Abstract
The objective of the present study was to determine if reactive oxygen species (ROS) are required as secondary messengers for fibronectin fragment stimulated matrix metalloproteinase (MMP) production in human articular chondrocytes. Cultured cells were stimulated with 25μg/ml of the α5β1 integrin-binding 110kDa fibronectin fragment (FN-f) in the presence and absence of various antioxidants including Mn(III) tetrakis(4-benzoic acid)porphyrin (MnTBAP). FN-f stimulation significantly increased intracellular levels of ROS in articular chondrocytes. Pretreatment of cells with 250μM MnTBAP or 40mM N-Acetyl-L-cysteine, but not inhibitors of nitric oxide synthase, completely prevented FN-f stimulated MMP-3, -10 and -13 production. MnTBAP also blocked FN-f induced phosphorylation of the MAP kinases and NF-κB-associated proteins and blocked activation of an NF-κB promoter-reporter construct. Overexpression of catalase, superoxide dismutase, or glutathione peroxidase also inhibited FN-f stimulated MMP-13 production. Pre-incubation of chondrocytes with rotenone, an inhibitor of the mitochondrial electron transport chain, or nordihydroguaiaretic acid (NDGA), a selective 5-lipoxygenase inhibitor, partially prevented FN-f stimulated MMP-13 production and decreased MAP kinase and NF-κB phosphorylation. These results show that increased production of ROS but not nitric oxide are obligatory secondary messengers in the chondrocyte FN-f signaling pathway that leads to the increased production of MMPs, including MMP-13.
Keywords: Reactive oxygen species, integrins, matrix metalloproteinase, antioxidants, signal transduction, mitogen activated protein kinase, nuclear factor-κB
Osteoarthritis (OA) is a multifactorial disease characterized by a progressive loss of matrix proteins in human articular cartilage and subsequent chondrocyte cell death, with age as the strongest risk factor [1]. The free radical theory of aging suggests that an accumulation of reactive oxygen species (ROS) causes irreparable damage to cells and tissues over time; however, the exact mechanism remains poorly understood [2]. Previous studies implicate ROS as playing an important role in cartilage destruction in arthritis [3]. Excessive ROS production can contribute to chondrocyte death [4–7]. However, controversy exists as to the exact role of cell death in the development of OA with the vast majority of cell death likely occurring in later stages of the disease [8]. We hypothesized that ROS have additional effects on articular cartilage well before cell death takes place.
ROS have physiologic roles as secondary mediators in multiple cell signaling pathways including those initiated by growth factors, cytokines and extracellular matrix proteins [2, 9, 10]. Activation of c-Jun NH2-terminal kinase (JNK) by IL-1 and TNF-α in chondrocytes has been shown to require ROS as a signaling intermediate [11]. In synovial fibroblasts, ROS have also been shown to be required for signaling initiated through the α5β1 integrin that results in increased production of matrix metalloproteinase (MMP)-1 [12]. Stimulation of the α5β1 integrin on articular chondrocytes, with either integrin-activating antibodies or fibronectin fragments, also resulted in increased MMP production [13, 14] but the potential role of ROS in this signaling event in chondrocytes has not been determined. Importantly, the role of ROS in the activation of specific downstream signaling proteins in the α5β1 pathway that mediates MMP expression has also not been determined.
Defining the role of ROS in integrin signaling which regulates MMP production is important because excessive MMP production is a key mechanism by which cartilage matrix destruction occurs during the development of arthritis. In chondrocytes, the integrin signaling pathway which mediates increased MMP-13 production includes activation of the three major families of MAP kinases (ERK, JNK, and p38) and increased activity of the NFκB and AP-1 transcription factors [13–15]. In these studies the chondrocyte α5β1 integrin was stimulated using either an integrin-activating antibody or the 110kDa fibronectin fragment (FN-f) which contains the RGD binding site for α5β1. Stimulation of chondrocytes with fibronectin fragments is relevant to cartilage biology because similar fragments have been found in both RA and OA articular cartilage and synovial fluid [16]. In addition, a previous study provided evidence that anti-oxidants could inhibit the ability of a smaller (29kDa) FN-f to degrade articular cartilage explants [17].
The aim of the present study was to determine whether FN-f stimulated MMP production requires ROS as secondary messengers. We chose the 110kD FN-f because it contains the α5β1 integrin cell binding region [18, 19] and because previous studies in our lab using this fragment had shown similar results to chondrocytes stimulated with the α5β1-activating antibody JBS5 [13]. Here we found that ROS are obligatory components of the signal transduction cascade responsible for increased MMP production by cells treated with FN-f. Both antioxidants and overexpession of catalase (CAT) or glutathione peroxidase (GPx) completely block this pathway. These results suggest that ROS can have deleterious effects on human tissues through the increased production of physiologically relevant MMPs.
