Summary
Improgan, a chemical congener of cimetidine, is a highly effective non-opioid analgesic when injected into the CNS. Despite extensive characterization, neither the improgan receptor, nor a pharmacological antagonist of improgan has been previously described. Presently, the specific binding of 3H-cimetidine (3HCIM) in brain fractions was used to discover 4(5)-((4-iodobenzyl)thiomethyl)-1H-imidazole, which behaved in vivo as the first improgan antagonist. The synthesis and pharmacological properties of this drug (named CC12) are described herein. In rats, CC12 (50 – 500 nmol, icv) produced dose-dependent inhibition of improgan (200 – 400 nmol) antinociception on the tail flick and hot plate tests. When given alone to rats, CC12 had no effects on nociceptive latencies, or on other observable behavioral or motor functions. Maximal inhibitory effects of CC12 (500 nmol) were fully surmounted with a large icv dose of improgan (800 nmol), demonstrating competitive antagonism. In mice, CC12 (200-400 nmol, icv) behaved as a partial agonist, producing incomplete improgan antagonism, but also limited antinociception when given alone. Radioligand binding, receptor autoradiography, and electrophysiology experiments showed that CC12's antagonist properties are not explained by activity at 25 sites relevant to analgesia, including known receptors for cannabinoids, opioids or histamine. The use of CC12 as an improgan antagonist will facilitate the characterization of improgan analgesia. Furthermore, because CC12 was also found presently to inhibit opioid and cannabinoid antinociception, it is suggested that this drug modifies a biochemical mechanism shared by several classes of analgesics. Elucidation of this mechanism will enhance understanding of the biochemistry of pain relief.
Keywords: antinociception, improgan, morphine, cannabinoids, cimetidine, analgesia
1. Introduction
Reports of antinociception following the injection of certain histamine H2 antagonists into the rodent CNS (Netti et al., 1984) led to the discovery and characterization of a family of analgesic drugs which act independently of the H2 receptor (Li et al., 1996, Hough et al., 1997, Hough, 2004). The prototype drug, named improgan (Fig. 1), was originally described as a cimetidine-like drug which lacked H2 receptor antagonist properties (Ganellin, 1982). When administered by intracerebroventricular (icv) injection to rats, improgan is highly effective in attenuating thermal (Li et al., 1996), mechanical (Li et al., 1997a) and neuropathic (in preparation) nociceptive responses at doses which do not produce toxicity, change locomotor activity, or impair rotorod performance (Li et al., 1997a). Thus, improgan-like drugs have a preclinical profile suggestive of highly effective analgesics. Significant impediments to the clinical development of improgan-like analgesics are the failure to identify the improgan antinociceptive target, and the lack of penetration of the blood-brain barrier by these drugs. Progress on the latter was recently reported (Hough et al., 2005).
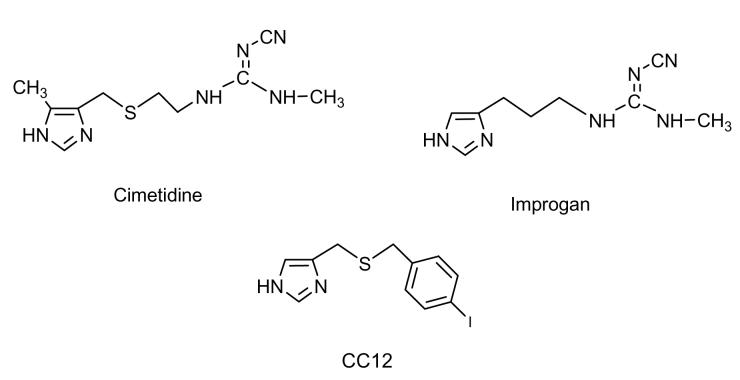
Figure 1.
Chemical structures of cimetidine, improgan, and CC12. When administered into the brain, cimetidine is an H2 receptor antagonist with antinociceptive properties. Improgan is a cimetidine congener lacking H2 receptor affinity, but which retains antinociceptive activity. CC12 is reported presently to be an improgan antagonist.
Although information has been gained by mapping the relevant CNS sites and circuits underlying improgan antinociception (Hough et al., 2001b, Svokos et al., 2001, Nalwalk et al., 2004, Nalwalk et al., 2005), the mechanism of action of this compound remains unknown. Improgan shows no significant affinity at 60 ion channels or G protein-coupled receptors (Hough et al., 2001a). Experiments with pharmacological antagonists (Li et al., 1997b) and knock-out mice exclude roles for known histamine (Mobarakeh et al., 2003) and opioid (Hough et al., 2000a) receptors. The improgan target may be an unknown histamine receptor (Hough et al., 1999), but this has not been established. Improgan also lacks affinity at known cannabinoid receptors, although cannabinoid mechanisms may participate in improgan signaling (Hough et al., 2002; Nalwalk et al., 2006). The search for improgan's mechanism has been hampered by a lack of appropriate pharmacological tools. For example, although some structure-activity studies have been performed (Hough et al., 1997, Hough et al., 2000b, Hough et al., 2006), no pharmacological antagonist of improgan has yet been discovered.
The search by our laboratory for in vitro models of improgan antinociception led us recently to investigate the radioligand binding of 3H-cimetidine (3HCIM) to rat brain homogenates. Although many laboratories have used 3HCIM binding as an index of histamine H2 receptor affinity, it was shown many years ago that the H2 receptor is not responsible for this specific binding (Smith et al., 1980;Warrander et al., 1983). The identity of the brain's 3HCIM binding site was never established, and the effects of improgan on 3HCIM binding have not been reported. Because icv-administered cimetidine produces antinociception (Li et al., 1996), the 3HCIM binding site may be an antinociceptive target. If so, then drugs with high affinity for this binding site should be analgesics, or behave as analgesic antagonists. Presently, we describe for the first time the synthesis and pharmacological properties of 4(5)-((4-iodobenzyl)thiomethyl)-1H-imidazole (herein named CC12, Fig. 1). We report here that CC12 has high affinity for the 3HCIM binding site and that this drug behaves as an improgan antagonist.
2. Methods
2.1. Animals
Male Sprague-Dawley rats (175 - 230 g) and male albino Swiss-Webster mice (25-40 g, both from Taconic Farms, Germantown, NY) were maintained on a 12-h light/ dark cycle (lights on from 7:00am to 7:00pm) and provided with food and water. Rats were housed in groups of 3-4 until the time of surgery and individually thereafter. Mice were housed in groups of 4-6. All animal experiments were approved by the Institutional Animal Care and Use Committee of Albany Medical College.
2.2. Drugs and solutions
Cimetidine (base, Tocris, Ellisville, MO) and improgan (base, synthesized as previously described (Hough et al., 2000a) were dissolved in dilute HCl, neutralized to pH 5.5 to 6, and diluted with either buffer or saline. CC12 hydrochloride was dissolved in saline (pH=4.5). Except for Fig. 7 studies, CC12 base was dissolved in dilute HCl and titrated to pH 4.5. This pH was the highest permitting the drug to remain in solution, and icv injections of this vehicle solution had no adverse effects. For experiments in Fig. 7, CC12 base and WIN-55,212-2 (Sigma-Aldrich, dosed as mesylate salt) were dissolved in DMSO and diluted with water to 60% DMSO. Several labs (including ours) have used this concentration of DMSO as a diluent for icv studies without adverse effects. Morphine sulfate (RBI/Sigma, dosed as salt) was dissolved in saline. SR141716A (base, kindly provided by the National Institute on Drug Abuse drug supply program) was dissolved in DMSO and diluted with buffer. [D-Ala2, N-Me-Phe4, Gly5-ol]-Enkephalin (DAMGO, acetate salt, Sigma-Aldrich) and naloxone (hydrochloride salt, Sigma-Aldrich) were dissolved in buffer.
Figure. 7.
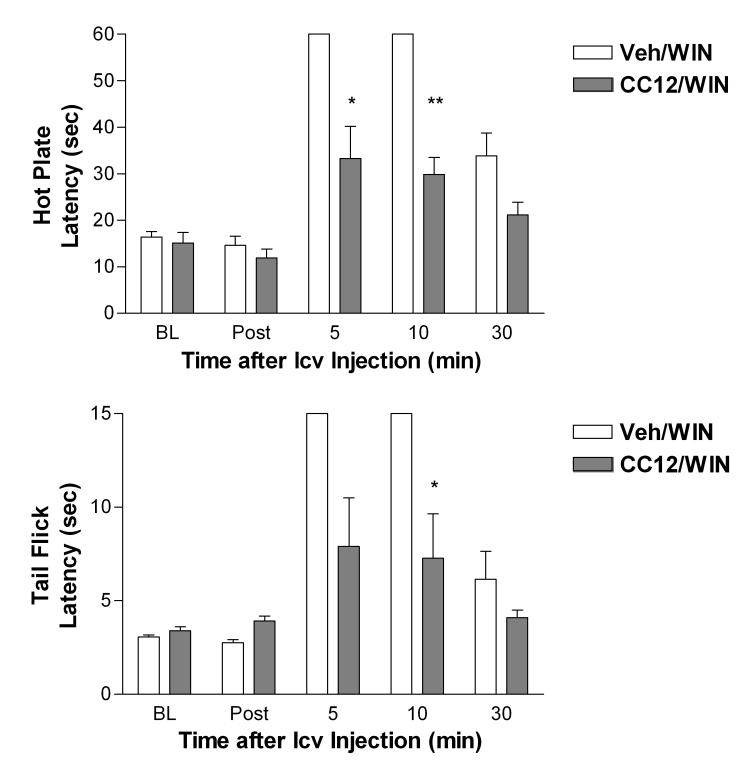
Antagonism of cannabinoid antinociception by CC12 in the rat. Subjects were tested and injected as in Fig. 3A. The first icv injection was CC12 (500 nmol) dissolved in 60% DMSO or vehicle (60% DMSO, Veh). The second icv injection was WIN55,212 (WIN, 20 μg) dissolved in the same vehicle. Latencies (sec, ordinate, mean ± SEM for 5 subjects per group) are shown at various post-icv injection times (abscissa) for the hot plate (top) and tail flick (bottom) tests. *,** P < 0.05, 0.01, respectively vs. Veh/WIN groups at the same time.
