Abstract
Background:
The use of radiofrequency energy to treat damaged anterior cruciate ligaments is gaining popularity. However, complete rupture of the ligament after treatment has been reported.
Purpose:
To evaluate the effect of thermal energy applied arthroscopically to normal, intact anterior cruciate ligaments in mature dogs.
Study Design:
Controlled laboratory study.
Methods:
Monopolar radiofrequency energy was applied to the normal anterior cruciate ligament of 1 knee in 18 dogs. The contralateral anterior cruciate ligament (also normal) was sham treated. Force-plate gait analysis was performed preoperatively and at 4, 8, 12, 16, 26, and 36 weeks after surgery. Anterior cruciate ligament rupture was detected by a sudden onset of nonweightbearing and a positive drawer sign.
Results:
All treated ligaments ruptured approximately 55 days after surgery (mean, 55 days; standard error, 1.6).
Conclusions:
Although monopolar radiofrequency energy may have some potential in the treatment of lax anterior cruciate ligaments, in the application described here the result was a highly predictable deterioration and rupture of all treated anterior cruciate ligaments.
Clinical Relevance:
On the basis of these findings, we strongly recommend that strict selection and application criteria be used when considering use of this modality on anterior cruciate ligaments that are stretched or partially disrupted, or both. Use of this modality should be followed by adherence to a highly conservative rehabilitation protocol.
The use of monopolar radiofrequency energy to shrink joint capsule has been previously studied and reported.4-7, 9, 11, 15 The shrinkage effect of this modality takes place via thermally induced changes in collagen structure.8 More recently, monopolar radiofrequency energy has been applied to ACLs that are stretched or partially disrupted to remove excess laxity and restore joint stability.19 Strict selection criteria and carefully controlled, prolonged rehabilitation protocols have been recommended. There are recent reports of complications resulting from application of radiofrequency energy to the ACL, PCL, or both, including one report of subsequent complete rupture of the ACL and PCL after monopolar radiofrequency energy treatment and another report of subsequent complete disruption of an ACL graft.16, 18
We undertook this study to evaluate the effect of surgically applied monopolar radiofrequency energy treatment on normal ACLs in a canine model.3, 10 Animals were examined at intervals up to 9 months postoperatively. The postoperative sequelae are presented here. This is the first such animal study, to our knowledge.
MATERIALS AND METHODS
All aspects of this study were performed in accordance with institutional and National Institutes of Health regulations governing the treatment of vertebrate animals. The study was initiated after approval by the University of Wisconsin-Madison Animal Care Committee. Eighteen skeletally mature female research hounds, weighing 21 to 30.5 kg each (mean, 25.9 kg; SEM, 0.9), were used in this study. Standard lateral to medial and caudal to cranial extended knee radiographs were obtained to rule out preexisting joint disease. All animals were also examined preoperatively for lameness by using force-plate gait analysis.
Each animal was anesthetized and the joint was explored arthroscopically through a stab incision in the lateral patellofemoral joint capsule. A stab incision was then made in the medial joint capsule at a slightly more proximal level, and a 2-mm diameter monopolar radiofrequency energy probe (TAC-C probe, Smith & Nephew Endoscopy, Inc., Menlo Park, California) was introduced into the joint. We applied energy to the ACL using a monopolar radiofrequency energy generator (ORA 50, Smith & Nephew Endoscopy, Inc.) with temperature and power settings of 70°C and 25 W, respectively (Fig. 1).3, 10 Energy was applied in a transverse sweeping motion at a rate of approximately 1 to 2 mm/sec across the ligament from its origin to its insertion, with no overlap between passes. The entire dorsal surface of the ligament was treated, leaving no untreated tissue. Treated ligament tissue took on a dull, off-white color, whereas untreated tissue retained a glistening white, fibrous appearance (Fig. 1). The joint was lavaged for 60 seconds with sterile saline just before closure. An identical operation was performed on the contralateral knee, with the exception that no energy was applied to the ACL. Postoperatively, no stabilization techniques were applied to the knees. Animals were confined to 1.2 × 2.4 meter kennels for the duration of the study.
