Abstract
Contemporary phytase research is primarily concerned with ameliorating the problem of inadequate digestion of inositol hexakisphosphate (phytate; InsP6) in monogastric farm animal feed, so as to reduce the pollution that results from the high phosphate content of the manure. In the current study we pursue a new, safe and cost-effective solution. We demonstrate that the rate of hydrolysis of InsP6 by recombinant avian MINPP (0.7 μmol/mg protein/min) defines it as by far the most active phytase found to date in any animal cell (the corresponding activity of recombinant mammalian MINPP is only 0.006 μmol/mg protein/min). Although avian MINPP has less than 20% sequence identity with microbial phytases, we create a homology model of MINPP in which it is predicted that the structure of the phytase active site is well-conserved. This model is validated by site-directed mutagenesis and by use of a substrate analogue, scyllo-InsP6, which we demonstrate is only a weak MINPP substrate. In a model chicken cell line, we overexpressed a mutant form of MINPP that is secretion-competent. This version of the enzyme was actively secreted without affecting either cell viability or the cellular levels of any inositol phosphates. Our studies offer a genetic strategy for greatly improving dietary InsP6 digestion in poultry.
Keywords: Phytase, InsP6, MINPP, Chickens
1. Introduction
The phytases hydrolyze InsP6 to less phosphory-lated inositol phosphates. These enzymes, when categorized by differences in catalytic mechanisms, fall into four different groups: the “purple acid phosphatase” family, β-propeller phytases, “P-loop” phytases and histidine acid phosphatases (Oh et al., 2004; Lei and Porres, 2003; Mullaney and Ullah, 2003; Chu et al., 2004). The latter is the largest family, which is designated by the RHGXRXP active site. However, there are many histidine acid phosphatases that have little or no activity against InsP6.
All of the cloned enzymes that are widely-recognized to be phytases originate from plants and micro-organisms (Oh et al., 2004; Lei and Porres, 2003; Mullaney and Ullah, 2003). Animal cells do contain InsP6 (Shears, 2001), and an InsP6-phosphatase, namely, the multiple inositol polyphosphate phosphatase, or MINPP (Craxton et al., 1997; Chi et al., 1999). However, mammalian MINPP has very weak activity against InsP6. For example, the rat enzyme metabolizes InsP6 at a rate of only approximately 6 nmol/mg protein/min (Nogimori et al., 1991). In contrast, phytases from a range of micro-organisms have between 103-fold and 104-fold higher specific activities (Wyss et al., 1999a; Mullaney et al., 2002). Furthermore, phylogenetic analysis (Chi et al., 1999) has indicated that MINPP is not a close relative of genuine phytases. Thus, there is a consensus in the recent literature that animal cells do not contain enzymes that are physiologically active and specific phytases (Lei and Porres, 2003; Mullaney and Ullah, 2003; Simon and Igbasan, 2002).
Current research in the phytase field is dominated by efforts to safely and economically improve the digestion of InsP6 in the diets of poultry, pigs, and other monogastrics (Mullaney and Ullah, 2003). This situation has arisen because soybeans, grains and other plant seeds are used as the primary protein source in the animal feed (Mullaney et al., 2000). Approximately 80% of the organic phosphate in these seeds resides in InsP6, but the digestive lumen of these animals contains little or no phytase activity (Abelson, 1999; Mullaney et al., 2000). Thus, nutritional requirements necessitate that inorganic phosphate be added to the feed. This use of phosphate is accelerating consumption of the planet’s non-renewable reserves of this material. Moreover, excess inorganic phosphate that is excreted in the manure, plus undigested phytate, is washed off the farmland to imperil adjacent waterways with eutrophication (Abelson, 1999; Mullaney et al., 2000; Correll, 1999); this situation has been called the planet’s “phosphate crisis” (Abelson, 1999). It was recently estimated that even if this waterway pollution could be stopped immediately, the restoration of oligotrophy in contaminated aquatic systems might take more than 1000 years to achieve (Carpenter, 2005). Thus, under legislative pressure, poultry diets now include crude preparations of microbial phytase (Mullaney et al., 2000). Unfortunately, phytase supplementation adds a substantial expense to the animal feed (Bosch et al., 1998). There is also the problem that the widespread use of fungal phytases is increasingly promoting occupational asthma and rhinitis (Baur et al., 2002). Phytase also deteriorates during its exposure to the high temperatures involved in feed processing, limiting its effectiveness (Oh et al., 2004; Haefner et al., 2005; Ullah et al., 2000).
Recently, one group (Golovan et al., 2001) took a novel approach to improving dietary InsP6 digestion. They created transgenic pigs that constitutively secrete microbial phytase from their salivary glands (Golovan et al., 2001). It was noted that the ongoing secretion of phytase directly into the digestive tract was more efficient at digesting InsP6 than the addition of phytase to feed (Golovan et al., 2001). However, the expression of microbial genes in animals does present potential problems. Paramount among these is the need to convince a skeptical public that the “foreign” protein will not be deleterious to human health (Ward, 2001). One of the concerns frequently expressed by opponents of genetic manipulation technologies is the possibility that a novel protein present in the transgenic organism might possess allergenic properties (Ward, 2001). This is one of the reasons that these transgenic pigs have not yet been commercially exploited (Forsberg et al., 2003), despite the potential financial benefits of eliminating dietary phytase supplementation.
