Abstract
Recombinant human Growth Hormone (GH) is used to treat growth hormone deficiency in children and adults, and wasting in AIDS patients. GH has a circulating half-life of only a few hours in humans and must be administered to patients by daily injection for maximum effectiveness. Previous studies showed that longer-acting forms of GH could be created by modification of GH with multiple 5 kDa amine-reactive polyethylene glycols (PEGs). Eight of nine lysine residues and the N-terminal amino acid were modified to varying extents by amine-PEGylation of GH. The amine-PEGylated GH product comprised a complex mixture of multiple PEGylated species that differed from one another in mass, in vitro bioactivity and in vivopotency. In vitro bioactivity of GH was reduced 100- to 1,000-fold by extensive amine-PEGylation of the protein. Here we describe a homogeneously modified, monoPEGylated GH protein that possesses near complete in vitro bioactivity, a long half-life and increased potency in vivo. The monoPEGylated GH was created by substituting cysteine for threonine-3 (T3C) of GH, followed by modification of the added cysteine residue with a single 20 kDa cysteine-reactive PEG. The PEG-T3C protein has an approximate 8-fold longer half-life than GH following sc administration to rats. Every other day or every third day administration of PEG-T3C stimulates increases in body weight and tibial epiphysis growth comparable to that produced by daily administration of GH in hypophysectomized rats. Long-acting, monoPEGylated GH analogs such as PEG-T3C are promising candidate for future testing in humans.
Keywords: growth hormone, polyethylene glycol, rat, bone, growth
Introduction
Growth Hormone (GH) is a 22 kDa protein secreted by the pituitary gland. GH stimulates metabolism of bone, cartilage and muscle and is the body’s primary hormone for stimulating somatic growth during childhood. Recombinant human GH is used to treat children with short stature resulting from GH inadequacy, Turner’s Syndrome and renal failure. GH also is used to treat metabolic complications of GH-deficiency in adults and wasting in AIDS patients. A limitation of current GH treatments is the fact that GH must be administered by daily sc injection for maximum effectiveness due to its short circulating half-life (1).
Several second-generation therapeutic proteins with extended half-lives and improved potencies in vivo have been created by modifying the proteins with polymers such as polyethylene glycol (PEG) (2-5). Covalent attachment of PEG to a protein increases the protein’s effective size and reduces its rate of clearance rate from the body. A previous report (6) described modification of GH with amine-reactive PEGs, which typically attach to proteins at lysine residues or at the N-terminal amino acid. GH contains nine lysines in addition to the N-terminal F1 amino acid. Modification of GH with 5 kDa amine-reactive-PEGs yielded a heterogeneous mixture of PEG-GH proteins containing from 2 to 7 PEGs, each of which had different in vitro and in vivo properties (6). In vitro biological activity of amine-PEGylated GH decreased with increasing numbers of PEGs attached to the protein, whereas half-life and in vivo potency increased with increasing numbers of PEGs attached to the protein. Eight lysine residues in addition to the N-terminal amino acid were modified to varying extents by the PEG reagent. The different PEGylated species could not be separated cleanly from one another by conventional column chromatography methods. GH conjugates containing 5 or more 5 kDA-PEGs were determined to be the most useful for increasing the protein’s half-life, but these conjugates possessed only 1% of the in vitro bioactivity of unmodified GH (6). Despite possessing significantly reduced in vitro biological activity, the multi-PEGylated GH proteins stimulated weight gain and bone growth, and could be administered less often than non-modified GH in a rat growth hormone-deficiency model (6).
In this report we describe a new class of rationally designed PEG-GH conjugates prepared by targeted attachment of a large 20 kDa-PEG to a single, non-essential site in the protein. Targeting of the PEG moiety was achieved by introducing an unpaired “free” cysteine residue into GH by site-directed mutagenesis, followed by modification of the added cysteine residue with a cysteine-reactive PEG [site-specific PEGylation (7)]. We demonstrate that the monoPEGylated GH cysteine analog retains high in vitro bioactivity, has a longer circulating half-life than GH in rats, and is more potent than GH at stimulating weight gain and bone growth in GH-deficient rats.
Materials and Methods
Cloning a cDNA encoding Growth Hormone and Construction of GH (T3C)
A cDNA encoding human GH was amplified from human pituitary single-stranded cDNA (CLONTECH, Inc., Palo Alto, CA), using the PCR method (8). A periplasmically secreted form of GH was created by using PCR to fuse DNA encoding the signal sequence of the Escherichia coli STII gene (9) to the coding sequence for mature GH. The T3C analog was constructed using site-directed PCR-based mutagenesis methods (10, 11). The DNA sequence of the entire T3C gene was determined to verify the presence of the T3C mutation and the absence of any secondary mutations. Genes encoding STII-GH and STII-GH (T3C) were subcloned into expression plasmid pCYB1 (New England BioLabs, Beverly, MA) and used to transform E. coli strain W3110.
Expression and purification of wild type GH and T3C
Overnight E. coli cultures were diluted to an optical density of ∼0.025 at 600 nm in LB media containing 100 μg/mL ampicillin and incubated at 37 C. When optical densities of the cultures reached 0.25-0.5, IPTG was added to a final concentration of 0.5 mM to induce expression of the GH proteins. After overnight induction, E. coli cells expressing wild type GH were subjected to osmotic shock (12). The osmotic shock supernatant containing GH was applied to a 5 ml HiTrap Q Sepharose column (GE Healthcare, Piscataway, NJ) and bound proteins eluted with a 50-250 mM linear NaCl gradient. Column fractions were analyzed by SDS-PAGE and fractions enriched for GH were pooled, concentrated and fractionated on a Superdex 200 HR 10/30 sizing column (GE Healthcare). Fractions containing GH were pooled and stored at -80 C.
