Abstract
BACKGROUND
Pancreatic cancer is a multifactorial disease with metastasis-prone and therapy-resistant nature. The authors hypothesized that genetic variants of glutathione S-transferase (GST) affect detoxification of carcinogens and anticancer agents in the human pancreas and, thus, the risk and survival of pancreatic cancer.
METHODS
Genotypes of GSTM1, GSTT1, and GSTP1 were determined in 352 patients with pancreatic ductal adenocarcinoma and in a control group of 315 healthy, non-Hispanic whites (frequency-matched by age and sex). Survival analysis was performed in a subset of 290 patients. Epidemiological and clinical information was obtained. A multiple unconditional logistic regression model, a Cox proportional hazards model, and log-rank tests were used for statistical analysis.
RESULTS
No significant main effects of any of 3 GST genes on the risk of pancreatic cancer were observed. Subgroup analysis showed that older individuals (aged 62 years) who carried the GSTP1*C (105Val-114Val) containing genotype tended to have a reduced risk compared with younger individuals who carried the non-*C genotype (for sex and pack-years of smoking, the adjusted odd ratio was 0.54; 95% confidence interval [95% CI], 0.29-1.02). In a survival analysis of 138 patients who received 5-flurorouracil, patients who carried the GSTP1*C containing genotype had a significantly longer survival than patients who carried the non-*C genotype (multivariate hazard ratio, 0.45; 95% CI, 0.22-0.94).
CONCLUSIONS
The GSTP1*C variant conferred a possible protective effect against pancreatic cancer in older individuals and a significant survival advantage in patients who received 5-florouracil. The current findings must be confirmed before further inferences can be made.
Keywords: glutathione S-transferase M1 (GSTM1), GSTT1, GSTP1, polymorphism, haplotype, risk, survival, pancreatic cancer
It is estimated that, in 2006, approximately 33,730 Americans will develop pancreatic cancer, and 32,300 will die from this disease.1 The mortality rate of pancreatic cancer (10.5 per 100,000 population) is almost equal to its incidence rate (11.0 per 100,000 population) for both sexes and all ethnic groups.2 Cigarette smoking is the only established environmental risk factor for pancreatic cancer. Very little is known about how genetic and environmental factors interact to foster the development of this malignancy.3 Clinically, treatment of pancreatic cancer remains a challenge, and there is great heterogeneity in the way patients respond to chemotherapy. Therefore, it is important to understand how genetic factors contribute to both predisposition to and clinical outcome of this disease.
The cytosolic glutathione S-transferases (GSTs) (Enzyme Commission no. 2.5.1.18) are a family of phase II isoenzymes that are important parts of the cell defense against numerous harmful chemicals that are produced endogenously and exogenously. GSTs catalyze the addition of reduced glutathione (GSH) to a variety of electrophilic compounds, including tobacco carcinogens4-6 and anticancer agents.7 Eight families of soluble GSTs in humans have been identified,8 and they have overlapping substrate specificities. GST-μ,GST-Θ, and GST-π are encoded by the GSTM1, GSTT1, and GSTP1 genes, respectively; and these 3 genes have been examined extensively in association with genetic susceptibility9,10 and clinical outcomes of several types of human cancer.11-15 Homozygous deletion of the GSTM1 and GSTT1 genes (null genotype) has been associated with the loss or lack of enzyme activity and increased vulnerability to cytogenetic damage.16,17 It has been shown that the exon 5 A1404G, which encodes an isoleucine (Ile)→valine (Val) exchange at codon 105 (Ile105Val) (reference single nucleotide polymorphism [rs] no. 947894), and exon 6 C2294T, which encodes an Ala→Val exchange at codon 114 (Ala114Val) (rs no. 1799811) of the GSTP1 gene, confer lower levels of metabolizing activity toward a variety of carcinogens and anticancer agents.18-20 GST-μ,GST-Θ,and GST-π all are expressed in the human gastrointestinal tract.21 In particular, GST-π appears to be one of the constitutive elements of the human pancreas.22
In the current study, we investigated the association between GST gene polymorphisms and the risk of pancreatic cancer as well as the clinical outcome of patients with cancer. We hypothesized that these polymorphisms influence the detoxification of carcinogens, reactive oxygen species, and anticancer drugs, thereby influencing susceptibility to pancreatic cancer as well as the clinical outcome of patients.23
MATERIALS AND METHODS
Study Population
We investigated the association between polymorphisms and pancreatic cancer risk in a hospital-based, case-control study. Patients and controls were recruited consecutively from January 20, 2000 to December 20, 2004. Patients with pathologically confirmed primary pancreatic ductal adenocarcinoma (International Classification of Diseases for Oncology code C25.3; World Health Organization, 2000) were recruited from the Gastrointestinal Center at The University of Texas M. D. Anderson Cancer Center (M. D. Anderson). Controls were healthy, cancer-free individuals who were recruited from friends and genetically unrelated family members (usually spouses or in-laws) of patients who were diagnosed with cancers other than pancreatic cancer. No study participants had a prior history of cancer (except for nonmelanoma skin cancer). Patients and controls were frequency-matched by age, sex, and race. Informed consent to participate in interviews and to provide a blood sample was obtained from each participant. The study was approved by the M. D. Anderson Institutional Review Board.
During the study period, 561 potential patients were approached, and 442 patients (78.8%) consented to participate in the study. Fifty-one of those 442 patients (11.5%) were excluded because they failed to donate a blood sample after signing their informed consent. In total, 483 potential controls were approached, and 372 individuals (77.0%) consented to participate in the study. Fifteen of those 372 controls (4.0%) were excluded because of incomplete questionnaire data. Consequently, 391 patients and 357 controls met the eligibility criteria. Because of the known ethnic variations in pancreatic cancer risk and genotype distribution, the case-control analysis was focused on non-Hispanic white individuals only, including 352 patients (90.0%) and 315 controls (88.2%). Survival analysis was conducted among 290 non-Hispanic white patients who received chemotherapy or chemoradiation at M. D. Anderson, and their complete medical records were available.