In the current study we demonstrate that chicken MINPP is an unexpectedly active phytase. Our results offer the proposal that genetically-modified poultry that constitutively secrete MINPP into their digestive tract could show improved digestion of dietary InsP6. Importantly, this can be achieved without expressing a “foreign” protein in the animals.
2. Methods
2.1. Materials
Na–InsP6 was purchased from Sigma. The pentamagnesium InsP6 salt was prepared as previously described (Torres et al., 2005). The scyllo-InsP6 was a gift from Dr. Max Tate at the University of Adelaide, Australia. Radiochemicals were purchased from Perkin-Elmer Life Sciences.
2.2. Culture of leghorn male hepatoma (LMH) cells
The LMH cells (ATCC #CRL-2117) were maintained in DMEM medium (Gibco-BRL) supplemented with 10% fetal bovine serum, 100I U/ml penicillin (Gibco-BRL), and 100 μg/ml streptomycin (Gibco-BRL). Cultures were incubated at 37 °C in a humidified atmosphere containing 5% CO2. One day prior to transfection with MINPP, cells were seeded into 60 mm dishes at a density of 0.8 × 106 to 1 × 106 cells/dish. In some experiments, cells were radiolabeled with [3H]inositol (10 μCi/ml) for 4 days.
2.3. Vector constructions, site-directed mutagenesis
cDNA encoding residues 19–445 of avian MINPP or residues 31–483 of human MINPP (i.e. lacking the N-terminal ER targeting sequences and the C-terminal ER retention signals (Romano et al., 1998; Caffrey et al., 1999)) were amplified by PCR and cloned into pGEM T-vector (Promega) and transformed into E. coli TOP 10F′ to screen for positive colonies. Avian and human MINPPcDNAs were introduced into the Pichia pastoris expression vector pPICZαA (Invitrogen) at, respectively, the EcoR I and Xba I sites, and the EcoR I and Kpn I sites. These genes were under the control of AOX1 promotor, led by a signal peptide α-factor and was in-frame with the C-terminal poly[His] epitope tag. Site-directed mutagenesis (QuikChangeTM; Stratagene) was performed using the avian MIPP plasmid as template, with the following sense primers (antisense were complements): T27G (5′-GGCGGCTACTTCGGCGGCAAGTCCCGCTA-CGAG-3′), Q78A (5′-CTACCCCACGGCCGGGGC-AATCCGCCGCCTGGCC-3′ (mutagenic residues underlined)). Constructs were transformed into E. coli TOP10F′ that was plated on LB medium containing 25 μg/ml zeocin to select positive colonies to prepare DNA for yeast transformation.
2.4. Expression and purification of recombinant MINPP in yeast
P. pastoris X33 (Invitrogen) was grown in YPD medium and transformed by electroporation (1.5 kV, 25 μF, 200 Ω; ECM 630 Electro Cell Manipulator, Genetronics, San Diego, CA) with 5 μg of plasmid DNA linearized using Sac I restriction enzyme. After incubation for 3 h at 30 °C in 1 M sorbitol without agitation, cells were plated on YPD medium with 200 μg zeocin/ml to screen integration of the transformed gene into the 5′AOX1 region of the host chromosomal DNA. After 3 days, transformants were incubated in Invitrogen’s BMGY media (contains 0.5%, v/v, glycerol) for 24 h. After the culture reached a density of about 3 × 108 cells/ml, the cells were transferred to Invitrogen’s BMMY media (contains 0.5%, v/v, methanol) for 5 days to induce protein expression. The culture medium containing secreted MINPP was centrifuged (6000 × g; 30 min; 4 °C) to remove cells, and then protein in the supernatant was precipitated with ammonium sulfate (70% saturation). The pellet was dissolved in 50 mM Tris–HCl (pH 8.0) and dialyzed overnight, before purification on a Ni-sepharose column (1.5 cm × 8.5 cm; Amersham Bioscience) using an imidazole gradient generated with an ÄKTA FPLC system (Amersham Pharmacia Biotech). Fractions exhibiting highest activities were pooled, dialyzed against 50 mM Tris–HCl (pH 7.4), and stored at −80 °C until required. Where indicated, deglycosylation of MINPP was achieved upon its incubation with 0.3 units of endoglycosidase Hf for 2 h at 37 °C according to the manufacturer’s instructions (New England Biolabs, Beverly, MA).
2.5. Expression of MINPP in LMH cells
cDNA encoding residues 1–445 avian MINPP was amplified by PCR, digested with Hind III and Xba I, and ligated into the corresponding sites of mammalian expression vector, pcDNA 3.1(+) (Invitrogen) driven by the cytomegalovirus (CMV) promoter and containing the neomycin resistance gene. The construct were used to transiently transfect LMH cells using FuGENE 6 (Roche Molecular Biochemicals) at 70–80% cell confluency. Transfection efficiencies were monitored by cotransfecting the pRL-TK plasmid (Promega) and measuring the Renilla luciferase activity. Cell viability of transfected cells was examined using a cell counting Kit-8 (Donjindo, Tokyo, Japan).