Materials and methods
Reagents
Dulbecco’s modified eagle medium (DMEM), Ham’s F-12, phosphate buffered saline (PBS), gentamicin, penicillin G sodium-streptomycin sulfate-amphotericin B and fetal bovine serum (FBS) were purchased from GibcoBRL (Gaithersburg, MD). Pronase, NG-Monomethyl-L-arginine (L-NMMA), L-N6-(1-iminoethyl)lysine (L-NIL), MK-886, 5,8,11,14-eicosatetraynoic acid (ETYA) and manganese (III)-tetrakis (4-benzoic acid) porphyrin (MnTBAP) were obtained from Calbiochem (La Jolla, CA). Collagenase-P was purchased from Boehinger-Mannheim (Germany). Nordihydroguaiaretic acid (NDGA), N-Acetyl-L-cysteine (NAC) and rotenone were from Sigma (St. Louis, MO). The 110kDa FN-f was kindly provided by Dr. Kenneth Ingram, American Red Cross, Rockville, MD. Antibodies against MMP-2, -3, -10, and -13 were purchased from Chemicon (Temecula, CA). All other antibodies used in this study were purchased from Cell Signaling Technology (Beverly, MA). Human MnSOD, CAT, and GPx cDNA expression vectors were obtained from Dr. Larry Oberly from the University of Iowa. The NFκB promoter-luciferase reporter plasmid (pNF-kB-Luc) was from Clontech (Mountain View, CA) and the control renilla plasmid (pRL-TK) and dual luciferase reporter assay system were purchased from Promega (Madison, WI).
Tissue acquisition and chondrocyte isolation
Human ankle articular cartilage was obtained within 72 hours of death through the Gift of Hope Organ and Tissue Donor Network (Elmhurst, IL) or from the National Disease Research Interchange (Philadelphia, PA). Only human donors with no known history of joint disease were used for tissue collection. Each donor specimen was graded for gross degenerative changes based on a modified version of the 5-point scale of Collins [20] and only samples of grade 0 or 1 were used for this study. Enzymatic isolation of chondrocytes and subsequent culture for signaling studies were performed as previously described [13]. Cells were cultured at high density and not passaged in order to maintain the differentiated chondrocyte phenotype.
Quantification of intracellular ROS production using confocal microscopy
The ROS-sensitive dye 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (DCFDA) (Molecular Probes, Eugene, OR) was solubilized in EtOH and diluted to a final concentration of 5μM in DMEM/F-12 without phenol red. Sub-confluent monolayers were established on 18-mm circular glass coverslips 2 days before experimental use, then loaded with DCFDA for 5 minutes before FN-f stimulation. Microscope slides containing either a 50μl droplet of 25μg/ml FN-f or a 50μl droplet of PBS as a control were first positioned and focused on the confocal microscope using untreated cells as a positional reference, and then circular coverslips containing cells were inverted onto the droplet. A picture was immediately taken for time 0, then every 30 seconds for 2 minutes. Fluorescent intensity was quantified using Quantity One software (Bio-Rad Laboratories, Hercules, CA).
Chondrocyte stimulation and immunoblot analysis
Media was changed to serum-free DMEM-F12 with antibiotics for 18 hours (overnight) and again 2 hours before addition of 25μg/ml FN-f. For anti-oxidant experiments, cells were preincubated with anti-oxidants for 30 minutes before stimulation. For analysis of MMP production, cultures were treated for 24 hours with FN-f and then MMPs released into the conditioned media were evaluated by immunoblotting with MMP antibodies as previously described [13, 14]. Conditioned media was also analyzed with the RayBio Matrix Metalloproteinase Antibody Array I according to the manufacture’s protocol (RayBiotech, Inc., Atlanta, GA).
For cell signaling studies, chondrocytes were treated with FN-f for the indicated time periods and then media was removed and a cell lysate was prepared after washing the cells once with ice-cold PBS containing 0.1mM Na3VO4. Cell lysates were prepared in a solubilization buffer containing: 20mM Tris-HCl (pH 7.5), 150mM NaCl, 1mM Na2EDTA, 1mM EGTA, 1% Triton, 2.5mM sodium pyrophosphate, 1mM β-glycerophosphate, 1mM Na3VO4, 1mM phenylmethylsulfonyl fluoride (PMSF) as previously described [13, 14]. Samples containing equal amounts of total protein were separated by SDS-PAGE and immunoblotted with phosphospecific protein antibodies followed by stripping and reprobing with non-phosphospecific antibodies to confirm equal protein loading as described [13, 14]. Band density was quantified using Quantity One software (Bio-Rad Laboritories, Hercules, CA).
MMP-13 ELISA
In selected experiments, MMP-13 production was assessed in conditioned media using the Fluorokine E Human Active MMP-13 Fluorescent Assay according to the manufacture’s protocol (R&D Systems, Inc., Minneapolis, MN). The ELISA is based on a monoclonal antibody that captures both active and pro-MMP-13, and a fluorogenic peptide that contains a MMP-13 cleavage site. Activity present after pre-treatment of the samples with p-Aminophenylmercuric acetate (APMA, a chemical activator of MMPs) represents the total MMP-13 present in the conditioned culture media. Activity present in media samples not treated with APMA represents only the active MMP-13 present in the conditioned culture media.
Plasmid transfection of human articular chondrocytes
Cells were electroporated with the Human Chondrocyte Nucleofector Kit system from Amaxa Biosystems (Germany) as previously described in detail [21]. Briefly, confluent cells were removed from monolayer culture with 0.2% Pronase and 0.025% bacterial collagenase in serum-free DMEM/F-12 media for 4 hours at 37°C and washed three times with PBS. Cells were then transfected with 4μg of the respective plasmid DNA and plated in a 6-well plate with 20% FBS for 48 hours before treatment with FN-f. Control cells were exposed to the same conditions in the presence of 4μg of empty vector. Cell survival after transfection was measured at the time of FN-f treatment in a parallel set of cultures using using ethidium bromide homodimer 1 to stain dead cells and calcein AM to visualize live cells as previously described [4, 22]. Survival after transfection with 4μg of plasmid was on average 67% compared to 82% in non-transfected cells treated under the same conditions. There was no significant difference between survival of cells transfected with empty vector and those transfected with the plasmids containing anti-oxidant enzyme constructs. Transfection experiments to measure NFκB promoter activity were performed as previously described [15].
