, and expressed as the ratio of labeling at the indicated time, Pn, to the initial activity prior to addition of agonist or control proteins, Pi. ZPse and ZP3 had similar effects and results are pooled for presentation (ZP3 and ZPse,  ). PI3 kinase antagonists (100 nM wortmannin and 10 uM LY294002) had similar inhibitory effects on the ZP3/ZPse-evoked responses and data are pooled for presentation (
). PI3 kinase antagonists (100 nM wortmannin and 10 uM LY294002) had similar inhibitory effects on the ZP3/ZPse-evoked responses and data are pooled for presentation ( ). Similarly, control proteins (10 ug/ml ZP1 or ZP2; 20 ug/ml BSA, a1-acid glycoprotein) failed to evoke a phosphoinositide response and these results are pooled for presentation (
). Similarly, control proteins (10 ug/ml ZP1 or ZP2; 20 ug/ml BSA, a1-acid glycoprotein) failed to evoke a phosphoinositide response and these results are pooled for presentation ( ).
).
Stimulation with ZPse or with ZP3 produced a rapid labeling of PI(3,4,5)P3 pools. In the continuous presence of agonists a half-maximal incorporation of 32P occurred within 30 sec; a peak response of >2.5-fold stimulation in steady state labeling occurred within 3-5 min; and peak levels were maintained for >10 min (Fig. 2A, B). Agonist-dependent lipid kinase activity preceded exocytosis, which occurred with a half-maximal response within 5-7.5 min (see Fig. 3D), similar to previous observations (O’Toole et al., 2000). Moreover, both wortmannin and LY294002 inhibited the ZP3/ZPse-dependent production of PI(3,4,5)P3 at the same concentrations that inhibited the evoked acrosome reaction (Fig. 1A). In contrast, proteins that did not induce acrosome reactions (BSA, α1-acid glycoprotein, ZP1, ZP2) failed to drive PI(3,4,5)P3 production (Fig. 2A, B).
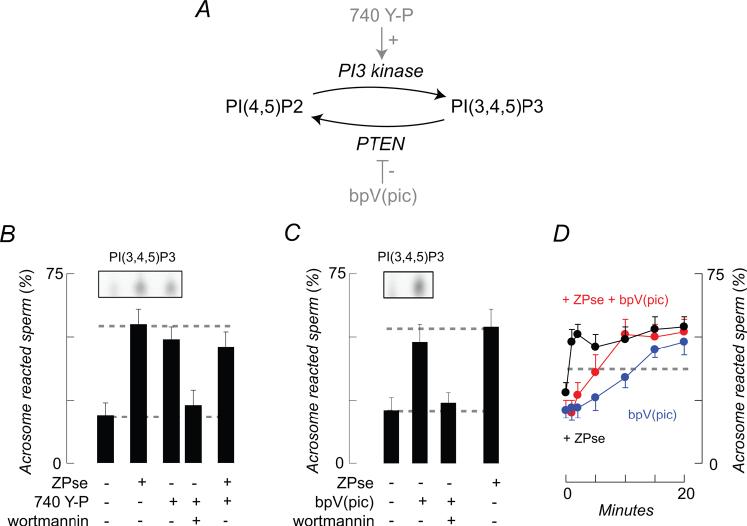
Figure 3.
Effects of a D3-phosphoinositide regulation on acrosome reactions. (A) D3 phosphinositide kinase/phosphatase cycle, illustrating the targets of the PI3 kinase activator, 740 Y-P, and the lipid phosphatase inhibitor, pbV(pic). (B) 740 Y-P stimulated acrosome reactions in the absence of ZP3/ZPse and exocytosis was inhibited by 100 nM wortmannin. 740 Y-P effects were quantitatively similar to and were not additive with those of ZPse. Inset: 740 Y-P treatment also enhanced 32P-labeling of PI(3,4,5)P3 as determined by thin layer chromatography. (C) pbV(pic) produced increased 32P-labeling of PI(3,4,5)P3 (inset) and induced wortmannin-sensitive acrosome reactions. (D) bpV(pic) accelerates the time course of the ZPse-induced acrosome reaction:  , bpV(pic);
, bpV(pic);  , ZP3/ZPse;
, ZP3/ZPse;  , pbV(pic) + ZP3/ZPse. (B-D) Data represents the mean (± SD) of 5-7 separate experiments with >300 sperm assessed/experiment. Dashed lines in B-C represent minimum acrosome reaction levels in buffer-treated sperm and maximal signals in ZP3/ZPse-treated sperm, respectively. Dashed line in D represents the half-maximal acrosome reaction response. ZP3 and ZPse doses were 5 ug/ml and 20 ug/ml, respectively.
, pbV(pic) + ZP3/ZPse. (B-D) Data represents the mean (± SD) of 5-7 separate experiments with >300 sperm assessed/experiment. Dashed lines in B-C represent minimum acrosome reaction levels in buffer-treated sperm and maximal signals in ZP3/ZPse-treated sperm, respectively. Dashed line in D represents the half-maximal acrosome reaction response. ZP3 and ZPse doses were 5 ug/ml and 20 ug/ml, respectively.
PI3 kinase isoforms differ in their substrate preference and form a variety of D3 phosphorylated products. We were unable to detect a ZP3- or ZPse-evoked increase in the labeling of either PI(3)P (Fig. 2A) or PI(3,4)P2 (data not shown), even in the presence of inhibitors of phosphoinositide phosphatases such as PTEN. We also could not detect the ZP3- or ZPse-dependent formation of PI(3,5)P2 (data not shown), a phosphoinositide that may be regulated by PI3 kinase indirectly (Vanhaesebroeck et al., 1997).
Induction of sperm acrosome reactions by D3-phosphoinositides
If PI3 kinase plays a role in the acrosome reaction then stimulation of enzyme activity in the absence of ZP3 should also drive exocytosis. Capacitated sperm were treated with 740 Y-P, a membrane-permeating phosphopeptide that binds to the p85 regulatory subunit of class IA PI3 kinase isoforms and so activates lipid kinase activity in the absence of agonist or of receptor engagement (Derossi et al., 1998; Williams and Doherty, 1999)(Fig. 3A). This peptide produced a 2.2-fold stimulation of acrosome reactions, a response similar to that to ZPse (Fig, 3B). Two observations link the 740 Y-P-induced acrosome reaction to a stimulation of PI3 kinase. First, peptide effects were attenuated by 100 nM wortmannin (Fig. 3B). Second, 740 Y-P treatment resulted in a ∼2.5-fold increase in 32P-incorporation into PI(3,4,5)P3 (Fig. 3B inset). Interestingly, we were not able to detect elevation of other phosphoinositides in response to 740 Y-P (data not shown) and similarly had found that PI(3,4,5)P3 was the only phosphoinositide which was elevated to detectable levels in response to ZP3 (see above).