2.6. Enzyme assays
Hydrolysis of 1 mM InsP6 by recombinant MINPP expressed in P. pastoris was measured at 40 °C by assaying inorganic phosphate release in 0.1–0.2 ml 50 mM acetate buffer (pH 5.0; unless otherwise stated). Phytase activity in LMH culture medium, and in cell lysates (prepared as described by Deleu et al., 2006), was analyzed 48 h after transfection with MINPP (see above). Assays were performed for 2 h at 40 °C using 0.5 mM [3H]InsP6; reactions were analyzed by HPLC (Chi et al., 2000) using a 250 mm × 4.6 mm Synchropak Q100 column connected to a 500TR Flow Scintillation analyzer (Packard Instruments) with a 1:3, v/v, mixture of HPLC eluate: scintillation fluid (MonoFlow 4; National Diagnostics). Data were processed using Flo-1 for Windows (v 3.61; background correction = 5 C.P.M. curve smoothing = 11-point), and then exported into SigmaPlot (v 8.0) as an ASCII file. Total Pi release (i.e. phytase activity) was estimated from the concentrations of the InsP6 substrate and InsPn products.
2.7. Inositol phosphates in LMH cells
Following quenching of [3H]inositol-labeled LMH cells with perchoric acid as previously described (Shears, 1997; Chi et al., 2000) the levels of [3H]inositol phosphates were determined by HPLC using a 250 mm × 4.6 mm Synchropak Q100 column; fractions of 1 ml were collected and mixed with 3 ml MonoFlow 4 scintillation fluid prior to counting. Data were normalized to the level of [3H]inositol lipid in each cell extract (Shears, 1997).
3. Results and discussion
3.1. Expression and characterization of recombinant avian MINPP
It is difficult to obtain high yields of pure recombinant MINPP by heterologous expression in either E. coli (Chi et al., 1999; Caffrey et al., 1999) or in insect cells (data not shown); this has previously hindered detailed MINPP studies. We have now expressed avian MINPP (Romano et al., 1998) in the methylotrophic yeast, P. pastoris, with the addition of a C-terminal poly[His] epitope tag (Fig. 1A). The success of this expression system is based upon a strong, tightly regulated alcohol oxidase (AOX1) promoter. High yield of protein is also aided by the fact that the yeast can be grown to high cell densities in inexpensive media using methanol as the sole carbon source. The recombinant avian MINPP that was secreted into the culture media was purified by Ni-sepharose affinity chromatography.
Fig. 1.

Characteristics of recombinant MINPP. (A) Schematic of MINPP constructs used in this study: top, wild-type MINPP; middle, ΔADEL-MINPP construct expressed in LMH cells; bottom, recombinant MINPP expressed in P. pastoris. (B and C) SDS-PAGE of recombinant avian MINPP was carried out using an Xcell II Mini-Cell and 4–12% NuPage Bis-Tris gels (Invitrogen) according to manufacturer’s recommendations. Proteins were visualized with SimplyBlueSafeStain (Invitrogen). In panel C, the left-hand lane shows endoglycosidase alone, the right-hand lane shows endoglycosidase and MINPP. (D) Phytase activity of avian MINPP before and after endoglycosidase treatment. (E) pH profile of phytase activity by MINPP using the following buffers: 50 mM glycine–HCl (pH 3); 50 mM sodium acetate (pH 4–5); 50 mM Bis-Tris–HCl (pH 6–7); 50 mM Tris–HCl (pH 7.4–8.5). (F) Phytase activities of MINPP and A. niger (ficuum) phytase against Mg- and Na-salts of InsP6. (G) HPLC profile of [Mg2+][3H]InsP6 (20 μM) at zero time (dotted line) and the end-point (solid line) after overnight incubation with 35 μg MINPP.
Native rat MINPP (Nogimori et al., 1991) and recombinant human MINPP (Table 1) only hydrolyze InsP6 at a very slow rate: 6 nmol/mg protein/min. It was an unexpected observation that recombinant avian MINPP has >100-fold higher phytase activity than its mammalian counterpart (Table 1). SDS-PAGE analysis of the recombinant enzyme revealed a single, diffuse band with an apparent mean size of 64 kDa (Fig. 1B), somewhat larger than the predicted size of 50.7 kDa for our MINPP construct. This difference is apparently due to glycosylation, since endoglycosidase reduced the apparent size of the phosphatase to a relatively sharp band of 48 kDa (Fig. 1C). Glycosylation did not have a significant impact upon MINPP activity (Fig. 1B–D).
Table 1.
InsP6 phosphatase (phytase) activity of MINPP
| Enzyme | Km (μM) | Vmax (nmol/mg/min) |
|---|---|---|
| W.T. avian MINPP | 140 ± 12 | 715 ± 31 |
| Q78A avian MINPP | 60 ± 2 | 625 ± 13 |
| T27G avian MINPP | 92 ± 2 | 30 ± 0.4 |
| W.T. human MINPP | 90 ± 1 | 6.2 ± 0.4 |
The kinetic parameters are for the hydrolysis of Mg–InsP6. These data were determined as described in Section 2. Most previous studies have used Na–InsP6 as a substrate. Thus, for comparative purposes, we determined the kinetic parameters of wild-type enzyme against Na–InsP6: the Km value was 200 ± 6 μM. The Vmax value was 501 ± 14 nmol/mg/min. All data represent means and standard errors from four experiments.