Statistical analyses
Data was analyzed with a one-way ANOVA to detect a difference in group means with a post-hoc Bonferroni correction using the Window’s-based Statistical Package for the Social Sciences (SPSS) software (SPSS Inc., Chicago, IL). A p-value < 0.05 was considered significant.
Results
FN-f stimulated chondrocytes display increased levels of ROS
The redox sensitive dye DCFDA was used to detect FN-f stimulated ROS production in chondrocytes cultured in monolayer. FN-f displayed a 4.5-fold increase in DCFDA intensity at 30 seconds whereas PBS alone displayed a 2.2-fold increase in DCFDA intensity at the same time point (Fig. 1). The signal intensity began to decrease after the 30 second time point in both control and FN-f treated cells possibly due to photobleaching (data not shown).
Fig. 1.

Increased reactive oxygen species production with fibronectin fragment stimulation detected using confocal microscopy. Chondrocytes cultured on 18-mm circular glass coverslips were loaded with 5μM DCFDA for 5 minutes and then stimulated with 25μg/ml FN-f or PBS as a control. Cells were imaged by confocal microscopy immediately for time 0, and then at every 30 seconds for two minutes. The images represent the typical result obtained from 3 different experiments.
Effect of antioxidants on FN-f stimulated MMP production
The contribution of ROS to FN-f-mediated MMP production was determined by pretreating chondrocytes with antioxidants before FN-f stimulation. Various inhibitors were tested for this purpose: Tiron, a chemical scavenger of O2•− [23], MnTBAP, a cell-permeable superoxide dismutase (SOD) mimetic and peroxynitrite scavenger [24], N-Acetyl-L-cysteine (NAC), a broad spectrum antioxidant [25], and L-NMMA, a competitive inhibitor of all three isoforms of nitric oxide synthase [26].
Established chondrocyte monolayers were serum-starved for 24 hours and pretreated with the various antioxidants 30 minutes prior to FN-f stimulation. Initial experiments were performed with MnTBAP and it significantly inhibited FN-f stimulated MMP-13 production at dose of 250μM (Fig. 2A). An MMP protein array was then used to efficiently scan the production of multiple MMPs on a single immunoblot. Media from unstimulated control cultures contained MMP-3, TIMP-1, TIMP-2, and a low level of MMP-10 (Fig. 2B). Treatment with FN-f increased the amounts of MMP-10, MMP-13, and TIMP-1 without noticeable changes in MMP-3 or TIMP-2. There was also a faint positive detection of MMP-1. Pre-treatment with MnTBAP followed by FN-f reduced the levels of MMP-1, 3, 10, and 13, and TIMP-1 (Fig. 2B). Addition of MnTBAP alone reduced the levels of MMP-3 and MMP-10 but not the TIMPs.
Fig. 2.

Inhibition of fibronectin fragment stimulated MMP production with anti-oxidants. Confluent monolayers were serum-starved for 24 hours, pretreated with anti-oxidants for 30 mins., and then treated with 25μg/ml FN-f for an additional 24 hour incubation period. Conditioned media was collected and used for immunoblotting. A, Dose response with 15.625, 31.25, 62.5, 125, and 250μM MnTBAP, respectively. Conditioned media was immunoblotted with an anti-MMP-13 polyclonal antibody. B, Conditioned media from confluent monolayers treated with or without either 25μg/ml FN-f or 250μM MnTBAP was analyzed using MMP array membranes. The positions of the antibodies on the array are shown below the membranes. C, Media was collected from chondrocytes treated with: (1) Control; (2) 25μg/ml FN-f; (3) 250μM MnTBAP + 25μg/ml FN-f; (4) 250 μM Tiron + 25μg/ml FN-f; (5) 40mM NAC + 25μg/ml FN-f; (6) 50μM L-NMMA + 25μg/ml FN-f. After a 24 hour incubation period, conditioned media was immunoblotted with either MMP-2, MMP-3, MMP-10, or MMP-13 polyclonal antibodies.
To corroborate the protein array data and to determine if other antioxidants would have similar effects, MMP-2, -3, -10, and -13 were analyzed by immunoblot in media samples from cells pre-treated with either MnTBAP, Tiron, NAC, or L-NMMA followed by FN-f stimulation. MMP-2 production was not affected by any of the treatments (Fig. 2C) and therefore served as a gel loading control. MnTBAP and NAC but not Tiron or L-NMMA inhibited FN-f stimulated MMP-3, -10, and -13 production. The FN-f stimulation of MMP-3 is consistent with previous studies [27, 28] and suggests that the protein array (Fig. 2B) may have been less sensitive in detecting an increase in MMP-3 in the conditioned media. No cell death was observed in any conditions tested using the LIVE/DEAD Cell Viability Assay (data not shown).