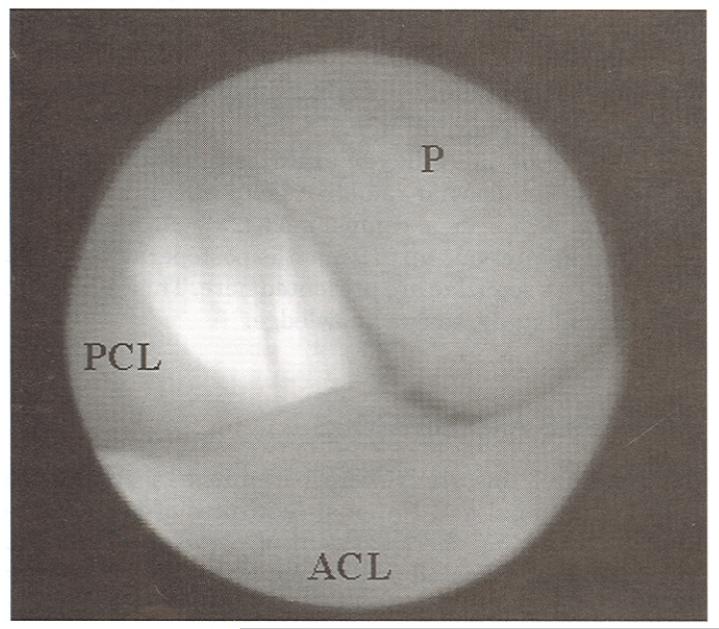
Figure 1.
A canine ACL after treatment with monopolar radiofrequency energy (70°C, 25 W). The monopolar radiofrequency energy probe (P) is pictured lightly touching the surface of the ACL, and the PCL is visible in the background.
Gait analysis was performed with the use of a force plate (OR6–6-1000 Biomechanics Platform with SGA6–4 Signal Conditioner/Amplifier, Advanced Medical Technology Inc., Newton, Massachusetts) preoperatively and at 4, 8, 12, 16, 26, and 36 weeks after surgery. The force plate was connected to a commercially available satellite data acquisition system (VETDATA v2.03, Acquire v5.0, Mininet v4.0, and Update v1.1 from Sharon Software Inc., Dewitte, Michigan), as previously described.2, 10, 12 Peak vertical force was recorded for each limb. Dogs were observed daily for lameness throughout the study. Acute onset of lameness and a positive anterior drawer sign with knee palpation were clear indications of ACL rupture in each dog. The animals were sacrificed at 16 (N = 7), 26 (N = 6), or 36 (N = 5) weeks after rupture of the treated ACL.
Two-way analysis of variance was used to evaluate differences in peak vertical force over time and between treatments. One-way analysis of variance was used to compare peak vertical values between time points. Tukey's post hoc analysis was used to evaluate significant differences between time points and Student's t-tests were used to compare peak vertical force values between treatments at each time point. Significance was set at P < 0.05.
RESULTS
All treated ACLs ruptured approximately 55 days after surgery (mean, 55 days; SEM, 1.6). The exact dates of ACL rupture were not available for four dogs, so information on those four was not used to calculate the mean rupture date. Rupture was detected by sudden onset of nonweight-bearing (lameness), significant joint effusion, and the presence of a positive drawer sign on palpation. Complete dissolution of all treated ACLs was confirmed at necropsy (Fig. 2). Pathologic joint changes apparent at necropsy in joints with treated ligaments were characteristic of the well-documented osteoarthritic changes noted in canine models with ACL transections.3, 13, 17, 20 There were no remnants of the ligament in the majority of the joints with treated ACLs (15 of 18). Three of the 18 joints had small granulation beds at the origin and insertion points of the ACL (Fig. 2). Joints in which no energy had been applied to the ACL were free of pathologic changes, and the ACL and PCL were intact.