Monogastric animals, such as chickens, cannot hydrolyze dietary InsP6 (see Section 1). However, the data described above indicate that avian cells do contain an active phytase. This raises the possibility that a genetically-modified chicken that secreted MINPP into the digestive tract might then be able to digest InsP6. Several additional MINPP traits suggest the enzyme could be well-suited to this task: one factor is the pH profile of MINPP, which showed near-maximal activities over a fairly broad range of pH 4.5–7.5 (Fig. 1E). This closely matches the pH of the chicken’s digestive tract (pH 4.5–7.5 (Herpol and van Gembergen, 1967; Faner, 1942; Winget et al., 1962), with the exception of pH 3.5 for the proventriculus (Winget et al., 1962)). Second, a recent and comprehensive re-evaluation of the nature of soluble InsP6 in biological systems has concluded that the pentamagnesium salt is the most biologically-relevant form of InsP6 (Torres et al., 2005). We routinely used Mg–InsP6 in our assays, after determining that MINPP was 25% more active against Mg–InsP6 compared to Na–InsP6 (Fig. 1F; Table 1). The opposite activity profile was shown by phytase from A. niger (ficuum) (Fig. 1F), one of the commercial poultry feed additives (Haefner et al., 2005). While avian MINPP has a lower specific activity than does A. niger (ficuum) phytase (Fig. 1F) it should be noted that when the latter is processed as a feed additive, which involves temperatures as high as 80–100 °C, more than 90% of activity may be lost (Oh et al., 2004; Haefner et al., 2005; Ullah et al., 2000).
A third criterion for an effective phytase is the end-point of its hydrolytic action. This is variable. Some microbial phytases convert InsP6 to InsP3 (Haefner et al., 2005), although others yield InsP1 (Wyss et al., 1999a) (i.e. five of the six phosphates in InsP6 are liberated). Our HPLC analysis revealed that MINPP converted 80% of InsP6 to InsP1 (Fig. 1G). Finally, the activity of MINPP towards some other organic phosphates, relative to InsP6, was as follows: para-nitrophenylphosphate = 17 ± 0.4%, ATP = 11 ± 1%, ribose 1-phosphate = 0.3 ± 0.01%. Microbial phytases show similar substrate specificity (Wyss et al., 1999a).
3.2. Homology modeling of MINPP
By determining the structural and functional relationships that exist between proteins from different organisms, we can gain insight into their evolutionary origins and physiological roles. We reasoned that insight into the three-dimensional structure of MINPP might rationalize its unexpectedly-high phytase activity. We searched for potential structural templates upon which to model MINPP using Gen-THREADER (http://bioinf.cs.ucl.ac.uk). Microbial phytases were the most significant templates to emerge from the pairwise alignments in the Gen-THREADER screen, but even then, their overall sequence identity with MINPP was not more than 17%, which falls into a so-called “twilight zone” that can thwart homology modeling. Nevertheless, three candidate structural templates (PDB entries 1IHP, 1QFX and 1QWO; Fig. 2A) were validated in a multiple sequence alignment arranged using T-COFFEE (http://igs-server.cnrs-mrs.fr/~cnotred/Projects_home_page/t_coffee_home_page.html); this comprised five microbial phytases, plus avian MINPP, and nine homologes of MINPP from mammals, Drosophila and Anopheles (not shown). There was a high degree of concordance between the T-COFFEE alignment of avian MINPP, 1IHP, 1QFX and 1QWO, as compared to each of the GenTHREADER pairwise alignments. For example, of the 16 secondary structure elements in 1QFX which are 5 or more residues in length, 13 were aligned to MINPP in precisely the same manner by both T-COFFEE and GenTHREADER. At these aligned positions, the aminoacid side-chains in MINPP could be modeled onto our consensus template backbone (Fig. 2B). Loops between secondary structural elements were modeled using one of the template structures where possible. The InsP6 substrate was modeled by rigid superposition of the coordinates of phytase from E. coli in complex with InsP6 (PDB entry 1DKP) onto those of 1IHP, based on structural alignment of their α/β domains. This modeling approach indicates that the MINPP active site (Fig. 2B) is strikingly similar to that of microbial phytases, despite the low sequence identity.
Fig. 2.

Homology modeling of the catalytic domains of avian MINPP. (Panel A) Regions within MINPP that comprise the putative catalytic domain are shown here aligned with phytases from Aspergillus ficuum (niger) (1IHP), Aspergillus fumigatus (1QWO) and acid phosphatase from Aspergillus niger (1QFX). Fully conserved residues in the alignment are marked with a cross. These alignments, which were independently obtained using either GenTHREADER or T-COFFEE, were the basis for constructing a model of the active site of MINPP with bound phytate using SwissPDBViewer (Panel B). The latter model predicts the residues in MINPP that interact with the InsP6 substrate; these are in bold type in the panel A alignment. The scissile 3-phosphate is coordinated by R68, H69, R72, R156 and H332. T27 and K28 contact the 4-phosphate. Q78 and K282 contact the 5-phosphate, K154 contacts the 1-phosphate, and K286 contacts the 6-phosphate.