Immunoblot data on media samples was supplemented with an ELISA based on the cleavage of a fluorogenic peptide substrate for a more precise quantification of FN-f induced MMP-13 production including production of the active enzyme. As expected, FN-f stimulated total MMP-13 levels to approximately 15-fold above control (Fig. 3A) and also stimulated active MMP-13 levels to approximately 4-fold above control (Fig. 3B). MnTBAP pre-treatment completely prevented the increased MMP-13 production. There was only slight, and not statistically significant, decrease in MMP-13 production using 50μM of either or both of the NO synthetase inhibitors L-NMMA (IC50 = 700nM for cNOS; IC50 = 3.9μM for iNOS; IC50 = 650nM for nNOS) and L-NIL (IC50 = 3.3μM for iNOS; IC50 = 92μM for cNOS). Importantly, despite the increase in TIMP-1 noted on the array membrane, active MMP-13 was produced in response to FN-f and the activity was significantly inhibited by pre-treatment with MnTBAP but not by the NO synthetase inhibitors (Fig. 3B).
Fig. 3.

Effect of MnTBAP and NO inhibitors on FN-f stimulated MMP-13 production using an ELISA for quantification. Serum-free chondrocyte cultures were pretreated with either 250μM MnTBAP, 25μM L-NIL, or 100μM L-NMMA or both L-NIL and L-NMMA for 30 minutes before the addition of 25μg/ml FN-f. Conditioned media was collected after 24 hours and assayed for total (A) and active (B) MMP-13 as described in the Methods. Results represent the mean and SEM of 3 separate experiments using primary chondrocytes derived from 3 different donors.
Effect of MnTBAP on the ability of FN-f to stimulate the MAP kinase and NF-κB pathways
We have previously reported that the activity of both the MAP kinases and NF-κB is required for FN-f stimulated MMP-13 production [13–15]. Pre-treatment of chondrocytes with MnTBAP inhibited the ability of FN-f to stimulate phosphorylation of ERK, JNK, p38, IκBα, IKKα, and the p65 subunit of NF-κB (Fig. 4A). There appeared to be some increase in the basal (time zero) phosphorylation of ERK in MnTBAP treated cultures but no further increase was seen with FN-f treatment. In addition, treatment of chondrocytes with MnTBAP resulted in inhibition of FN-f stimulated NFκB activity measured by transfecting chondrocytes with an NFκB promoter - luciferase reporter construct (Fig. 4B).
Fig. 4.

Effect of MnTBAP on FN-f stimulated MAP kinase and NF-κB activation. A, Chondrocytes in serum-free media were stimulated for the indicated time period with 25μg/ml FN-f. Cells were pretreated with the 250μM MnTBAP for 30 minutes before the addition of FN-f. After stimulation, cell lysates were prepared and immunoblotted with antibodies to the phosphorylated form (phospho) of the indicated proteins. Equal protein loading in each lane was confirmed by immunoblotting with antibodies to non-phosphospecific (total) ERK and p38. B, Chondrocytes were co-transfected with pRL-TK and pNFκB-Luc luciferase reporter vectors. After 48 hours, transfected cells were serum starved and treated with: 1) no treatment as a negative control; 2) 10ng/ml IL-1β for 45 minutes as a positive control for NFκB activation; 3) 25μg/ml FN-f stimulation for 45 minutes; 4) 250μM MnTBAP for 30 minutes prior to 25μg/ml FN-f stimulation for 45 minutes. Cell lysates were assayed using a dual-luciferase reporter system. The firefly luciferase measurements (RLUs) reported were corrected by subtracting the background (from assayed non-transfected cells) and the samples’ respective renilla measurements.
Effect of inhibition of the mitochondrial electron transport chain and 5-lipoxygenase on FN-f stimulated MMP-13 production and MAP kinase and NF-κB activation
The mitochondria are a principal source of ROS in mammalian cells. Rotenone was used to inhibit the mitochondrial electron transport chain at its terminal complex as a means to reduce the intracellular production of ROS. Pre-incubation with rotenone reduced FN-f stimulated MMP-13 production 2.8-fold (Fig. 5A) and the blocked the phosphorylation of the JNK and p38 MAP kinases with little effect on ERK (Fig. 5B). This was accompanied by a reduction in IκBα phosphorylation and phosphorylation of the p65 subunit of NF-κB but not IKKα (Fig. 5B).
Fig. 5.

Effect of lipoxygenase and mitochondrial electron transport chain inhibitors on FN-f stimulated MMP-13 production and MAP kinase and NF-κB activation. Confluent monolayers were serum-starved for 24 hours in DMEM/F-12, pretreated with either 1μM MK-886, 5μM NDGA, 25μM ETYA or 25μM rotenone for 30 minutes, and then treated with 25μg/ml FN-f. A, After pretreatment with inhibitors, FN-f was added for 24 hours, and conditioned media was immunoblotted with an anti-MMP-13 polyclonal antibody. B, After pretreatment with inhibitors, FN-f was added for 30 minutes, and whole cell lysates were immunoblotted as described in Fig.4. Band density was quantified using Quantity One software (Bio-Rad Laboritories, Hercules, CA).
Lipoxygenases (LOX) have been shown to produce ROS and were therefore attractive targets for intracellular ROS production in response to integrin stimulation [29]. Pre-treatment of chondrocytes with the 5-lipoxygenase (5-LOX) inhibitor nordihydroguaiaretic acid (NDGA) (IC50 = 200nM) caused a modest reduction in MMP-13 production and reduced phosphorylation of JNK but was less effective than either MnTBAP or rotenone (Figs. 5A & 5B). This effect was not seen with the other LOX inhibitors ETYA and MK-886. ETYA at the 10μM concentration tested here primarily inhibits 12-LOX (IC50 = 300nM) and 15-LOX (IC50 = 200nM) over 5-LOX (IC50 = 10μM) and cyclooxygenase (COX) (IC50 = 8μM). MK-866 prevents 5-LOX from binding to the 5-lipoxygenase-activating protein (FLAP) (IC50 = 102nM) thus suggesting a FLAP-independent mechanism. No cell death was observed in any of the conditions tested (data not shown).