740 Y-P drives acrosome reactions in a subpopulation of mouse sperm (Fig. 3B). In this regard, it is understood that not all sperm in a population complete the capacitation process and that ZP3 initiates exocytosis only in capacitated sperm (Ward and Storey, 1984; Arnoult et al., 1999; Florman and Ducibella, 2006). The effects of ZP3/ZPse and 740 Y-P were found to be non-additive (Fig. 3B), indicating that these agents act on the same subpopulation and suggesting that sperm must be capacitated in order to respond to 740 Y-P. This possibility was addressed directly in a series of experiments. A 20 min incubation with 740 Y-P produced a 2.4-fold increase in the level of acrosome reactions in sperm populations that had been incubated under capacitating conditions, from 17±2% to 41±4% (buffer-treated controls rose from 14±2% to 18±3%), whereas similar treatments of uncapacitated populations had no significant effects (untreated, 5±2%, 740 Y-P-treated, 8±3%; n=3, >300 sperm counted/experiment).
As an alternative test of the hypothesis that PI3 kinase products are required for the acrosome reaction, we examined the effects of inhibitors of PTEN. PTEN is a phosphoinositide D3-phosphatase that utilizes PI(3,4,5)P3 as a substrate and so antagonizes the effects of PI3 kinase (Maehama et al., 2001); Fig. 3A). When capacitated sperm were treated with bpV(pic), a membrane-permeating bisperoxovanadium inhibitor of the lipid phosphatase activity of PTEN (Schmid et al., 2004) and, likely, of other phosphoinositide phosphatases, we observed an enhanced labeling of PI(3,4,5)P3 (Fig. 3C, inset). In addition, bpV(pic) drove the acrosome reactions to a level similar to that evoked by ZP3 or ZPse, and this phosphatase inhibitor-induced exocytosis was in turn inhibited to basal levels by 100 nM wortmannin (Fig. 3C). Similar results were observed with three other related bisperoxovanadium inhibitors of PTEN family phosphatases: pbV(OHpic), pbV(bipy), and bpV(phen)(data not shown; (Schmid et al., 2004).
We next examined the effects of bpV(pic) on the time course of ZP3/ZPse-induced acrosome reactions (Fig. 3D). In sperm treated with ZP3 or ZPse acrosome reactions were first observed following an initial lag of ∼2 min and half-maximal and maximal responses were apparent at 6 and 10 min, respectively, as described previously (O’Toole et al., 2000). bpV(pic)-induced exocytosis was slower, with half-maximal and maximal levels reached at ∼12 min and ∼20 min. However, when sperm were treated with bpV(pic) and then with ZPse acrosome reactions occurred without apparent time lag and reached half-maximal and maximal response levels in <30 sec and 1 min, respectively. These observations suggest that PI(3,4,5)P3 levels are maintained by the dynamic balance between lipid kinase and phosphatase activities and distortion of this level can produce exocytosis.
Distribution of PI3 kinase and PTEN in mouse sperm
Sperm are highly polarized cells (Fig. 4A) in which the ZP3 reception and signal processing machinery are located in the head, and particularly in the the acrosomal crescent and equatorial segment regions (Fig. 4A)(Bleil and Wassarman, 1986; Florman et al., 1989; Fukami et al., 2003). If ZP3 activates phosphoinositide-dependent pathways then the necessary lipid kinases, as well as associated phosphatases, should exhibit a similar localization. The distribution of PI3 kinase subunits is illustrated in Fig. 4B and summarized in 4C. Class 1A subunits (catalytic subunits-p110 α, δ; regulatory subunits-p55, p85α/β) were detected in the acrosomal crescent and in flagellum, while class 1B p110γ was observed only in the acrosomal crescent (Fig. 4B,C). In contrast, the class 1A p110β catalytic subunit was not detected by either microscopic or immunoblot methods (Fig. 4B,C).
Figure 4.
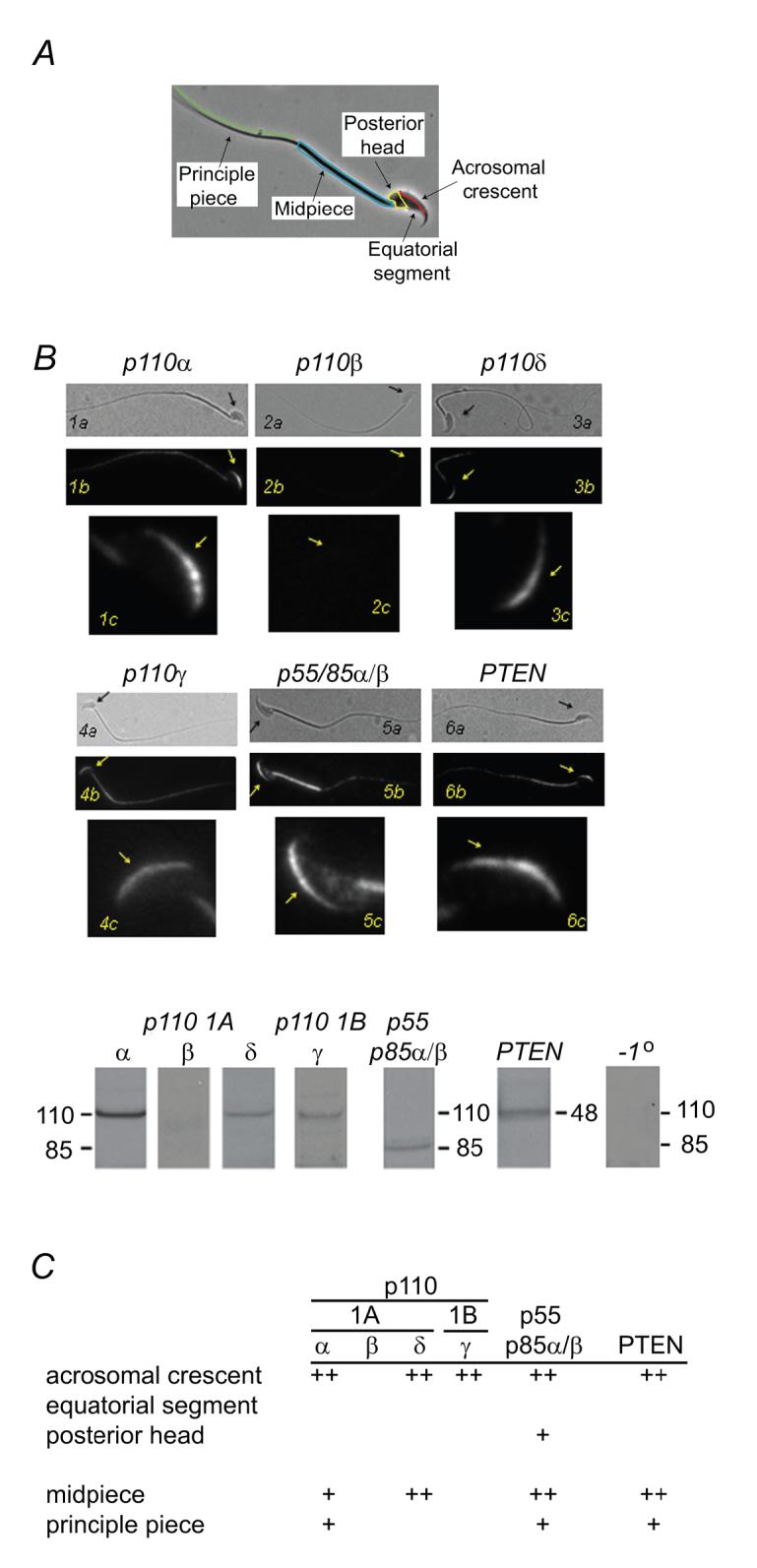