Exposure and Clinical Data Collection
Information on demographics and smoking exposure was collected by personal interview using a structured questionnaire, as described previously.24 The participants were classified as ever-smokers or never-smokers according to whether they had ever smoked >100 cigarettes in their lifetime. Cumulative smoking exposure was estimated in pack-years, which were based on the average number of daily cigarette intake and the number of years smoked.
Clinical information of 290 patients was abstracted from the patients’ medical records, as described previously.25 A comprehensive medical record review was performed to obtain information on neoadjuvant and/or adjuvant chemotherapy/chemoradiation for patients with resectable tumors and on first- and second-line definitive treatments for patients with unresectable tumors. None of these patients underwent tumor resection before they came to M. D. Anderson. Treatment regimens during the entire disease process were collapsed into 3 groups: 1) gemcitabine (GEM)-cisplatin (CDDP) (including oxaliplatin)-based therapy, including GEM/CDDP alone or followed by GEM/radiation; 2) GEM-based therapy, including GEM alone, GEM/radiation, and GEM/investigational agents; and 3) 5-fluorouracil (5-FU)-based therapy, including 5-FU/radiation, 5-FU/other agents, and 5-FU/other agents/radiation. The majority of patients who received 5-FU (88%) received concurrent radiotherapy. Capecitabine, 1 of the synthesized 5-FU prodrugs, was the most common agent administered in 5-FU-based therapy (received by approximately 95% of patients). Treatment was started within 1 month of pathologic diagnosis of pancreatic cancer.
Survival Measurement
The subset of 290 patients was followed through December 30, 2005. The primary endpoint was overall survival (OS) duration from the date of pathologic diagnosis of pancreatic cancer to the date of death from any cause. Patients who were alive at the end of the study period were censored on the last date of follow-up. Each patient’s vital status was obtained and cross-checked with at least 1 of the following: inpatient medical record, the M. D. Anderson Tumor Registry, and the Social Security Death Index.25
Genotyping
Genomic DNA was extracted from peripheral blood mononuclear cells that were obtained from the participants by using the Qiagen DNA isolation kit (Qiagen, Valencia, CA). The DNA aliquot was stored at 4°C for immediate use. GSTM1 and GSTT1 null genotypes were identified by using multiplex polymerase chain reaction, as described previously.26 The experiment reagents were purchased from PGC Scientifics (Frederick, MD). The GSTP1 polymorphisms were identified by using the Masscode system (BioServe Biotechnologies, Ltd., Laurel, MD). We analyzed a random 10% of samples in duplicate for quality control. Any discrepancy in genotype calls (0.2% of the duplicate) was resolved by using additional genotyping. Unsuccessful genotyping occurred most often in GSTP1 assays (<6% of samples) and was caused by inadequate quality or quantity of DNA.
Statistical Analyses
The Intercooled Stata 9.0 software program (Stata Corporation, College Station, TX) and SPSS 12.0 software (SPSS Inc., Chicago, IL) were used for statistical analyses. All of the statistical tests were 2-tailed, and P values ≤.05 were considered statistically significant. The Pearson chi-square test was used to examine differences in the distribution of the risk factors and genotypes between patients and controls. The SNPAlyze software program (DYNACOM Company, Ltd., Mobara, Japan) was used to test for Hardy-Weinberg equilibrium of GSTP1 genotypes and linkage disequilibrium of the 2 alleles. The effect of different allele combinations of the GSTP1 gene was evaluated by using haplotype analysis. Haplotypes were reconstructed from the genotype data by using the PHASE software program (version 2).27 A diplotype (combined genotype) was assigned for each participant.
Odds ratios (ORs) and 95% confidence intervals (95% CIs) were estimated by using an unconditional logistic regression model to examine the association between genotype and pancreatic cancer risk. Potential confounding factors were adjusted in the multivariate model when relevant (ie, when their removal from the multivariate model caused the β parameter estimates to change by >10%). Potential multiplicative interactions between gene-gene, gene and age (<62 years vs ≥62 years; 62 years was the median age in the control group), smoking (never-smoker vs ever-smoker), and pack-years of smoking (light smoker vs heavy smoker; the median number of pack-years in ever-smokers among controls was used as the cut-off point) were explored by generating a cross-product term in the unconditional logistic regression models. The likelihood-ratio test was used to test the significance of the interaction term in the models.
Survival curves were estimated using the Kaplan-Meier product-limit method. Differences in the survival curves were tested by using the log-rank test. Factors that showed a significant effect on survival in the log-rank test were included in the multivariate Cox proportional hazards model to estimate hazard ratios (HRs) and the 95% CIs. Stratified analyses were performed to evaluate the association between genotypes and OS by age, sex, tumor resection, clinical stage, and treatment modality, as defined beforehand. Bonferroni correction was used to adjust the statistical significance level when multiple testing was performed in which α = .05/k, with k equal to the number of independent hypotheses tested.
RESULTS
Characteristics of the Study Participants
There were no significant differences in distribution according to age (10-year intervals) or sex between patients and controls (P .37 and P .40, respectively). The mean age was 61.7 years =(range, 37-87 years) for patients and 61.4 years (range, 32-88 years) for controls. Women accounted for 43.8% and 47.0% of patients and controls, respectively. Ever-smokers were more prevalent among patients (62.5%) than among controls (53.3%; P = .02), with an unadjusted OR of 1.46 (95% CI, 1.06-2.01). The prevalence of heavy smoking (≥22 pack-years) was 38.1% in patients and 27.0% in controls (P = .01; data not shown).