The proposed MINPP catalytic site comprises a catalytic cleft at the interface between a large α/β domain and a small α domain (Fig. 2B); this is a highly-conserved structural feature of phytases (Kostrewa et al., 1997). We identified 11 putative MINPP active site residues that interact with InsP6 (Fig. 2B). Six of these (R68, H69, R72, K154, R156 and H332) reside in the α/β domain; three more are present in the α domain (Q78, K282 and K286). The conservation in MINPP of the phytase α-domain architecture is significant, because it is this domain, and its basic aminoacid residues, that imposes phytase specificity upon the otherwise promiscuous RHGXRXP histidine acid phosphatase motif (Kostrewa et al., 1997). Moreover, A. niger (ficuum) phytase (1IHP) also distinguishes itself from other histidine acid phosphatases by having an N-terminal lid with which it closes the catalytic site (Kostrewa et al., 1997); a similar structure is predicted to be present in MINPP (containing T27 and K28; Fig. 2B). Finally, our 3D-model indicated that three of the five disulfide bridges in 1IHP (C192–C442; C48–C391; C241–C259), which impart stability upon the phytase (Mullaney and Ullah, 2005), are also conserved in our homology model of MINPP (data not shown).
3.3. Verification of the homology model
There are data in the literature obtained by site-directed mutagenesis that are consistent with the model structure depicted in Fig. 2B. First, two of the predicted avian MINPP active site residues (H69, H332, Fig. 2) are conserved in mammalian MINPPs; earlier studies showed that these residues are catalytically essential (Chi et al., 1999; Caffrey et al., 1999). Second, studies with phytases (Kostrewa et al., 1997) have shown the importance to catalysis of three other residues that are conserved in the MINPP active site (R68, R72, R156; Fig. 2A and B). In addition to these five residues, we targeted two additional, putative substrate-binding residues in MINPP (T27, Q78; Fig. 2B) that are not conserved in phytases (Fig. 2A). A T27G mutant of MINPP lost 96% of its activity towards InsP6 (Table 1) and the other, weakly metabolized inorganic phosphates (data not shown). As well as verifying our prediction by homology modeling (Fig. 2B) that T27 is catalytically important, this observation formally demonstrates that high phytase activity is truly an inherent property of MINPP, and not due to an inadvertent contaminant in our recombinant enzyme preparations. A Q78A mutation showed a 13% lower Vmax, but a two-fold greater substrate affinity (Table 1); overall the catalytic efficiency (Vmax/Km) was doubled. The Q78A mutant also showed some differences in its specificity profile compared to wild-type enzyme: relative to InsP6, the activity towards some other organic phosphates was as follows: para-nitrophenylphosphate = 7 ± 0.2% (2.4-fold less than wild-type, see above), ATP = 12 ± 0.1% (same as wild-type), ribose 1-phosphate = 3 ± 0.05% (10-fold higher than wild-type). Thus, Q78 makes subtle but nevertheless important contributions to both activity and specificity. Interestingly, Q78 (Fig. 2) aligns with one of the residues in A. niger (ficuum) phytase known to impart substrate specificity, namely, K68 (Kostrewa et al., 1997). Two additional A. niger (ficuum) phytase residues that define its specificity, K71 and K277 (Kostrewa et al., 1997), align with R81 and K282 in MINPP (Fig. 2).
Finally, our homology model makes a prediction about the relative ease of access of scyllo-InsP6 and myo-InsP6 to the active site. In myo-InsP6 the 2-phosphate group is axial to the plane of the inositol ring. In scyllo-InsP6 this group is equatorial, and it is predicted to sterically clash with H332. We tested this prediction by incubating MINPP with scyllo-InsP6. The latter is indeed a poor substrate: the value for the Km was 1740 ± 360 μM (12-fold lower affinity than that for myo-InsP6; Table 1). The value of the Vmax for scyllo-InsP6 was 90 ± 4 nmol/mg protein/min (eight-fold lower than that for myo-InsP6; Table 1). These data further validate our homology model of the MINPP active site. Microbial phytases have also been shown to only weakly hydrolyze scyllo-InsP6 (Cosgrove, 1966).
3.4. Overexpression of a secretion-competent MINPP construct
The above results raise the possibility that genetically-modified poultry that constitutively secrete MINPP into their digestive tract could effectively hydrolyze dietary InsP6. Yet, MINPP is intracellular; is there a means by which we can force it to be secreted from cells? The answer to this question arises from the observation that, in most animal cells, MINPP resides inside the endoplasmic reticulum (ER) (Ali et al., 1993; Chi et al., 1999).
Although ER lumen residents tend to exit from this organelle in the bulk flow, they are efficiently retrieved from a pre-Golgi compartment and returned to the ER (Wilson et al., 1993). This retrograde transport process is driven by a specific receptor, ERD2, which recognizes the canonical C-terminal tetrapeptide of the ER resident, XDEL (Wilson et al., 1993). We examined if a truncated form of MINPP, from which just this C-terminal tetrapeptide was eliminated (ΔADEL-MINPP), might permit it to escape the ER and enter the secretory pathway. For these experiments, we transiently transfected the immortalized avian hepatocellular carcinoma (LMH) cell line (Kawaguchi et al., 1987) with ΔADEL-MINPP, under the control of the CMV promoter.
