Abstract
We recently showed that the Drosophila transforming acidic coiled-coil (D-TACC) protein is located in the centrosome, interacts with microtubules, and is required for mitosis in the Drosophila embryo. There are three known human TACC proteins that share a conserved, C-terminal, coiled-coil region with D-TACC. These proteins have all been implicated in cancer, but their normal functions are unknown. We show that all three human TACC proteins are concentrated at centrosomes, but with very different characteristics: TACC1 is weakly concentrated at centrosomes during mitosis; TACC2 is strongly concentrated at centrosomes throughout the cell cycle; and TACC3 is strongly concentrated in a more diffuse region around centrosomes during mitosis. When the C-terminal TACC domain is overexpressed in HeLa cells, it forms large polymers in the cytoplasm that can interact with both microtubules and tubulin. The full-length TACC proteins form similar polymers when overexpressed, but their interaction with microtubules and tubulin is regulated during the cell cycle. At least one of the human TACC proteins appears to increase the number and/or stability of centrosomal microtubules when overexpressed during mitosis. Thus, the TACC domain identifies a family of centrosomal proteins that can interact with microtubules. This may explain the link between the TACC genes and cancer.
Keywords: centrosome, cancer, mitosis
Both centrosomes and the microtubules they organize play crucial roles in many cell processes (1–3). Despite their importance, however, surprisingly little is known about how centrosomes interact with microtubules at the molecular level.
Considerable progress has been made recently in understanding how γ-tubulin ring complexes in the centrosome are involved in microtubule nucleation (4, 5). The interaction between centrosomes and microtubules, however, appears to be more complicated than just a simple nucleation (6–9). To understand better how centrosomes interact with microtubules, we and others have biochemically isolated a number of proteins from Drosophila embryos that interact with microtubules in vitro and concentrate at centrosomes in vivo (10–13). We have previously shown that one of these proteins, Drosophila transforming acidic coiled-coil (D-TACC), is essential for mitotic spindle function in the early Drosophila embryo (14). In embryos where D-TACC function is perturbed, spindle and astral microtubules are abnormally short and weak, and this leads to failures in nuclear migration and chromosome segregation.
The C-terminal region of D-TACC is predicted to form a coiled-coil that is similar to that found in the mammalian TACC-containing proteins. The normal functions of the three known mammalian TACC proteins are unknown, but several observations suggest that the proteins may contribute to cancer: the human TACC genes are all in genomic regions that are rearranged in certain cancer cells; TACC3 is up-regulated in some cancer cell lines; and the overexpression of TACC1 transforms mouse fibroblasts (15, 16). Very recently, TACC2 has also been identified as a potential tumor suppressor protein called AZU-1; the expression of the protein is down-regulated in many breast carcinoma cell lines and primary tumors, and restoring TACC2/AZU-1 protein to normal levels reduces the malignant phenotype of cells both in culture and in vivo (17). A recently identified Xenopus protein called maskin, which is related to TACC3, has been shown to be involved in regulating the translation of specific mRNAs in the developing frog embryo (18).
We previously showed that the conserved C-terminal region (which we call the TACC domain) of D-TACC can direct a heterologous fusion protein to centrosomes and microtubules in Drosophila embryos (14). Moreover, we showed that the human TACC2 protein is also concentrated at centrosomes in human cells. We therefore postulated that all of the TACC proteins might interact with microtubules and be concentrated at centrosomes via their TACC domains. Here, we provide evidence that this is the case. We show that human TACC1 and TACC3 are concentrated at centrosomes, although only during mitosis. We demonstrate that, when the TACC domain is overexpressed in human cells in culture, it forms large polymeric structures in the cytoplasm that can interact with both microtubules and tubulin. When the full-length TACC proteins are overexpressed, similar polymers form, but their interaction with microtubules and tubulin is now cell-cycle regulated. Moreover, in cells that overexpress the TACC3 protein, the number of centrosomal microtubules appears to be increased. These findings suggest that the TACC domain is a conserved motif that can interact with centrosomes and microtubules and that the TACC proteins may play a conserved role in organizing centrosomal microtubules.
Materials and Methods
TACC cDNAs.
The sequences of the TACC cDNAs have the following GenBank accession numbers: AFO49910 (TACC1); AFO95791 (TACC2); and AF093543 (TACC3). The numbering of amino acids refers to the predicted proteins encoded by these cDNAs. Recently, we identified a larger TACC2 cDNA that encodes a larger protein of 1,026 aa. In preliminary experiments, this larger protein behaved in a very similar manner to the shorter protein (not shown).
Antibody Production.