2.3. Chemical synthesis of CC12
Two different chemical forms of CC12 (salt and base) were prepared in two different labs by different methods. Results with the two substances were indistinguishable.
2.3.1. Preparation of CC12 hydrochloride (Curragh Chemistries)
To a mixture of 4-iodobenzenemethanethiol (1.36g, 5.44 mmol, (Ryu et al., 2004)) in anhydrous ethanol (15 ml) at room temperature under N2 was added sodium methoxide (0.295g, 5.44 mmol). The mixture was heated to 70 °C and 4-chloromethylimidazole hydrochloride (0.41g, 2.72 mmol) added. The reaction was heated at reflux for 10 h, cooled, and added to 10% aqueous HCl. The aqueous layer was washed with ethyl acetate (3× 20ml), separated, made basic with 1N NaOH solution (pH>11), and then extracted with dichloromethane (75ml). The organic layer was separated, dried (MgSO4), filtered, and evaporated in vacuo to give crude product. The crude reaction mixture was protected by treatment with trityl chloride (5.44 mmol) in the presence of triethylamine (2 equiv) in dichloromethane. The trityl protected imidazole was purified by flash silica chromatography using dichloromethane/ethyl acetate (3:7). After purification, the trityl group was removed by treatment with 4N HCl in dioxane at reflux for 1 h. The crude product was cooled and triturated with ether to obtain 400mg of a white solid, 4(5)-((4-iodobenzyl)thiomethyl)-1H-imidazole hydrochloride. 1H NMR (300MHz, D2O): δ 8.40 (s, 1H), 7.61(m, 2H), 7.03 (m, 3H), 3.78(s, 2H), 3.70 (s, 2H).
2.3.2 Preparation of CC12 base (Rensselaer Polytechnic Institute)
A mixture of 4-iodobenzenemethanethiol (0.626g, 2.50 mmol, Ryu et al., 2004) and 4-(hydroxymethyl)-imidazole hydrochloride (1.72g, 12.5 mmol, 98%) was refluxed in glacial acetic acid under N2 for 4 days. Acetic acid was removed in vacuo and H2O (50 mL) was added to the resulting residue. The aqueous solution was treated with NaOH (1 M) to pH = 9, and then extracted with CH2Cl2 (3×). The combined organic phases were dried over Na2SO4, concentrated and purified with CombiFlash (A: CH2Cl2, B: CH2Cl2/CH3OH/NH4OH 40:9:1; B%: 12.5 → 50%) to give the target compound as an off-white solid (0.287g, 0.869mmol, 35%): 1H NMR (300 MHz, CDCl3) δ7.64 (m, 1H), 7.61 (m, 2H), 7.05 (bd, 2H, J = 7.8 Hz), 6.90 (bs, 1 H), 3.61 (bs, 4H); IR (nujol) νmax 2969, 2950, 2617, 1283, 994, 827, 645, 508 cm−1; GC-MS m/z 330 (M+), 217, 82; Anal. Calcd. for C11H11IN2S: C 40.01, H 3.36, N 8.48. Found: C 39.97, H 3.14, N 8.35.
2.4. 3HCIM membrane binding
3HCIM binding experiments were performed with the following modifications from Smith et al. (1980). Homogenates from rat whole brains (10 volumes, 100mM Tris-HCl, 0.5 mM EDTA, pH 7.4, prepared with a Polytron) were centrifuged (26,000 × g, 15 min), supernatants decanted and pellets resuspended with a glass-Teflon homogenizer in the original volume of buffer. Homogenates were re-centrifuged, and crude membrane pellets stored at – 80°C. On the day of assay, pellets were resuspended in assay buffer (100 mM Tris-HCl, pH 7.4) and centrifuged (26,000 × g, 10 min). Pellets resuspended in assay buffer were incubated in a total volume of 0.1 ml (400 μg protein) containing 50 nM 3[H]-cimetidine (22 – 25 Ci/mmol, Amersham Biosciences Corp., Piscataway, NJ), competing ligand, and assay buffer for 60 min on ice. Samples were rapidly filtered through glass fiber GF/B filters, and rinsed three times with 1.5 ml of ice-cold assay buffer. Filters were then placed in 5 ml of Ecoscint scintillation fluid and counted in a scintillation counter.
2.5. Surgery
For intracerebroventricular (icv) injections in rats, animals were anesthetized with pentobarbital sodium and supplemented with isofluorane. Cannulas were stereotaxically implanted into the left lateral ventricle and anchored to the skull with three stainless steel screws and cranioplast cement (Crane and Glick, 1979). The coordinates for the cannula (in mm from bregma, Paxinos and Watson, 1986) were: anterior-posterior −0.8, medial-lateral + 1.5, dorsoventral −3.3. After surgery, the animals were individually housed with food and water available and were allowed to recover for at least 5 to 7 days before testing. Each animal was used for a single experiment.
2.6. Rat nociceptive testing
Two nociceptive tests were used. For the hot plate test (Eddy and Leimbach, 1953), animals were placed on a 52° C surface and the latency to hind paw lift or lick was recorded with a maximal exposure of 60 s. Baseline latencies were 10 to 15 s. For the tail flick test (D'Amour and Smith, 1941), the ventral surface of the tail (a randomly selected location 2-5 cm from the tip) was exposed to radiant heat, and the latency for tail movement was recorded. The heat source was set so that baseline latencies were generally between 3 and 4 s with a 15-s cutoff. The heat source was not adjusted for individual animals.
2.7. Rat icv injections and procedures
Subjects were baseline tested with a single hot plate test, followed by three tail flick tests performed at one-min intervals, with the third test used as the baseline score. Animals were then gently secured by wrapping with a laboratory pad, the stylet was removed, and the injection cannula inserted. This cannula extends 1 mm beyond the guide to penetrate the lateral ventricle. Unless noted otherwise, rat icv injections were performed manually over a five-min period with a volume of 5 μl. One min after the end of the infusion, the injection cannula was clipped approximately 2 mm above the juncture with the guide cannula. At a specified interval after the first injection, a single hot plate followed by a single tail flick test was performed, followed by a second icv injection. The injection cannula was clipped as done after the first injection, and single hot plate and tail flick latencies were subsequently recorded at specified intervals. Successful icv injections were assured by following the movement of an air bubble in the tubing between the syringe and the cannula and by the absence of leakage. After testing, animals received pentobarbital sodium (100 mg/kg, i.p.) and India Ink (5 μl, icv). Proper distribution of the ink in the cerebroventricular system indicated successful icv injections. Data from animals with poor placements or unsuccessful injections were excluded.
2.8. Mouse icv injections and nociceptive testing
Mouse testing was performed with the hot water tail immersion test (Li et al., 1997a). Subjects were restrained in a conical polypropylene tube. The tail (2–3 cm) was immersed into a 55°C water bath and the latency to sudden tail movement or removal of the tail from the water was recorded. Unless specified otherwise, cutoff latencies were 8 s. After baseline testing, animals were lightly anesthetized with ether. A microliter syringe was connected to a 26-gauge needle with polyethylene 20 tubing. The needle was inserted into the lateral ventricle through a stereotaxically drilled Plexiglas plate as described previously (Glick et al., 1975). Drug solutions (2 μl) were manually injected over a 1-min period, and the needle removed after an additional min. Animals regained consciousness within 3 min after the injection. After the specified intervals, animals were retested, briefly anesthetized a second time, received a second i.c.v. injection, and were retested as described.
2.9. [35S]-GTP-γS receptor autoradiography
This method was performed essentially according to Happe et al. (2001) to assess CC12's activity on cannabinoid CB1 and μ opioid receptors. Rats were euthanized with carbon dioxide. Brains were rapidly removed, frozen, and serial 16 micron sections were taken at the levels of globus pallidus and thalamus, placed on subbed slides, and stored at −20°. Sections were then treated at room temperature as described (Happe et al., 2001) in the following steps: rehydration (assay buffer: 50 mM glycylglycine, 3 mM MgCl2, 1 mM EGTA, 100 mM NaCl, pH 7.5, 10 min), pre-incubation (assay buffer plus 2 mM GDP, 30 min), and incubation (assay buffer containing 0.08 – 0.1 nM [35S]-GTP-γS [Perkin Elmer/NEN, Boston, MA], 2mM GDP, 0.2 mM dithiothreitol, along with specified agonists and antagonists, 2 h). Sections were then washed (2 × 3 min, 50 mM glycylglycine, pH 7.4 containing 0.2 mM dithiothreitol, followed by water), dried and exposed to Kodak Biomax film for 18 h. The film was developed, scanned, and analyzed (Imagequant, Molecular Dynamics, Sunnyvale, CA). Results were quantified by comparison with 14C standards which were placed on each film. Basal and non-specific binding were determined in the absence of agonist and in the presence of 1 mM unlabeled GTPγS, respectively.
2.10. Receptor/enzyme screening
CC12 was screened at sites identified in Table 1. These were performed as indicated by either the NIMH Psychopharmacology Drug Screening Program (PSP, see http://kidb.cwru.edu/nimh/binding.php for methods), MDS Pharma Services (MDS, Bothell, WA, http://discovery.mdsps.com/Catalog/), or the Leiden Amsterdam Center for Drug Research (LACDR, Lim et al., 2005).
Table 1.