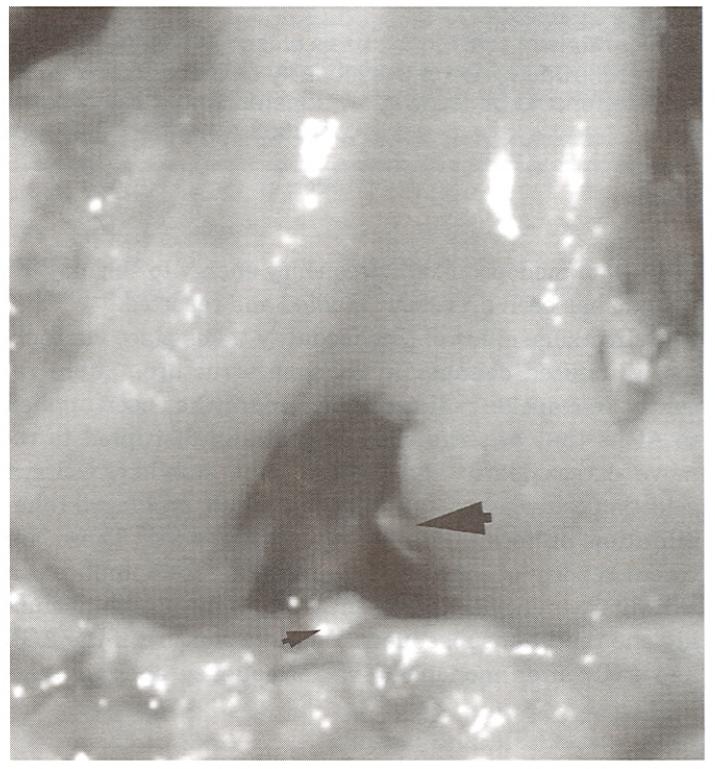
Figure 2.
Canine stifle demonstrating complete dissolution of the ACL 16 weeks after treatment with monopolar radiofrequency energy. There are small granulation beds at the origin (large arrow) and insertion (small arrow) points of the ligament.
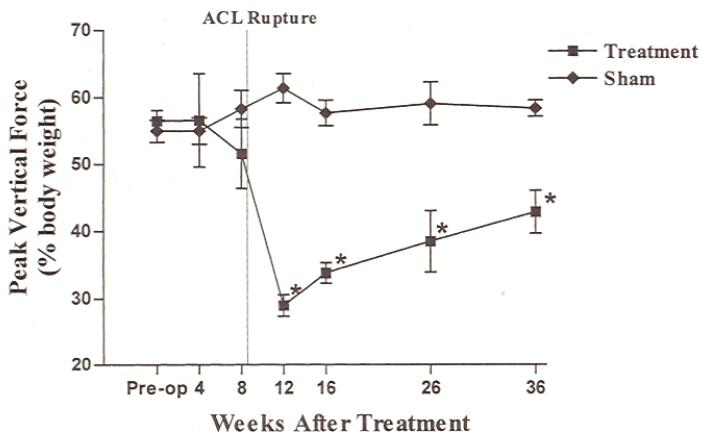
Peak vertical force values in limbs with treated ACLs decreased significantly between the 8- and 12-week evaluation points, which corresponded to the mean rupture date (Fig. 3).10 The forces were significantly lower in limbs with treated ACLs than in limbs with sham-treated ACLs for the remainder of the study. Preoperative and 4-week peak vertical force values were significantly higher than 12-, 16-, and 26-week values in limbs with treated ACLs. Peak vertical force values in limbs with treated ACLs were significantly higher at the 8-week time point than the values at 12 and 16 weeks. There were no significant differences in peak vertical force values between time intervals in limbs with sham-treated ACLs.
Figure 3.
Peak vertical force (percent body weight) in limbs with treated and untreated ACLs over the course of the study. There was a significant decrease in peak vertical force in limbs with treated ACLs between 8 and 12 weeks after surgery. The mean rupture date is indicated by a dotted line. The asterisks indicate a significant difference between limbs with treated ACLs and those with untreated ACLs (P < 0.05).
DISCUSSION
The use of monopolar radiofrequency energy in the treatment of glenohumeral joint instability has been reported to result in beneficial clinical effects.6, 7 This success has encouraged its use in the treatment of injuries in other collagenous tissues, including the ACL. Radiofrequency energy has been used successfully to treat ACL injuries when followed by a prolonged rehabilitation period.19 The therapy, however, has not been without complications.16, 18 In this study, we documented the complete dissolution of normal, intact canine ACLs after thermal treatment of the entire ligament surface without postoperative joint protection.
The procedure and model for application of radiofrequency energy to the ACL in this study differed from those used in the clinical situation in a number of ways. The ACLs and associated joints in this study were clinically normal before treatment. The entire surface of the ACLs was treated, leaving no untreated areas on the ligaments. In addition, there was no joint protection after treatment. The application was performed in a quadruped (canine) model, which of course has a different knee joint angle and applied ACL forces than in the human knee. We chose this model and procedure to evaluate the results of radiofrequency energy treatment of the ACL in an unprotected model so that negative influences on ligament structure would be readily apparent, both histologically and ultrastructurally. Given the differences between the canine stifle and the human knee joint, as well as the intentional treatment of the entire ligament (leaving no untreated tissue) and the absence of any attempt to protect the treated tissues, any comparisons between this study and potential human application must be made with extreme caution.