Antibodies were raised in rabbits against bacterially expressed and purified glutathione S-transferase (19) or maltose-binding protein (New England BioLabs) fusion proteins that contained the following regions of the TACC proteins: TACC1, amino acids 1–323; TACC2, amino acids 689-1026; and TACC3, amino acids 73–265. Rabbit antisera were raised by Eurogentec (Brussels), and we affinity purified and stored the antibodies as described (20).
Immunofluoresence.
Human HeLa or primary fibroblast cells (MHF 181 primary foreskin fibroblasts) were cultured, fixed with methanol, and stained with antibodies as described (21). All affinity-purified primary antibodies were used at 1–2 μg/ml. DM1A anti-α-tubulin antibody and GTU-88 anti-γ tubulin antibody (Sigma) were used at 1:500 dilution. Appropriate Cy5-, Cy3- (Jackson), or Alexa488- (Molecular Probes) coupled secondary antibodies were used at 1:500 dilution. With the transfected cells, we performed several control experiments to eliminate the possibility that the fluorescence from the green fluorescent protein (GFP)-fusion proteins was “bleeding through” into other channels. All imaging was performed by using a Bio-Rad 1024 scanning confocal microscope. Images were imported into Adobe photoshop.
Western Blotting.
Whole-cell extracts were made by pelleting HeLa cells and boiling the pellet in SDS sample buffer. The extracts were separated by SDS/PAGE and blotted to nitrocellulose as described (22, 23). Blots were incubated with primary antibodies at 1–2 μg/ml final concentration, and antibody binding was detected by using a Supersignal kit (Pierce), according to the manufacturer's instructions.
DNA Constructs.
Transient transfection experiments were performed with various regions of the TACC proteins subcloned into either pEYFP or pEGFP vectors (CLONTECH), producing YFP-TACC or GFP-TACC fusion proteins. Several regions of the TACC proteins were also subcloned into the pCMV-Tag2 (Stratagene), pSEM, or pCDNA3 (Invitrogen) vectors, producing either FLAG-TACC fusion proteins or untagged TACC proteins. Similar results were obtained with the different regions of the TACC proteins whether they were expressed on their own or as fusion proteins with GFP or FLAG, except that the GFP-fusion proteins routinely appeared to be expressed at higher levels. The following regions of the TACC proteins were used (numbers for TACC2 refer to the recently discovered larger TACC2 cDNA): full-length TACC proteins (TACC1, amino acids 1–805; TACC2, 397-1026; TACC3, 1–838); TACC domains (TACC1, amino acids 588–804; TACC2, 771-1026; TACC3, 604–838); TACC proteins missing the TACC domain (TACC1, amino acids 3–562; TACC2, 452–765; TACC3, 1–593).
Transient Transfection and Drug Treatment.
The mammalian Profection transfection kit (Promega) was used according to the manufacturer's instructions. In the case of drug treatments, taxol was added to 2 μM and nocodazole to 1 μM for 4–26 h, either immediately after the cells had been transfected or for 12 h before the cells were transfected (in which case the whole transfection procedure was carried out in the presence of the drug). Similar results were obtained using either protocol. To observe cells in mitosis, we synchronized HeLa cells in G2 with a double thymidine block. The cells were transfected during the last 6–12 h of the second block and fixed 12–14 h later, when the majority of the cells should have been in mitosis. To test whether microtubules were required to maintain the localization of the TACC proteins at the centrosomes, cells were synchronized in G2 and then released for 12 h; nocodazole was then added to 25 μM for 1 h before fixation.
Electron Microscopy.
Cells were fixed and processed for thin-section electron microscopy and thin-section immunoelectron microscopy as described (24).
Results
The TACC Proteins Are Differentially Distributed in the Cell.
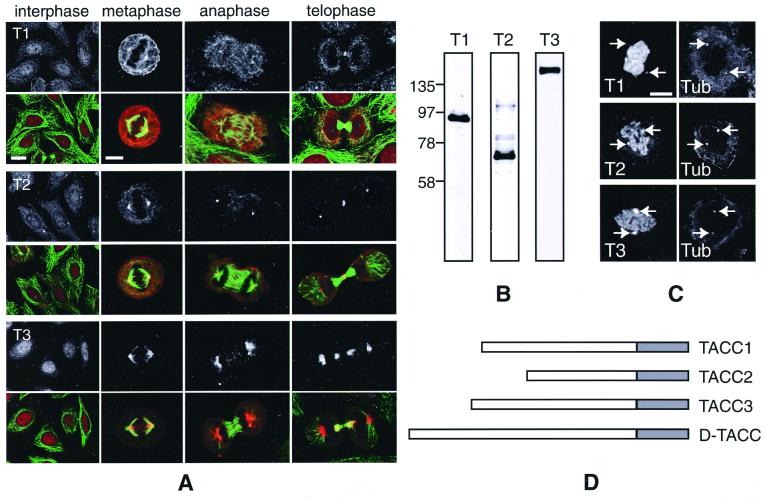
The three human TACC proteins are related to each other in a ≈200-aa region. This TACC domain region is predicted to form a coiled-coil (Fig. 1D; see figure 7 in ref. 14 for a sequence alignment and coiled-coil predictions of the TACC domains). Although the three known human TACC genes appear to have been generated by gene duplication events, there is very little amino acid homology between the TACC proteins outside of the TACC domain (15, 16). We previously showed that TACC2 is strongly associated with centrosomes, and more weakly with mitotic spindles, in both HeLa cells and primary fibroblasts (14). To investigate the localization of human TACC1 and TACC3, we raised and affinity-purified antibodies against both of these proteins. In Western blots of HeLa cell extracts, the anti-TACC1 antibodies recognized a major band of ≈85 kDa, the anti-TACC2 antibodies a major band of ≈73 kDa, and the anti-TACC3 antibodies a major band of ≈150 kDa (Fig. 1B).