Activity of CC12 on Receptors Related to Analgesia and/or Histamine
| Receptor/Target | Potential Anti- Analgesic Action |
Methodsh |
pA2 or % Inhib. (10 μM)g |
|---|---|---|---|
| μ opioid | Antaga | PSP/AMC | 0% / 5.3 (Fig. 8) |
| δ opioid | Antag a | PSP | 20% |
| CB1 | Antag a | AMC | 5.2 (Fig. 8) |
| NK1 and relatedk | complex f | PSP-HTS | inactivej |
| NT1 | complex e,f | PSP/MDS | 16% / 2% |
| 5HT2A | uncertain | PSP | 42% |
| 5HT2B | uncertain | PSP | 47% |
| 5HT2C | uncertain | PSP | 63% |
| NMDA (MK801 binding) | Antaga,b | PSP | 1% |
| GABAA (agonist binding) | Agonist c,d | MDS | 6% |
| GABAA (responses) | Agonist c,d | AMC | inactivei |
| Kappa opioid | Agonist c,d | PSP | 50% |
| ORL1 | Agonist c,d | MDS | 11% |
| CCK2 (CCKB) | Agonist c | MDS | 0% |
| H1 | Antag a,f | PSP/MDS | 41% / 31% |
| H2 | Antag a,f | MDS | 33% |
| H3 | complexf | LACD | 7.3 ± 0.1 |
| H4 | unknown | LACD | 6.6 ± 0.1 |
| eNOSl | uncertain | MDS | 0% |
| nNOSl | uncertain | MDS | 0% |
| FAAHl | activator | MDS | 8% |
CC12 was screened at the receptors/enzymes shown. For each receptor, column 2 shows the theoretical action on that receptor which would be required to inhibit analgesic activity. For example, CC12 would need to be a μ opioid antagonist in order to block opioid analgesia.
Antagonism at this receptor reduces antinociceptive responses.
Blockade of this receptor blocks excitatory transmission in brain stem antinociceptive circuits (Heinricher et al., 2001b).
Activation of this receptor reduces opioid and, in several cases, cannabinoid antinociception (Zonta et al., 1981; Vaughan et al., 1999; Pan et al., 1997; Heinricher et al., 1997, 2001a).
Activation of this receptor hyperpolarizes “off” cells in the rostral ventral medulla (Pan et al., 1997; Heinricher et al., 1997).
Activation of NT1 stimulates RVM “on” cell firing, facilitating nociception (Neubert et al., 2004)
Activation of this receptor can have opposing actions on nociceptive assays depending on agonist dose, route, CNS region and/or nociceptive test.
Values are mean ± SEM for 3 experiments performed in triplicate (for pA2 values) or mean percent inhibition of binding (10 μM) in triplicates from a single experiment.
Assays performed as described in Methods at Albany Medical College (AMC), Leiden Amsterdam Center for Drug Design (LACD), NIMH Psychopharmacology Screening Program (PSP), the same program's high throughput screening program (PSP-HTS), or MDS Pharma Services (MDS).
CC12 (up to 500 μM) did not display any GABAA agonist properties when tested exactly as described previously (Cannon et al., 2004) in HEK293 cells expressing recombinant α1β2χ2 receptors (n=12).
Inactive as agonist, antagonist or allosteric modulator at 10 μM.
CC12 (10 μM) was also inactive at NK2, NK3, vassopressin V1 and V2, and oxytocin receptors by the same methods.
Abbreviations: eNOS, nNOS: endothelial and neuronal forms of nitric oxide synthase; FAAH: fatty acid amide hydrolase.
2.11. Data analysis
Nociceptive data are expressed in latencies (s, mean ± SEM). Latencies were analyzed by one- or two-factor repeated measures analysis of variance, followed by Bonferroni post-hoc testing (Statistica, Statsoft, Tulsa, OK). Dose-response curves from receptor autoradiography experiments and competition curves from radioligand binding experiments were fitted by iterative non-linear regression (Graphpad Prism 4.0, San Diego, CA).
3. Results
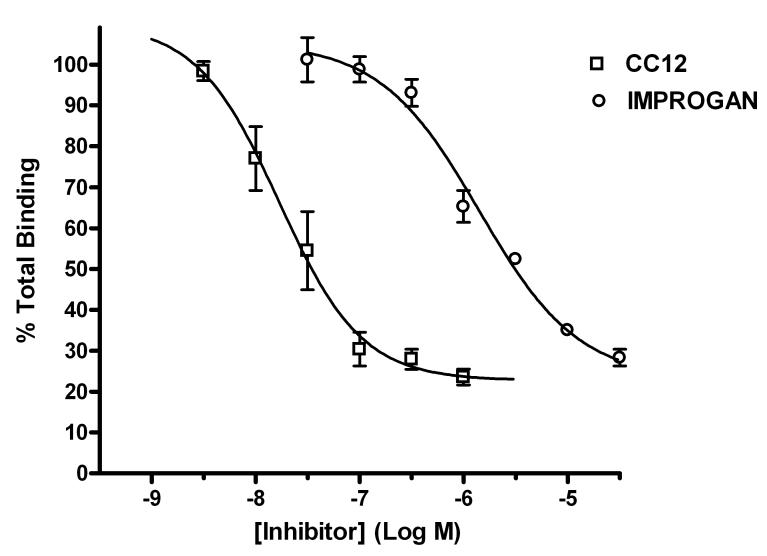
Preliminary studies explored the possible relevance of 3HCIM binding to improgan action. Membrane fractions from rat brain homogenates exhibited 3HCIM specific binding with characteristics similar to those previously published (Smith et al., 1980, data not shown). Improgan produced concentration-dependent inhibition of 3HCIM binding (Fig. 2). Non-linear regression of the inhibitory curve yielded an estimated IC50 value of 1.4 μM (calculated Ki = 793 nM), with a Hill coefficient of −0.91. Exploration of the biological significance of 3HCIM binding would be facilitated by studying the effects of drugs with higher affinity for the binding site. Synthesis and testing of CC12 revealed this new compound to be a potent inhibitor of 3HCIM binding in brain homogenates, with an estimated IC50 of 16.8 nM (calculated Ki = 9.5 nM), and Hill coefficient of −1.09 (Fig. 2).
Figure 2.
Inhibition of 3H-cimetidine (3HCIM) binding to brain homogenates by improgan and CC12. Crude membrane fractions of rat brain (400 μg protein) were incubated with 3HCIM (50 nM) in the presence of varying concentrations of unlabeled drugs. Percent total binding (ordinate) is shown at each concentration of competing drug (abscissa). Each data point represents the mean (± SEM) from three separate experiments, with values from each experiment determined in triplicate (or in two cases duplicate). Data were fit to sigmoidal dose-response curves by non-linear regression. Examples of the mean total and non-specific binding were 6,337 cpms (0.26 pmol)/tube and 1,502 cpms (0.06 pmol)/tube, respectively.
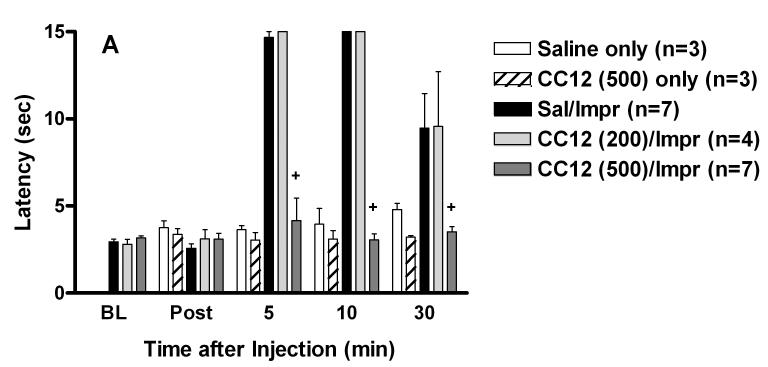
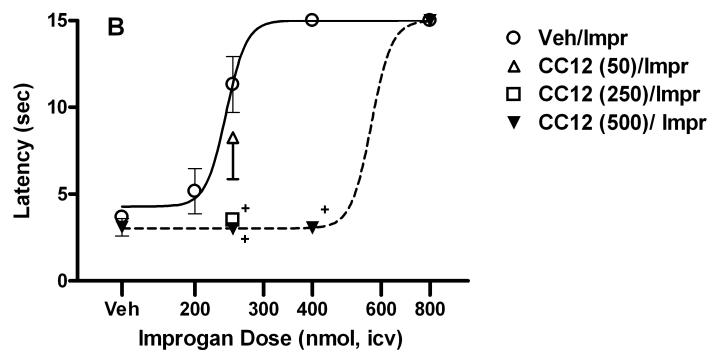
The effects of CC12 were then investigated in vivo. When tested alone in rats, icv injections of up to 500 nmol of CC12 had no effect on tail flick (Fig. 3A) or hot plate (not shown) responding. ANOVA of the single-injected groups of Fig. 3A (between groups: saline or CC12, within groups [repeated measures]: time) showed no significant (P > 0.05) main effects of CC12 (df=1, F=3.19), time (df=3, F=1.22) or CC12 by time interactions (df=3, F=1.05). Open field observations of rats receiving CC12 (up to 500 nmol, icv) with or without other drugs found no effects on motor function or other behaviors. To investigate a possible relationship between CC12 and improgan, the effects of the former were studied on nociceptive latencies in the presence and absence of the latter (Fig. 3). In rats, improgan induced maximal suppression of nociceptive tail flick responses 5 and 10 min following icv administration, effects which were completely blocked by CC12 (Fig. 3A). ANOVA of the double-injected groups of Fig. 3A (between groups: dose of CC12, within groups [repeated measures]: time) showed significant (P<0.001) main effects of CC12 (df=2, F= 45.34), time (df=4, F= 63.18) with a significant (P < 0.001) treatment by time interaction (df=8, F= 16.14). Post-hoc testing showed a significant difference in improgan antinociception between animals pretreated with 500 nmol CC12 and those receiving saline at 5, 10 and 30 min. Improgan antinociception was dose-dependent, and CC12 produced a dose-dependent inhibition of improgan effects (Fig. 3B). Doses of 250 and 500 nmol (but not 50 nmol, icv) of CC12 completely antagonized the effects of a moderate antinociceptive dose of improgan (250 nmol). The effect of the maximal inhibitory dose of CC12 (500 nmol) was completely reversed by a large dose (800 nmol) of improgan, demonstrating surmountability (Fig. 3B). The shift in the improgan dose-response curve after this antagonist treatment was estimated to be 2.4-fold. ANOVA of the two dose-response curves of Fig. 3B (10 min tail flick, between groups #1: pre-treatment with CC12 [500 nmol] vs vehicle, between groups #2: dose of improgan) found highly significant (P <0.001) main effects of improgan dose (df=3, F= 21.33) and CC12 pretreatment (df=1, F= 28.02), with a significant (P < 0.001) improgan dose by CC12 interaction (df=3, F= 9.84). Combinations of improgan and CC12 produced effects on the hot plate nociceptive test (not shown) which were virtually identical with those seen on the tail flick test (Fig. 3).