Genotype and Haplotype
Table 1 shows the genotype distribution in patients and controls. The prevalence of the genotype or variant allele was within the ranges commonly reported in whites of European ancestry.28,29 The GSTP1 genotype distribution was in Hardy-Weinberg equilibrium in both patients and controls (P > .30). Ile105Val and Ala114Val were in complete linkage disequilibrium (P < .0001; D′ = 1.0 for both patients and controls). Three haplotypes putatively were inferred—105Ile-114Ala (65.9%), 105Val-114Ala (25.9%), and 105Val-114Val (8.2%)— which corresponded to the GSTP1*A, GSTP1*B, and GSTP1*C alleles, respectively.19,30 The GSTP1*D allele (105Ile-P114Val) was not found in our study population. The haplotype distribution did not differ between patients and controls (P = .64). Six diplotypes (variant combinations) were reconstructed: GSTP1 *A/*A, *A/*B, *A/*C, *B/*B, *B/*C, and *C/*C. It is noteworthy that the GSTP1*A/*C diplotype was related to a lower risk of pancreatic cancer compared with the GSTP1*A/*A diplotype (OR, 0.62; 95% CI, 0.37-1.03) (Table 1). We defined the genotype as GSTP1*C-containing if the 105Val-114Val allele combination was present; we classified all other genotypes as non-GSTP1*C reference genotypes.29
TABLE 1.
Distribution of Glutathione S-transferase Genotypes in Pancreatic Cancer Patients and Controls
| No. (%) |
|||
|---|---|---|---|
| Genotype | Patients (n = 352) | Controls (n = 315) | Unadjusted OR [95% CI] |
| GSTM1 | |||
| Wild type | 187 (54.0) | 157 (49.8) | 1.00 |
| Null | 159 (46.0) | 158 (50.2) | 0.84 [0.62-1.16] |
| GSTT1 | |||
| Wild type | 271 (78.6) | 248 (78.7) | 1.00 |
| Null | 74 (21.4) | 67 (21.3) | 1.01 [0.68-1.49] |
| GSTP1 Ile105Val | |||
| Ile/Ile | 151 (44.7) | 117 (39.3) | 1.00 |
| Ile/Val | 141 (41.7) | 146 (49.0) | 0.75 [0.54-1.04] |
| Val/Val | 46 (13.6) | 35 (11.7) | 1.02 [0.62-1.68] |
| Ile/Val and Val/Val | 187 (55.3) | 181 (60.7) | 0.80 [0.58-1.10] |
| Val allele frequency | 0.34 | 0.36 | |
| GSTP1 Ala114Val | |||
| Ala/Ala | 286 (85.4) | 242 (81.2) | 1.00 |
| Ala/Val | 46 (13.7) | 55 (18.5) | 0.71 [0.46-1.08] |
| Val/Val | 3 (0.9) | 1 (0.3) | 2.53 [0.26-24.6] |
| Ala/Val and Val/Val | 49 (14.6) | 56 (18.8) | 0.74 [0.49-1.13] |
| Val allele frequency | 0.08 | 0.10 | |
| GSTP1 diplotype* | |||
| *A/*A | 161 (45.8) | 127 (40.3) | 1.00 |
| *A/*B | 117 (33.2) | 113 (35.9) | 0.82 [0.58-1.16] |
| *A/*C | 32 (9.1) | 41 (13.0) | 0.62 [0.37-1.03] |
| *B/*B | 25 (7.1) | 19 (6.0) | 1.04 [0.55-1.97] |
| *B/*C | 14 (4.0) | 14 (4.5) | 0.79 [0.36-1.71] |
| *C/*C | 3 (0.8) | 1 (0.3) | 2.34 [0.24-23.0] |
OR indicates odds ratio; 95% CI, 95% confidence interval; GST, glutathione S-transferase; GSTM1, GST M1 polymorphism; GSTT1, GST T1 polymorphism; GSTP1, GST P1 polymorphism; Ile, isoleucine; Val, valine; Ala, alanine.
* A, 105Ile-114Ala; *B, 105Val-114Ala; *C, 105Val-P114Val.
Potential GSTP1 Polymorphism and Age Interaction in the Risk-association Study
We observed a potential interaction between age and the GSTP1 Ile105Val polymorphism in modifying the risk of pancreatic cancer (Pinteraction = .05) (Table 2). Furthermore, older individuals (aged ≥62 years) who carried the GSTP1*C (105Val-114Val)-containing genotype had a nonsignificant reduced risk of pancreatic cancer compared with younger individuals who carried the non-*C genotype (OR adjusted for sex and pack-years of smoking, 0.54; 95% CI, 0.29-1.02). The interaction effect between GSTP1*C and age also was not statistically significant (P = .22).
TABLE 2.
Glutathione S-transferase P1 Polymorphisms and Age Interaction in Modifying Pancreatic Cancer Risk
| Genotype | Age, y | No. of patients/controls | Adjusted OR* | P† |
|---|---|---|---|---|
| GSTP1 Ile105Val | ||||
| Ile/Ile | <62 | 57/57 | 1.00 | |
| Ile/Ile | ≥62 | 89/57 | 1.61 (0.98-2.65) | |
| Ile/Val and Val/Val | <62 | 93/79 | 1.20 (0.74-1.92) | |
| Ile/Val and Val/Val | ≥62 | 89/101 | 0.92 (0.57-1.47) | .05 |
| GSTP1 Ala114Val | ||||
| Ala/Ala | <62 | 128/109 | 1.00 | |
| Ala/Ala | ≥62 | 158/133 | 0.99 (0.70-1.40) | |
| Ala/Val and Val/Val | <62 | 29/26 | 0.94 (0.52-1.70) | |
| Ala/Val and Val/Val | ≥62 | 20/30 | 0.52 (0.28-0.98) | .22 |
| GSTP1 genotypes | ||||
| Non-*C | <62 | 135/119 | 1.00 | |
| Non-*C | ≥62 | 168/140 | 1.04 (0.74-1.45) | |
| *C | <62 | 29/26 | 0.98 (0.54-1.76) | |
| *C | ≥62 | 20/30 | 0.54 (0.29-1.02) | .22 |
OR indicates odds ratio; GSTP1, glutathione S-transferase P1 polymorphism; Ile, isoleucine; Val, valine; Ala, alanine; *C, 105Val114Val.
ORs were adjusted for sex and for the number of smoking pack-years (continuous).