Figure 1.
A comparison of the known human TACC proteins. (A) Fixed HeLa cells at interphase, metaphase, anaphase, and telophase were stained with anti-TACC antibodies (Top; red in color panels) and anti-tubulin antibodies (green in color panels). In this panel, and in all subsequent panels, T1 denotes TACC1, T2 denotes TACC2, and T3 denotes TACC3. Scale bars: interphase, 10 μm; mitotic cells, 5 μm. (B) Western blots of HeLa cell extracts probed with affinity-purified anti-TACC1 (lane 1), anti-TACC2 (lane 2), and anti-TACC3 (lane 3) antibodies. (C) Nocodazole-treated cells were stained with the anti-TACC antibodies (Left) and with a mixture of anti-α-tubulin and anti-γ-tubulin antibodies (Right) to monitor the location of the centrosomes and to confirm that microtubules were depolymerized. All of the TACC antibodies stained the centrosomes (arrows) even though there were no visible microtubules in the cell. Note that the chromosomes in these cells were counterstained with propidium iodide, and the signal from this fluorophore “bleeds through” into the channel used to detect the TACC antibodies. (Scale bar = 4 μm.) (D) A schematic diagram of the TACC proteins. The shaded boxes represent the conserved 200-aa TACC domain that is predicted to form a coiled-coil.
In contrast to TACC2 antibodies, the TACC1 and TACC3 antibodies did not stain centrosomes in interphase HeLa cells. Instead, they diffusely stained both the cytoplasm and the nucleus, with TACC3 being slightly concentrated in the nucleus (Fig. 1A). In mitotic HeLa cells, however, all of the TACC antibodies stained centrosomes to varying extents: the TACC1 antibodies weakly stained centrosomes, the TACC2 antibodies strongly stained centrosomes, whereas the TACC3 antibodies strongly stained a more diffuse region around the centrosomes. All of the antibodies stained the mitotic spindle to varying extents (TACC3 antibodies being the strongest and TACC1 antibodies the weakest), and they also stained a ring-like structure at the cleavage furrow during cytokinesis. In primary fibroblasts, all three TACC proteins had a similar distribution to that shown here in HeLa cells (not shown).
To test whether the concentration of the TACC proteins at the centrosome during mitosis required microtubules, we treated HeLa cells with nocodazole to depolymerize the microtubules. All three of the TACC proteins remained concentrated at centrosomes in the treated mitotic cells (Fig. 1C), suggesting that microtubules are not required to maintain their localization at the centrosome. Interestingly, even in the absence of microtubules, the TACC proteins appeared to maintain their characteristic association with centrosomes: TACC1 was only weakly concentrated at centrosomes, TACC2 was strongly concentrated at centrosomes, and TACC3 was strongly concentrated in a more diffuse region around the centrosome.
The TACC Domain Forms Large Polymers in the Cytoplasm.
We showed previously that the TACC domain of D-TACC can direct a heterologous fusion protein to centrosomes and microtubules in Drosophila embryos (14). We therefore overexpressed the TACC domain of each human TACC protein as a GFP fusion to assess its contribution to localizing the TACC proteins to centrosomes and spindles. Surprisingly, all of the expressed TACC domains assembled into large structures in the cytoplasm in virtually all of the transfected HeLa cells in which they were expressed (Fig. 2A). Similar structures formed when the Drosophila TACC domain was expressed in HeLa cells or in a Drosophila cell line (not shown). These structures did not form in HeLa cells when we expressed GFP on its own or in GFP fusion proteins containing the full-length TACC proteins that lacked the C-terminal TACC domain (not shown).
Figure 2.