Overexpression of antioxidant enzymes inhibits FN-f induced MMP-13
If endogenous production of ROS is required for FN-f stimulation of MMP-13 production, we hypothesized that overexpression of specific anti-oxidant enzymes would be inhibitory. Chondrocytes were transfected with plasmids designed to overexpress three different anti-oxidant enzymes: superoxide dismutase, catalase, or glutathione peroxidase. Either glutathione peroxidase or catalase overexpression was able to block FN-f induced MMP-13 production while SOD overexpression was less effective (Fig. 6). However, complete inhibition was seen when catalase was co-expressed with SOD. These results suggest that intracellular H2O2 may be the causative ROS responsible for FN-f induced MMP-13 production.
Fig. 6.
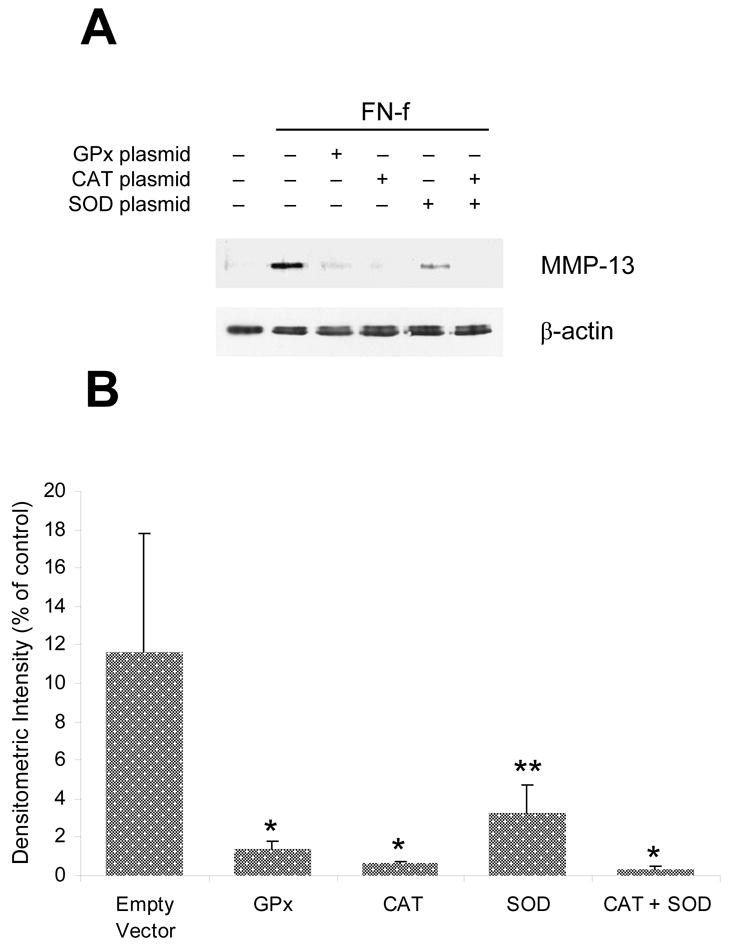
Inhibition of fibronectin fragment stimulated MMP-13 production by overexpression of anti-oxidant enzymes. Primary human articular chondrocytes were transiently transfected with either GPx, CAT, and/or SOD wild-type plasmids. Cells were transfected with 4μg of the indicated plasmid (2μg CAT + 2μg SOD for the combination) or an empty vector control plasmid and then plated and maintained for 48 hours in 20% FBS. After serum-starvation for 24 hours, cells were stimulated for an additional 24 hours with FN-f. A, Immunoblot analysis of conditioned media with a polyclonal MMP-13 antibody and of the cell lysates from the same wells with a β-actin antibody. B, Densitometric analysis of immunoblots showing the mean and SEM of 3 separate experiments using primary chondrocytes derived from 3 different donors. * p < 0.001; **p<0.025.
Discussion
There is accumulating evidence that ROS serve as signaling intermediates in a number of intracellular pathways initiated through a variety of receptors including growth factor, cytokine, and integrin receptors [2, 9–11]. In the present study, we focused on the chondrocyte α5β1 integrin pathway which is stimulated in response to FN-f and results in increased MMP production. We found that ROS levels in the cell were rapidly increased after exposure to FN-f and that inhibition of ROS with anti-oxidants inhibited FN-f stimulation of MAP kinase phosphorylation as well as phosphorylation of the p65 subunit of NFκB. Importantly, the signaling inhibition was associated with an inhibition of NFκB promoter activity and MMP production, in particular the production of MMP-13.