Figure. 3.
Time-action (A) and dose-response (B) curves for antagonism of improgan antinociception by CC12 in rats. A) Subjects were tested for baseline responses (BL), received an icv injection of either saline (Sal) or CC12 (dose in nmol in parentheses), were re-tested 15 min after the beginning of the first icv injection (Post), then immediately received a second icv injection of improgan (Impr, 400 nmol). Animals were re-tested at the times shown (min, abscissa) after the end of the second icv injection. Animals from “Saline only” and “CC12 only” groups were tested once for baseline responses (shown at Post), received a single icv injection, and were re-tested. Nociceptive tail flick latencies (sec, ordinate, mean ± SEM for the number of subjects specified) are shown for each treatment group. +P <0.01 vs. Sal/Impr at the same time. B) Subjects were injected and tested as in 3A. Tail flick latencies shown were recorded 10 min after the end of the improgan infusion (sec, ordinate, mean ± SEM). Data from a total of 61 subjects are shown (n=3-12 per group). Data from subjects receiving 400 nmol of improgan are shown in both 3A and 3B. Some of the data from the Sal/Impr (200) groups were reported elsewhere (Hough et al., 2006). +P < 0.01 vs. Sal/Imp group.
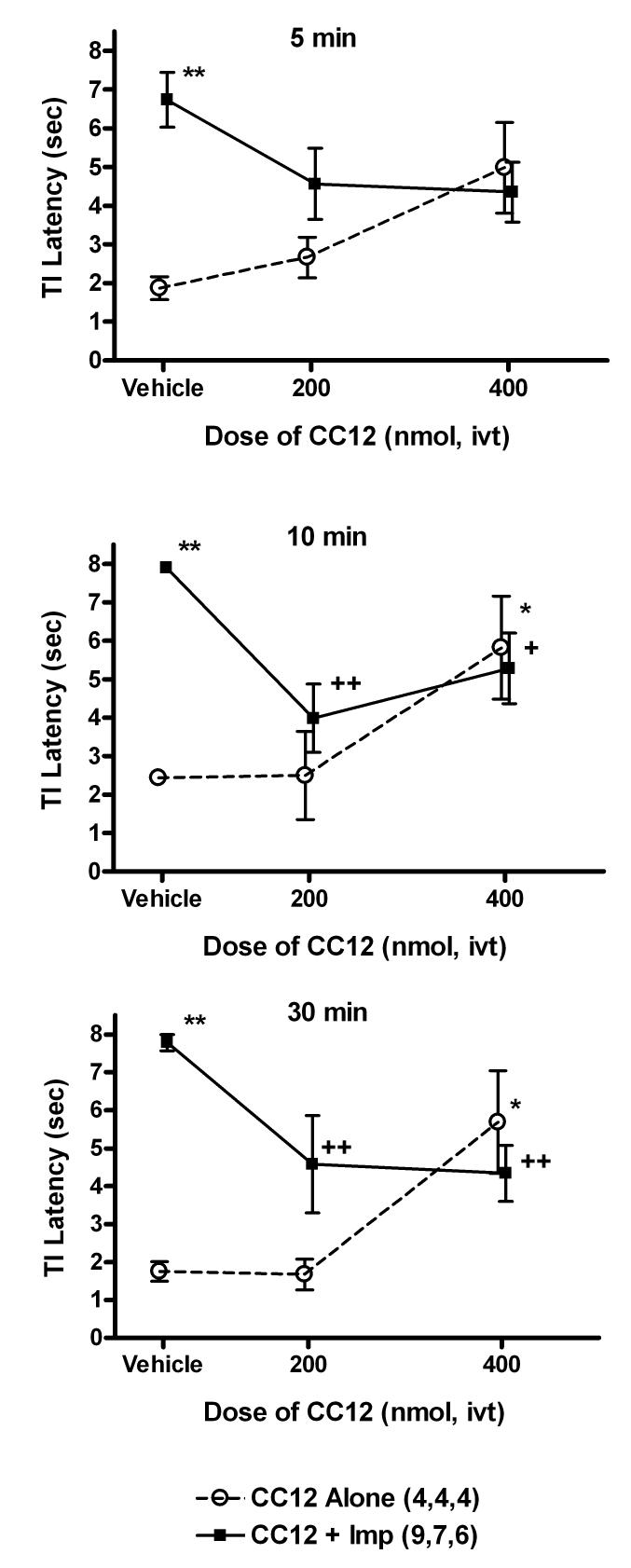
Improgan - CC12 interactions were also studied in mice. Icv administration of improgan induced a maximal antinociceptive effect over the subsequent 10 – 30 min period (Fig. 4). Icv pretreatment with CC12 (200 nmol) inhibited the improgan effect at the 10 and 30 min time point (Fig. 4). Pretreatment with a higher dose of CC12 (400 nmol) showed the same degree of incomplete antagonism of improgan seen with the lower CC12 dose (Fig. 4). Although pretreatment with the lower dose of CC12 alone had no significant effect on nociceptive latencies, the higher dose given alone produced significant, but incomplete antinociception. This degree of antinociceptive agonism was very similar to the level of improgan antagonism (i.e. latencies around 5.5 sec), consistent with a partial agonist action for CC12 (Fig. 4). ANOVA of the data of Fig. 4 (between groups: dose of CC12, ivt improgan; within groups [repeated measures]: time) showed significant (P<0.02 or lower) main effects of CC12 (df=2, F=4.65), improgan (df=1, F=13.25), and time (df=4, F= 43.46), with significant (P < 0.001) CC12 by improgan (df=2, F=9.20), improgan by time (df=4, F=7.14), and CC12 by improgan by time (df=8, F=4.72) interactions. Mice showed no unusual behavioral or motor changes following icv CC12 administration.
Figure 4.
Antagonism of improgan antinociception by CC12 in the mouse. Mice were baseline tested and received an icv injection of CC12 (200 or 400 nmol) or pH 4.5 saline vehicle (Vehicle), indicated on abscissa. Ten min later, they were again tested, received icv improgan (Imp, 145 nmol) or saline, and were re-tested at 5 (top), 10 (middle) and 30 (below) min following the end of the second icv injection. Nociceptive tail immersion latencies (sec, ordinate, mean ± SEM for the number of subjects specified) are shown for each treatment group. *,**P<0.05, 0.01, respectively vs Vehicle/Saline at the same time. +,++P <0.05, 0.01, respectively vs. Sal/Impr at the same time.
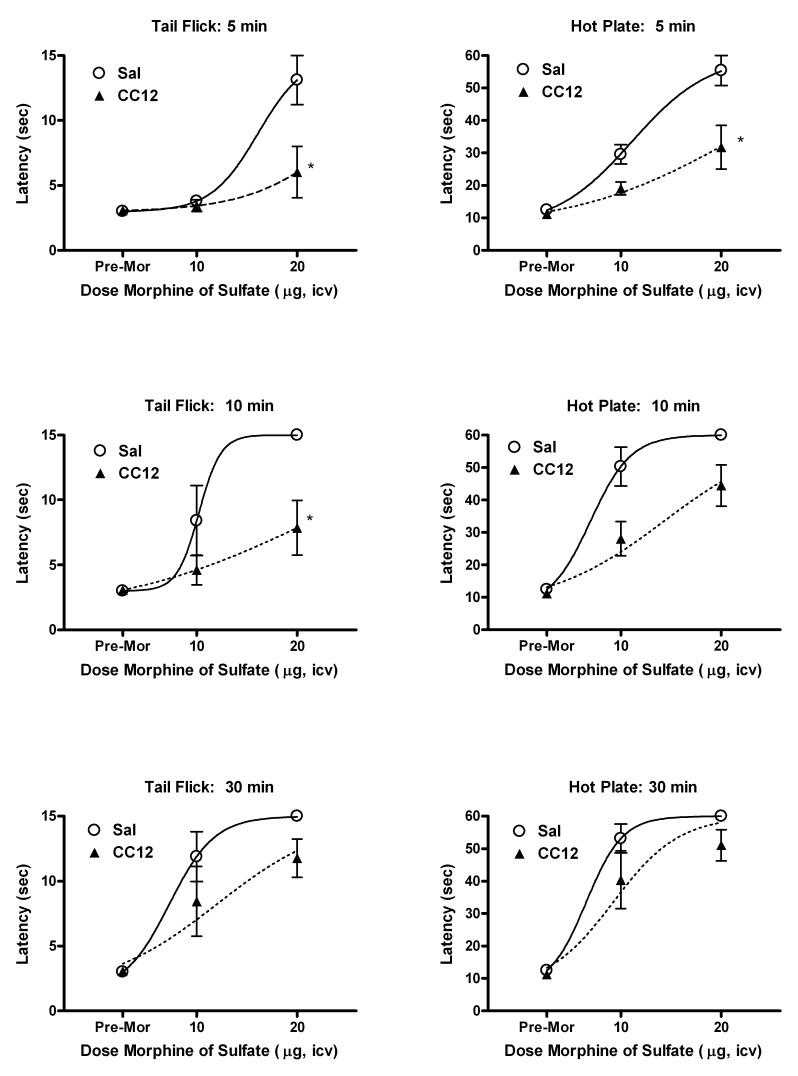
To assess the selectivity of CC12's antagonism , the effects of this drug were studied on morphine (MOR) antinociception. Surprisingly, CC12 pre-treatment (500 nmol, icv) significantly attenuated antinociception 5 and 10 min after icv MOR treatment in rats (Fig. 5). MOR dose-response curves were shifted downward at both time points. Thirty min following MOR, the inhibition by CC12 seemed less pronounced. Nearly identical patterns of CC12-MOR interactions were observed on the hot plate and tail flick nociceptive tests. ANOVA of the tail flick data of Fig. 5 (between groups #1: CC12 treatment, between groups #2: dose of MOR, within groups [repeated measures]: time) showed significant (P<0.01) main effects of CC12 (df=1, F=8.86), MOR (df=1, F=10.47), and time (df=4, F=33.86) with significant (P<0.01) time by CC12 (df=3, F=2.95) and time by MOR (df=3, F=4.52) interaction terms. Statistical analysis of the hot plate data revealed remarkably similar results (not shown).