The behavior of the overexpressed TACC domains is shown in normal cells (Top) and in cells where microtubules are stabilized by taxol (Middle) or depolymerized by nocodazole (Bottom). The TACC domain is visualized by the fluorescence of the GFP tag (Left). Microtubules were stained with anti-tubulin antibodies (Center). A merged image is shown (Right): in this and all subsequent merged panels, the GFP-TACC fusion protein is shown in green and tubulin in red. The arrow highlights the weak association of tubulin with a TACC domain structure in an untreated cell. In nocodazole-treated cells, the unpolymerized tubulin is concentrated around the TACC domain structures, whereas in taxol the structures stretch out along the microtubule bundles. Only the TACC2 TACC domain is shown, as the TACC domains of TACC1 and TACC3 behave identically. (Scale bar = 10 μm.)
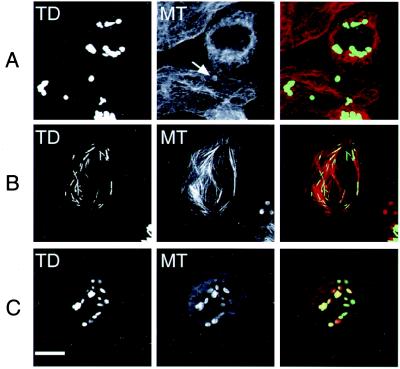
When we examined these structures by thin-section electron microscopy, we found that they were not random aggregates of protein but were highly ordered polymeric structures consisting of many layers of a regularly spaced electron-dense matrix (Fig. 3). Interestingly, the interlayer spacing of these polymers is about 35 nm, which is close to the expected length of the TACC domains coiled-coil (approximately 30 nm for a 200-aa α-helix).
Figure 3.
The large TACC-containing structures are highly ordered polymers. (A) Immuno-electron microscopy of cells overexpressing the GFP-TACC domain fusion proteins. Cells were fixed with formaldehyde and stained with anti-GFP antibodies, followed by Nanogold-labeled secondary antibodies and silver intensification. In thin sections, the silver particles stain the edges of large, globular, cytoplasmic structures that were composed of a regularly spaced, electron dense, polymer (the cell shown here is overexpressing the TACC2 TACC domain). The staining is largely confined to the margins of these structures probably because the formaldehyde fixation has highly cross-linked the structures, impeding internal antibody access. A 2× higher magnification view of part of this structure is shown in B. The ordered morphology of these structures is better preserved in glutaraldehyde-fixed cells shown in C and, at 2× higher magnification, in D. This cell is expressing a GFP full-length TACC2 fusion protein, and the largest diameter of this TACC structure is about half the size of this cell's nucleus. (Scale bar = 0.5 μm.)
The TACC Domain Can Interact with Microtubules and Tubulin.
As the TACC polymers are easily recognizable, they provide an excellent system to study interactions between the TACC proteins and other cellular components in living cells. When we overexpressed the TACC domains in HeLa cells, the microtubule cytoskeleton was usually not dramatically perturbed, and the TACC domain polymers did not appear to interact significantly with microtubules (Fig. 2A). When we treated the transfected cells with taxol to produce large bundles of stabilized microtubules, however, the usually rounded TACC polymers often reorganized into large rod-like fibers that stretched out along the microtubule bundles (Fig. 2B). Thus, the TACC domain polymers can interact with microtubules under certain conditions.
In addition, we noticed that in nontaxol-treated cells that overexpressed a TACC domain, small amounts of tubulin appeared to accumulate around the periphery of the polymer structures (Fig. 2A, arrow). To investigate further this potential interaction with unpolymerized tubulin, we treated the transfected cells with nocodazole to depolymerize the microtubules. In these cells, both anti-α (Fig. 2C) and anti−β−tubulin (not shown) antibodies strongly stained the periphery of the TACC domain polymers, suggesting that the depolymerized tubulin dimers could interact with the TACC domain polymers. We obtained similar results when microtubules were depolymerized by cooling (not shown). No interactions were detected between the polymers and γ-tubulin in either normal or drug-treated cells (data not shown), suggesting that this interaction with tubulin/microtubules is specific.
We have attempted to investigate the interaction between the TACC domain and microtubules/tubulin in vitro by using purified components. In both microtubule spin-down and gel-filtration experiments, however, we were unable to detect any interaction between the purified TACC domains and microtubules or tubulin (not shown). We believe that the TACC domain proteins interact with microtubules in a complex with at least one other protein (see Discussion).
The Interaction of the TACC Domain with Microtubules and Tubulin Depends on Its Protein Context.