It is useful to view ROS on a sliding scale – lower levels are required for normal cellular homeostasis while higher levels can participate in pathological conditions [2]. After exposure to various stimuli, chondrocytes have been shown to produce a variety of ROS including NO, superoxide, H2O2, and peroxynitrite (ONOO−), the latter which can form by the reaction of NO with superoxide [30–33]. In the present study, we did not directly identify which specific ROS was produced in response to FN-f stimulation of α5β1 but the results with the inhibitors suggest that the species mediating the observed signaling events and the increase in MMP production was likely H2O2. Of the inhibitors we have tested in the present study, MnTBAP was most effective in blocking signaling and blocking the increase in MMPs. MnTBAP is not only an SOD mimetic and peroxynitrite scavenger [24], it also possess catalase activity and so converts H2O2 to water [34]. Because MnTBAP scavenges ONOO−, we cannot rule out the possible contribution of ONOO−, formed by NO reacting with O2−, but against this are the findings of a lack of significant inhibition by NO synthase inhibitors and the finding that overexpression of catalase or glutathione peroxidase was more effective than MnSOD overexpression at inhibiting FN-f induced MMP-13 production.
The present results are consistent with previous work in synovial fibroblasts which demonstrated a requirement for ROS production during α5β1 stimulation of MMP-1 production [12] and a study in bovine cartilage explants where N- acetylcysteine (NAC) was shown to block MMP-3 production induced by the 29kD FN-f [17]. However, since we only studied the 110kD FN-f, we do not know if the requirement for ROS in the signaling induced by the 29kD FN-f follows a similar mechanism. But similar to our findings in chondrocytes, the studies in synovial fibroblasts found ROS were required for activation of NFκB and the increase in MMP-1 was inhibited with NAC but not with NO synthase inhibitors.
However, in synovial fibroblasts the increase in MMP-1 also required expression and release of IL-1 which acted in an autocrine manner to stimulate MMP-1 production while we have shown IL-1 is not required for FN-f stimulation of MMP-13 or cytokine production by chondrocytes [13, 15, 21]. Another difference between α5β1 signaling in synovial fibroblasts and in chondrocytes is that rotenone, which inhibits mitochondrial ROS production by blocking complex I in the electron transport chain, completely inhibited MMP-1 production in fibroblasts [35] while in chondrocytes rotenone showed partial but not complete inhibition. Also, in chondrocytes Tiron was less effective than NAC or MnTBAP. These findings suggest differences in sources and types of ROS in chondrocytes in response to integrin signaling compared to fibroblasts or differences in their matrices and/or matrix receptors that affect ROS signaling. Partial inhibition by NDGA in chondrocytes indicates that 5-lipoxygenase may be another source of ROS which would be consistent with integrin signaling studies in NIH-3T3 fibroblasts where ROS production was blocked by NDGA but not rotenone [29]. However, because of the lack of specificity of NDGA these results should be interpreted with caution. It is also not clear why ETYA was effective at inhibiting FN-f induced JNK and p65 phosphorylation but did not appear to reduce MMP-13 production. Together, the findings in different cell types indicate that there are likely multiple pathways for ROS generation downstream from integrin activation.
Regulation of MAP kinase activation has been shown to be redox-sensitive and we found that anti-oxidants blocked FN-f stimulated phosphorylation of all 3 MAP kinases with JNK phosphorylation being particularly sensitive. ROS have been shown to activate JNK through the oxidative inactivation of endogenous JNK inhibitors such as the JNK phosphatases [36]. Treatment of chondrocytes with MnTBAP appeared to cause a modest increase in ERK phosphorylation in the absence of FN-f and rotenone had much less inhibitory affect on ERK phosphorylation as it did on JNK and p38. These findings suggest redox regulation of the ERK pathway may be different than JNK and p38.
Consistent with our previous results using chemical MAP kinase inhibitors [13] and overexpression of dominant negative MAP kinase constructs [14], we found that anti-oxidant inhibition of MAP kinase phosphorylation was associated with inhibition of MMP-13 production. Using an MMP array, we also found that the anti-oxidant MnTBAP inhibited FN-f stimulation of MMP-1 and MMP-10 as well. TIMP-1 levels in the media were also increased by FN-f and were reduced to basal control levels by MnTBAP whereas TIMP-2 did not change with MnTBAP and/or FN-f. The MMP-13 ELISA assay results demonstrated that FN-f was able to stimulate production of active MMP-13, which was consistent with our previous work [13], and MnTBAP inhibited production of both total and active enzyme.
MMP-13 is increased in OA cartilage and is thought to play a key role in the degradation of type II collagen [37–39]. For this reason targeting signaling pathways which regulate MMP-13 expression would represent a potential therapeutic approach for slowing or stopping cartilage destruction in arthritis. The results from the present study suggest that specific anti-oxidants may be useful in this regard. Because of additional evidence that excessive ROS levels may contribute to cartilage loss by other mechanisms as well [3], further studies of these compounds for their ability to block cartilage destruction are warranted.