The T3C protein was purified from the induced E. coli cell pellets by treating the cell pellets with a mild detergent solution (B-Per™, Pierce Chemical Company, Rockford, IL) according to the manufacturer’s directions. The mixture was centrifuged, the supernatant discarded and the pellet suspended in 8 M urea, 20 mM Tris, 20 mM cysteine, pH 9 (40 mL/600 mL culture). After 2 h of mixing at room temperature, the solution was diluted into 160 mL of 15% glycerol, 20 mM Tris, pH 8, 40 μM copper sulfate and held at 4 C overnight. The refold mixture was then clarified by centrifugation and the supernatant loaded onto a 5 mL HiTrap Q-Sepharose column equilibrated in 20 mM Tris, pH 8.0, 10% glycerol. The T3C protein was recovered by elution with a 20-column volume gradient from 0-500 mM NaCl in 20 mM Tris, pH 8, 10% glycerol. Fractions were analyzed by non-reducing SDS-PAGE and fractions enriched for the T3C protein were pooled and stored at -80 C. Protein concentrations of purified T3C and PEG-T3C were measured using a Bradford Dye Binding assay (Bio-Rad Laboratories, Richmond, CA), using bovine serum albumin as the standard. The Bradford method measures only the protein content of PEG-T3C and not the PEG component.
PEGylation of T3C
Purified T3C protein was diluted to 100 μg/mL with 100 mM Tris, pH 8.0 and incubated with a 15-fold molar excess of 20 kDa vinylsulfone PEG (Nektar, Inc.) and an 8-fold molar excess of TCEP (Pierce Chemical Company). After 2 h at room temperature the reaction mixture was applied to a 1 mL Q-Sepharose column equilibrated in 20 mM Tris pH 8.0. The column was eluted with a 0-0.5 M NaCl gradient in 20 mM Tris pH 8, 10% glycerol. Fractions containing monoPEGylated T3C protein were identified by non-reducing SDS-PAGE, pooled and stored at -80 C. Samples of the purified PEG-T3C protein were applied to a Bio-Sil 400 size-exclusion HPLC column (Bio-Rad Laboratories) and eluted with an isocratic gradient of PBS, pH 7.5. Molecular weight standards used to calibrate the size-exclusion column were obtained from Bio-Rad Laboratories.
Selection of Stably Transfected FDC-P1 Cells Expressing the Rabbit GH Receptor
A cDNA encoding the rabbit GH receptor was amplified from rabbit liver poly (A)+ mRNA (CLONTECH, Inc.) using the RT-PCR technique (13), cloned into expression vector pCDNA3.1 (+) (Invitrogen Corporation, Carlsbad, CA) and used to transfect mouse FDC-P1 cells (American Type Culture Collection, Manassas, VA). Stably transformed cells were selected in RPMI media containing 10% horse serum, 50 μg/mL penicillin, 50 μg/mL streptomycin, 2 mM glutamine, 400 ug/mL Geneticin (Invitrogen Corporation) and 5 nM human pituitary GH (obtained from the National Hormone and Pituitary Program of the National Institute of Diabetes and Digestive and Kidney Diseases). Five cell lines that showed a proliferative response to GH were selected by limiting dilution. The GH-R4 cell line was used for the assays presented here. GH-R4 cells were propagated in RPMI 1640 media containing 10% horse serum, 50 units/mL penicillin, 50 μg/mL streptomycin, 2 mM glutamine, 400 μg/mL Geneticin and 2-5 nM human pituitary GH or recombinant human GH prepared by us.
In Vitro Bioassay
GH-R4 cells were suspended in assay media (phenol red-free RPMI 1640, 10% horse serum, 2 mM glutamine, 50 units/mL penicillin, 50 μg/mL streptomycin, 400 μg/mL Geneticin) at a concentration of 0.5-1 × 105 cells/mL. Fifty μL of the cell suspension were added to wells of a 96 well flat bottomed tissue culture plate. Serial 3-fold dilutions of the protein samples were prepared in assay media and added to microtiter wells in a volume of 50 μL. Bioactivities of the proteins were assayed over the concentration range of 0.085 to 185 ng/mL. Concentrations of PEG-T3C exclude the contribution of PEG to the mass of the protein. Protein samples were assayed in triplicate wells. Plates were incubated at 37 C in a humidified 5% CO2 tissue culture incubator for 3 d, at which time 20 μL of CellTiter 96 Aqueous One solution (Promega Corporation, Madison, WI) was added to each well. Absorbance of the wells at 490 nm, which is proportional to cell number, was measured 1-4 h later. Control wells contained media but no cells. Recombinant wild type GH or pituitary-derived GH were assayed in parallel as controls.
Pharmacokinetic Experiments
All animal experiments were performed with the approval of BolderPATH’s Institutional Animal Care and Use Committee and in accordance with accepted standards of humane animal care. Male Sprague Dawley rats, weighing approximately 320g each, were obtained from Harlan Sprague Dawley (Indianapolis, IN). Rats were injected and bled essentially as described (14). Blood samples (0.3 -0.4 mL) were drawn from the rats at selected time points for serum preparation. Serum samples were stored at -80 C until use. A pre-dose blood sample was drawn one day prior to injection of the test compounds. Rats received injections of recombinant human GH (Nutropin®, Genentech, Inc.) or PEG-T3C. Serum levels of the test compounds were quantitated using human GH ELISA kits (Diagnostic Systems Laboratories, Inc., San Antonio, TX). Serial dilutions of the serum samples were analyzed in duplicate in the ELISAs. Standard curves for detecting GH and PEG-T3C in the ELISA were prepared and used to adjust the final serum concentrations reported in the figures. Pharmacokinetic parameters were analyzed using the WinNonlin software program (Pharsight, Inc., Mountain View, CA) using non-compartmental methods.
Hypox Rat Experiments
HYPOX male Sprague-Dawley rats were purchased from Harlan Sprague-Dawley, Inc. (Indianapolis, IN) and weighed about 90 g (Experiment 1) or 100 g (Experiment 2) at study initiation. Rats were acclimated for 13 d and animals gaining more than 4 g during acclimation were culled from the study. Body weight measurements were taken at 09:30 every day. Rats (4-5/group) were randomized by weight to the various test groups, which were given daily, every other day, or every third day sc injections of vehicle solution [Delbucco’s PBS containing 200 μg/mL rat serum albumin (Sigma Chemical Company)], recombinant human GH (Nutropin®), or various doses of PEG-T3C, depending upon the experiment and test group. The PEG-T3C doses excluded the contribution of PEG to the mass of the protein. Protein solutions were prepared in vehicle solution. Animals were treated for 9 days. On day 10, the animals were sacrificed and their tibias harvested and fixed in 10% neutral buffered formalin. The fixed tibias were decalcified in 5% formic acid and split at the proximal end in the frontal plane. The tibias were processed for paraffin embedding, sectioned at 8 microns and stained with toluidine blue. The width of the tibial epiphysis was measured on the left tibia (5 measurements per tibia). Statistical analyses between samples were compared using a student’s T-test with significance set at p < 0.05.