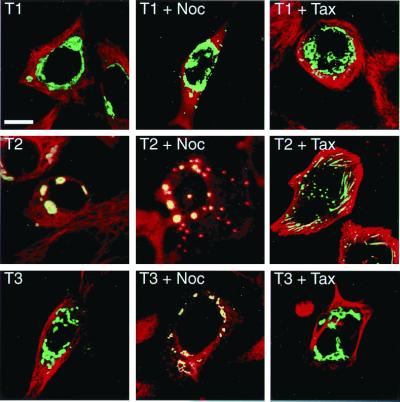
We next overexpressed the full-length TACC proteins as GFP fusions in HeLa cells to test whether they, too, could form similar cytoplasmic polymers. All of the full-length TACC proteins formed large structures in the cytoplasm (Fig. 4 Left), and thin-section electron microscopy of TACC2-expressing cells revealed that these polymers had a very similar organization to the overexpressed TACC domain polymers (Fig. 3 C and D). Unlike the TACC domains on their own, however, the morphology of these TACC structures varied somewhat between the different TACC proteins; the TACC1 structures in particular were often less compacted than the other TACC structures, and they were almost always clustered around the nucleus. Similar polymers formed when the full-length TACC proteins were expressed without the GFP tag or as FLAG-tagged fusion proteins (not shown).
Figure 4.
The behavior of the overexpressed full-length TACC proteins. In normal transfected cells (Left), all of the overexpressed TACC proteins (green) form large structures in the cytoplasm. Tubulin is shown in red in the merged images; when tubulin is concentrated around the TACC structures, they appear to be yellow. Tubulin is not highly concentrated around the TACC1 or TACC3 polymers but is concentrated around the TACC2 polymers. In nocodazole-treated cells (Center), the TACC1 polymers do not interact with tubulin and TACC2 polymers strongly interact with tubulin, whereas the TACC3 polymers weakly interact with tubulin. In taxol-treated cells (Right), only the TACC2 polymers interact with the stabilized microtubules. (Scale bar = 10 μm.)
The presence of the polymers again provided an opportunity to analyze the interactions between the TACC proteins and the microtubule cytoskeleton. Whereas the majority of cells transfected with TACC1 or TACC3 had a normal-looking microtubule cytoskeleton, those overexpressing TACC2 were often rounded and had a slightly disorganized microtubule network. Moreover, the TACC2 polymers accumulated more tubulin in or around them than did TACC1 and TACC3 polymers (Fig. 4 Left). This difference was even more apparent in nocodazole-treated cells where most of the cytoplasmic tubulin appeared to be sequestered around the TACC2 polymers, whereas the TACC3 polymers interacted more weakly with tubulin, and the TACC1 polymers did not appear to interact with tubulin at all (Fig. 4 Center). In taxol-treated cells, only the overexpressed TACC2 protein polymers spread out along the taxol-stabilized microtubules; the TACC1 and TACC3 polymers did not appear to significantly interact with microtubules in these cells (Fig. 4 Right).
The Interaction of the TACC3 Polymers with Microtubules Is Cell Cycle Regulated, and TACC3 Appears to Stabilize Centrosomal Microtubules in Mitosis.
As only the endogenous TACC2 protein is normally present in centrosomes in interphase cells, it may not be surprising that TACC1 and TACC3 polymers failed to interact with tubulin in interphase cells. To test whether the TACC polymers could interact with microtubules in mitotic cells, we transfected synchronized HeLa cells with full-length TACC constructs and fixed them at a time point when they should have been in mitosis (Fig. 5A). Although many of the nontransfected cells were found to be in mitosis at the time of fixation, very few of the transfected cells were in mitosis. This suggests that the presence of the TACC polymers may affect cell cycle progression. We suspect, however, that the presence of these huge TACC polymers could indirectly perturb many cellular processes, and indeed all of the transfected cells die within 4–5 days of transfection. Thus, we cannot conclude that the overexpression of the TACC proteins directly affects cell cycle progression.
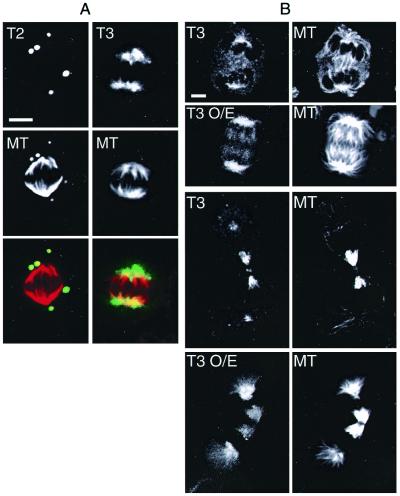
Figure 5.
The behavior of overexpressed TACC2 and TACC3 in mitotic cells. (A) The TACC2 polymers (green in merged image) remain highly compacted throughout mitosis but strongly interact with the unpolymerized tubulin (red in merged image) in the cell. During mitosis, the TACC3 polymers are much less compacted than in interphase, and they are strongly concentrated around the poles of the mitotic spindle. (Scale bar = 5 μm.) (B) TACC3 appears to increase the number of centrosomal microtubules. A comparison between transfected cells overexpressing TACC3 (T3 O/E) and nontransfected cells stained with anti-TACC3 (Left) and anti-tubulin antibodies (Right). In both anaphase (Upper) and telophase (Lower), the centrosomes in TACC3 overexpressing cells appear to be associated with many more microtubules. Note that all of these images were taken from transfected and nontransfected cells on the same coverslip with identical settings on the confocal microscope so that meaningful comparisons could be made between them.