We found that, 48 h after transfection with MINPP, intracellular phytase activity increased several-fold above that in control cells (Fig. 3B). However, it was the extracellular medium from the cultures of ΔADEL-MINPP transfected cells that contained the majority of total phytase activity (Fig. 3B). In contrast, no extracellular phytase activity was observed in medium taken from vector-transfected cells (Fig. 3B), consistent with earlier demonstrations that wild-type, avian MINPP is not normally secreted (Romano et al., 1998). A representative analysis of the products of InsP6 dephosphorylation is shown in Fig. 3C. Within the experimental time-frame, little InsP5 accumulated, but considerable quantities of InsP4 and InsP3 were detected. Thus, the transfection led to considerable overexpression of ΔADEL-MINPP which, in the absence of an ER-retention signal, efficiently entered the secretory pathway. There was no detectable effect of transfection with ΔADEL-MINPP upon cell viability (Fig. 3A) indicating that release of MINPP into the culture medium is not due to cell lysis.
Fig. 3.
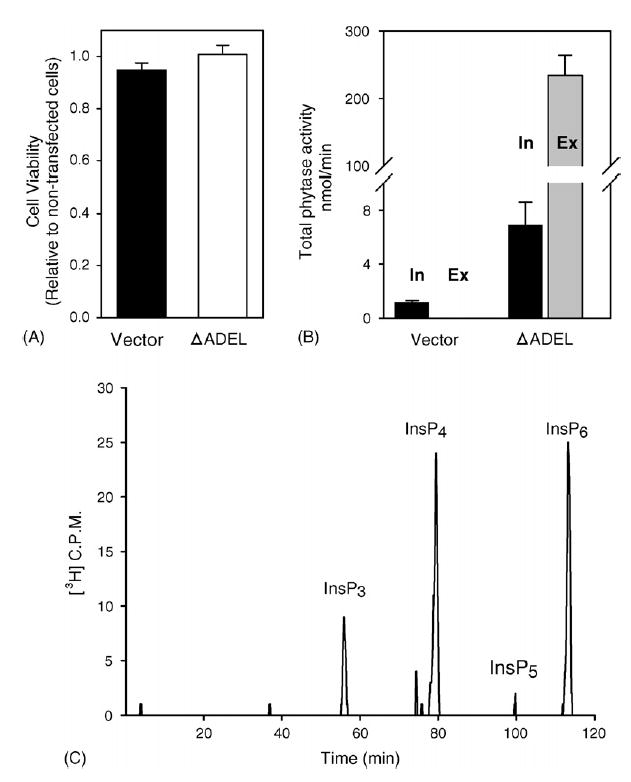
Transient expression of ΔADEL-MINPP in LMH cells. Cells were assayed 48 h after transfection with either vector or ΔADEL-MINPP (2 μg cDNA). (A) Cell viability (means and standard errors from four experiments). (B) Total intracellular (‘In’, black bars) and extracellular (‘Ex’, gray bars) phytase activity. Note that no phytase activity was detected in the extracellular medium used to culture vector-transfected cells. Data are means and standard errors from four experiments. (C) Aliquots (30 μl) of culture medium from cells in which MINPP was overexpressed were assayed for phytase activity as described in Section 2. A representative experiment is shown, typical of 8.
In view of the high phytase activity of MINPP (Fig. 3), it was important to determine if there was any effect upon cellular levels of its substrates, as the enzyme migrated through the secretory pathway. We therefore analyzed the effects of ΔADEL-MINPP upon the entire inositol phosphate spectrum in LMH cells (Fig. 4A). This was achieved by first labeling the cells with [3H]inositol, and then using HPLC to resolve the various cellular [3H]inositol phosphates. We found an inositol phosphate profile that was typical of that seen in numerous, earlier experiments with mammalian cells (Shears, 1997): relatively high levels of InsP1, InsP5 and InsP6, with much lower levels of other isomers (Fig. 4A). [3H]-labeled standards were used to assign InsP5 as the 1,3,4,5,6-isomer, and to define Ins(1,4,5)P3, Ins(1,3,4)P3 and Ins(1,4,5,6)P4.
Fig. 4.
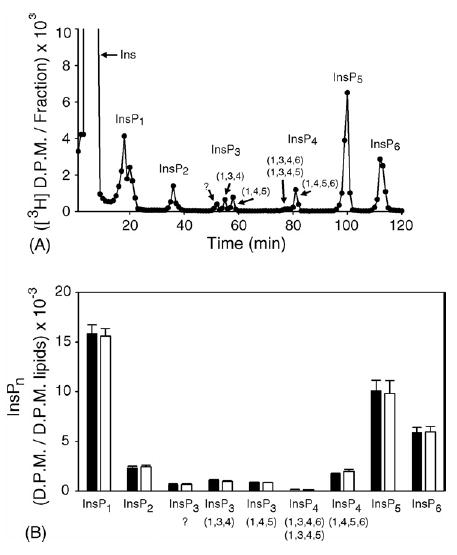
Effect of ΔADEL-MINPP upon the inositol phosphate profile in LMH cells (Panel A) [3H]inositol-radiolabeled cells were assayed 48 h after transfection with vector alone (2 μg cDNA). The question mark above the InsP3 peak indicates its identity was unknown. (Panel B) Levels of inositol phosphates in cells that were transfected with either vector (black bars) or ΔADEL-MINPP (2 μg cDNA; white bars). Data represent means and standard errors from four experiments.