Figure 5.
Antagonism of MOR antinociception by icv CC12 in rats. Subjects were tested and injected twice as described in Fig. 3A. The first icv injection was either CC12 (500 nmol, triangles) or pH 4.5 saline vehicle (circles), followed by re-testing (“Pre-Mor”), followed by icv MOR sulfate (doses in μg shown on abscissa). Tail flick (left) and hot plate (right) latencies (ordinates, mean ± SEM for 5-8 subjects per group) are shown at 5 (top), 10 (middle) and 30 (bottom) min intervals following MOR treatment. *P < 0.05 vs. Sal at the same time and dose.
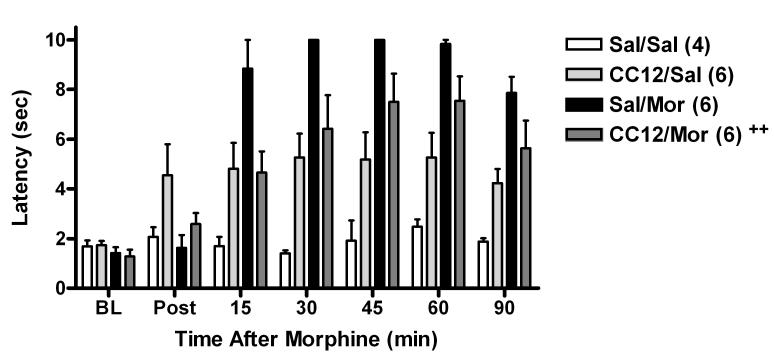
CC12-MOR interactions were also studied in mice. In these experiments (Fig. 6), systemic dosing with both drugs was employed to assess the brain-penetrating properties of CC12. Pretreatment with CC12 (100 mg/kg, s.c.) produced a significant inhibition of antinociception produced by MOR (3.2 mg/kg, s.c. given 15 min later, Fig. 6). ANOVA (Fig. 6 data, between groups #1: CC12 vs saline, between groups #2: MOR vs saline, within groups [repeated measures]: time) showed significant (P<0.001) main effects of MOR (df=1, F=30.2) and time (df=6, F=29.5), with significant (P<0.001) MOR by CC12 (df=1, F=18.2), MOR by time (df=6, F=15.4), and MOR by CC12 by time (df=6, F=4.26) interaction terms. A separate ANOVA of subjects not receiving MOR (between groups: CC12 vs saline, within groups [repeated measures]: time) found a slight but significant antinociceptive effect of CC12 given alone (main effect of CC12: df=2, F=10.4, P<0.05, with no other significant factors). No significant effects of CC12 in the presence or absence of MOR were detected by post-hoc testing.
Figure 6.
Antagonism of MOR antinociception by systemically-administered CC12 in the mouse. Mice were tested for baseline (BL) responses, then received CC12 (base, 100 mg/kg, s.c.) or pH 4.5 saline (Sal). Fifteen min later, subjects were again tested (Post), and received MOR sulfate (3.2 mg/kg, s.c.) or saline (Sal), and were re-tested at the times shown (abscissa). Nociceptive latencies (ordinate, mean ± SEM for the number of subjects in parentheses) for each treatment group are shown. In this figure only, the cutoff latency used was 10 sec. ++ Significant (P < 0.001) CC12 by MOR by time interaction term was found by ANOVA, confirming antagonism of MOR by CC12 (see text).
Additional studies were performed to assess the effects of CC12 on cannabinoid antinociception in rats. On both the hot plate and tail flick tests, CC12 pretreatment reduced the antinociception produced by the cannabinoid agonist WIN 55,212 (20 μg, icv) by approximately 50% by (Fig. 7). ANOVA of hot plate data of Fig. 7 (between groups: CC12 treatment, within groups [repeated measures]: time) found highly significant (P<0.01) main effects of CC12 (df=1, F= 24.22), time (df=4, F= 64.61) and a significant (P<0.01) CC12 by time interaction (df=4, F= 24.22). Analysis of the tail flick data gave virtually identical results.
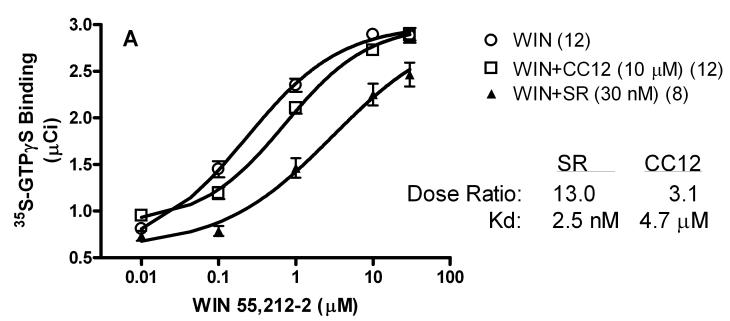
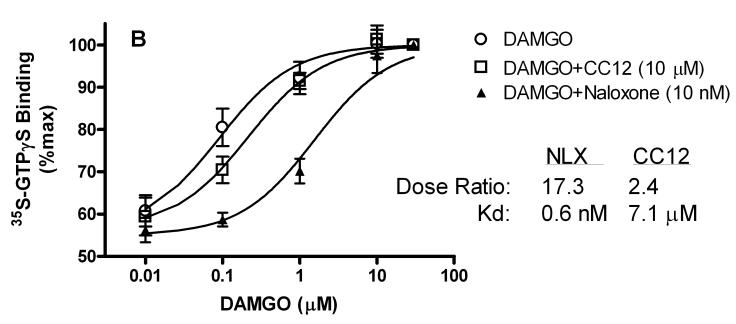
To search for the CC12 mechanism, the actions of this compound on cannabinoid CB1 and μ opioid receptors were determined in GTPγS autoradiography experiments with slices from rat brain (Fig. 8A). Concentration-response curves with cannabinoid (Fig. 8A) and opioid (Fig. 8B) agonists were predictably shifted to the right by low concentrations of reference antagonists for these receptors, yielding calculated Kd values of 2.5 nM and 0.6 nM for SR141716A and naloxone, respectively (Fig. 8). Under these conditions, a very large concentration of CC12 (10 μM) produced only 2-3 fold shifts in agonist curves, giving estimated Kd values of 4.7 and 7.1 μM on CB1 and μ opioid receptors, respectively.
Figure 8.
CB1 receptor (A, top) and mu opioid receptor (B, bottom) activity of CC12. Frozen sections from globus pallidus (top) or thalamus (bottom) were incubated as described in the presence of 35S-GTPγS, candidate antagonists, and increasing concentrations of either the CB1 agonist WIN55,212-2 (WIN, top, abscissa), or the mu agonist DAMGO (bottom, abscissa). A) Specific binding of 35S-GTPγS (μCi, mean ± SEM) is shown for the number of brain sections given in parentheses. B) In the thalamic sections, binding was normalized to 30 μM responses for each dose-response curve (n=5-6). For each antagonist/receptor combination, the dose ratio (shifted EC50/control EC50) is given along with the calculated Kd values. SR141716A (SR) and naloxone (NLX) were used as reference antagonists.
Other screening found little or no activity of CC12 at a variety of receptors related to antinociception (Table 1). Radioligand binding confirmed a lack of activity at μ receptors, and also found low affinities at delta, kappa and ORL1 sites. CC12 had very little or no activity at a variety of receptors for peptides (NK1, NK2, NK3, NT1, CCK2), serotonin (5HT2A 5HT2B 5HT2C), and amino acids (NMDA, GABAA). Particular attention was paid to the potential activity of CC12 on GABAA receptors. In binding assays, the drug failed to significantly modify either MK801 or GABA binding. In addition, electrophysiological testing found no agonism, no antagonism and no allosteric modulation of recombinant GABAA receptors by CC12, even at very high concentrations (Table 1). CC12 was also inactive on two forms of nitric oxide synthase, and on fatty acid amide hydrolyase, the enzyme of endocannabinoid metabolism (Table 1). On histamine receptors, CC12 had negligible activity at H1, H2, and H4 sites, and showed moderate antagonist activity at H3 receptors (Kd = 50 nM).
4. Discussion
3HCIM has been used extensively as a radioligand for the H2 receptor (e.g. Miyamoto and Nishio, 1993, Rivera et al., 1994) , but it is clear that the binding sites labeled with this ligand are not H2 receptors. Smith et al. (1980) showed that imidazole- containing H2 antagonists bind to this site, but H2 antagonists lacking imidazole do not. Furthermore, Warrander et al. (1983) reported the existence of compounds which lack H2 antagonist activity, but have high affinity for 3HCIM binding sites. Both of these findings have been recently replicated in our lab (manuscript in preparation). Although the identity and biological significance of 3HCIM binding remain unclear, the activity of the compounds reported by Warrander et al. (1983) led to the synthesis and testing of CC12.
Our lab's interest in the brain 3HCIM binding site emanates from the possibility that this site represents the molecular target for improgan antinociception. The present findings, showing that CC12 inhibits 3HCIM binding with high affinity (Ki = 9.5 nM) and blocks improgan antinociception, support this hypothesis. However, improgan's affinity for this site (Ki = 793 nM) seems low as compared with its antinociceptive icv ED50 (200 nmol, Hough et al., 2006). Relative to other icv-administered analgesics in rats, improgan is 6.7-fold less potent than morphine (ED50 = 30 nmol; Ki = 6.5 nM, Spetea et al., 2004)) and 1.9-fold more potent than THC (ED50 = 373 nmol; Ki = 110 nM , Lichtman et al., 1996, Breivogel and Childers, 2000). Synthesis and testing of a number of compounds with varying affinity for the binding site are needed to rigorously test this hypothesis. These studies are underway.