In the few mitotic cells that overexpressed the TACC3 protein, the polymers were less compacted than in interphase cells, and the protein was strongly concentrated in a diffuse region around the spindle poles in a manner similar to the endogenous TACC3. Strikingly, in the mitotic cells that overexpressed the TACC3 protein, there appeared to be many more microtubules associated with the spindles when compared with nontransfected cells on the same coverslip that were at similar stages of mitosis (Fig. 5B). This suggests that the extra TACC3 may increase the number and/or stability of centrosomal microtubules. In the rare mitotic cells that overexpressed TACC1, however, the polymers did not detectably associate with tubulin or centrosomes (not shown). Similarly, the TACC2 polymers remained compacted throughout mitosis and did not associate with the centrosomes, although tubulin was still strongly concentrated in or around these polymers (Fig. 5A). Although the number of transfected cells in mitosis was small, there did not appear to be any obvious delay at any particular stage of mitosis in cells transfected with any of the TACC proteins.
Discussion
The TACC Proteins Are a Conserved Family of Centrosome- and Microtubule-Interacting Proteins.
We previously showed that the D-TACC protein interacted with microtubules in Drosophila embryo extracts and was concentrated at centrosomes in embryos. We also showed that the conserved C-terminal region of D-TACC could target a heterologous fusion protein to centrosomes and microtubules, and that the human TACC2 protein was concentrated at centrosomes in human cells. We proposed that the TACC domain was a conserved microtubule- and centrosome-interacting domain.
The data we present here support this proposal, although the three known human TACC proteins appear to interact with centrosomes and microtubules in unique ways. Unlike TACC2, both TACC1 and TACC3 are not concentrated at centrosomes in interphase but are distributed in the cytoplasm and nucleus, with TACC3 being concentrated in the nucleus of many cells. In mitosis, all three TACC proteins interact with centrosomes and microtubules but in different ways: TACC1 is only weakly concentrated at centrosomes and on spindles, TACC2 is strongly concentrated at centrosomes and more weakly associates with spindles, whereas TACC3 is strongly concentrated in a more diffuse region that surrounds the centrosome, and it has the strongest interaction with the mitotic spindle. An interaction with microtubules does not, however, appear to be required to maintain the association of the TACC proteins with centrosomes, as they remain concentrated at centrosomes even in the presence of microtubule depolymerizing agents. Thus, the TACC proteins all appear to be genuine centrosomal proteins (25) that can also associate with microtubules.
It is not clear how the TACC domain interacts with centrosomes or microtubules. We have so far been unable to observe a strong interaction between any of the bacterially expressed and purified TACC domains and purified tubulin (ref. 14 and M. J. Lee and J.W.R., unpublished observations). In the case of D-TACC, however, the bacterially expressed TACC domain binds strongly to microtubules when it is mixed with embryo extracts (14). Our preliminary data suggest that D-TACC binds to microtubules in a complex with at least one other protein and that this is also true of the human TACC proteins (M. J. Lee, F.G., and J.W.R., unpublished observations). Surprisingly, however, if we perform similar microtubule spin-down experiments with mitotic or interphase HeLa cell extracts, we do not detect a strong interaction between any of the endogenous TACC proteins and microtubules (F.G., unpublished observations). This may represent a real difference between the human TACCs and D-TACC; as in mitotic HeLa cells treated with taxol, many supernumary asters form in the cytoplasm, but the TACC proteins do not interact with these asters and remain concentrated around the centrosomes (F.G., unpublished observations). Thus, the endogenous human TACC proteins may only associate strongly with microtubules when they are in the context of the centrosome or spindle.
The TACC Proteins Can Form Large Polymers.
When the TACC proteins are overexpressed in HeLa cells, they all form large structures in the cytoplasm. Ultrastructural analysis of these structures revealed that they were highly ordered polymers, consisting of interwoven layers of a regularly spaced, electron-dense, matrix. The formation of these polymers is TACC domain dependent; fusion proteins that lack the TACC domain do not form these structures, and the TACC domain from any of the human TACC proteins (and also from D-TACC) is sufficient to drive the formation of similar polymers when overexpressed in HeLa cells. Surprisingly, the overexpressed TACC domain polymers do not normally appear to interact with centrosomes or microtubules in transfected cells. We suspect that this is because transient transfection leads to a massive overexpression of the TACC domain proteins, and this drives the formation of large, compact cytoplasmic polymers that may be unable to interact efficiently with centrosomes or microtubules. In support of this possibility, when microtubules are stabilized with taxol, the TACC domain polymers reorganize and spread out along the bundles of stabilized microtubules, perhaps because the bundles of microtubules give the compacted polymers a greater surface area of interaction.