RESULTS
Expression, Purification and Bioactivity of GH and the GH (T3C) Analog
Recombinant human GH and the human GH cysteine analog T3C (cysteine substituted for threonine-3) were secreted to the E. coli periplasm using the bacterial STII signal sequence. GH was readily released from the E.coli periplasm as a soluble, monomeric protein following osmotic shock treatment of the induced cells, and was purified from the osmotic shock supernatant using a combination of Q-Sepharose and size-exclusion chromatography. In contrast, the T3C analog was recovered in both the soluble and insoluble fractions following osmotic shock treatment of induced cells. The T3C protein present in the osmotic shock supernatant consisted of multiple molecular weight species when analyzed under non-reducing SDS-PAGE conditions, suggesting that a large proportion of the soluble T3C protein was present in an aggregated form (data not shown). To optimize recovery of the T3C protein we developed a procedure for denaturing and refolding the T3C protein starting from detergent lysates of induced cells. The refolded T3C protein was purified by Q-Sepharose column chromatography. The purified T3C protein co-migrated with pituitary GH and GH under reducing and non-reducing SDS-PAGE conditions, suggesting that the T3C protein was similarly folded and disulfide-bonded (data not shown). Peptide mapping studies confirmed that the two native disulfide bonds in GH (cysteine-53 to cysteine-165 and cysteine-182 to cysteine-189) are intact in the purified T3C protein (data not shown). The T3C mutein was modified with a 20 kDa-vinylsulfone PEG as described in Materials and Methods. The PEGylation reaction yielded only monoPEGylated protein, which was separated from non-PEGylated protein and excess PEG reagent by Q-Sepharose column chromatography. Control experiments indicated that wild type GH did not PEGylate under conditions used to PEGylate T3C (data not shown). The purified PEG-T3C protein migrates with an apparent molecular mass of 52,500 by non-reducing SDS-PAGE (Fig. 1). By size-exclusion HPLC, the PEG-T3C protein migrates with an effective mass of 330,000, which is about 15-fold larger than the effective mass of GH determined by size-exclusion chromatography [22,000 (6)].
Figure 1.
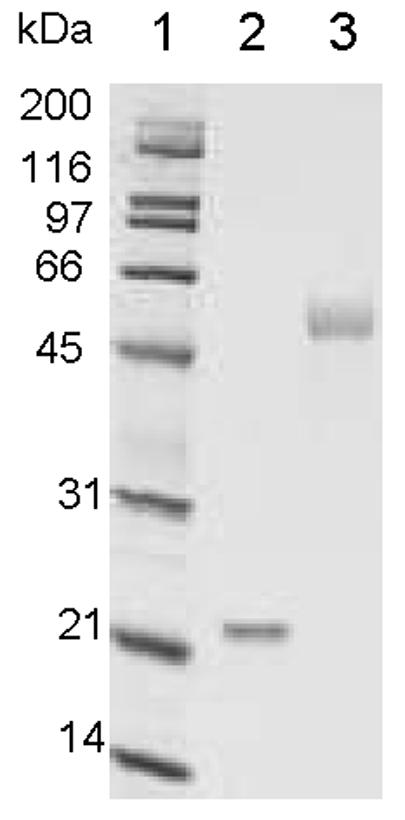
Non-Reducing SDS-PAGE analysis of purified PEG-T3C. Lane 1, molecular weight markers; lane 2, pituitary GH; lane 3, PEG-T3C. Proteins were stained with Coomassie Blue.
In Vitro Bioactivities of T3C, PEG-T3C and GH
In vitro bioactivities of the purified T3C and PEG-T3C proteins were measured using a cell line, GH-R4 cells, that proliferates in response to human GH. The GH-R4 cell line was created by stably transforming the mouse FDC-P1 cell line with a cDNA encoding the rabbit GH receptor (15). The concentrations of PEG-T3C used in the bioassays exclude the contribution of the PEG to the mass of the protein. T3C and PEG-T3C stimulated GH-R4 cell proliferation to the same maximal extent as the GH control proteins, although more PEG-T3C was required to achieve maximal cell proliferation compared to the other proteins. The mean EC50 of the unmodified T3C protein in this assay was 1.4 ± 0.9 ng/mL, which was not significantly different from the mean EC50s for recombinant GH prepared by us (0.9 ± 0.2 ng/mL) and human pituitary-derived GH (1.0 ± 0.2 ng/mL). The mean EC50 for the PEG-T3C protein was 3.7 ± 1.2 ng/mL, which was significantly higher than the mean EC50s for the GH control proteins and unmodified T3C (p < 0.05). Representative dose-response curves for the T3C and PEG-T3C proteins and pituitary GH are shown in Figure 2.
Figure 2.