The present results show that in rats, CC12 antagonizes antinociception at doses that lack other obvious effects. It is worth noting that reversal of antinociception requires the reinstatement of nocifensive reflexes (e.g. tail flick), effects which are not consistent with motor inhibition. Furthermore, CC12 does not decrease nociceptive thresholds when given alone, indicating that the antagonist actions are not explained by net hyperalgesic effects (Fig. 3). The dose-dependent inhibition of improgan antinociception by CC12, and the shift to the right in the improgan dose-response curve produced by this drug (Fig. 3) show that CC12 behaves in vivo as a competitive antagonist of improgan in the rat. The magnitude of the shift produced by 500 nmol of CC12 (2.4-fold, Fig. 3) permits the calculation of an in vivo icv Kd value of 370 nmol.
Fig. 4 confirms earlier studies showing that improgan produces antinociception in mice (Li et al., 1997a). Inhibition of this effect by icv CC12 adds to the evidence supporting CC12's antagonism of improgan. The finding that systemically-administered CC12 inhibits systemically-administered MOR in mice (Fig. 6) shows that the antagonism does not require icv injections (e.g. is not a result of drug solution mixing/precipitation in the ventricle). Data of Fig. 6 also make it likely that CC12 has at least some CNS-penetrating properties. This brain penetration by CC12 may be limited by the presence of this drug's imidazole group, similar to known properties of imidazole-containing H2 (Young et al., 1988) and H3 (Sakurai et al., 1994), antagonists. This limited CNS penetration probably explains the need for the large systemic dose of CC12 (100 mg/kg, Fig. 6).
Unlike findings in the rat, even large doses of CC12 given to mice did not completely inhibit improgan actions (Fig. 4). CC12 also had antinociceptive actions when given alone to mice but not to rats (Fig. 4 vs. Fig. 3). This pattern of incomplete inhibition of improgan, along with the partial antinociceptive actions of the drug given alone, suggest that CC12 behaves as a partial agonist in the mouse. The slight, but statistically signficant antinociceptive effects of systemic CC12 given alone to mice (Fig. 6) are also consistent with this conclusion. If CC12 is a partial agonist in the mouse brain (i.e. if it is an agonist with low efficacy, which requires many receptors to enable agonist effects), then receptor densities could account for the observed species difference. In rat brain, a low receptor density might allow the drug to behave as a pure antagonist, whereas a higher receptor concentration in mouse brain could permit some agonist (antinociceptive) actions.
Improgan antagonists have not been previously known. However, earlier studies found that longer-chain analogs of improgan (Hough et al., 2006) or N-substituted lipophilic congeners of the closely-related burimamide (Hough et al., 1997) showed evidence of partial agonist activity. The lipophilic p-iodobenzyl moiety in CC12 (Fig. 1) resembles the hydrophobic nature of these earlier derivatives. The discovery of a competitive antagonist of improgan supports (but does not prove) the idea that CC12 and improgan share a molecular site of action.
It is important to establish antagonist specificity in order to show a role for independent receptors in drug action. Just as opioid antagonists attenuate opioid, but not cannabinoid antinociception (Welch et al., 1995) and vice versa (Compton et al., 1996), it was predicted that CC12 would inhibit the antinociception produced by improgan, but not that produced by opioids or cannabinoids. However, results showing the opposite (CC12 blocked improgan [Figs. 3,4], MOR [Fig. 5,6], and WIN 55,212 [Fig. 7]) imply the opposite conclusion: that all three antinociceptive agents share a biochemical substrate. As estimated from the size of the respective shifts in the analgesic dose-response curves, CC12 blocks improgan (2.4-fold shift, Fig. 3) and MOR (approximately a 2-fold shift, Fig. 5) with similar potencies, consistent with a shared site of action. Since CC12 is neither an opioid nor a cannabinoid antagonist (Fig. 8), and because an action at either receptor cannot account for blockade of both types of analgesia, it is likely that this drug acts at a site beyond these receptors to attenuate analgesic signaling.
Discovery of CC12's target may illuminate a common analgesic signaling pathway, but this may or may not facilitate identification of improgan's receptor. If CC12's site of action is shown to be same as improgan's molecular target, then improgan could be acting to boost analgesic signaling in a manner opposite that of CC12. Although this has not been established, the competitive antagonism of improgan by CC12 (Fig. 3) and the apparent non-competitive antagonism of MOR by CC12 (Fig. 5) seem consistent with this hypothesis (Black et al., 1980). However, alternative explanations are possible. For example, there is accumulating evidence that improgan may act through a cannabinoid mechanism. The inhibition of improgan antinociception by low doses of the CB1 antagonist SR141716A (Hough et al., 2002), the lack of affinity of improgan for CB1 receptors (Hough et al., 2006), and the reduction in improgan responses following development of cannabinoid tolerance (Nalwalk et al., 2006) suggest that an indirect mechanism utilizing cannabinoid receptors (e.g. endocannabinoid release) remains possible. If so, then CC12's antagonism of improgan would be consistent with downstream inhibition of cannabinoid antinociception (Fig. 7). In this case, CC12's antagonism of improgan would not result from receptor antagonism.
Since the site of CC12's anti-analgesic activity has not been found, it may be useful to estimate an in vitro affinity of CC12 for its target. For example, it can be calculated from Sterious and Walker (2003) that an icv dose of 3 nmol of the opioid antagonist naltrexone shifts the μ opioid analgesic dose-response curve in rats two-fold. Similarly, Lichtman and Martin (1997) allows an icv in vivo Kd value to be calculated as 32 nmol for the CB1 antagonist SR141716A on rat cannabinoid analgesic curves. The present results, which give an in vivo icv Kd value of 370 nmol for CC12, suggest that CC12's in vitro Kd value on its receptor is approximately 123.3- and 11.6-fold higher, respectively than that of naltrexone and SR141716A on the μ opioid and CB1 receptors, respectively. Calculations using literature Ki values for these antagonists on their receptors (0.74 [Gharagozlou et al., 2003] and 6.2 nM [Wiley et al., 2001], respectively, for naltrexone and SR141716A) suggest that CC12's Kd value is likely to be 70 – 90 nM. Although this estimate requires many assumptions which have not been verified, the estimate seems useful in the search for CC12's molecular target(s).
To account for CC12's anti-analgesic activity, several well-known regulators of antinociceptive circuits have been studied (Table 1). Because GABAA agonists inhibit the antinociception produced by opioids (Zonta et al., 1981), cannabinoids (Vaughan et al., 1999) and improgan (Hough et al., 2001b), both binding and electrophysiological methods were used to confirm that CC12 does not activate GABAA channels. Similarly, activation of kappa opioid (Pan et al., 1997) or ORL1 (Heinricher et al., 1997) receptors hyperpolarize rostral ventral medullary “off” cells, and can block opioid and cannabinoid antinociception. However, CC12 has little or no activity at these sites. NMDA activation has been implicated in both opioid and non-opioid antinociception (Spinella et al., 1996, Sacerdote et al., 1985), but CC12 lacks activity at these sites as well. CC12 also has no activity at several other receptors and enzymes related to pain-relieving mechanisms. Thus, the findings of Table 1 suggest that the anti-analgesic activity of CC12 is not explained by currently known receptor systems and therefore may have a novel mechanism.
Table 1 is a large, but certainly not comprehensive list of antinociceptive targets. For example, 5HT3 and other receptors in the spinal cord are capable of nociceptive modulation (Zeitz et al., 2002). However, mapping studies have shown that improgan acts in the brain stem, but not in the spinal cord (Nalwalk et al., 2004), making many of these receptors unlikely targets for CC12. Cholinergic and serotonergic mechanisms in the brain could also potentially be important for CC12 actions, but several of these have been shown to not participate in improgan analgesia (Nalwalk et al., 2005). Additional work is needed to unequivocally identify the CC12 site of action.
The antagonism of improgan by CC12 is not likely to be mediated by any of the known histamine (H1-H4) receptors (Table 1). Improgan antinociception does not require activation of any of these receptors (Li et al., 1997b, Mobarakeh et al., 2003). The negligible activity of CC12 at H1 and H2 receptors is consistent with this conclusion. At H4 receptors, both improgan (Zhu et al., 2001) and CC12 have low affinity. The affinity of CC12 for the H3 receptor is noteworthy (Ki = 50 nM), and consistent with H3 pharmacophore studies (Esbenshade et al., 2006). However, improgan antinociception is not blocked by either H3 agonists or H3 antagonists (Li et al., 1997b), and is not reduced in H3 receptor deficient mutant mice (Mobarakeh et al., 2003). Thus, CC12 is a moderately potent H3 antagonist, but it does not reduce improgan antinociception by an action at these receptors.
CC12 has not been previously described in the literature. However, a closely related substance, 4(5)-(benzylthiomethyl)-1H-imidazole (which has the structure of CC12 without the iodo- substituent), was reported to be a weak inhibitor of histamine synthesis (Swett and Yellin, 1970), and an inhibitor of microsomal drug oxidation (Wilkinson et al., 1974;Wilkinson et al., 1972). Interactions between imidazole-containing histamine antagonists and some cytochrome P-450 proteins are well known (Szutowski et al., 2002, Alves-Rodrigues et al., 1996). The modulation of a cytochrome P-450 protein by CC12 could be the basis for this drug's anti-analgesic action, since a P-450 pathway has been implicated in supraspinal opioid analgesic signaling (Vaughan et al., 1997). Unpublished preliminary results confirm that CC12 inhibits some forms of cytochrome P-450, but the large number of P-450 proteins (Nelson et al., 2004) and the analgesic (vs. anti-analgesic) activity of some P-450 inhibitors (Ho et al., 1978, Lehman and Peterson, 1978) present challenges to establishing this mechanism of action. The presently-described anti-analgesic actions of CC12 will help elucidate the mechanisms by which improgan, as well as other analgesics, act to reduce nociceptive transmission.