Abbreviations
- MnTBAP
Mn(III) tetrakis(4-benzoic acid)porphyrin
- MMP
matrix metalloproteinase
- FN-f
110 kDa fibronectin fragment
- MAP kinases
mitogen activated protein kinase
- NF-κB
nuclear factor -κB
- JNK
c-Jun NH2-terminal kinase
- IKK
IκB kinase
Footnotes
This work was supported by grant AR49003 from the National Institute of Health. We gratefully acknowledge the Gift of Hope Organ and Tissue Donor Network, the National Disease Research Interchange (NDRI) and the donor families for providing tissue and the assistance of Dr. Arkady Margulis for collecting donor tissues. We also thank Dr. Larry Oberly for providing plasmids and Dr. Kenneth Ingram for fibronectin fragments.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Loeser RF, Shakoor N. Aging or osteoarthritis: which is the problem? Rheum Dis Clin North Am. 2003;29:653–673. doi: 10.1016/s0889-857x(03)00062-0. [DOI] [PubMed] [Google Scholar]
- 2.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 3.Henrotin YE, Bruckner P, Pujol JP. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthritis Cartilage. 2003;11:747–755. doi: 10.1016/s1063-4584(03)00150-x. [DOI] [PubMed] [Google Scholar]
- 4.Del Carlo M, Jr, Loeser RF. Nitric oxide--mediated chondrocyte cell death requires the generation of additional reactive oxygen species. Arthritis Rheum. 2002;46:394–403. doi: 10.1002/art.10056. [DOI] [PubMed] [Google Scholar]
- 5.Del Carlo M, Jr, Loeser RF. Increased oxidative stress with aging reduces chondrocyte survival: Correlation with intracellular glutathione levels. Arthritis Rheum. 2003;48:3419–3430. doi: 10.1002/art.11338. [DOI] [PubMed] [Google Scholar]
- 6.Kurz B, Lemke A, Kehn M, Domm C, Patwari P, Frank EH, Grodzinsky AJ, Schunke M. Influence of tissue maturation and antioxidants on the apoptotic response of articular cartilage after injurious compression. Arthritis Rheum. 2004;50:123–130. doi: 10.1002/art.11438. [DOI] [PubMed] [Google Scholar]
- 7.Jallali N, Ridha H, Thrasivoulou C, Underwood C, Butler PE, Cowen T. Vulnerability to ROS-induced cell death in ageing articular cartilage: the role of antioxidant enzyme activity. Osteoarthritis Cartilage. 2005;13:614–622. doi: 10.1016/j.joca.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Aigner T, Kim HA, Roach HI. Apoptosis in osteoarthritis. Rheum Dis Clin North Am. 2004;30:639, 653. doi: 10.1016/j.rdc.2004.04.002. xi. [DOI] [PubMed] [Google Scholar]
- 9.Nathan C. Specificity of a third kind: reactive oxygen and nitrogen intermediates in cell signaling. J Clin Invest. 2003;111:769–778. doi: 10.1172/JCI18174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamata H, Hirata H. Redox regulation of cellular signalling. Cell Signal. 1999;11:1–14. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- 11.Lo YY, Wong JM, Cruz TF. Reactive oxygen species mediate cytokine activation of c-Jun NH2- terminal kinases. J Biol Chem. 1996;271:15703–15707. doi: 10.1074/jbc.271.26.15703. [DOI] [PubMed] [Google Scholar]
- 12.Kheradmand F, Werner E, Tremble P, Symons M, Werb Z. Role of Rac1 and oxygen radicals in collagenase-1 expression induced by cell shape change. Science. 1998;280:898–902. doi: 10.1126/science.280.5365.898. [DOI] [PubMed] [Google Scholar]
- 13.Forsyth CB, Pulai J, Loeser RF. Fibronectin fragments and blocking antibodies to alpha2beta1 and alpha5beta1 integrins stimulate mitogen-activated protein kinase signaling and increase collagenase 3 (matrix metalloproteinase 13) production by human articular chondrocytes. Arthritis Rheum. 2002;46:2368–2376. doi: 10.1002/art.10502. [DOI] [PubMed] [Google Scholar]
- 14.Loeser RF, Forsyth CB, Samarel AM, Im HJ. Fibronectin fragment activation of proline-rich tyrosine kinase PYK2 mediates integrin signals regulating collagenase-3 expression by human chondrocytes through a protein kinase C-dependent pathway. J Biol Chem. 2003;278:24577–24585. doi: 10.1074/jbc.M304530200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Im HJ, Pacione C, Chubinskaya S, Van Wijnen AJ, Sun Y, Loeser RF. Inhibitory effects of insulin-like growth factor-1 and osteogenic protein-1 on fibronectin fragment- and interleukin-1beta-stimulated matrix metalloproteinase-13 expression in human chondrocytes. J Biol Chem. 2003;278:25386–25394. doi: 10.1074/jbc.M302048200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Homandberg GA, Wen C, Hui F. Cartilage damaging activities of fibronectin fragments derived from cartilage and synovial fluid. Osteoarthritis Cartilage. 1998;6:231–244. doi: 10.1053/joca.1998.0116. [DOI] [PubMed] [Google Scholar]
- 17.Homandberg GA, Hui F, Wen C. Fibronectin fragment mediated cartilage chondrolysis. I. Suppression by anti-oxidants. Biochim Biophys Acta. 1996;1317:134–142. doi: 10.1016/s0925-4439(96)00046-4. [DOI] [PubMed] [Google Scholar]
- 18.Pytela R, Pierschbacher MD, Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985;40:191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- 19.Werb Z, Tremble PM, Behrendtsen O, Crowley E, Damsky CH. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989;109:877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muehleman C, Bareither D, Huch K, Cole AA, Kuettner KE. Prevalence of degenerative morphological changes in the joints of the lower extremity. Osteoarthritis Cartilage. 1997;5:23–37. doi: 10.1016/s1063-4584(97)80029-5. [DOI] [PubMed] [Google Scholar]