PI3 kinase and PTEN distribution in mouse sperm. (A) Surface domains of mouse sperm. (B, C) Immunolocalization of PI3 kinase catalytic and regulatory subunits and of PTEN. For each panel >100 sperm were examined. A typical labeling pattern is illustrated in (B) and results are summarized in (C). (B) Panel shows paired phase contrast and fluorescence images (a, b; scale bar, 10 μm) and an enlarged fluorescence image of the sperm head (c; scale bar, 5 μm). Arrows facilitate orientation. Immunoblots of PI3 kinase catalytic and regulatory subunits, of PTEN, and of a control (no primary antibody). (C) Summary of immunolocalization results. Class 1A (catalytic subunits: p110α, -δ; a regulatory subunit, p85α) and IB (catalytic subunit, p110γ) PI3 kinase isoforms are detected in sperm and specifically in the acrosomal crescent region, as is PTEN. A third class IA PI3 kinase catalytic subunit (p110β) was not detected. ++, strong labeling; +, weak labeling; blank, no labeling detected.
An antibody generated against recombinant full-length human PTEN labeled mouse sperm in the acrosomal crescent of the head and in the flagellar principle piece. This antibody specifically recognized a 60 kDa protein, the anticipated size of murine PTEN (Fig. 4B,C).
Downstream pathways controlled by ZP3-stimulated phosphoinositides
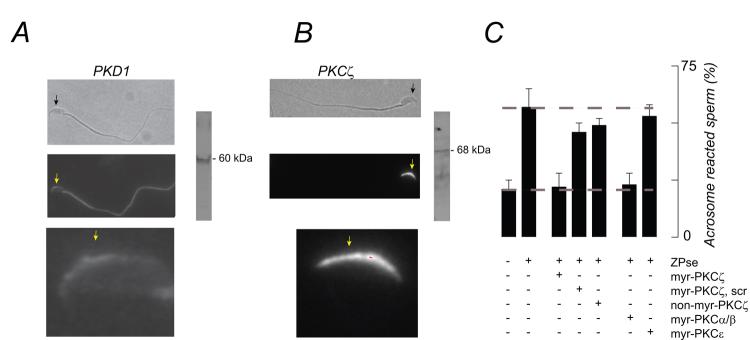
PI(3,4,5)P3 acts through a number of downstream effectors, including PDK1, the 3-phosphoinositide-dependent protein kinase (Vanhaesebroeck and Alessi, 2000). PDK1 is expressed in sperm (Aquila et al., 2004) and found here to be localized in the acrosomal crescent region of the head as well as in the flagellum (Fig. 5A). This enzyme then co-distributes with pools of PI3 kinase (Fig. 4B,C) and provides a plausible PI(3,4,5)P3 target.
Figure 5.
PI3 kinase effectors, PDK1 and PKCζ, in mouse sperm. (A) PDK1 is present in the acrosomal crescent and in the flagellum. (B) PKCζ in restricted to the acrosomal crescent. (A, B) Panels show paired phase contrast and fluorescence images (scale bar, 10 μm), and a magnified fluorescence image (scale bar, 5 μm). Arrows facilitate orientation. Images are representative of 50-100 sperm observed. (C) Effects of PKC isoform-specific pseudosubstrate antagonists on the ZPse- or ZP3-evoked acrosome reaction (20 and 5 ug/ml, respectively). Myristolated (myr-) inhibitors directed against ζ, α/β, and ε isoforms were tested, as well as a myr-scrambled (scr-) PKCζ sequence and a non-myristolated PKCζ reagent. All inhibitors were used at 50 uM. Data represents the mean (± SD) of 3-5 separate experiments, with >300 sperm assessed/experiment. Dashed lines represent the levels of acrosome reaction in sperm treated with buffer or with ZPse or ZP3 in the absence of inhibitors and represent minimal and maximal responses, respectively. ZPse and ZP3 data are pooled.
Since PKD1 is the activation loop kinase for members of the AGC family of protein kinases (Newton, 2003) we examined the role of several members of that protein kinase class in ZP3 signaling. One PI(3,4,5)P3/PDK1 effector, protein kinase C (PKC) ζ, was restricted to the acrosomal crescent (Fig. 5B). When sperm were pretreated with a myristolated pseudosubstrate inhibitor of this atypical PKC the ZP3/ZPse-induced acrosome reaction was completely blocked (Fig. 5B).
Negative controls in these studies included: (i) a non-myristolated PKCζ pseudosubstrate peptide, which should not penetrate living sperm, (ii) a myristolated-scrambled PKCζ peptide, which should not target PKCζ, and (iii) a myristolated PKCε pseudosubstrate peptide, which is directed against an isoform that was not detectable in mouse sperm by either immunoblot or immunofluorescence methods (data not shown). These agents inhibited the ZPse-evoked acrosome reaction by 27%, 34%, and 21% respectively (Fig. 5C). As a positive control we examined the effects of a myristolated pseudosubstrate peptide targeted to PKCα/β, as it had previously been shown that one of these phorbol ester-sensitive isoforms played an essential role in the acrosome reaction (Lee et al., 1987). Here, we found that a myristolated inhibitor directed against α/β isoforms completely inhibited the egg-evoked acrosome reaction (Fig. 5B). These data then suggest that both PI(3,4,5)P3-independent (PKCα/β) and -dependent (PKCζ) isoforms participate in ZP3 signaling.