P value for likelihood ratio test for interaction between age (<62 years vs ≥62 years; 62 years was the median age in the control group) and GSTP1 polymorphisms.
Clinical Characteristics of the Patients
The characteristics of the subset of 290 patients who were included in the survival analysis (age, sex, and smoking status) were similar to the characteristics of the entire patient group. Of those 290 patients, 84 patients (29.0%) had localized tumors, 51 patients (17.6%) had potentially resectable tumors, 94 patients (32.4%) had locally advanced tumors, and 61 patients (21.0%) had metastatic tumors. During the study period, 69.3% of the patients died, and the median survival duration was 11.8 months. The median follow-up duration for the surviving patients was 24.5 months. The log-rank test showed that female sex, tumor resected, early-stage disease (localized or potentially resectable vs locally advanced or metastatic), low baseline serum CA19-9 level (categorized by quartile distribution), and a good performance status (<3) were significant, positive prognostic factors for OS at an α = .05 level of significance. These factors were included in the Cox regression model to estimate the adjusted HR.
Genotype and Survival According to Treatment Modalities
We observed no statistically significant associations between any of the polymorphisms and the clinical characteristics, treatment modalities, or OS of patients (log-rank test; all P values ≥.40). Because the association between polymorphisms and OS did not differ according to the first-line or second-line therapy, the analysis was based on whether patients ever received GEM/CDDP-, GEM-, or 5-FU-based therapy (Table 3).
TABLE 3.
Glutathione S-transferase P1 Polymorphisms and Overall Survival by Treatment Modality in Patients With Pancreatic Cancer
| GEM/CDDP-based treatment |
GEM-based treatment |
5-FU-based treatment |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polymorphism | No. of patients | No. of deaths | MS, Months | HR (95% CI)* | P† | No. of patients | No. of deaths | MS, Months | HR (95% CI)* | P† | No. of patients | No. of deaths | MS, Months | HR (95% CI)* | P† |
| Overall | 150 | 105 | 15.9 | 146 | 109 | 17 | 138 | 87 | 18.6 | ||||||
| GSTP1105 | |||||||||||||||
| Ile/Ile | 62 | 45 | 16.2 | 1.00 | 64 | 44 | 18.1 | 1.00 | 62 | 45 | 15.8 | 1.00 | |||
| Ile/Val | 56 | 42 | 12.8 | 1.06 (0.67-1.69) | 66 | 51 | 14.5 | 1.03 (0.67-1.57) | 49 | 27 | 22.2 | 0.64 (0.38-1.09) | |||
| Val/Val | 27 | 15 | 24.5 | 0.74 (0.40-1.37) | 13 | 11 | 19.6 | 0.92 (0.46-1.83) | 20 | 9 | 35.8 | 0.40 (0.18-0.89) | |||
| Ile/Val and Val/Val | 83 | 57 | 14.4 | 0.99 (0.62-1.42) | .02 | 79 | 62 | 16.2 | 1.00 (0.67-1.51) | .41 | 69 | 36 | 23.3 | 0.56 (0.34-0.92) | .02 |
| GSTP1114 | |||||||||||||||
| Ala/Ala | 118 | 83 | 15.9 | 1.00 | 122 | 90 | 18.3 | 1.00 | 118 | 76 | 18.6 | 1.00 | |||
| Ala/Val and Val/Val | 25 | 16 | 14.5 | 106 (0.59-1.92) | .46 | 18 | 16 | 8.5 | 1.58 (0.88-2.82) | .02 | 18 | 9 | 22.3 | 0.45 (0.24-1.02) | .24 |
| GSTP1 genotype | |||||||||||||||
| Non-*C | 125 | 90 | 15.8 | 1.00 | 129 | 94 | 18.3 | 1.00 | 117 | 78 | 16.2 | 1.00 | |||
| *C | 25 | 15 | 18.2 | 1.10 (0.60-2.30) | .30 | 17 | 15 | 11.8 | 1.44 (0.80-2.61) | .02 | 19 | 9 | 22.3 | 0.45 (0.22-0.94)‡ | .11 |
GEM indicates gemcitabine; CDDP, cisplatin; 5-FU, 5-fluorouracil; MS, medial survival; HR, hazard ratio; 95% CI, 95% confidence interval; GSTP1, glutathione S-transferase P1 polymorphism; Ile, isoleucine; Val, valine; ALA, alanine, *C, 105Val-114Val.
HRs were adjusted for sex, tumor resection, tumor stage, CA19-9 value, and performance status.
P value for log-rank test.
P = .025 (not significant at a Bonferroni-adjusted α level of .017).
Among the patients who received GEM/CDDP-based treatment, GSTP1 Val105Val carriers had a longer median survival time (MST) than patients who carried the other 2 genotypes (log-rank test; P= .02). In the patients who received GEM-based treatment, the GSTP1 114Val carriers survived significantly shorter than the Ala114Ala carriers (log-rank test; P= .02). However, the associations were not statistically significant after other clinical predictors were considered. The GSTP1 Ile105Val polymorphism was associated significantly with OS in 138 patients who received 5-FU-based therapy. The MST was 15.8 months (95%CI, 13.1-20.9 months) for patients who carried the Ile/Ile genotype, 22.2 months (95%CI, 16.0-37.9 months) for patients who carried the Ile/ Val genotype, and 35.8 months (95%CI, 14.4 months to not achieved) for patients who carried the Val/Val genotype (log-rank test; P .02) (Fig 1A). Compared with the Ile105Ile genotype, the adjusted HR was 0.64 (95% CI, 0.38-1.09; P= .06) for the Ile105Val genotype and 0.40 (95% CI, 0.18-0.89; P= .015) for the Val105 Val genotype. The GSTP1 Ile105Val polymorphism had no effect on OS in patients who did not receive 5-FU (Fig.1B). Furthermore, the GSTP1*C carriers survived significantly longer than patients who carried the non-*C genotypes (adjusted HR, 0.45; 95% CI, 0.22-0.94; P= .025) (Table 3). However, after Bonferroni correction for multiple testing, the different survival function by GSTP1 genotype (*C vs non-*C) was not statistically significant (P = .025) at an α level of .17 (α = .05/3, assuming 3 independent hypotheses because of 3 treatment regimens). Tumor resection, tumor stage, the GSTP1 Ile105Val polymorphism, and the serum CA19-9 level remained significant independent prognostic factors for survival among the patients who received 5-FU.