The lack of ACL remnants in knees with treated ligaments is not unusual. There is often little or no evidence of ruptured ACLs in canine knees that have undergone spontaneous ACL rupture. The reason for this is the retraction of the ends of the ruptured ligament and their gradual resorption by collagenase activity of the synovia.14 The earliest time of gross evaluation of the knees in this study was 16 weeks after surgery, or approximately 8 weeks after rupture. This was likely enough time for normal enzymatic, proteolytic, and phagocytic mechanisms to remove the damaged tissue.
There may be multiple reasons for the rupture of treated ACLs in this study. The restricted blood supply and associated limited healing capacity of the canine ACL is well documented.1 It is possible that the treatment technique and the power setting used resulted in thermally mediated changes that extended deeper than the treated superficial surface, causing major, catastrophic changes in the ligament structure. This, coupled with the fact that there was no protection of the treated ligament, likely caused ligament rupture. The decrease in stiffness and the increase in relaxation properties in thermally treated joint capsular tissue have been documented in vitro and in vivo.5, 9 It is likely that these types of changes occurred in the treated tissue in this study. Given the changes in structure, biomechanical properties, and lack of protection of treated joints, it is not surprising that the ligaments failed.
One of the most interesting findings in this study was the consistency of the time point at which the ligaments ruptured, as was indicated by a sudden onset of clinical signs.3, 10 There was some variation in the canine subjects, and surgeries were performed at different times. The mechanism by which the ACL degenerated and ruptured, however, was clearly chronologically similar in all of the animals. The similarities in the degeneration process between animals makes it possible that, in addition to the mechanical changes in ligament structure, treatment may incite a specific inflammatory response directed against the ligament. This possibility deserves further consideration and study, and this canine model may be appropriate for insight into the process of naturally occurring ligament degeneration in humans.
This study demonstrated a very consistent pattern of degeneration and rupture of the normal canine ACL after treatment with monopolar radiofrequency energy.3, 10 The entire ligament surface was treated and there was no postoperative protection of treated tissues. These factors likely contributed to the dissolution of the ligaments. On the basis of these findings, we strongly recommend that there be strict selection and application criteria of this modality when used in subjects with stretched or partially disrupted ACLs. Strict adherence to a highly conservative rehabilitation protocol is imperative. Further studies are necessary to determine the precise mechanism of ACL failure caused by monopolar radiofrequency energy.
ACKNOWLEDGMENTS
The authors thank John Bogdanske, Jennifer Devitt, and Daria Schwartz for their assistance in all aspects of this study. This study was funded in part by Smith & Nephew Endoscopy, Inc., Menlo Park, California, and the National Institutes of Health–National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Footnotes
Both authors have a commercial affiliation with a product named in this study. Funding was received from commercial parties related to products mentioned in the study. Funding information is noted in the “Acknowledgments” section.