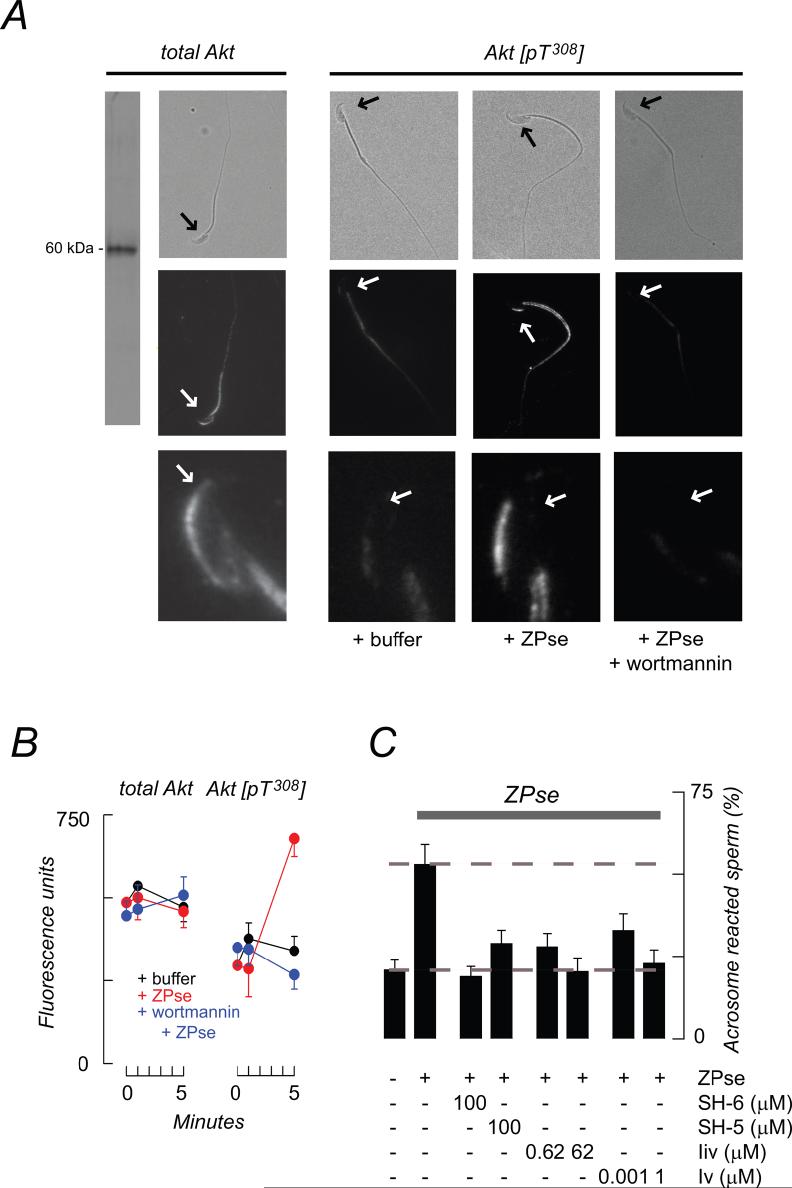
A second PDK1-regulated protein kinase, Akt, is also present in sperm. We observed Akt in the acrosomal crescent, posterior head, and flagellum of mouse sperm, with midpiece immunoreactivity greater than that of principle piece (Fig. 6A, total Akt). Akt is activated by phosphorylation of residues T308 and S473, and PKD1 has been identified as the Akt-T308 kinase (Chan et al., 1999). Local Akt stimulation was monitored using a T308-phosphopeptide antibody. ZP3/ZPse-stimulation produced a 2.3-fold increase in activated Akt in the sperm head (and, to a lesser extent, in the flagellum) within 5 min and that this was completely inhibited by pretreatment with wortmannin (Fig. 6A,B). This stimulatory response was not apparent within the first 1 min (Fig. 6B) and so lags behind the appearance of PI(3,4,5)P3 (see Fig. 2B,C), as expected if phosphoinositide production is a preliminary event to Akt activation. There was no corresponding increase in the amount of total Akt (Fig. 6B), consistent with the lack of active protein synthesis in sperm.
Figure 6.
Role of Akt in the ZP3-evoked acrosome reaction. (A) Akt is present in the acrosomal crescent, posterior head, and flagellum (total Akt). Treatment with ZPse or ZP3 (5 and 20 ug/ml, respectively; data pooled for presentation) resulted in enhanced T308 phosphorylation of Akt. Paired phase contrast and fluorescence images (a,b; scale bar, 10 μm), and magnified fluorescence images (c; scale bar, 5 μm) are shown. Arrows facilitate orientation. Data are quantified in panel B. (B) Effects of ZPse and ZP3 total and active Akt. Pixel intensities were integrated across the sperm head (mean ± SD, triplicate experiments with >25 sperm observed/group in each experiment). ZP3/ZPse treatment produced a 2-3-fold increase in pT308-Akt and this was reduced to basal levels by 100 nM wortmannin. During these treatments there were no significant changes in the total Akt levels. (C) Akt inhibitors block the ZP3/ZPse-induced acrosome reaction. Dashed lines indicate acrosome reaction levels in buffer-treated controls and in ZP3/ZPse-treated samples. Data represent the mean (± SD) of 3-4 separate experiments (>200 sperm observed/group in each experiment).
We next tested whether Akt played a role in sperm exocytosis using three classes of Akt inhibitors that block kinase activity by different mechanisms: the phosphatidylinositol analogs, SH-5 and SH-6, which are expected to act with modest selectivity (Kozikowski et al., 2003); and somewhat more specific agents such as the tricyclic nucleoside inhibitor V (Triciribine; Yang et al., 2004) and the bezimidazole inhibitor IV (Kau et al., 2003). These agents inhibited ZP3/ZPse-induced acrosome reactions in a dose-dependent fashion with maximal inhibition of 75-100% (Fig. 6C).