We next found that, while in transit through the membrane-delimited secretory pathway, ΔADEL-MINPP remained insulated from cytosolic InsP6 (Fig. 4B). In fact, the entire inositol phosphate profile was unaffected by overexpression of ΔADEL-MINPP (Fig. 4B). This result is valuable because it shows that avian cells can be made to secrete considerable quantities of MINPP without detriment to cell function. In addition, these data are of biological interest, because it has been a point of some debate as to the conditions under which MINPP might normally access its inositolphosphate substrates. The two alternate possibilities have been that either the endoplasmic reticulum can become permeable to inositol phosphates under certain conditions, or that MINPP can somehow be released from the endoplasmic reticulum. The most obvious escape route is for MINPP to move from the endoplasmic reticulum to the Golgi network, but as a consequence of the data in the current report, we can see that would not, by itself, increase access of the enzyme to its substrates. These data indicate that changes in MINPP expression cannot acutely regulate the turnover of InsP5 and InsP6 in intact cells.
3.5. General conclusions
In this study, we have shown that avian MINPP is a considerably more active phytase than its mammalian homologues (Table 1). Although MINPP shares less than 20% sequence identity with phytases, homology modeling of MINPP (Fig. 2) predicts that the enzyme has a conserved, phytase-like active site. It is therefore possible that there is a much stronger evolutionary link between microbial phytases and avian MINPP than has previously been appreciated. The latter conclusion is consistent with the concept that protein structures are typically more highly conserved than are their aminoacid sequences (Sánchez and Sali, 1998). However, it is equally plausible that the functional similarities between MINPP and microbial phytases is a consequence of convergent evolution.
Our studies with avian MINPP represent the first molecular characterization of an active phytase in any animal. Yet, even in cells in which avian MINPP activity was genetically increased five- to six-fold (Fig. 3), and even though MINPP left the confines of the endoplasmic reticulum and moved through the secretory pathway, there was no effect upon cellular levels of any inositol phosphates (Fig. 4). This is a striking demonstration of the inability of MINPP to acutely regulate any of the signaling activities that have been attributed to Ins(1,3,4,5,6)P5 and InsP6. While this is also the conclusion to arise from earlier work with the mammalian enzyme (Chi et al., 2000), it is especially striking in the case of avian MINPP because the latter has much greater catalytic activity than its mammalian counterpart. This impermeability of the secretory pathway to cytosolic inositol phosphates also rationalizes why overexpression of a secreted microbial phytase in the salivary glands of pigs was without apparent detriment to salivary gland function (Forsberg et al., 2003; Golovan et al., 2001).
Finally, our data lead us to propose that a genetically-modified chicken that expresses ΔADEL-MINPP in intestinal epithelial cells would deliver significant quantities of phytase activity into the digestive tract. The specific activity of recombinant avian MINPP (Table 1) is 30–300-fold lower than that of recombinant microbial phytases (Wyss et al., 1999b; Mullaney et al., 2002). However, this comparison overstates the difference in specific activities at the site of action (the digestive tract), because up to 90% of dietary phytase activity is lost due to thermal inactivation during feed processing (Oh et al., 2004; Haefner et al., 2005; Ullah et al., 2000). Moreover, it has been shown that, at least in pigs, constitutive secretion of phytase directly into the digestive tract provides a more efficient InsP6-digesting capability as compared to adding phytase to the diet (Golovan et al., 2001). Our proposal to create a strain of genetically-modified chickens would also have the advantage of not incurring any persistent expenses, unlike the supplementation of chicken feed with either phytase, inorganic phosphate (Wodzinski and Ullah, 1996; Bosch et al., 1998), or even phytase-producing bacterial broth (Lan et al., 2002). Our strategy could improve conservation of the planet’s diminishing phosphate reserves, and a significant reduction in phosphate-mediated waterway pollution could also be anticipated. This genetic approach would also offer a safe alternative option to farm workers who currently face the hazard of occupational asthma and rhinitis brought on by exposure to fungal phytases (Baur et al., 2002). Finally, as MINPP is endogenous to poultry, we would anticipate our proposal to be tolerated by those who believe it may be hazardous to introduce “foreign” genes into farm animals.