FIGURE 1.
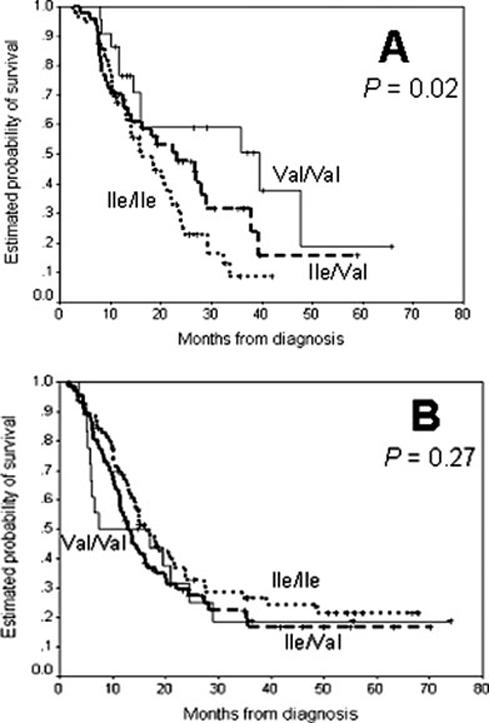
The probability of survival is illustrated according to the presence of the glutathione S-transferase P1 (GSTP1) isoleucine (Ile)→valine (Val) exchange at codon 105 (Ile105Val) polymorphism in patients who received 5-fluorouracil (5-FU)-based chemotherapy/chemoradiation (A) and patients who received therapies other than 5-FU (B). P values were determined from log-rank tests.
There was no significant interactive effect between GSTP1 polymorphisms and smoking status or pack-years of smoking. In addition, there was no suggestive association between GSTM1 and GSTT1 genotypes and the risk or survival of pancreatic cancer, either in combination with other polymorphisms or with other exposure factors (data not shown).
DISCUSSION
To our knowledge, this study is the first to suggest a role of GSTP1 polymorphisms in pancreatic pathogenesis. We observed that the GSTP1*C allele potentially was related to a reduced risk of pancreatic cancer in older individuals and was associated significantly with a reduced hazard of dying in patients who received 5-FU-based treatment. It is unlikely that the GSTM1 and GSTT1 null genotypes play a major role in pancreatic pathogenesis.
Individuals with reduced GST enzymatic activity may be at a greater risk for cancer because of decreased detoxification of tobacco carcinogens. Results from a previous study on pancreatic cancer indicated an interaction between the GSTT1 null genotype and heavy smoking in risk modification.31 Despite the finding in the current study that smoking is a significant risk factor for pancreatic cancer, we did not detect any gene-smoking interaction effect. Instead, we observed a nonsignificant, negative association between the risk of pancreatic cancer and the presence of the GSTP1*C allele exclusively in older individuals. The GSTP1*C variants encode the amino acids 105Val [in the central part of the hydrophobic substrate binding site (H site)] and 114Val (at the beginning of helix 5 outside the H site). Such amino acid substitutions may limit free access of the substrate to the H site, the efficient conjugation with glutathione, and the thermostability of the enzyme.32,33 Several studies have reported an age-specific effect of the GSTP1 polymorphism on the risk of cancer.34,35 The underlining biologic mechanism remains unclear. The possible interaction between age and GSTP1 genotype should be interpreted with caution. Previous studies of the association between GSTP1 polymorphisms and cancer risk generated mixed results.36-38 The discrepancy may be explained in part by the variations in study size, control selection, and genotyping methods as well as by different levels of metabolizing activity conferred by particular alleles toward the electrophilic compounds that are major etiologic agents in certain organs.
Using various study systems, it has been shown that different GSTP1 alleles have different degrees of protection against the cytotoxicity of different anticancer agents.39 For example, the GSTP1*A allele has significantly higher activity against 1-chloro-2,4-dinitrobenzene,19 thiotepa,39,40 and chlorambucil41; whereas GSTP1*B and GSTP1*C have higher conjugation activity toward cisplatin, carboplatin,39 and epoxides of benzo[a]pyrene.42 Expression of GST-π is up-regulated significantly in pancreatic adenocarcinoma,43 indicating its potential role in acquired resistance to certain anticancer drugs. Among the various anticancer agents involved in pancreatic cancer treatment, CDDP is the only one shown to be the substrate for GST-π. However, we did not observe that GSTP1 polymorphisms modified the survivorship of patients who received CDDP-based therapy, but we did observe a reduced hazard of dying associated with the GSTP1*C variant allele in patients who received 5-FU or 5-FU after CDDP. Although capecitabine is a 5-FU prodrug that undergoes complex metabolic pathways and nuclear transport activity before exerting its cytotoxic activity,44 no established data have demonstrated that GSTs are involved in the conjugation of 5-FU and its intermediate metabolites. Nevertheless, results from a previous study indicated an increased survival associated with the GSTP1 105Val variant in patients with advanced colorectal cancer who received 5-FU/oxaliplatint.12 Results from another study45 indicated an increase in GST-π expression in human gastrointestinal cancer cells after sequential administration of CDDP and 5-FU. The same study45 also showed that resistance of a gastrointestinal cancer cell line to 5-FU was related to the expression of multidrug resistance-associated protein. Multidrug resistance-associated protein and GST-π are common cofactors in drug resistance. Goto et al.46 demonstrated that GSH and GST-π are not important to the intracellular metabolism of 5-FU; however, those authors observed nuclear GST-π accumulation in cancer cells in response to treatment with several anticancer drugs, including 5-FU. This evidence lent some indirect support to the association between GSTP1 polymorphisms and survival in patients who received treatment with 5-FU. We speculated that the GSTP1*C allele may affect cellular sensitivity to 5-FU through the drug-resistance mechanism. GST-π is a known regulator of the activity of Jun N-terminal kinase (JNK), which is activated transiently in response to oxidative stress.47 The *C allelic variant may interfere with the regulatory role of GST-π on JNK activity and, ultimately, in the modulation of apoptosis, depending on the redox status of the cell.32 Another possibility is that the GSTP1 gene polymorphisms are in linkage disequilibrium with other nearby genes that determine the biologic properties of 5-FU.