Finally, it is recognized that ZP3 drives a complex signaling mechanism in sperm which includes elements other than PI3 kinase (reviewed by Florman and Ducibella, 2006), and so might activate Akt and PKCζ through mechanisms other than PI(3,4,5)P3. To assess this, we activated PI3 kinase of capacitated sperm in the absence of ZP3 using 740 Y-P. This produced a >2.5 fold elevation in the fraction of acrosome reacted sperm (control, 19 ± 5%; 740 Y-P, 55 ± 7%; n=3). Pretreatment of sperm with antagonists of either Akt (1 μM inhibitor V) or PKCζ (50 μM myr-pseudosubstrate peptide) inhibited this PI3 kinase-dependent acrosome reaction by 91% (22 ± 3% acrosome reacted) and 82% (25 ± 4% acrosome reacted), respectively. These observations further suggest that the PI3 kinase induced acrosome reaction is mediated by PKCζ and Akt.
Discussion
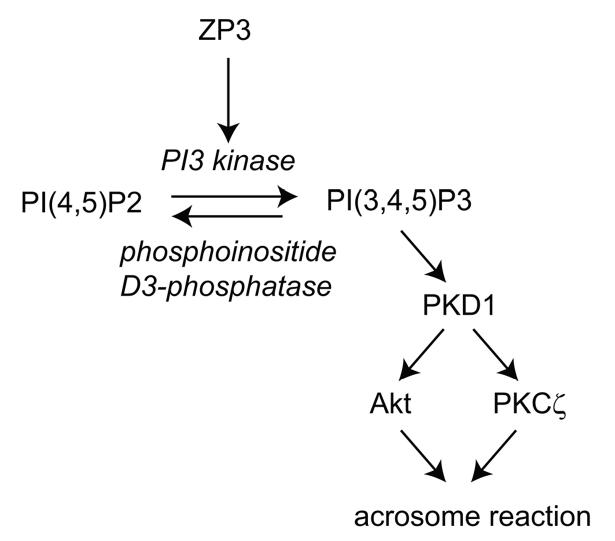
The principle observations of this study are that ZP3 stimulation of capacitated mouse sperm leads to the production of PI(3,4,5)P3 and that the protein kinases Akt and PKCζ function as downstream effectors of phosphoinositide signaling (Fig. 7). This pathway plays an essential role in the induction of the acrosome reaction by the zona pellucida and it is required for successful fertilization in vitro.
Figure 7.
Model of phosphoinositide signaling during the ZP3-evoked acrosome reaction. PI(3,4,5)P3 levels are regulated by a constitutively active D3-phosphorylation/dephosphorylation cycle. ZP3 stimulation during sperm contact with the zona pellucida drives this cycle towards enhanced production of PI(3,4,5)P3. PI(3,4,5)P3 provides binding sites for PDK1 and facilitates it’s activation of the downstream targets, Akt and PKCζ. These downstream effectors are both required to drive sperm exocytosis.
The PI(3,4,5)P3-regulatory machinery appear to consist of a class 1 PI3 kinase and a phosphoinositide D3-phosphophatase. Focus on a class 1 kinase is based on three observations. First, ZP3 stimulation drives the production of PI(3,4,5)P3 in sperm. Class I enzymes utilize PI(4,5)P2 as a substrate and are the major source of PI(3,4,5)P3, whereas other classes either cannot utilize this substrate efficiently or do so only under specialized conditions in vitro (Virbasius et al., 1996; Domin et al., 1997; Vanhaesebroeck et al., 2001; Cantley, 2002). Second, it is consistent with the effects of 740 Y-P, which selectively activates class IA enzymes through binding to SH2 domains of the p85 regulatory subunit (Derossi et al., 1998). Finally, this conclusion is also consistent with the presence of class I phosphoinositide kinases in the anterior sperm head where they would be in the vicinity of the acrosome and of other elements of the ZP3 signal transducing machinery (Bleil and Wassarman, 1986; Florman et al., 1989; Walensky and Snyder, 1995; Ramalho-Santos et al., 2000; Fukami et al., 2001; Jungnickel et al., 2001). Missing at present is supportive data from targeted gene deletion or disruption studies, since mice with a targeted deletion of either p110α or p110b catalytic subunits are not viable and fail to produce sperm (Vanhaesebroeck et al., 2005), while conditional mutants in which gene disruption is restricted to the male germ lineage are not yet available.
Sperm PI(3,4,5)P3 levels are also regulated by a phosphoinositide D-3 phosphatase activity. The strongest evidence is that treatment of sperm with lipid phosphatase inhibitors results in an accumulation of PI(3,4,5)P3 but not in other phosphoinositides, thus demonstrating a D3-phosphatase activity. A protein labeled by a PTEN antibody, and with an Mr similar to that of murine PTEN, is localized in the acrosomal region of the head. However, sperm or the precursor spermatogenic cells also express a number of related lipid phosphatases, including PTEN2/TPEP/TPIP, that share sequence homology and may plausibly serve as targets of PTEN antibodies (Wu et al., 2001; Worby and Dixon, 2005; Murata et al., 2005). These PTEN-related proteins are also likely to be sensitive to the bisperoxovanadium PTEN inhibitors used here. Yet, in many cases these PTEN-related proteins also are phosphoinositide D-3 phosphatases that utilize PI(3,4,5)P3 substrate (Wu et al., 2001; Worby and Dixon, 2005; Murata et al., 2005), and thus would act similarly to PTEN to control PI(3,4,5)P3 levels.
In typical resting cells PI(3,4,5)P3 is present at very low levels (Vanhaesebroeck et al., 2001). In contrast, sperm display detectable levels of this phosphoinositide prior to stimulation with ZP3 and a relatively small agonist-dependent enhancement. This pattern is unanticipated although not without precedent (Hutchcroft et al., 1995). It should be noted then that the sperm that is contacting the zona pellucida is not in a quiescent state with regard to D-phosphoinositide metabolism. It has activated and then hyperactivated flagellar motility (Suarez and Pacey, 2006) and has undergone capacitation (Florman and Ducibella, 2006), all processes that have been linked to PI3 kinase (Luconi et al., 2001, 2004; Nagdas et al., 2002; Nauc et al., 2004). These other processes provide a background and likely account for the low fold-stimulation of PI(3,4,5)P3 following application of ZP3.
The regulation of multiple processes in sperm by PI(3,4,5)P3 suggests fine control over levels and location of this phosphoinositide. For example, capacitation, which entails events associated with the development of ZP3 sensitivity (Florman and Ducibella, 2006), must proceed without crosstalk resulting in inadvertent initiation of exocytosis. The need to isolate these regulatory pathways is essential since premature completion of the acrosome reaction leads to the trapping of sperm within the cumulus oophorus surrounding the egg (Cummins and Yanagimachi, 1986) and is expected to reduce fertility. These considerations, taken together with observations that D3-phosphoinositide phosphatase inhibitors drive exocytosis, suggests that ZP3-independent exocytosis is suppressed in capacitated sperm by D3-phosphoinositide phosphatase acitivity. Coupling of PI3 kinase with an associated phosphatase in a constitutive cycle permits rapid, finely-tuned responses, as has been shown in a number of metabolic pathways (Newsholme and Start, 1973). The molecular mechanism by which ZP3 drives this cycle towards enhanced production of PI(3,4,5)P3 was not the subject of this study and may entail regulation of both kinase and phosphatase activities.