5. Acknowledgements
We thank Dr. Mark Fleck, Albany Medical College for evaluation of CC12 on GABAA receptors. We also thank Konstantina Svokos and Amanda Carpenter for excellent technical assistance. Prof. Rob Leurs (Vrije University, Amsterdam), Dr. Mary Heinricher (Oregon Health Sciences University), and Dr. Milt Teitler (Albany Medical College) provided valuable comments on the manuscript. This work was supported by grants from the National Institute on Drug Abuse (DA-03816, DA-015915, and DA-07307).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- Alves-Rodrigues A, Leurs R, Wu TS, Prell GD, Foged C, Timmerman H. [3H]-thioperamide as a radioligand for the histamine H3 receptor in rat cerebral cortex. British Journal of Pharmacology. 1996;118:2045–2052. doi: 10.1111/j.1476-5381.1996.tb15642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JW, Jenkinson DH, Kenakin TP. Antagonism of an indirectly acting agonist: block by propranolol and sotalol of the action of tyramine on rat heart. Eur.J.Pharmacol. 1980;65:1–10. doi: 10.1016/0014-2999(80)90202-2. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Childers SR. Cannabinoid agonist signal transduction in rat brain: comparison of cannabinoid agonists in receptor binding, G-protein activation, and adenylyl cyclase inhibition. J Pharmacol Exp Ther. 2000;295:328–336. [PubMed] [Google Scholar]
- Cannon KE, Fleck MW, Hough LB. Effects of cimetidine-like drugs on recombinant GABAA receptors. Life Sci. 2004;75:2551–2558. doi: 10.1016/j.lfs.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Compton DR, Aceto MD, Lowe J, Martin BR. In vivo characterization of a specific cannabinoid receptor antagonist (SR141716A): inhibition of delta 9-tetrahydrocannabinol-induced responses and apparent agonist activity. J.Pharmacol.Exp.Ther. 1996;277:586–594. [PubMed] [Google Scholar]
- Crane LA, Glick SD. Simple cannula for repeated intracerebral drug administration in rats. Pharmacol.Biochem.Behav. 1979;10:799–800. doi: 10.1016/0091-3057(79)90336-8. [DOI] [PubMed] [Google Scholar]
- D'Amour FE, Smith DL. A method for determining loss of pain sensation. J.Pharmacol.Exp.Ther. 1941;72:74–79. [Google Scholar]
- Eddy NB, Leimbach D. Synthetic analgesics, II Dithienylbutenyl and dithienylbutylamines. J.Pharmacol.Exp.Ther. 1953;107:385–393. [PubMed] [Google Scholar]
- Esbenshade TA, Fox GB, Cowart MD. Histamine H3 receptor antagonists: preclinical promise for treating obesity and cognitive disorders. Mol.Interv. 2006;6:77–88. doi: 10.1124/mi.6.2.5. [DOI] [PubMed] [Google Scholar]
- Ganellin CR. Chemistry and Structure-Activity Relationships of Drugs Acting at Histamine Receptors. In: Ganellin CR, Parsons ME, editors. Pharmacology of Histamine Receptors. John Wright & Sons, Ltd.; Bristol: 1982. pp. 10–102. [Google Scholar]
- Gharagozlou P, Demirci H, David CJ, Lameh J. Activity of opioid ligands in cells expressing cloned mu opioid receptors. BMC.Pharmacol. 2003;3:1. doi: 10.1186/1471-2210-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick SD, Crane AM, Barker LA, Mittag TW. Effects of N-hydroxyethyl-pyrrolidinium methiodide, a choline analogue, on passive avoidance behaviour in mice. Neuropharmacol. 1975;14:561–564. doi: 10.1016/0028-3908(75)90121-5. [DOI] [PubMed] [Google Scholar]
- Happe HK, Bylund DB, Murrin LC. Agonist-stimulated [35S]GTPgammaS autoradiography: optimization for high sensitivity. Eur.J.Pharmacol. 2001;422:1–13. doi: 10.1016/s0014-2999(01)01043-3. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, McGaraughty S, Grandy DK. Circuitry underlying antiopioid actions of orphanin FQ in the rostral ventromedial medulla. J.Neurophysiol. 1997;78:3351–3358. doi: 10.1152/jn.1997.78.6.3351. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, McGaraughty S, Tortorici V. Circuitry underlying antiopioid actions of cholecystokinin within the rostral ventromedial medulla. J.Neurophysiol. 2001a;85:280–286. doi: 10.1152/jn.2001.85.1.280. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Schouten JC, Jobst EE. Activation of brainstem N-methyl-D-aspartate receptors is required for the analgesic actions of morphine given systemically. Pain. 2001b;92:129–138. doi: 10.1016/s0304-3959(00)00480-2. [DOI] [PubMed] [Google Scholar]
- Ho TK, LaBella FS, Pinsky C. Opiate properties of SKF 525A. Can.J.Physiol Pharmacol. 1978;56:550–554. doi: 10.1139/y78-088. [DOI] [PubMed] [Google Scholar]
- Hough LB. Improgan-Like Analgesics: A Family of Compounds Derived From Histamine Antagonists. Med.Chem.Res. 2004;13:78–87. [Google Scholar]
- Hough LB, De Esch IJ, Janssen E, Phillips J, Svokos K, Kern B, Trachler J, Abood ME, Leurs R, Nalwalk JW. Antinociceptive activity of chemical congeners of improgan: Optimization of side chain length leads to the discovery of a new, potent, non-opioid analgesic. Neuropharmacology. 2006;51:447–456. doi: 10.1016/j.neuropharm.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Hough LB, Nalwalk JW, Barnes WG, Leurs R, Menge WM, Timmerman H. A Third Legacy for Burimamide: Discovery and Characterization of Improgan and a New Class of NonOpioid Analgesics Derived from Histamine Antagonists. In: Watanabe T, Timmerman H, Yanai K, editors. Histamine Research in the New Millenium. Elsevier; Amsterdam: 2001a. pp. 237–242. [Google Scholar]
- Hough LB, Nalwalk JW, Chen Y, Schuller A, Zhu Y, Zhang J, Menge WMPB, Leurs R, Timmerman H, Pintar JE. Improgan, a cimetidine analog, induces morphine-like antinociception in opioid receptor-knockout mice. Brain Res. 2000a;880:102–108. doi: 10.1016/s0006-8993(00)02776-1. [DOI] [PubMed] [Google Scholar]
- Hough LB, Nalwalk JW, Leurs R, Menge WMPB, Timmerman H. Antinociceptive activity of impentamine, a histamine congener, after CNS administration. Life Sci. 1999;64:PL79–PL86. doi: 10.1016/s0024-3205(98)00571-2. [DOI] [PubMed] [Google Scholar]
- Hough LB, Nalwalk JW, Leurs R, Menge WMPB, Timmerman H. Antinociceptive activity of derivatives of improgan and burimamide. Pharmacol.Biochem.Behav. 2000b;65:61–66. doi: 10.1016/s0091-3057(99)00187-2. [DOI] [PubMed] [Google Scholar]
- Hough LB, Nalwalk JW, Li BY, Leurs R, Menge WMPB, Timmerman H, Cioffi C, Wentland M. Novel qualitative structure-activity relationships for the antinociceptive actions of H2 antagonists, H3 antagonists and derivatives. J.Pharmacol.Exp.Ther. 1997;283:1534–1543. [PubMed] [Google Scholar]
- Hough LB, Nalwalk JW, Lu Q, Shan Z, Svokos K, Wentland MP, Montero MJ. Antinociceptive, brain-penetrating derivatives related to improgan, a non-opioid analgesic. Eur.J.Pharmacol. 2005;522:38–46. doi: 10.1016/j.ejphar.2005.08.040. [DOI] [PubMed] [Google Scholar]
- Hough LB, Nalwalk JW, Menge WM, Leurs R, Timmerman H. Significance of GABAergic systems in the action of improgan, a non-opioid analgesic. Life Sci. 2001b;68:2751–2757. doi: 10.1016/s0024-3205(01)01080-3. [DOI] [PubMed] [Google Scholar]
- Hough LB, Nalwalk JW, Stadel R, Timmerman H, Leurs R, Paria BC, Wang X, Dey SK. Inhibition of improgan antinociception by the cannabinoid (CB)(1) antagonist N(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-p yrazole-3-carboxamide (SR141716A): lack of obligatory role for endocannabinoids acting at CB(1) receptors. J.Pharmacol.Exp.Ther. 2002;303:314–322. doi: 10.1124/jpet.102.036251. [DOI] [PubMed] [Google Scholar]
- Lehman T, Peterson GR. Naloxone-reversible analgesic action of SKF 525-A in mice. Psychopharmacology (Berl) 1978;59:305–308. doi: 10.1007/BF00426639. [DOI] [PubMed] [Google Scholar]
- Li BY, Nalwalk JW, Barker LA, Cumming P, Parsons ME, Hough LB. Characterization of the antinociceptive properties of cimetidine and a structural analog. J.Pharmacol.Exp.Ther. 1996;276:500–508. [PubMed] [Google Scholar]
- Li BY, Nalwalk JW, Finkel JM, Glick SD, Hough LB. SKF92374, a cimetidine analog, produces mechanical and thermal antinociception in the absence of motor impairment. Analgesia. 1997a;3:15–20. [Google Scholar]
- Li BY, Nalwalk JW, Hough LB. Effects of naltrexone and histamine antagonists on the antinociceptive activity of the cimetidine analog SKF92374 in rats. Brain Res. 1997b;748:168–174. doi: 10.1016/s0006-8993(96)01288-7. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Cook SA, Martin BR. Investigation of brain sites mediating cannabinoid-induced antinociception in rats: evidence supporting periaqueductal gray involvement. Journal of Pharmacology & Experimental Therapeutics. 1996;276:585–593. [PubMed] [Google Scholar]