- 21.Pulai JI, Chen H, Im HJ, Kumar S, Hanning C, Hegde PS, Loeser RF. NF-{kappa}B Mediates the Stimulation of Cytokine and Chemokine Expression by Human Articular Chondrocytes in Response to Fibronectin Fragments. J Immunol. 2005;174:5781–5788. doi: 10.4049/jimmunol.174.9.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pulai JI, Del Carlo M, Jr, Loeser RF. The alpha5beta1 integrin provides matrix survival signals for normal and osteoarthritic human articular chondrocytes in vitro. Arthritis Rheum. 2002;46:1528–1535. doi: 10.1002/art.10334. [DOI] [PubMed] [Google Scholar]
- 23.Moreno-Manzano V, Ishikawa Y, Lucio-Cazana J, Kitamura M. Selective involvement of superoxide anion, but not downstream compounds hydrogen peroxide and peroxynitrite, in tumor necrosis factor-alpha- induced apoptosis of rat mesangial cells. J Biol Chem. 2000;275:12684–12691. doi: 10.1074/jbc.275.17.12684. [DOI] [PubMed] [Google Scholar]
- 24.Szabo C, Day BJ, Salzman AL. Evaluation of the relative contribution of nitric oxide and peroxynitrite to the suppression of mitochondrial respiration in immunostimulated macrophages using a manganese mesoporphyrin superoxide dismutase mimetic and peroxynitrite scavenger. FEBS Lett. 1996;381:82–86. doi: 10.1016/0014-5793(96)00087-7. [DOI] [PubMed] [Google Scholar]
- 25.Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci. 2003;60:6–20. doi: 10.1007/s000180300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakuma I, Stuehr DJ, Gross SS, Nathan C, Levi R. Identification of arginine as a precursor of endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1988;85:8664–8667. doi: 10.1073/pnas.85.22.8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie DL, Hui F, Meyers R, Homandberg GA. Cartilage chondrolysis by fibronectin fragments is associated with release of several proteinases: stromelysin plays a major role in chondrolysis. Arch Biochem Biophys. 1994;311:205–212. doi: 10.1006/abbi.1994.1228. [DOI] [PubMed] [Google Scholar]
- 28.Stanton H, Ung L, Fosang AJ. The 45 kDa collagen-binding fragment of fibronectin induces matrix metalloproteinase-13 synthesis by chondrocytes and aggrecan degradation by aggrecanases. Biochem J. 2002;364:181–190. doi: 10.1042/bj3640181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiarugi P, Pani G, Giannoni E, Taddei L, Colavitti R, Raugei G, Symons M, Borrello S, Galeotti T, Ramponi G. Reactive oxygen species as essential mediators of cell adhesion: the oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion. J Cell Biol. 2003;161:933–944. doi: 10.1083/jcb.200211118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henrotin Y, Deby-Dupont G, Deby C, De Bruyn M, Lamy M, Franchimont P. Production of active oxygen species by isolated human chondrocytes. Br J Rheumatol. 1993;32:562–567. doi: 10.1093/rheumatology/32.7.562. [DOI] [PubMed] [Google Scholar]
- 31.Studer R, Jaffurs D, Stefanovic-Racic M, Robbins PD, Evans CH. Nitric oxide in osteoarthritis. Osteoarthritis Cartilage. 1999;7:377–379. doi: 10.1053/joca.1998.0216. [DOI] [PubMed] [Google Scholar]
- 32.Hiran TS, Moulton PJ, Hancock JT. Detection of superoxide and NADPH oxidase in porcine articular chondrocytes. Free Radic Biol Med. 1997;23:736–743. doi: 10.1016/s0891-5849(97)00054-3. [DOI] [PubMed] [Google Scholar]
- 33.Tiku ML, Shah R, Allison GT. Evidence linking chondrocyte lipid peroxidation to cartilage matrix protein degradation: Possible role in cartilage aging and the pathogenesis of osteoarthritis. J Biol Chem. 2000;275:20069–20076. doi: 10.1074/jbc.M907604199. [DOI] [PubMed] [Google Scholar]
- 34.Day BJ, Fridovich I, Crapo JD. Manganic porphyrins possess catalase activity and protect endothelial cells against hydrogen peroxide-mediated injury. Arch Biochem Biophys. 1997;347:256–262. doi: 10.1006/abbi.1997.0341. [DOI] [PubMed] [Google Scholar]
- 35.Werner E, Werb Z. Integrins engage mitochondrial function for signal transduction by a mechanism dependent on Rho GTPases. J Cell Biol. 2002;158:357–368. doi: 10.1083/jcb.200111028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen YR, Shrivastava A, Tan TH. Down-regulation of the c-Jun N-terminal kinase (JNK) phosphatase M3/6 and activation of JNK by hydrogen peroxide and pyrrolidine dithiocarbamate. Oncogene. 2001;20:367–374. doi: 10.1038/sj.onc.1204105. [DOI] [PubMed] [Google Scholar]
- 37.Reboul P, Pelletier JP, Tardif G, Cloutier JM, Martel-Pelletier J. The new collagenase, collagenase-3, is expressed and synthesized by human chondrocytes but not by synoviocytes. A role in osteoarthritis. J Clin Invest. 1996;97:2011–2019. doi: 10.1172/JCI118636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitchell PG, Magna HA, Reeves LM, Lopresti-Morrow LL, Yocum SA, Rosner PJ, Geoghegan KF, Hambor JE. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97:761–768. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Billinghurst RC, Wu W, Ionescu M, Reiner A, Dahlberg L, Chen J, van Wart H, Poole AR. Comparison of the degradation of type II collagen and proteoglycan in nasal and articular cartilages induced by interleukin-1 and the selective inhibition of type II collagen cleavage by collagenase. Arthritis Rheum. 2000;43:664–672. doi: 10.1002/1529-0131(200003)43:3<664::AID-ANR24>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]