Although the large polymers formed in transfected cells are clearly a consequence of overexpression, the ability to form such polymers is highly unusual. Several components of the yeast spindle pole body, however, form large polymeric structures when overexpressed, and their ability to polymerize is thought to be important for their function (24, 26, 27). The essential vertebrate spindle component NuMA has also been shown to form large rod-like polymers when overexpressed in human cells (28). Further experiments will be required, however, to determine whether the TACC proteins normally form polymeric structures within cells.
The Functions of the TACC Proteins.
The results presented here raise the possibility that the human TACC proteins and D-TACC could perform similar functions. When, for example, TACC3 is overexpressed in cells, it is strongly concentrated around centrosomes in mitosis, and the centrosomes appear to associate with more microtubules than normal. We observe similar effects when D-TACC is overexpressed in Drosophila embryos (J.W.R. and K. Jeffers, unpublished work). This effect is not, however, seen in mitotic cells that overexpress TACC1 or TACC2. This may be because the large cytoplasmic polymers formed by the overexpressed TACC1 and TACC2 proteins cannot fulfil the normal functions of the endogenous proteins. Unlike the TACC3 polymers, TACC1 and TACC2 polymers remain compacted throughout mitosis, and they do not mimic the localization of the endogenous proteins. On the other hand, our results indicate that the three human TACC proteins interact with centrosomes and microtubules in different ways, suggesting that they could perform distinct and potentially nonredundant functions in cells.
If the human TACC proteins are involved in regulating the interaction between centrosomes and microtubules, this could explain the proposed links between the TACC genes and cancer. In Drosophila embryos, decreasing the levels of D-TACC at the centrosome leads to failures in pronuclear fusion, nuclear migration, and chromosome segregation and ultimately to embryonic death. Conversely, increasing the levels of D-TACC also leads to chromosome segregation defects and to a significant reduction in embryonic viability (J.W.R. and K. Jeffers, unpublished work). Thus, perturbing the levels of the human TACC proteins could cause similar defects in human cells, and this could contribute to the large-scale genetic instability that is a common feature of many, if not all, cancers (29–35). This could also explain the apparent paradox of why the TACC proteins have been suggested to be transforming proteins (15, 16), whereas TACC2 has also been isolated as a tumor suppressor protein (17); in Drosophila, increasing or decreasing the levels of D-TACC leads to an increase in genetic instability.
Acknowledgments
We thank Kim Jeffers for technical assistance and Douglas Kershaw for cutting serial thin sections. We thank Dávid Szüts and members of the Raff lab for comments on the manuscript. This work was supported by a Wellcome Trust Prize studentship and Overseas Research Scholarship Scheme Award (to F.G.), a Wellcome Trust Senior Research Fellowship (to J.W.R.), a Marie Curie European Fellowship (to C.K.), the Medical Research Council (to J.K.), and US Army Medical Research Grant BC980338 (to I.S. and J.C.).
Abbreviations
- D-TACC
Drosophila transforming acidic coiled-coil
- GFP
green fluorescent protein
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Glover D M, Gonzalez C, Raff J W. Sci Am. 1993;268:62–68. doi: 10.1038/scientificamerican0693-62. [DOI] [PubMed] [Google Scholar]
- 2.Kellogg D R, Moritz M, Alberts B M. Annu Rev Biochem. 1994;63:639–674. doi: 10.1146/annurev.bi.63.070194.003231. [DOI] [PubMed] [Google Scholar]
- 3.Desai A, Mitchison T J. Annu Rev Cell Dev Biol. 1997;13:83–117. doi: 10.1146/annurev.cellbio.13.1.83. [DOI] [PubMed] [Google Scholar]
- 4.Moritz M, Braunfeld M B, Sedat J W, Alberts B, Agard D A. Nature (London) 1995;378:638–640. doi: 10.1038/378638a0. [DOI] [PubMed] [Google Scholar]