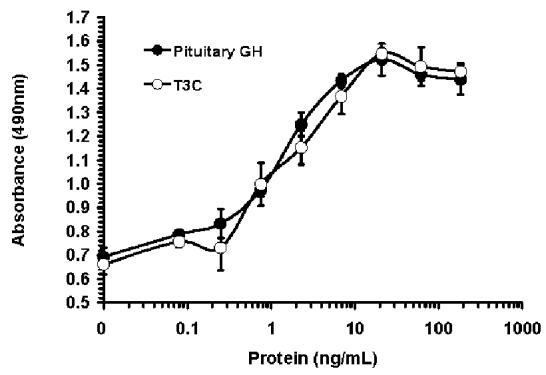
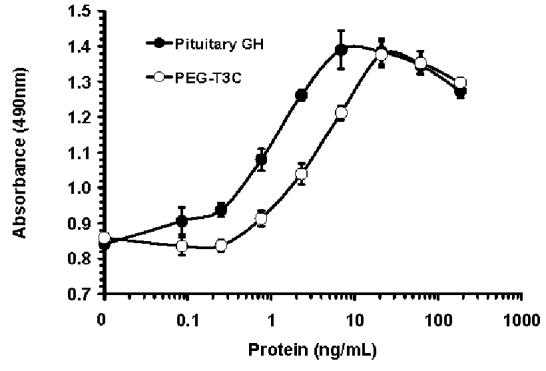
Dose response curves for pituitary GH, T3C and PEG-T3C for stimulating proliferation of GH-R4 cells. Data are means ± SD for triplicate wells from representative experiments. Panel (A) compares T3C and pituitary GH. Panel (B) compares PEG-T3C and pituitary GH. Proteins shown in the same panel were assayed on the same day. Absorbance values on the Y-axis are proportional to cell number. The PEG-T3C concentrations shown on the X-axis of (B) exclude the contribution of PEG to the mass of PEG-T3C.
Pharmacokinetic Analyses
Circulating half-lives of a commercial recombinant human GH product (Nutropin®) and PEG-T3C were determined following iv and sc administration to rats. Rats received the same dose of each protein (100 μg protein/kg). The PEG-T3C dose excluded the contribution of PEG to the mass of the protein. Following iv administration (Fig. 3A), serum levels of GH decreased rapidly (> 1,000-fold in 10 h) and the protein could not be detected 10 h post-injection. Similar findings were reported previously (6). In contrast, serum levels of PEG-T3C exhibited a biphasic curve, with an initial rapid decrease followed by a slower secondary elimination phase. Serum levels of PEG-T3C were detectable for up to 72h post-injection. The terminal half-life of GH was 0.34 h (measured from 0.25 to 4h) and the terminal half-life of PEG-T3C was 10 h (measured from 10 to 72 h post-injection).
Figure 3.
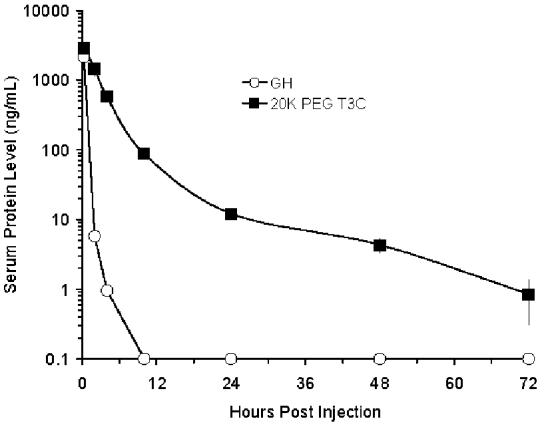
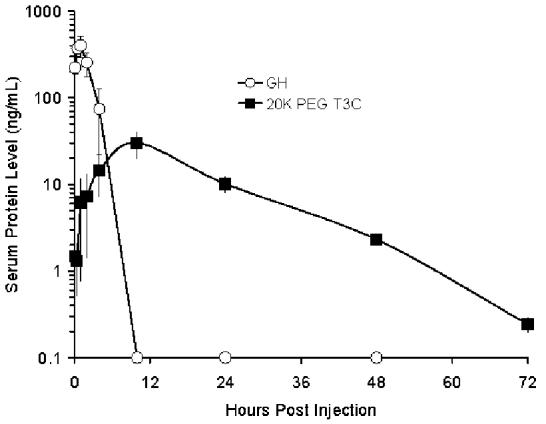
Pharmacokinetic properties of recombinant GH (Nutropin) and PEG-T3C following a single iv (A) and sc (B) injection in rats. Serum levels of the proteins were measured by ELISA. Data are means ± SD for 3 rats/group. Rats were dosed at 100 μg protein/kg. The dose of PEG-T3C excludes the contribution of PEG to the mass of PEG-T3C.
Following sc injection (Fig. 3B), GH serum levels reached a maximum 1 h post-injection and decreased to undetectable levels by 10 h. The terminal half-life of GH was 1.2 h (measured from 1-4 h post-injection), which is similar to the 1.35 h sc half-life reported previously for GH (6). In contrast, PEG-T3C protein was absorbed more slowly and did not reach maximum circulating levels until 10 h post-injection. Peak serum levels of PEG-T3C (30 ng/mL) were about 7.4 % of the peak serum levels of GH (403 ng/mL). The PEG-T3C protein was cleared from the circulation more slowly than GH, and had an elimination half-life of 9 h (measured from 10 to 72 h post-injection). Whether the immunoreactive PEG-T3C protein detected in the circulation 10-72 h post-administration is intact or degraded PEG-T3C has not been determined. However, the improved potency of PEG-T3C versus GH in the HYPOX rat studies presented below suggests that at least some of the immunoreactive PEG-T3C protein detected at these later time points is functional protein.
Comparative Efficacy of GH and PEG-T3C in HYPOX Rats
We performed two experiments to compare the relative abilities of GH and PEG-T3C to stimulate somatic growth in HYPOX rats, which is a well-characterized animal model of GH deficiency (6, 16). Growth parameters measured were weight gain and bone growth (tibial epiphysis width). We did not measure IGF-I levels in the animals because a previous study indicated that IGF-I levels in HYPOX rats do not increase significantly in response to daily GH administration and thus, are not a reliable indicator of GH activity in this model (6). We also did not want to stress the rats and potentially compromise the efficacy studies by subjecting the rats to repeated bleedings for IGF-I level measurements. In both studies the PEG-T3C doses used exclude the contribution of PEG to the mass of the protein. In the first experiment rats received every other day (dosing on days 1, 3, 5, 7 and 9) sc injections of 0.4, 2 or 10 μg of PEG-T3C or 10 μg of GH. Control rats received sc injections of 10 μg of PEG-T3C or GH using an every day dosing regimen (dosing on days 1-9). Additional control rats (placebo groups) received injections of vehicle solution only. There were 5 rats per group except for the PEG-T3C every day dosing group, which contained 4 rats. Rats were treated with the test compounds for 9 d and body weights were measured daily. On d 10 the rats were sacrificed and the widths of their tibial epiphyses determined. Cumulative body weight gain and tibial epiphyses measurements for the different test groups are presented in Table 1. PEG-T3C stimulated dose-dependent increases in both body weight and tibial epiphyses width when administered using an every other day dosing regimen. Rats receiving 10μg PEG-T3C every other day gained significantly more weight than rats receiving 10μg GH every other day. Rats receiving 10μg PEG-T3C every other day gained as much weight as rats receiving 10μg GH every day or 10μg PEG-T3C every day, even though rats receiving every other day injections of PEG-T3C received only half as much total protein over 10 days as rats receiving every day injections of GH or PEG-T3C. Rats receiving 10μg GH every other day did not gain as much weight as rats receiving 10μg GH every day, although the differences between groups were not statistically significant. Rats receiving 2 μg or less PEG-T3C every other day did not gain as much weight as rats receiving 10μg GH every day. Similar relative results were obtained for tibial epiphyses measurements (Table 1).