The downstream events of ZP3 signal transduction include the activation of the protein kinases Akt and PKCζ (Fig. 7). This conclusion was based on the demonstration of Akt activation as well as on the effects of both Akt and PKCζ kinase inhibitors on the egg-evoked acrosome reaction. Both of these effectors appear essential for ZP3 signaling, as suggested by the failure of acrosome reactions when either protein kinase was inhibited separately. Akt is activated in other systems by phosphorylation at S473 by the rictor/mTOR complex (Sarbassov et al., 2005) and at T308 by PDK1 (Chan et al., 1999). The finding that ZP3 stimulates T308 phosphorylation of Akt suggests a ZP3-dependent activation of PDK1. Other PDK1-regulated protein kinases that may be activated during ZP3 signaling, as well as the substrates for PKCζ and Akt, remain to be identified.
The studies presented here then strongly suggest that D-3 phosphoinositide production is an essential step in the ZP3 signaling pathway leading to the acrosome reaction. Further, it may also be a sufficient step, as argued by the triggering of exocytosis by direct stimulation of PI3 kinase in the absence of ZP3. Previous studies of ZP3 signal transduction have focused largely on the roles of Ca2+ and of G proteins (reviewed by Florman and Ducibella, 2006). The question of how phosphoinositide mechanisms can be combined with those other pathways to produce a coherent understanding of the control of acrosome reactions during fertilization is not yet answered.
Acknowledgements
We wish to thank Drs. Bayard Storey and Pablo Visconti for valuable discussions. This work was supported by a grant from the National Institute of Child Health and Human Development (HD46948).
Abbreviations List
- PI3 kinase
1-phosphatidylinositol-3-kinase
- PI(3,4,5)P3
phosphatidylinositol(3,4,5)-triphosphate
- ZPse
zona pellucida soluble extract
Footnotes
Mammalian sperm are released from males in an infertile, or uncapacitated, state and acquire the ability to undergo zona pellucida-evoked acrosome reactions and to fertilize eggs within the female reproductive tract or in an appropriate culture medium. This functional maturation is known as capacitation (reviewed by Florman and Ducibella, 2006). Unless otherwise noted, all experiments here use sperm preparations that were capacitated in vitro.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amann RP, Hammerstedt RH. Detection of differences in fertility. J. Androl. 2002;23:317–325. [PubMed] [Google Scholar]
- Aquila S, Sisci D, Gentile M, Middea E, Catalano S, Carpino A, Rago V, Ando S. Estrogen receptor (ER)alpha and ER beta are both expressed in human ejaculated spermatozoa: evidence of their direct interaction with phosphatidylinositol-3-OH kinase/Akt pathway. J. Clin. Endocrinol. Metab. 2004;89:1443–1451. doi: 10.1210/jc.2003-031681. [DOI] [PubMed] [Google Scholar]
- Arnoult C, Kazam IG, Visconti PE, Kopf GS, Villaz M, Florman HM. Control of the low voltage-activated calcium channel of mouse sperm by egg ZP3 and by membrane hyperpolarization during capacitation. Proc. Natl. Acad. Sci. U. S. A. 1999;96:6757–6762. doi: 10.1073/pnas.96.12.6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE. Rapid vesicular translocation and insertion of TRP channels 5. Nat. Cell Biol. 2004;6:709–720. doi: 10.1038/ncb1150. [DOI] [PubMed] [Google Scholar]
- Bleil JD, Wassarman PM. Sperm-egg interactions in the mouse: sequence of events and induction of the acrosome reaction by a zona pellucida glycoprotein. Dev. Biol. 1983;95:317–324. doi: 10.1016/0012-1606(83)90032-5. [DOI] [PubMed] [Google Scholar]
- Bleil JD, Wassarman PM. Autoradiographic visualization of the mouse egg’s sperm receptor bound to sperm. J. Cell Biol. 1986;102:1363–1371. doi: 10.1083/jcb.102.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4. Nat. Rev. Mol. Cell Biol. 2002;3:267–277. doi: 10.1038/nrm782. [DOI] [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Chan TO, Rittenhouse SE, Tsichlis PN. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu. Rev. Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- Cousin MA, Malladi CS, Tan TC, Raymond CR, Smillie KJ, Robinson PJ. Synapsin I-associated phosphatidylinositol 3-kinase mediates synaptic vesicle delivery to the readily releasable pool. J. Biol. Chem. 2003;278:29065–29071. doi: 10.1074/jbc.M302386200. [DOI] [PubMed] [Google Scholar]
- Cummins JM, Yanagimachi R. Development of ability to penetrate the cumulus oophorus by hamster spermatozoa capacitated in vitro, in relation to the timing of the acrosome reaction. Gamete Res. 1986;15:187–212. [Google Scholar]
- Czech MP. Dynamics of phosphoinositides in membrane retrieval and insertion. Annu. Rev. Physiol. 2003;65:791–815. doi: 10.1146/annurev.physiol.65.092101.142522. [DOI] [PubMed] [Google Scholar]
- De Blas G, Michaut M, Trevino CL, Tomes CN, Yunes R, Darszon A, Mayorga LS. The intraacrosomal calcium pool plays a direct role in acrosomal exocytosis. J. Biol. Chem. 2002;277:49326–49331. doi: 10.1074/jbc.M208587200. [DOI] [PubMed] [Google Scholar]
- Derossi D, Williams EJ, Green PJ, Dunican DJ, Doherty P. Stimulation of mitogenesis by a cell-permeable PI 3-kinase binding peptide. Biochem. Biophys. Res. Commun. 1998;251:148–152. doi: 10.1006/bbrc.1998.9444. [DOI] [PubMed] [Google Scholar]
- DiNitto JP, Cronin TC, Lambright DG. Membrane recognition and targeting by lipid-binding domains. Sci. STKE. 2003. 2003:re16. doi: 10.1126/stke.2132003re16. [DOI] [PubMed] [Google Scholar]