- 5.Zheng Y X, Wong M L, Alberts B, Mitchison T. Nature (London) 1995;378:578–583. doi: 10.1038/378578a0. [DOI] [PubMed] [Google Scholar]
- 6.Merdes A, Ramyar K, Vechio J D, Cleveland D W. Cell. 1996;87:447–458. doi: 10.1016/s0092-8674(00)81365-3. [DOI] [PubMed] [Google Scholar]
- 7.Karsenti E. Semin Cell Biol. 1991;2:251–260. [PubMed] [Google Scholar]
- 8.Hyman A A, Karsenti E. Cell. 1996;84:401–410. doi: 10.1016/s0092-8674(00)81285-4. [DOI] [PubMed] [Google Scholar]
- 9.Compton D A. J Cell Sci. 1998;111:1477–1481. doi: 10.1242/jcs.111.11.1477. [DOI] [PubMed] [Google Scholar]
- 10.Kidd D, Raff J W. J Cell Sci. 1997;110:209–219. doi: 10.1242/jcs.110.2.209. [DOI] [PubMed] [Google Scholar]
- 11.Kellogg D R, Oegema K, Raff J, Schneider K, Alberts B M. Mol Biol Cell. 1995;6:1673–1684. doi: 10.1091/mbc.6.12.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kellogg D R, Field C M, Alberts B M. J Cell Biol. 1989;109:2977–2991. doi: 10.1083/jcb.109.6.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oegema K, Marshall W F, Sedat J W, Alberts B M. J Cell Sci. 1997;110:1573–1583. doi: 10.1242/jcs.110.14.1573. [DOI] [PubMed] [Google Scholar]
- 14.Gergely F, Kidd D, Jeffers K, Wakefield J G, Raff J W. EMBO J. 2000;19:241–252. doi: 10.1093/emboj/19.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Still I H, Hamilton M, Vince P, Wolfman A, Cowell J K. Oncogene. 1999;18:4032–4038. doi: 10.1038/sj.onc.1202801. [DOI] [PubMed] [Google Scholar]
- 16.Still I H, Vince P, Cowell J K. Genomics. 1999;58:165–170. doi: 10.1006/geno.1999.5829. [DOI] [PubMed] [Google Scholar]
- 17.Chen H M, Schmeichel K L, Mian I S, Lelievre S, Petersen O W, Bissell M J. Mol Biol Cell. 2000;11:1357–1367. doi: 10.1091/mbc.11.4.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stebbins-Boaz B, Cao Q, de Moor C H, Mendez R, Richter J D. Mol Cell. 1999;4:1017–1027. doi: 10.1016/s1097-2765(00)80230-0. [DOI] [PubMed] [Google Scholar]
- 19.Smith D B, Johnson K S. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 20.Huang J, Raff J W. EMBO J. 1999;18:2184–2195. doi: 10.1093/emboj/18.8.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pines J, Hunter T. J Cell Biol. 1991;115:1–17. doi: 10.1083/jcb.115.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Towbin H, Staehlin T, Gordon J. Proc Natl Acad Sci USA. 1979;80:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Adams I R, Kilmartin J V. J Cell Biol. 1999;145:809–823. doi: 10.1083/jcb.145.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalt A, Schliwa M. Trends Cell Biol. 1993;3:119–128. doi: 10.1016/0962-8924(93)90174-y. [DOI] [PubMed] [Google Scholar]
- 26.Bullitt E, Rout M P, Kilmartin J V, Akey C W. Cell. 1997;89:1077–1086. doi: 10.1016/s0092-8674(00)80295-0. [DOI] [PubMed] [Google Scholar]
- 27.Kilmartin J V, Goh P Y. EMBO J. 1996;15:4592–4602. [PMC free article] [PubMed] [Google Scholar]
- 28.Saredi A, Howard L, Compton D A. J Cell Sci. 1996;109:619–630. doi: 10.1242/jcs.109.3.619. [DOI] [PubMed] [Google Scholar]
- 29.Doxsey S. Nat Genet. 1998;20:104–106. doi: 10.1038/2392. [DOI] [PubMed] [Google Scholar]
- 30.Brinkley B R, Goepfert T M. Cell Motil Cytoskeleton. 1998;41:281–288. doi: 10.1002/(SICI)1097-0169(1998)41:4<281::AID-CM1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 31.Li R, Yerganian G, Duesberg P, Kraemer A, Willer A, Rausch C, Hehlmann R. Proc Natl Acad Sci USA. 1997;94:14506–14511. doi: 10.1073/pnas.94.26.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lingle W L, Lutz W H, Ingle J N, Maihle N J, Salisbury J L. Proc Natl Acad Sci USA. 1998;95:2950–2955. doi: 10.1073/pnas.95.6.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghadimi B M, Sackett D L, Difilippantonio M J, Schrock E, Neumann T, Jauho A, Auer G, Ried T. Genes Chromosomes Cancer. 2000;27:183–190. [PMC free article] [PubMed] [Google Scholar]
- 34.Lingle W L, Salisbury J L. Am J Pathol. 1999;155:1941–1951. doi: 10.1016/S0002-9440(10)65513-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salisbury J L, Whitehead C M, Lingle W L, Barrett S L. Biol Cell. 1999;91:451–460. [PubMed] [Google Scholar]