Table 1.
Effects of every day (ED) or every other day (EOD) administration of vehicle solution, GH or PEG-T3C for 9 d on body weight gain and tibial epiphyses width in HYPOX rats.
| Compound | Dose and Frequency a | Cumulative Body Weight Gain (g) b | Tibial Epiphyses Width (μm) b |
|---|---|---|---|
| Vehicle | ED | -1.0 ± 0.7 | 206.8 ± 9.2 |
| GH | 10 μ g ED | 11.2 ± 1.0 c | 348.8 ± 8.6 c |
| PEG-T3C | 10 μ g ED | 14.3 ± 0.8 c,e | 333.0 ± 9.8 c |
| Vehicle | EOD | 0.6 ± 1.0 | 204.4 ± 8.6 |
| GH | 10 μ g EOD | 8.6 ± 1.1 d,g | 298.8 ± 10.1 d |
| PEG-T3C | 10 μ g EOD | 15.4 ± 0.7 d, e, f | 357.2 ± 7.7 d, f |
| PEG-T3C | 2 μ g EOD | 5.6 ± 0.5 d, e, g | 274.8 ± 9.0 d, e, f |
| PEG-T3C | 0.4 μ g EOD | -0.2 ± 0.7 e, f, g | 225.2 ± 10.0 d, e, f, g, |
ED rats were dosed on d 1-9. EOD rats were dosed on d 1, 3, 5, 7 and 9. Rats were sacrificed on d 10. The PEG-T3C doses exclude the contribution of PEG to the mass of PEG-T3C.
Means ± SE for 5 rats/group, except for the PEG-T3C ED group, which contained 4 rats. Body weights were measured over 10 d.
p < 0.05 vs ED vehicle
p < 0.05 vs EOD vehicle
p < 0.05 vs ED GH
p < 0.05 vs EOD GH
p < 0.05 vs ED PEG-T3C
The second experiment was performed as described above except that the rats received every third day (dosing on days 1, 4 and 7) sc injections of 2, 10 or 30 μg of PEG-T3C or 10 or 30 μg of GH. The every third day 30 μg dose provides rats with the same total amount of GH as the standard 10 μg per day dose of GH administered over 3 days. The 2 μg dose of PEG-T3C was included so that we could test three doses of PEG-T3C to look for dose-dependent effects on weight gain and bone growth. An every third day 2 μg GH dose group was not included as a comparator because the every other day dosing study suggested that the 10 μg GH dose, and thus a lower 2 μg GH dose, would not be effective when administered every third day. Control rats received every day sc injections of 10 μg of PEG-T3C or 10 or 30 μg of GH. Additional control rats received injections of vehicle solution. There were 5 rats per group. Cumulative weight gains for the different test groups are summarized in Table 2. As was seen in the every other day dosing experiment, every third day dosing of PEG-T3C stimulated dose-dependent increases in body weight and tibial epiphysis width. In contrast, every third day dosing of GH did not show dose-dependent effects on these growth parameters. Every third day administration of 10 or 30 μg PEG-T3C stimulated significantly greater increases in body weight than every third day administration of the same doses of GH (p< 0.05). Every third day administration of 30 μg PEG-T3C stimulated an approximately equivalent increase in body weight as every day injections of 10 or 30 μg of GH or 10 μg PEG-T3C. The weight gain stimulated by every third day dosing of 10 μg PEG-T3C was less than that stimulated by every day dosing with 10 or 30 μg of GH (p < 0.05), but not significantly different from that stimulated by every day dosing with 10 μg PEG-T3C. Similar relative results were obtained for tibial epiphyses width measurements for the different test groups (Table 2). Representative stained sections of the tibias of animals in this experiment are shown in Fig. 4.
Table 2.
Effects of every day (ED) or every third day (ETD) administration of vehicle solution, GH or PEG-T3C for 9 d on body weight gain and tibial epiphyses width in HYPOX rats
| Compound | Dose and Frequency a | Cumulative Body Weight Gain (g)b | Tibial Epiphyses Width (μm)b |
|---|---|---|---|
| Vehicle | ED | 0.8 ± 0.7 | 223 ± 15.1 |
| GH | 30 μ g ED | 21.3 ± 1.4c | 408.4 ± 14.2c |
| GH | 10 μ g ED | 16.2 ± 1.2 c,g | 399.6 ± 15.6 c |
| PEG-T3C | 10 μ g ED | 18.6 ± 2.2 c | 384.4 ± 13.0 c |
| Vehicle | ETD | 1.5 ± 1.4 | 231.6 ± 17.4 |
| GH | 30 μ g ETD | 6.8 ± 1.4 d, g, h, i | 315.2 ± 15.6 d, g, h, i |
| GH | 10 μ g ETD | 8.0 ± 1.6 d, g, h, ,i | 284.0 ± 6.9 d, g, h, i |
| PEG-T3C | 30 μ gETD | 17.5 ± 1.2 d, e, f | 428.4 ± 18.3 d, e, f |
| PEG-T3C | 10 μ g ETD | 12.3 ± 0.8 d, e, f, g, h | 329.2 ± 15.6 d, f, h |
| PEG-T3C | 2 μ g ETD | 8.0 ± 1.4 d, g, h, i | 263.2 ± 7.1 e, g, h, i |
ED rats were dosed on d 1-9. ETD rats were dosed on d 1, 4 and 7. Rats were sacrificed on d 10. The PEG-T3C doses exclude the contribution of PEG to the mass of PEG-T3C.
means ± SE for 5 rats/group. Body weights were measured over 10 d.
p < 0.05 vs ED vehicle
p < 0.05 vs ETD vehicle
p < 0.05 vs ETD GH 30μg
p < 0.05 vs ETD GH 10μg
p < 0.05 vs ED GH 30μg
p < 0.05 vs ED GH 10μg
p < 0.05 vs ED PEG-T3C
Figure 4.