REFERENCES
- 1.Arnoczky SP, Rubin RM, Marshall JL. Microvasculature of the cruciate ligament and its response to injury. An experimental study in dogs. J Bone Joint Surg. 1979;61A:1221–1229. [PubMed] [Google Scholar]
- 2.Budsberg SC, Jevens DJ, Brown J, et al. Evaluation of limb symmetry indices, using ground reaction forces in healthy dogs. Am J Vet Res. 1993;54:1569–1574. [PubMed] [Google Scholar]
- 3.Chu Q, Lopez MJ, Hayashi K, et al. Elevation of a collagenase generated type II collagen neoepitope and proteoglycan epitopes in synovial fluid following induction of joint instability in the dog. Osteoarthritis Cartilage. 2002;10:662–669. doi: 10.1053/joca.2002.0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hecht P, Hayashi K, Cooley AJ, et al. The thermal effect of monopolar radiofrequency energy on the properties of joint capsule: An in vivo histological study using a sheep model. Am J Sports Med. 1998;26:808–814. doi: 10.1177/03635465980260061201. [DOI] [PubMed] [Google Scholar]
- 5.Hecht P, Hayashi K, Lu Y, et al. Monopolar radiofrequency energy effects on joint capsular tissue: Potential treatment for joint instability. An in vivo mechanical, morphological, and biochemical study using an ovine model. Am J Sports Med. 1999;27:761–771. doi: 10.1177/03635465990270061301. [DOI] [PubMed] [Google Scholar]
- 6.Levitz CL, Dugas J, Andrews JR. The use of arthroscopic thermal capsulorrhaphy to treat internal impingement in baseball players. Arthroscopy. 2001;17:573–577. doi: 10.1053/jars.2001.24853. [DOI] [PubMed] [Google Scholar]
- 7.Levy O, Wilson M, Williams H, et al. Thermal capsular shrinkage for shoulder instability. Mid-term longitudinal outcome study. J Bone Joint Surg. 2001;83B:640–645. doi: 10.1302/0301-620x.83b5.11374. [DOI] [PubMed] [Google Scholar]
- 8.Lopez M, Hayashi K, Fanton GS, et al. The effect of radiofrequency energy on the ultrastructure of joint capsular collagen. Arthroscopy. 1998;14:495–501. doi: 10.1016/s0749-8063(98)70078-7. [DOI] [PubMed] [Google Scholar]
- 9.Lopez MJ, Hayashi K, Vanderby R, Jr, et al. Effects of monopolar radiofrequency energy on ovine joint capsular mechanical properties. Clin Orthop. 2000;374:286–297. doi: 10.1097/00003086-200005000-00026. [DOI] [PubMed] [Google Scholar]
- 10.Lopez MJ, Kunz D, Vanderby R, Jr, et al. A comparison of joint stability between anterior cruciate intact and deficient knees: A new canine model of anterior cruciate ligament disruption. J Orthop Res. 2003;21:224–230. doi: 10.1016/S0736-0266(02)00132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu Y, Hayashi K, Edwards RB, III, et al. The effect of monopolar radiofrequency treatment pattern on joint capsular healing. In vitro and in vivo studies using an ovine model. Am J Sports Med. 2000;28:711–719. doi: 10.1177/03635465000280051601. [DOI] [PubMed] [Google Scholar]
- 12.Markel MD, Wood SA, Bogdanske JJ, et al. Comparison of healing of allograft/endoprosthetic composites with three types of gluteus medius attachment. J Orthop Res. 1995;13:105–114. doi: 10.1002/jor.1100130116. [DOI] [PubMed] [Google Scholar]
- 13.Marshall KW, Chan AD. Bilateral canine model of osteoarthritis. J Rheumatol. 1996;23:344–350. [PubMed] [Google Scholar]
- 14.Moore KW, Read RA. Rupture of the cranial cruciate ligament in dogs—Part I. Compend Contin Educ Pract Vet. 1996;18:223–233. [Google Scholar]
- 15.Orbzut SL, Hecht P, Hayashi K, et al. The effect of radiofrequency energy on the length and temperature profiles of the glenohumeral joint capsule. Arthroscopy. 1998;14:395–400. doi: 10.1016/s0749-8063(98)70007-6. [DOI] [PubMed] [Google Scholar]
- 16.Perry JJ, Higgins LD. Anterior and posterior cruciate ligament rupture after thermal treatment. Arthroscopy. 2000;16:732–736. doi: 10.1053/jars.2000.17981. [DOI] [PubMed] [Google Scholar]
- 17.Pond MJ, Nuki G. Experimentally induced osteoarthritis in the dog. Ann Rheum Dis. 1973;32:387–388. doi: 10.1136/ard.32.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sekiya JK, Golladay GJ, Wojtys EM. Autodigestion of a hamstring anterior cruciate ligament autograft following thermal shrinkage. A case report and sentinel of concern. J Bone Joint Surg. 2000;82A:1454–1457. doi: 10.2106/00004623-200010000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Thabit G., III The arthroscopic monopolar radiofrequency treatment of chronic anterior cruciate ligament instability. Oper Tech Sports Med. 1998;6:157–160. [Google Scholar]
- 20.Visco DM, Hill MA, Widmer WR, et al. Experimental osteoarthritis in dogs: A comparison of the Pond-Nuki and medial arthrotomy methods. Osteoarthritis Cartilage. 1996;4:9–22. doi: 10.1016/s1063-4584(96)80003-3. [DOI] [PubMed] [Google Scholar]