In the current study, we failed to demonstrate any effect of GSTM1 and GSTT1 polymorphisms on pancreatic cancer risk and survival either individually or in combination with other factors. The lack of an association with risk was consistent with previous findings.48,49 The predominant expression of GST-π in pancreatic cells may explain why polymorphisms ofGSTP1, but not of GSTM1 or GSTT1, exerted certain effects on risk and survival of pancreatic cancer in this study.50,21 Alternatively, deficiency of an individual GST-l and GST-Θ isoenzyme may be compensated by other isoforms. We may have missed weak effects conferred by GSTM1 and GSTT1 polymorphisms, because our genotyping assay could not distinguish between wild-type and heterozygous individuals.51
Our survival analysis was limited in a number of ways. 1) Although we adjusted significant predictors of clinical outcome of pancreatic cancer in the multivariate models, we do not know whether residual confounding effects of tumor resection or tumor stage or uncontrolled effects of multiple chemotherapy agents or nutritional status would bias the study observations. 2) We were unable to monitor the cytotoxic effects in patients who received chemotherapy. Indeed, clinical outcomes reflect the balance of the drug response of cancer cells and cytotoxic effects on normal cells. 3) It is not feasible to evaluate GST expression at the messenger RNA or protein levels or enzyme activity in the pancreas during the course of treatment. Therefore, genotype and phenotype associations cannot be established. 4) After Bonferroni correction for multiple testing, the association between the GSTP1*C allele and survival in patients who received treatment with 5-FU was not statistically significant. Therefore, the preliminary finding from this hypothesis-generating study must be replicated in other studies. 5) Our observations were made among non-Hispanic white patients and may not be applicable to other ethnic groups.
In summary, in this report, we have data suggesting that genetic polymorphisms of GSTP1 may be among the mechanisms that modify the risk of pancreatic cancer in older individuals and affect the survival of patients who receive 5-FU-based treatment. Because our major findings were based on post-hoc hypotheses, they must be confirmed in larger, well-characterized populations. Both risk and survival are phenotypic consequences of the interplay of multiple genes in different pathways. Further in-depth studies will be required to improve our understanding of the roles of genetic variations and molecular regulatory machineries of GST-π and other players in pancreatic cancer development and clinical management.
Acknowledgments
We thank the study participants. We acknowledge Yingqiu Du, Ping Chang, Jijiang Zhu, Yanan Li, and Cynthia Zhang for the laboratory work and Rabia Khan, Kaustubh Mestry, Ajay Nooka, and Hui Liu for the fieldwork. We also thank the clinical staff for their assistance with patient recruitment. and Don Norwood for scientific editing.
Footnotes
Supported by National Institutes of Health grants CA84581 and CA98380, National Institute of Environmental Health Sciences Center grants P30 ES07784, National Institutes of Health Cancer Center Support (Core) grants CA16672, and a research grant from The University of Texas M. D. Anderson Cancer Center.
REFERENCES
- 1.American Cancer Society . Cancer Facts and Figures: 2006. American Cancer Society; Atlanta, Ga: 2006. [Google Scholar]
- 2.Ries L, Eisner M, Kosary C, Hankey B, Miller B, Clegg L. SEER Cancer Statistics Review, 1975-2002. National Cancer Institute; Bethesda, Md: 2005. [Google Scholar]
- 3.Li D, Jiao L. Molecular epidemiology of pancreatic cancer. Int J Gastrointest Cancer. 2003;33:3–14. doi: 10.1385/IJGC:33:1:3. [DOI] [PubMed] [Google Scholar]
- 4.Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 5.Landi S. Mammalian class theta GST and differential susceptibility to carcinogens: a review. Mutat Res. 2000;463:247–283. doi: 10.1016/s1383-5742(00)00050-8. [DOI] [PubMed] [Google Scholar]
- 6.Salama SA, Abdel-Rahman SZ, Sierra-Torres CH, Hamada FA, Au WW. Role of polymorphic GSTM1 and GSTT1 genotypes on NNK-induced genotoxicity. Pharmacogenetics. 1999;9:735–743. [PubMed] [Google Scholar]
- 7.Tew KD, Monks A, Barone L, et al. Glutathione-associated enzymes in the human cell lines of the National Cancer Institute Drug Screening Program. Mol Pharmacol. 1996;50:149–159. [PubMed] [Google Scholar]
- 8.Strange RC, Spiteri MA, Ramachandran S, Fryer AA. Glutathione-S-transferase family of enzymes. Mutat Res. 2001;482(12):21–26. doi: 10.1016/s0027-5107(01)00206-8. [DOI] [PubMed] [Google Scholar]
- 9.Rebbeck TR. Molecular epidemiology of the human glutathione S-transferase genotypes GSTM1 and GSTT1 in cancer susceptibility. Cancer Epidemiol Biomarkers Prev. 1997;6:733–743. [PubMed] [Google Scholar]
- 10.Strange RC, Fryer AA. The glutathione S-transferases: influence of polymorphism on cancer susceptibility. IARC Sci Publ. 1999:231–249. [PubMed] [Google Scholar]
- 11.Yang G, Shu XO, Ruan ZX, et al. Genetic polymorphisms in glutathione-S-transferase genes (GSTM1, GSTT1, GSTP1) and survival after chemotherapy for invasive breast carcinoma. Cancer. 2005;103:52–58. doi: 10.1002/cncr.20729. [DOI] [PubMed] [Google Scholar]