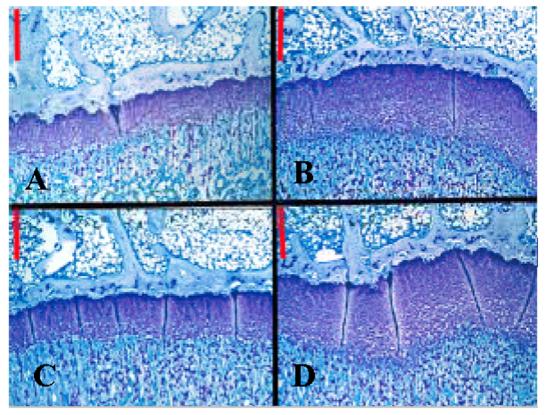
Representative stained sections of tibial epiphyses of HYPOX rats receiving sc injections of (A) Vehicle ETD; (B) GH (Nutropin) 30 μg ED; (C) GH (Nutropin) 30 μg ETD; and (D) PEG-T3C 30 μg ETD. Rats were sacrificed on d 10 of treatment and their tibias harvested, fixed, sectioned and stained with toluidine blue. Note that the tibial epiphyses (horizontal, purple-staining bands) are thicker in (B) and (D) than in (A) and (C). Red bar in each section = 325 □M.
Discussion
The studies presented here indicate that it is possible to use site-specific PEGylation technology to create a monoPEGylated GH analog that retains high in vitro biological activity and has a longer half-life and significantly improved potency in vivo compared to GH. The T3C analog was identified from a screen of over 40 GH cysteine analogs as one of several GH cysteine analogs that appears to fold properly, as judged by co-migration with GH under reducing and non-reducing SDS-PAGE conditions, peptide mapping studies and in vitro bioactivity measurements (Fig. 2 and unpublished results). T3C also reacts efficiently with cysteine-reactive PEG reagents and retains high in vitro bioactivity when PEGylated. Alanine is present in at the third amino acid position in bovine, ovine, porcine and avian GH proteins, suggesting that this position is tolerant of some amino acid changes (17). A human GH analog containing an alanine substitution for T3 [GH (T3A)] bound the human growth hormone receptor in solid phase assays with approximately the same affinity as wild type human GH; however in vitro biological activity of the human GH (T3A) protein was not reported (18). The high in vitro potency of PEG-T3C was unexpected in light of the fact that the neighboring amino acids F1 and I4 are believed to be critical for GH receptor dimerization, which is required for intracellular signaling (19). Alanine substitutions for F1 and I4 increased the EC50 for GH receptor dimerization by 5-fold and 55-fold, respectively (20). The modest 3- to 4-fold reduction in in vitro biological activity of the PEG-T3C protein may be due to the PEG molecule interfering with the proper interactions of F1 and I4 with the GH receptor. Alternatively, the reduced in vitro bioactivity of PEG-T3C may be due to the large PEG slowing diffusion and association of PEG-T3C with the GH receptor on cells, as has been postulated to explain the reduced in vitro bioactivities of amine-PEGylated GH proteins (6). The finding that PEG-T3C stimulated the same level of maximal stimulation of GH-R4 cell proliferation as GH suggests that PEG-T3C is capable of activating intracellular signaling pathways in GH-R4 cells to the same extent as GH, at least with respect to cell proliferation. Detailed in vitro and in vivo studies on a variety of GH-responsive cell lines and tissues will be required to determine whether PEG-T3C is capable of stimulating the full range of cellular responses stimulated by GH.
The PEG-T3C protein has significantly different structural and biological properties than previously described amine-PEGylated GH proteins (6). In vitro bioactivity of PEG-T3C protein (EC50 of ∼ 4 ng/mL) appears to be approximately 100-fold greater than that of the best studied amine-PEGylated GH (EC50 of ∼ 400 ng/mL), which contained an average of five to six 5 kDA-PEGs per protein (6). This conclusion must be considered tentative because bioactivities of the proteins have not been compared directly in the same bioassay. We attribute the apparent improved in vitro bioactivity of PEG-T3C to the use of a single large 20 kDa-PEG and the ability to target attachment of the PEG to a unique, largely non-essential site in the protein. Previous studies showed that chemical modification of lysine residues significantly reduces receptor binding and bioactivity of GH (18, 21-23). F1, K38, K70, K140 and K145 exhibited moderate to high reactivity with amine-PEG reagents, K41, K168, K172 and K158 exhibited weak reactivity with amine-PEG reagents and K115 was unreactive with amine-PEG reagents (6). Certain of these amine-PEG-reactive residues (F1, K38, K41, K70, K168, K172 and K158) make direct contacts with the GH receptor, or are located in regions of the protein that make direct contacts with the GH receptor (24). Amine-PEGylated GH also comprises a heterogeneous mixture of PEGylated GH species containing from 2 to 7 small 5 kDA-PEG molecules per protein (6). The different amine-PEGylated GH species possess different in vitro and in vivo properties. In contrast, the monoPEGylated GH (T3C) protein is homogeneously modified with PEG at a single unique site (T3C) and possesses reproducible biological properties, which may make it (and other monoPEGylated GH proteins like it) more suitable for development as a human therapeutic, where reproducibility of bioactivity and half-life are critical.