- Lichtman AH, Martin BR. The selective cannabinoid antagonist SR 141716A blocks cannabinoid-induced antinociception in rats. Pharmacol.Biochem.Behav. 1997;57:7–12. doi: 10.1016/s0091-3057(96)00121-9. [DOI] [PubMed] [Google Scholar]
- Lim HD, van Rijn RM, Ling P, Bakker RA, Thurmond RL, Leurs R. Evaluation of histamine h1-, h2-, and h3-receptor ligands at the human histamine h4 receptor: identification of 4-methylhistamine as the first potent and selective h4 receptor agonist. J.Pharmacol.Exp.Ther. 2005;314:1310–1321. doi: 10.1124/jpet.105.087965. [DOI] [PubMed] [Google Scholar]
- Miyamoto A, Nishio A. Characterization of histamine receptors in isolated pig basilar artery by functional and radioligand binding studies. Life Sci. 1993;53:1259–1266. doi: 10.1016/0024-3205(93)90570-s. [DOI] [PubMed] [Google Scholar]
- Mobarakeh JI, Nalwalk JW, Watanabe T, Sakurada S, Hoffman M, Leurs R, Timmerman H, Silos-Santiago I, Yanai K, Hough LB. Improgan antinociception does not require neuronal histamine or histamine receptors. Brain Res. 2003;974:146–152. doi: 10.1016/s0006-8993(03)02572-1. [DOI] [PubMed] [Google Scholar]
- Nalwalk JW, Svokos K, Hough LB. Cannabinoid-improgan cross-tolerance: Improgan is a cannabinomimetic analgesic lacking affinity at the cannabinoid CB(1) receptor. Eur.J.Pharmacol. 2006;549:79–83. doi: 10.1016/j.ejphar.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Nalwalk JW, Svokos K, Leurs R, Hough LB. Absence of 5-HT3 and cholinergic mechanisms in improgan antinociception. Pharmacol.Biochem.Behav. 2005;80:505–510. doi: 10.1016/j.pbb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Nalwalk JW, Svokos K, Taraschenko O, Leurs R, Timmerman H, Hough LB. Activation of brain stem nuclei by improgan, a non-opioid analgesic. Brain Res. 2004;1021:248–255. doi: 10.1016/j.brainres.2004.06.066. [DOI] [PubMed] [Google Scholar]
- Nelson DR, Zeldin DC, Hoffman SM, Maltais LJ, Wain HM, Nebert DW. Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics. 2004;14:1–18. doi: 10.1097/00008571-200401000-00001. [DOI] [PubMed] [Google Scholar]
- Netti C, Bossa R, Galatulas I, Sibilia V, Pecile A. Antinociceptive effect of centrally administered cimetidine and dimaprit in the rat. Pharmacology. 1984;28:262–267. doi: 10.1159/000137972. [DOI] [PubMed] [Google Scholar]
- Neubert MJ, Kincaid W, Heinricher MM. Nociceptive facilitating neurons in the rostral ventromedial medulla. Pain. 2004;110:158–165. doi: 10.1016/j.pain.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Pan ZZ, Tershner SA, Fields HL. Cellular mechanism for anti-analgesic action of agonists of the kappa-opioid receptor. Nature. 1997;389:382–385. doi: 10.1038/38730. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; Sydney: 1986. [Google Scholar]
- Rivera ES, Davio CA, Venturino A, Caro RA, Bergoc RM. Histamine receptors in an experimental mammary carcinoma. Biomed.Pharmacother. 1994;48:399–406. doi: 10.1016/0753-3322(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Ryu EK, Choe YS, Byun SS, Lee KH, Chi DY, Choi Y, Kim BT. Synthesis of radioiodine labeled dibenzyl disulfide for evaluation of tumor cell uptake. Bioorg.Med.Chem. 2004;12:859–864. doi: 10.1016/j.bmc.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Sacerdote P, Mantegazza P, Panerai AE. Analgesic effects of μ antagonists after naloxone non-reversible stress-induced analgesia. Brain Res. 1985;359:34–38. doi: 10.1016/0006-8993(85)91409-x. [DOI] [PubMed] [Google Scholar]
- Sakurai E, Gunji E, IIzuka Y, Hikichi N, Maeyama K, Watanabe T. The disposition of thioperamide, a histamine H3-receptor antagonist, in rats. J.Pharm.Pharmacol. 1994;46:209–212. doi: 10.1111/j.2042-7158.1994.tb03780.x. [DOI] [PubMed] [Google Scholar]
- Smith IR, Cleverley MT, Ganellin CR, Metters KM. Binding of [3H]cimetidine to rat brain tissue. Agents Actions. 1980;10:422–426. doi: 10.1007/BF01968040. [DOI] [PubMed] [Google Scholar]
- Spetea M, Friedmann T, Riba P, Schutz J, Wunder G, Langer T, Schmidhammer H, Furst S. In vitro opioid activity profiles of 6-amino acid substituted derivatives of 14-Omethyloxymorphone. Eur.J.Pharmacol. 2004;483:301–308. doi: 10.1016/j.ejphar.2003.10.049. [DOI] [PubMed] [Google Scholar]
- Spinella M, Cooper ML, Bodnar RJ. Excitatory amino acid antagonists in the rostral ventromedial medulla inhibit mesencephalic morphine analgesia in rats. Pain. 1996;64:545–552. doi: 10.1016/0304-3959(95)00192-1. [DOI] [PubMed] [Google Scholar]
- Sterious SN, Walker EA. Potency differences for D-Phe-Cys-Tyr-D-Trp-Arg-Thr-PenThr-NH2 as an antagonist of peptide and alkaloid μ-agonists in an antinociception assay. J.Pharmacol.Exp.Ther. 2003;304:301–309. doi: 10.1124/jpet.102.042093. [DOI] [PubMed] [Google Scholar]
- Svokos K, Nalwalk JW, Leurs R, Menge WM, Timmerman H, Hough LB. A role for spinal, but not supraspinal, alpha2 adrenergic receptors in the actions of improgan, a powerful, non-opioid analgesic. Brain Res. 2001;921:12–19. doi: 10.1016/s0006-8993(01)03191-2. [DOI] [PubMed] [Google Scholar]
- Swett LR, Yellin TO. Imidazole derivatives. Histidine decarboxylase inhibitors. J.Med.Chem. 1970;13:968–970. doi: 10.1021/jm00299a040. [DOI] [PubMed] [Google Scholar]
- Szutowski MM, Lukasik M, Wawer ZT, Chrobak K, Michalska M, Borzecka K, Brzezinski J. In vivo effect of 5- and 8-methoxypsoralens and cimetidine on R,S-warfarin metabolism in rat. J.Appl.Toxicol. 2002;22:327–332. doi: 10.1002/jat.867. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, Ingram MA, Connor MA, Christie MJ. How opioids inhibit GABA-mediated neurotransmission. Nature. 1997;390:611–614. doi: 10.1038/37610. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, McGregor IS, Christie MJ. Cannabinoid receptor activation inhibits GABAergic neurotransmission in rostral ventromedial medulla neurons in vitro. Br.J.Pharmacol. 1999;127:935–940. doi: 10.1038/sj.bjp.0702636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrander SE, Norris DB, Rising RJ, Wood TP. 3H-cimetidine and the H2-receptor. Life Sci. 1983;33:1119–1126. doi: 10.1016/0024-3205(83)90015-2. [DOI] [PubMed] [Google Scholar]
- Welch SP, Thomas C, Patrick GS. Modulation of cannabinoid-induced antinociception after intracerebroventricular versus intrathecal administration to mice: Possible mechanisms for interaction with morphine. J.Pharmacol.Exp.Ther. 1995;272:310–321. [PubMed] [Google Scholar]
- Wiley JL, Jefferson RG, Grier MC, Mahadevan A, Razdan RK, Martin BR. Novel pyrazole cannabinoids: insights into CB(1) receptor recognition and activation. J.Pharmacol.Exp.Ther. 2001;296:1013–1022. [PubMed] [Google Scholar]
- Wilkinson CF, Hetnarski K, Hicks LJ. Substituted Imidazoles as Inhibitors of Microsomal Oxidation and Insecticide Synergists. Pesticide Biochem.and Physiology. 1974;4:299–312. [Google Scholar]
- Wilkinson CF, Hetnarski K, Yellin TO. Imidazole derivatives--a new class of microsomal enzyme inhibitors. Biochem.Pharmacol. 1972;21:3187–3192. doi: 10.1016/0006-2952(72)90147-5. [DOI] [PubMed] [Google Scholar]
- Young RC, Mitchell RC, Brown TH, Ganellin CR, Griffiths R, Jones M, Rana KK, Saunders D, Smith IR, Sore NE, Wilks TJ. Development of a new physicochemical model for brain penetration and its application to the design of centrally acting H2 receptor histamine antagonists. J.Med.Chem. 1988;31:656–671. doi: 10.1021/jm00398a028. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Michalovich D, Wu H-L, Tan KB, Dytko GM, Mannan IJ, Boyce R, Alston J, Tierney LA, Li X, Herrity NC, Vawter L, Sarau HM, Ames RS, Davenport CM, Hieble JP, Wilson S, Bergsma DJ, Fitzgerald LW. Cloning, expression and pharmacological characterization of a novel human histamine receptor. Mol.Pharmacol. 2001;59:434–441. doi: 10.1124/mol.59.3.434. [DOI] [PubMed] [Google Scholar]
- Zeitz KP, Guy N, Malmberg AB, Dirajlal S, Martin WJ, Sun L, Bonhaus DW, Stucky CL, Julius D, Basbaum AI. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J.Neurosci. 2002;22:1010–1019. doi: 10.1523/JNEUROSCI.22-03-01010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonta N, Zambotti F, Vicentini L, Tammiso R, Mantegazza P. Effects of some GABAmimetic drugs on the antinociceptive activity of morphine and β-endorphin in rats. Naunyn Schmiedebergs Arch.Pharmacol. 1981;316:231–234. doi: 10.1007/BF00505654. [DOI] [PubMed] [Google Scholar]