- 12.Stoehlmacher J, Park DJ, Zhang W, et al. Association between glutathione S-transferase P1, T1, and M1 genetic polymorphism and survival of patients with metastatic colorectal cancer. J Natl Cancer Inst. 2002;94:936–942. doi: 10.1093/jnci/94.12.936. [DOI] [PubMed] [Google Scholar]
- 13.Hohaus S, Di Ruscio A, Di Febo A, et al. Glutathione Stransferase P1 genotype and prognosis in Hodgkin’s lymphoma. Clin Cancer Res. 2005;11:2175–2179. doi: 10.1158/1078-0432.CCR-04-1250. [DOI] [PubMed] [Google Scholar]
- 14.Ambrosone CB, Sweeney C, Coles BF, et al. Polymorphisms in glutathione S-transferases (GSTM1 and GSTT1) and survival after treatment for breast cancer. Cancer Res. 2001;61:7130–7135. [PubMed] [Google Scholar]
- 15.Okcu MF, Selvan M, Wang LE, et al. Glutathione S-transferase polymorphisms and survival in primary malignant glioma. Clin Cancer Res. 2004;10:2618–2625. doi: 10.1158/1078-0432.ccr-03-0053. [DOI] [PubMed] [Google Scholar]
- 16.Hayes JD, Strange RC. Glutathione S-transferase polymorphisms and their biological consequences. Pharmacology. 2000;61:154–166. doi: 10.1159/000028396. [DOI] [PubMed] [Google Scholar]
- 17.Norppa H. Cytogenetic biomarkers and genetic polymorphisms. Toxicol Lett. 2004;149(13):309–334. doi: 10.1016/j.toxlet.2003.12.042. [DOI] [PubMed] [Google Scholar]
- 18.Harris MJ, Coggan M, Langton L, Wilson SR, Board PG. Polymorphism of the Pi class glutathione S-transferase in normal populations and cancer patients. Pharmacogenetics. 1998;8:27–31. doi: 10.1097/00008571-199802000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Ali-Osman F, Akande O, Antoun G, Mao JX, Buolamwini J. Molecular cloning, characterization, and expression in Escherichia coli of full-length cDNAs of three human glutathione S-transferase Pi gene variants. Evidence for differential catalytic activity of the encoded proteins. J Biol Chem. 1997;272:10004–10012. doi: 10.1074/jbc.272.15.10004. [DOI] [PubMed] [Google Scholar]
- 20.Watson MA, Stewart RK, Smith GB, Massey TE, Bell DA. Human glutathione S-transferase P1 polymorphisms: relationship to lung tissue enzyme activity and population frequency distribution. Carcinogenesis. 1998;19:275–280. doi: 10.1093/carcin/19.2.275. [DOI] [PubMed] [Google Scholar]
- 21.de Bruin WC, Wagenmans MJ, Peters WH. Expression of glutathione S-transferase alpha, P1-1 and T1-1 in the human gastrointestinal tract. Jpn J Cancer Res. 2000;91:310–316. doi: 10.1111/j.1349-7006.2000.tb00946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coles BF, Anderson KE, Doerge DR, Churchwell MI, Lang NP, Kadlubar FF. Quantitative analysis of interindividual variation of glutathione S-transferase expression in human pancreas and the ambiguity of correlating genotype with phenotype. Cancer Res. 2000;60:573–579. [PubMed] [Google Scholar]
- 23.Evans WE, Relling MV. Pharmacogenomics: translating functional genomics into rational therapeutics. Science. 1999;286:487–491. doi: 10.1126/science.286.5439.487. [DOI] [PubMed] [Google Scholar]
- 24.Jiao L, Hassan MM, Bondy ML, Abbruzzese JL, Evans DB, Li D. The XPD Asp(312)Asn and Lys(751)Gln polymorphisms, corresponding haplotype, and pancreatic cancer risk. Cancer Lett. 2006 Feb 1; doi: 10.1016/j.canlet.2005.12.026. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li D, Frazier M, Evans DB, et al. Single nucleotide polymorphisms of RecQ1, RAD54L, and ATM genes are associated with reduced survival of pancreatic cancer. J Clin Oncol. 2006;24:1720–1728. doi: 10.1200/JCO.2005.04.4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiong P, Bondy ML, Li D, et al. Sensitivity to benzo(a)pyrene diol-epoxide associated with risk of breast cancer in young women and modulation by glutathione S-transferase polymorphisms: a case-control study. Cancer Res. 2001;61:8465–8469. [PubMed] [Google Scholar]
- 27.Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garte S, Gaspari L, Alexandrie AK, et al. Metabolic gene polymorphism frequencies in control populations. Cancer Epidemiol Biomarkers Prev. 2001;10:1239–1248. [PubMed] [Google Scholar]
- 29.Maugard CM, Charrier J, Pitard A, et al. Genetic polymorphism at the glutathione S-transferase (GST) P1 locus is a breast cancer risk modifier. Int J Cancer. 2001;91:334–339. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1057>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 30.Lo HW, Antoun GR, Ali-Osman F. The human glutathione S-transferase P1 protein is phosphorylated and its metabolic function enhanced by the Ser/Thr protein kinases, cAMP-dependent protein kinase and protein kinase C, in glioblastoma cells. Cancer Res. 2004;64:9131–9138. doi: 10.1158/0008-5472.CAN-04-0283. [DOI] [PubMed] [Google Scholar]
- 31.Duell EJ, Holly EA, Bracci PM, Liu M, Wiencke JK, Kelsey KT. A population-based, case-control study of polymorphisms in carcinogen-metabolizing genes, smoking, and pancreatic adenocarcinoma risk. J Natl Cancer Inst. 2002;94:297–306. doi: 10.1093/jnci/94.4.297. [DOI] [PubMed] [Google Scholar]