Potency studies in HYPOX rats demonstrated that PEG-T3C was as effective as daily GH but could be administered less frequently. Every other day administration of 10 μg of PEG-T3C was as effective at stimulating weight gain and bone growth as every day administration of 10 μg of GH, despite animals receiving only one-half as much PEG-T3C as GH during the course of the experiment. Efficacy of the 10 μg dose of PEG-T3C decreased compared to daily GH administration when this dose of PEG-T3C was administered every third day. However, administering the equivalent of 3 days worth of PEG-T3C (30 μg) as a single injection every third day proved as effective as daily administration of 10 μg of GH. By contrast, administering 3 days worth of GH (30 μg) as a single bolus injection every third day was not as effective as daily injections of 10 μg of GH. Our results with PEG-T3C are similar to results obtained with other PEGylated proteins, which typically are administered to patients at higher doses per injection than the unPEGylated proteins. For example, cancer chemotherapy patients receiving PEGylated G-CSF (Neulasta®, Amgen, Inc.) typically receive the equivalent of 20 days worth of G-CSF as a single injection (a single injection of 6 mg (∼ 100 μg/kg) of PEG-G-CSF vs a daily dose of 5 μg/kg of G-CSF, typically administered for 10-15 days). PEGylated interferon alpha products are administered at doses of 70-100 μg/wk (PEG-Intron®, Schering Corporation) or 180 μg/week (PEGASYS®, Roche, Inc.) as opposed to a thrice weekly dose of ∼ 11 μg (33 μg/wk) of interferon alpha.
PEG-T3C has a much larger effective mass than GH as measured by size-exclusion chromatography (330,000 versus 22,000, respectively). The large effective mass of PEG-T3C is typical of PEGylated proteins and is believed to be due to the elongated structure and large hydrodynamic radius of the attached PEG moiety (6, 25). The large effective masses of PEGylated proteins slow their filtration by the kidney and liver and contribute to their prolonged circulating half-lives (6, 25). Whether the larger effective mass of PEG-T3C alters its tissue distribution and relative effects on GH-responsive target organs in vivo compared to GH remains to be determined. The large mass of PEG-T3C does not appear to hinder its ability to stimulate weight gain and bone growth in HYPOX rats.
Most recombinant proteins have 3-to 7-fold longer circulating half-lives in humans than rats because humans metabolize proteins slower than rats (26). If PEG-T3C behaves similarly, it may be possible to administer PEG-T3C once per week to humans with comparable efficacy as daily administration of GH. This less frequent dosing regimen might be attractive to patients who must take daily injections of GH, typically for several years. The less frequent dosing regimen associated with PEG-T3C also may potentially improve efficacy due to improved patient compliance and increased in vivo potency of the molecule.
Acknowledgments
The publication’s contents are solely the responsibility of the authors and do not necessarily represent the official views of The National Institutes of Health. Human pituitary-derived GH and was generously provided by Dr. A.F. Parlow and the National Hormone and Pituitary Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
Abbreviations
- ED
every day
- EOD
every other day
- ETD
every third day
- G-CSF
granulocyte colony-stimulating factor
- HYPOX
hypophysectomized
- IPTG
isopropyl-β-D-thiogalactopyranoside
- PEG
polyethylene glycol
- STII
heat-stable enterotoxin gene
- TCEP
Tris (2-carboxyethylphosphine) hydrochloride.
Footnotes
Grant support: This work was supported by grants 1R43 DK54079 and 2R44 DK54079 to G.C. from The National Institutes of Health.
Disclosure Statement: GC, MR, DS, SC and DD are employees or former employees of Bolder BioTechnology, Inc. and have equity interests in the company. GC, MR and DD are inventors on issued and pending patents assigned to Bolder BioTechnology, Inc. EC has an equity interest in BolderPATH, Inc., which received contract research fees from Bolder Biotechnology, Inc. EC has an equity interest in Premier Laboratory LLC, which has received contract research fees from Bolder BioTechnology, Inc.
NIH statement: This is an un-copyedited author manuscript copyrighted by The Endocrine Society. This may not be duplicated or reproduced, other than for personal use or within the rule of “Fair Use of Copyrighted Materials” (section 107, Title 17, U.S. Code) without permission of the copyright owner, The Endocrine Society. From the time of acceptance following peer review, the full text of this manuscript is made freely available by The Endocrine Society at http://www.endojournals.org/. The final copy edited article can be found at http://www.endojournals.org/. The Endocrine Society disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties. The citation of this article must include the following information: author(s), article title, journal title, year of publication and DOI.
References
- 1.MacGillivray MH, Baptista J, Johnson A. Outcome of a four-year randomized study of daily versus three times weekly somatropin treatment in prepubertal naïve growth hormone-deficient children. J Clin Endocrinol Metab. 1996;81:1806–1809. doi: 10.1210/jcem.81.5.8626839. [DOI] [PubMed] [Google Scholar]
- 2.Abuchowski A, Kazo GM, Verhoest CR, van Es T, Kafkewitz D, Nucci ML, Viau AT, Davis FF. Cancer therapy with chemically modified enzymes. I. Antitumor proterties of polyethylene glycol-asparaginase conjugates. Cancer Biochem Biophys. 1984;7:175–186. [PubMed] [Google Scholar]