- Domin J, Pages F, Volinia S, Rittenhouse SE, Zvelebil MJ, Stein RC, Waterfield MD. Cloning of a human phosphoinositide 3-kinase with a C2 domain that displays reduced sensitivity to the inhibitor wortmannin. Biochem. J. 1997;326(Pt 1):139–147. doi: 10.1042/bj3260139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppig JJ. Mouse oocyte maturation, fertilization and preimplantation development in vitro. In: Richter JD, editor. A comparative methods approach to the study of oocytes and embryos. Oxford University Press; NY: 1999. pp. 3–9. [Google Scholar]
- Feng H, Sandlow JI, Sandra A. The c-kit receptor and its possible signaling transduction pathway in mouse spermatozoa. Mol. Reprod. Dev. 1998;49:317–326. doi: 10.1002/(SICI)1098-2795(199803)49:3<317::AID-MRD12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Feng HL, Sandlow JI, Zheng LJ. C-kit receptor and its possible function in human spermatozoa. Mol. Reprod. Dev. 2005;70:103–110. doi: 10.1002/mrd.20186. [DOI] [PubMed] [Google Scholar]
- Fisher HM, Brewis IA, Barratt CL, Cooke ID, Moore HD. Phosphoinositide 3-kinase is involved in the induction of the human sperm acrosome reaction downstream of tyrosine phosphorylation. Mol. Hum. Reprod. 1998;4:849–855. doi: 10.1093/molehr/4.9.849. [DOI] [PubMed] [Google Scholar]
- Florman HM, Ducibella T. Fertilization in Mammals. In: Neill JD, editor. Physiology of Reproduction. Elsevier; San Diego CA: 2006. pp. 55–112. [Google Scholar]
- Florman HM, Tombes RM, First NL, Babcock DF. An adhesion-associated agonist from the zona pellucida activates G protein-promoted elevations of internal Ca and pH that mediate mammalian sperm acrosomal exocytosis. Dev. Biol. 1989;135:133–146. doi: 10.1016/0012-1606(89)90164-4. [DOI] [PubMed] [Google Scholar]
- Fukami K, Nakao K, Inoue T, Kataoka Y, Kurokawa M, Fissore RA, Nakamura K, Motoya K, Mikoshiba K, Yoshida N, Takenawa T. Requirement of phospholipase Cδ4 for the zona pellucida-induced acrosome reaction. Science. 2001;292:920–923. doi: 10.1126/science.1059042. [DOI] [PubMed] [Google Scholar]
- Fukami K, Yoshida M, Inoue T, Kurokawa M, Fissore RA, Yoshida N, Mikoshiba K, Takenawa T. Phospholipase Cδ4 is required for Ca2+ mobilization essential for acrosome reaction in sperm. J. Cell Biol. 2003;161:79–88. doi: 10.1083/jcb.200210057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper CV, Publicover SJ. Reassessing the role of progesterone in fertilization--compartmentalized calcium signalling in human spermatozoa. Hum. Reprod. 2005;20:2675–2680. doi: 10.1093/humrep/dei158. [DOI] [PubMed] [Google Scholar]
- Holleran JL, Egorin MJ, Zuhowski EG, Parise RA, Musser SM, Pan SS. Use of high-performance liquid chromatography to characterize the rapid decomposition of wortmannin in tissue culture media. Anal. Biochem. 2003;323:19–25. doi: 10.1016/j.ab.2003.08.030. [DOI] [PubMed] [Google Scholar]
- Hutchcroft JE, Franklin DP, Tsai B, Harrison-Findik D, Varticovski L, Bierer BE. Phorbol ester treatment inhibits phosphatidylinositol 3-kinase activation by, and association with, CD28, a T-lymphocyte surface receptor. Proc. Natl. Acad. Sci. U. S. A. 1995;92:8808–8812. doi: 10.1073/pnas.92.19.8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungnickel MK, Marrero H, Birnbaumer L, Lemos JR, Florman HM. Trp2 regulates entry of Ca2+ into mouse sperm triggered by egg ZP3. Nat. Cell Biol. 2001;3:499–502. doi: 10.1038/35074570. [DOI] [PubMed] [Google Scholar]
- Kau TR, Schroeder F, Ramaswamy S, Wojciechowski CL, Zhao JJ, Roberts TM, Clardy J, Sellers WR, Silver PA. A chemical genetic screen identifies inhibitors of regulated nuclear export of a Forkhead transcription factor in PTEN-deficient tumor cells. Cancer Cell. 2003;4:463–476. doi: 10.1016/s1535-6108(03)00303-9. [DOI] [PubMed] [Google Scholar]
- Kozikowski AP, Sun H, Brognard J, Dennis PA. Novel PI analogues selectively block activation of the pro-survival serine/theonine kinase Akt. J. Amer. Chem. Soc. 2003;125:1144–1145. doi: 10.1021/ja0285159. [DOI] [PubMed] [Google Scholar]
- Lee MA, Kopf GS, Storey BT. Effects of phorbol esters and a diacylglycerol on the mouse sperm acrosome reaction induced by the zona pellucida. Biol. Reprod. 1987;36:617–627. doi: 10.1095/biolreprod36.3.617. [DOI] [PubMed] [Google Scholar]
- Liu DY, Baker HWG. Defective sperm-zona pellucida interaction: a major cause of failure of fertilization in clinical in-vitro fertilization. Hum. Reprod. 2000;15:702–708. doi: 10.1093/humrep/15.3.702. [DOI] [PubMed] [Google Scholar]
- Liu d.Y., Baker HW. Disordered zona pellucida-induced acrosome reaction and failure of in vitro fertilization in patients with unexplained infertility. Fertil. Steril. 2003;79:74–80. doi: 10.1016/s0015-0282(02)04555-7. [DOI] [PubMed] [Google Scholar]
- Luconi M, Carloni V, Marra F, Ferruzzi P, Forti G, Baldi E. Increased phosphorylation of AKAP by inhibition of phosphatidylinositol 3-kinase enhances human sperm motility through tail recruitment of protein kinase A. J. Cell Sci. 2004;117:1235–1246. doi: 10.1242/jcs.00931. [DOI] [PubMed] [Google Scholar]
- Luconi M, Marra F, Gandini L, Filimberti E, Lenzi A, Forti G, Baldi E. Phosphatidylinositol 3-kinase inhibition enhances human sperm motility. Hum. Reprod. 2001;16:1931–1937. doi: 10.1093/humrep/16.9.1931. [DOI] [PubMed] [Google Scholar]
- Maehama T, Taylor GS, Dixon JE. PTEN and myotubularin: novel phosphoinositide phosphatases. Annu. Rev. Biochem. 2001;70:247–279. doi: 10.1146/annurev.biochem.70.1.247. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Chattopadhyay A, Carpenter G, Jaffe LA. Evidence that phospholipase C from the sperm is not responsible for initiating Ca(2+) release at fertilization in mouse eggs. Dev. Biol. 2001;236:492–501. doi: 10.1006/dbio.2001.0329. [DOI] [PubMed] [Google Scholar]