- 32.Bernardini S, Bellincampi L, Ballerini S, et al. Glutathione S-transferase P1 *C allelic variant increases susceptibility for late-onset Alzheimer disease: association study and relationship with apolipoprotein E epsilon4 allele. Clin Chem. 2005;51:944–951. doi: 10.1373/clinchem.2004.045955. [DOI] [PubMed] [Google Scholar]
- 33.Johansson AS, Stenberg G, Widersten M, Mannervik B. Structure-activity relationships and thermal stability of human glutathione transferase P1-1 governed by the H-site residue 105. J Mol Biol. 1998;278:687–698. doi: 10.1006/jmbi.1998.1708. [DOI] [PubMed] [Google Scholar]
- 34.Gaspar J, Rodrigues S, Gil OM, et al. Combined effects of glutathione S-transferase polymorphisms and thyroid cancer risk. Cancer Genet Cytogenet. 2004;151:60–67. doi: 10.1016/j.cancergencyto.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Spitz MR, Schabath MB, Ali-Osman F, Mata H, Wu X. Association between glutathione S-transferase p1 polymorphisms and lung cancer risk in Caucasians: a case-control study. Lung Cancer. 2003;40:25–32. doi: 10.1016/s0169-5002(02)00537-8. [DOI] [PubMed] [Google Scholar]
- 36.Chan QK, Khoo US, Ngan HY, et al. Single nucleotide polymorphism of pi-class glutathione S-transferase and susceptibility to endometrial carcinoma. Clin Cancer Res. 2005;11:2981–2985. doi: 10.1158/1078-0432.CCR-04-2038. [DOI] [PubMed] [Google Scholar]
- 37.Hashibe M, Brennan P, Strange RC, et al. Meta- and pooled analyses of GSTM1, GSTT1, GSTP1, and CYP1A1 genotypes and risk of head and neck cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:1509–1517. [PubMed] [Google Scholar]
- 38.Cao W, Cai L, Rao JY, et al. Tobacco smoking, GSTP1 polymorphism, and bladder carcinoma. Cancer. 2005;104:2400–2408. doi: 10.1002/cncr.21446. [DOI] [PubMed] [Google Scholar]
- 39.Ishimoto TM, Ali-Osman F. Allelic variants of the human glutathione S-transferase P1 gene confer differential cytoprotection against anticancer agents in Escherichia coli. Pharmacogenetics. 2002;12:543–553. doi: 10.1097/00008571-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Srivastava SK, Singhal SS, Hu X, Awasthi YC, Zimniak P, Singh SV. Differential catalytic efficiency of allelic variants of human glutathione S-transferase Pi in catalyzing the glutathione conjugation of thiotepa. Arch Biochem Biophys. 1999;366:89–94. doi: 10.1006/abbi.1999.1217. [DOI] [PubMed] [Google Scholar]
- 41.Pandya U, Srivastava SK, Singhal SS, et al. Activity of allelic variants of Pi class human glutathione S-transferase toward chlorambucil. Biochem Biophys Res Commun. 2000;278:258–262. doi: 10.1006/bbrc.2000.3787. [DOI] [PubMed] [Google Scholar]
- 42.Hu X, Herzog C, Zimniak P, Singh SV. Differential protection against benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxideinduced DNA damage in HepG2 cells stably transfected with allelic variants of pi class human glutathione S-transferase. Cancer Res. 1999;59:2358–2362. [PubMed] [Google Scholar]
- 43.Trachte AL, Suthers SE, Lerner MR, et al. Increased expression of alpha-1-antitrypsin, glutathione S-transferase pi and vascular endothelial growth factor in human pancreatic adenocarcinoma. Am J Surg. 2002;184:642–647. doi: 10.1016/s0002-9610(02)01105-4. discussion, 647-648. [DOI] [PubMed] [Google Scholar]
- 44.Martino R, Malet-Martino M, Gilard V. Fluorine nuclear magnetic resonance, a privileged tool for metabolic studies of fluoropyrimidine drugs. Curr Drug Metab. 2000;1:271–303. doi: 10.2174/1389200003339036. [DOI] [PubMed] [Google Scholar]
- 45.Nishiyama M, Yamamoto W, Park JS, et al. Low-dose cisplatin and 5-fluorouracil in combination can repress increased gene expression of cellular resistance determinants to themselves. Clin Cancer Res. 1999;5:2620–2628. [PubMed] [Google Scholar]
- 46.Goto S, Kamada K, Soh Y, Ihara Y, Kondo T. Significance of nuclear glutathione S-transferase pi in resistance to anticancer drugs. Jpn J Cancer Res. 2002;93:1047–1056. doi: 10.1111/j.1349-7006.2002.tb02482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McIlwain CC, Townsend DM, Tew KD. Glutathione S-transferase polymorphisms: cancer incidence and therapy. Oncogene. 2006;25:1639–1648. doi: 10.1038/sj.onc.1209373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bartsch H, Malaveille C, Lowenfels AB, Maisonneuve P, Hautefeuille A, Boyle P. Genetic polymorphism of N-acetyltransferases, glutathione S-transferase M1 and NAD(P)H:-quinone oxidoreductase in relation to malignant and benign pancreatic disease risk. The International Pancreatic Disease Study Group. Eur J Cancer Prev. 1998;7:215–223. doi: 10.1097/00008469-199806000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Liu G, Ghadirian P, Vesprini D, et al. Polymorphisms in GSTM1, GSTT1 and CYP1A1 and risk of pancreatic adenocarcinoma. Br J Cancer. 2000;82:1646–1649. doi: 10.1054/bjoc.2000.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Collier JD, Bennett MK, Hall A, Cattan AR, Lendrum R, Bassendine MF. Expression of glutathione S-transferases in normal and malignant pancreas: an immunohistochemical study. Gut. 1994;35:266–269. doi: 10.1136/gut.35.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moore LE, Huang WY, Chatterjee N, et al. GSTM1, GSTT1, and GSTP1 polymorphisms and risk of advanced colorectal adenoma. Cancer Epidemiol Biomarkers Prev. 2005;14:1823–1827. doi: 10.1158/1055-9965.EPI-05-0037. [DOI] [PubMed] [Google Scholar]