- 3.Molineux G, Kinstler O, Briddell B, Hartley C, McElroy P, Kerzic P, Sutherland W, Stoney G, Kern B, Fletcher FA, Cohen A, Korach E, Ulich T, McNiece I, Lockbaum P, Miller-Messana MA, Gardner S, Hunt T, Schwab G. A new form of filgrastim with sustained duration in vivo and enhanced ability to mobilize PBPC in both mice and humans. Exp Hematol. 1999;27:1724–1734. doi: 10.1016/s0301-472x(99)00112-5. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y-S, Youngster S, Bausch J, Zhang R, McNemar C, Wyss DF. Identification of the major positional isomer of PEGylated interferon alpha-2b. Biochemistry. 2000;39:10634–10640. doi: 10.1021/bi000617t. [DOI] [PubMed] [Google Scholar]
- 5.Bailon P, Palleroni A, Schaffer CA, Spence CL, Fung W-J, Porter JE, Ehrlich GK, Pan W, Xu Z-X, Modi MW, Farid A, Berthold W. Rational design of a potent, long-acting form of interferon: a 40 kDa branched polyethylene glycol-conjugated interferon α2a for the treatment of hepatitis C. Bioconjugate Chem. 2001;12:195–202. doi: 10.1021/bc000082g. [DOI] [PubMed] [Google Scholar]
- 6.Clark R, Olson K, Fuh G, Marian M, Mortensen D, Teshima G, Chang S, Chu H, Mukku V, Canova-Davis E, Somers T, Cronin M, Winkler M, Wells JA. Long-acting growth hormones produced by conjugation with polyethylene glycol. J Biol Chem. 1996;271:21969–21977. doi: 10.1074/jbc.271.36.21969. [DOI] [PubMed] [Google Scholar]
- 7.Goodson RJ, Katre NV. Site-directed pegylation of recombinant interleukin-2 at its glycosylation site. Biotechnology. 1990;8:343–346. doi: 10.1038/nbt0490-343. [DOI] [PubMed] [Google Scholar]
- 8.Scharf SJ. Cloning with PCR. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR Protocols: A Guide to Methods and Applications. Academic Press; San Diego: 1990. pp. 84–91. Ch 11. [Google Scholar]
- 9.Picken RN, Mazaitis AJ, Maas WK, Rey M, Heyneker H. Nucleotide sequence of the gene for heat-stable enterotoxin II of Escherichia coli. Infect and Immun. 1983;42:269–275. doi: 10.1128/iai.42.1.269-275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higuchi R. Recombinant PCR. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR Protocols: A Guide to Methods and Applications. Academic Press; San Diego: 1990. pp. 177–183. Ch 22. [Google Scholar]
- 11.Horton RM. In Vitro Recombination and mutagnesis of DNA. SOIng together tailor-made genes. In: White BA, editor. Methods in Molecular Biology. Vol. 15. Humana Press; Totawa NJ: 1993. pp. 214–250. [DOI] [PubMed] [Google Scholar]
- 12.Koshland D, Botstein D. Secretion of beta-lactamase requires the carboxy end of the protein. Cell. 1980;20:749–760. doi: 10.1016/0092-8674(80)90321-9. [DOI] [PubMed] [Google Scholar]
- 13.Kawasaki ES. Amplification of RNA. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR Protocols: A Guide to Methods and Applications. Academic Press; San Diego: 1990. pp. 21–27. Ch 22. [Google Scholar]
- 14.Cox GN, Smith DJ, Carlson SJ, Bendele AM, Chlipala EA, Doherty DH. Enhanced circulating half-life and hematopoietic properties of a human granulocyte colony-stimulating factor (G-CSF)-immunoglobulin fusion protein. Exp Hematol. 2004;32:441–449. doi: 10.1016/j.exphem.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Rowlinson SW, Barnard R, Bastiras S, Robins AJ, Brinkworth R, Waters MJ. A growth hormone agonist produced by targeted mutagenesis at binding site 1. J Biol Chem. 1995;270:16833–16839. doi: 10.1074/jbc.270.28.16833. [DOI] [PubMed] [Google Scholar]
- 16.Groesbeck MD, Parlow AF. Highly improved precision of the hypophysectomized female rat body weight gain assay for growth hormone by increased frequency of injections, avoidance of antibody formation, and other simple modifications. Endocrinology. 1987;120:2582–2590. doi: 10.1210/endo-120-6-2582. [DOI] [PubMed] [Google Scholar]
- 17.Abdel-Meguid SS, Shieh H-S, Smith WW, Dayringer HE, Violand BN, Bentle LA. Three-dimensional structure of a genetically engineered variant of porcine growth hormone. Proc Natl Acad Sci USA. 1987;84:6434–6437. doi: 10.1073/pnas.84.18.6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cunningham BC, Wells JA. High-resolution epitope mapping of hGH-receptor interactions by alanine-scanning mutagenesis. Science. 1989;244:1081–1085. doi: 10.1126/science.2471267. [DOI] [PubMed] [Google Scholar]
- 19.Cunningham BC, Jhurani P, Ng P, Wells JA. Receptor and antibody epitopes in human growth hormone identified by homolog-scanning mutagenesis. Science. 1989;243:1330–1336. doi: 10.1126/science.2466339. [DOI] [PubMed] [Google Scholar]
- 20.Cunningham BC, Ultsch M, de Vos AM, Mulkerrin MG, Clauser KR, Wells JA. Dimerization of the extracellular domain of the growth hormone receptor by a single hormone molecule. Science. 1991;254:821–825. doi: 10.1126/science.1948064. [DOI] [PubMed] [Google Scholar]
- 21.de la Llosa P, Chene N, Martal J. Involvement of lysine residues in the binding of ovine prolactin and human growth hormone to lactogenic receptors. FEBS Letts. 1985;191:211–215. doi: 10.1016/0014-5793(85)80010-7. [DOI] [PubMed] [Google Scholar]
- 22.Martal J, Chene N, de la Llosa P. Involvement of lysine residues in the binding of hGH and bGH to somatotropic receptors. FEBS Letts. 1985;180:295–299. doi: 10.1016/0014-5793(85)81089-9. [DOI] [PubMed] [Google Scholar]
- 23.Teh L-C, Chapman GE. Determination of the effect of acetylation of specific lysine residues in human growth hormone on its affinity for somatogenic receptors by an affinity selection technique. Biochem Biophys Res Comm. 1988;150:391–398. doi: 10.1016/0006-291x(88)90533-5. [DOI] [PubMed] [Google Scholar]
- 24.de Vos AM, Ultsch M, Kossiakoff AA. Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science. 1992;255:306–312. doi: 10.1126/science.1549776. [DOI] [PubMed] [Google Scholar]
- 25.Knauf MJ, Bell DP, Hirtzer P, Zhen-Ping L, Young JD, Katre NV. Relationship of effective molecular size to systemic clearance in rats of recombinant interleukin-2 chemically modified with water-soluble polymers. J Biol Chem. 1988;263:15064–15070. [PubMed] [Google Scholar]
- 26.Mordenti J, Chen SA, Moore JA, Ferrailo BL, Green JD. Interspecies scaling of clearance and volume of distribution data for five therapeutic proteins. Pharmacol Res. 1991;8:1351–1359. doi: 10.1023/a:1015836720294. [DOI] [PubMed] [Google Scholar]