- Murata Y, Iwasaki H, Sasaki M, Inaba K, Okamura Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 2005;435:1239–1243. doi: 10.1038/nature03650. [DOI] [PubMed] [Google Scholar]
- Nagdas SK, Winfrey VP, Olson GE. Identification of ras and its downstream signaling elements and their potential role in hamster sperm motility. Biol. Reprod. 2002;67:1058–1066. doi: 10.1095/biolreprod67.4.1058. [DOI] [PubMed] [Google Scholar]
- Nauc V, de Lamirande E, Leclerc P, Gagnon C. Inhibitors of phosphoinositide 3-kinase, LY294002 and wortmannin, affect sperm capacitation and associated phosphorylation of proteins differently: Ca2+-dependent divergences. J. Androl. 2004;25:573–585. doi: 10.1002/j.1939-4640.2004.tb02828.x. [DOI] [PubMed] [Google Scholar]
- Newsholme EA, Start C. Regulation of metabolism. John Wiley and Sons; Toronto: 1973. [Google Scholar]
- Newton AC. Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem. J. 2003;370:361–371. doi: 10.1042/BJ20021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole CMB, Arnoult C, Darszon A, Steinhardt RA, Florman HM. Ca2+ entry through store-operated channels in mouse sperm is initiated by egg ZP3 and drives the acrosome reaction. Mol. Biol. Cell. 2000;11:1571–1584. doi: 10.1091/mbc.11.5.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalho-Santos J, Moreno RD, Sutovsky P, Chan AW, Hewitson L, Wessel GM, Simerly CR, Schatten G. SNAREs in mammalian sperm: possible implications for fertilization. Dev. Biol. 2000;223:54–69. doi: 10.1006/dbio.2000.9745. [DOI] [PubMed] [Google Scholar]
- Roldan ERS, Murase T, Shi Q-X. Exocytosis in spermatozoa in response to progesterone and zona pellucida. Science. 1994;266:1578–1581. doi: 10.1126/science.7985030. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Schmid AC, Byrne RD, Vilar R, Woscholski R. Bisperoxovanadium compounds are potent PTEN inhibitors. FEBS Lett. 2004;566:35–38. doi: 10.1016/j.febslet.2004.03.102. [DOI] [PubMed] [Google Scholar]
- Suarez SS, Pacey AA. Sperm transport in the female reproductive tract. Hum. Reprod. Update. 2006;12:23–37. doi: 10.1093/humupd/dmi047. [DOI] [PubMed] [Google Scholar]
- Sutton KA, Jungnickel MK, Wang Y, Cullen K, Lambert S, Florman HM. Enkurin is a novel calmodulin and TRPC channel binding protein in sperm. Dev. Biol. 2004;274:426–435. doi: 10.1016/j.ydbio.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Thaler CD, Cardullo RA. Biochemical characterization of a glycosylphosphatidylinositol-linked hyaluronidase on mouse sperm. Biochem. 1995;34:7788–7795. doi: 10.1021/bi00024a002. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Alessi DR. The PI3K-PDK1 connection: more than just a road to PKB. Biochem. J. 2000;346(Pt 3):561–576. [PMC free article] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Ali K, Bilancio A, Geering B, Foukas LC. Signalling by PI3K isoforms: insights from gene-targeted mice. Trends Biochem. Sci. 2005;30:194–204. doi: 10.1016/j.tibs.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ, Waterfield MD. Synthesis and function of 3-phosphorylated inositol lipids. Annu. Rev. Biochem. 2001;70:535–602. doi: 10.1146/annurev.biochem.70.1.535. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Leevers SJ, Panayotou G, Waterfield MD. Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem. Sci. 1997;22:267–272. doi: 10.1016/s0968-0004(97)01061-x. [DOI] [PubMed] [Google Scholar]
- Virbasius JV, Guilherme A, Czech MP. Mouse p170 is a novel phosphatidylinositol 3-kinase containing a C2 domain. J. Biol. Chem. 1996;271:13304–13307. doi: 10.1074/jbc.271.23.13304. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development. 1995;121:1129–1137. doi: 10.1242/dev.121.4.1129. [DOI] [PubMed] [Google Scholar]
- Walensky LD, Snyder SH. Inositol 1,4,5-trisphosphate receptors selectively localized to the acrosomes of mammalian sperm. J. Cell Biol. 1995;130:857–869. doi: 10.1083/jcb.130.4.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EH, Pacold ME, Perisic O, Stephens L, Hawkins PT, Wymann MP, Williams RL. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol. Cell. 2000;6:909–919. doi: 10.1016/s1097-2765(05)00089-4. [DOI] [PubMed] [Google Scholar]
- Ward CR, Storey BT. Determination of the time course of capacitation in mouse spermatozoa using a chlortetracycline fluorescence assay. Dev. Biol. 1984;104:287–296. doi: 10.1016/0012-1606(84)90084-8. [DOI] [PubMed] [Google Scholar]
- Weiner OD. Regulation of cell polarity during eukaryotic chemotaxis: the chemotactic compass. Curr. Opin. Cell Biol. 2002;14:196–202. doi: 10.1016/s0955-0674(02)00310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EJ, Doherty P. Evidence for and against a pivotal role of PI 3-kinase in a neuronal cell survival pathway. Mol. Cell Neurosci. 1999;13:272–280. doi: 10.1006/mcne.1999.0750. [DOI] [PubMed] [Google Scholar]
- Worby CA, Dixon JE. Phosphoinositide phosphatases: emerging roles as voltage sensors. Mol. Interv. 2005;5:274–277. doi: 10.1124/mi.5.5.5. [DOI] [PubMed] [Google Scholar]
- Wu Y, Dowbenko D, Pisabarro MT, Dillard-Telm L, Koeppen H, Lasky LA. PTEN 2, a Golgi-associated testis-specific homologue of the PTEN tumor suppressor lipid phosphatase. J. Biol. Chem. 2001;276:21745–21753. doi: 10.1074/jbc.M101480200. [DOI] [PubMed] [Google Scholar]
- Yang L, Dan HC, Sun M, Liu Q, Sun XM, Feldman RI, Hamilton AD, Polokoff M, Nicosia SV, Herlyn M, Sebti SM, Cheng JQ. Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res. 2004;64:4394–4399. doi: 10.1158/0008-5472.CAN-04-0343. [DOI] [PubMed] [Google Scholar]