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
References
- Abelson PH. A potential phosphate crisis. Science. 1999;283:2015. doi: 10.1126/science.283.5410.2015. [DOI] [PubMed] [Google Scholar]
- Ali N, Craxton A, Shears SB. Hepatic Ins(1,3,4,5)P4 3-phosphatase is compartmentalized inside endoplasmic reticulum. J Biol Chem. 1993;268:6161–6167. [PubMed] [Google Scholar]
- Baur X, Melching-Kollmuss S, Koops F, Strassburger K, Zober A. IgE-mediated allergy to phytase—a new animal feed additive. Allergy. 2002;57:943–945. doi: 10.1034/j.1398-9995.2002.23702.x. [DOI] [PubMed] [Google Scholar]
- Bosch D, Zhu M, Kornegay ET. Net returns from microbial phytase when crop applications of swine manure are limited by phosphorus. J Prod Agric. 1998;11:205–213. [Google Scholar]
- Caffrey JJ, Hidaka K, Matsuda M, Hirata M, Shears SB. The human and rat forms of multiple inositol polyphosphate phosphatase: functional homology with a histidine acid phosphatase up-regulated during endochondral ossification. FEBS Lett. 1999;442:99–104. doi: 10.1016/s0014-5793(98)01636-6. [DOI] [PubMed] [Google Scholar]
- Carpenter SR. Eutrophication of aquatic ecosystems: bistability and soil phosphorus. Proc Natl Acad Sci USA. 2005;102:10002–10005. doi: 10.1073/pnas.0503959102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi H, Tiller GE, Dasouki MJ, Romano PR, Wang J, O’Keefe RJ, Puzas JE, Rosier RN, Reynolds PR. Multiple inositol polyphosphate phosphatase: evolution as a distinct group within the histidine phosphatase family and chromosomal location of the human and mouse genes to chromosomes 10q23 and 19. Genomics. 1999;56:324–336. doi: 10.1006/geno.1998.5736. [DOI] [PubMed] [Google Scholar]
- Chi H, Yang X, Kingsley PD, O’Keefe RJ, Puzas JE, Rosier RN, Shears SB, Reynolds PR. Targeted deletion of Minpp1 provides new insight into the activity of multiple inositol polyphosphate phosphatase in vivo. Mol Cell Biol. 2000;20:6496–6507. doi: 10.1128/mcb.20.17.6496-6507.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu HM, Guo RT, Lin TW, Chou CC, Shr HL, Lai HL, Tang TY, Cheng KJ, Selinger BL, Wang AH. Structures of Selenomonas ruminantium phytase in complex with per-sulfated phytate: DSP phytase fold and mechanism for sequential substrate hydrolysis. Structure. 2004;12:2015–2024. doi: 10.1016/j.str.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Correll DL. Phosphorus: a rate limiting nutrient in surface waters. Poult Sci. 1999;78:674–682. doi: 10.1093/ps/78.5.674. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. Synthesis of the hexaphosphates of myo-, scyllo-, neo-, and D-inositol. J Sci Fd Agric. 1966;17:550–554. [Google Scholar]
- Craxton A, Caffrey JJ, Burkhart W, Safrany ST, Shears SB. Cloning and expression of rat hepatic multiple inositol polyphosphate phosphatase. Biochem J. 1997;328:75–81. doi: 10.1042/bj3280075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleu S, Choi K, Pesesse X, Cho J, Sulis ML, Parsons R, Shears SB. Physiological levels of PTEN control the size of the cellular Ins(1,3,4,5,6)P(5) pool. Cell Signal. 2006;18:488–498. doi: 10.1016/j.cellsig.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Faner DS. The hydrogen concentration in avian digestive tracts. Poult Sci. 1942;21:33–38. [Google Scholar]
- Forsberg CW, Phillips JP, Golovan SP, Fan MZ, Meidinger RG, Ajakaiye A, Hilborn D, Hacker RR. The Enviropig physiology, performance, and contribution to nutrient management advances in a regulated environment: the leading edge of a change in the pork industry. J Anim Sci. 2003;81 (E Suppl 2):E68–E77. [Google Scholar]
- Golovan SP, Meidinger RG, Ajakaiye A, Cottrill M, Wiederkehr MZ, Barney DJ, Plante C, Pollard JW, Fan MZ, Hayes MA, Laursen J, Hjorth JP, Hacker RR, Phillips JP, Forsberg CW. Pigs expressing salivary phytase produce low-phosphorus manure. Nat Biotechnol. 2001;19:741–745. doi: 10.1038/90788. [DOI] [PubMed] [Google Scholar]
- Haefner S, Knietsch A, Scholten E, Braun J, Lohscheidt M, Zelder O. Biotechnological production and applications of phytases. Appl Microbiol Biotechnol. 2005:1–10. doi: 10.1007/s00253-005-0005-y. [DOI] [PubMed] [Google Scholar]
- Herpol C, van Gembergen G. La signification du pH dans le tube digestif de gallus domesticus. Ann Biol Anim Biochim Biophys. 1967;7:33–38. [Google Scholar]
- Kawaguchi T, Nomura K, Hirayama Y, Kitagawa T. Establishment and characterization of a chicken hepatocellular carcinoma cell line, LMH. Cancer Res. 1987;47:4460–4464. [PubMed] [Google Scholar]
- Kostrewa D, Grüninger-Leitch F, Broger C, D’Arcy A, van Loon APGM. Crystal structure of phytase from Aspergillus ficuum at 2.5 Å resolution. Nat Struct Biol. 1997;4:185–190. doi: 10.1038/nsb0397-185. [DOI] [PubMed] [Google Scholar]
- Lan GQ, Abdullah N, Jalaludin S, Ho YW. Efficacy of supplementation of a phytase-producing bacterial culture on the performance and nutrient use of broiler chickens fed corn–soybean meal diets. Poult Sci. 2002;81:1522–1532. doi: 10.1093/ps/81.10.1522. [DOI] [PubMed] [Google Scholar]
- Lei XG, Porres JM. Phytase enzymology, applications, and biotechnology. Biotechnol Lett. 2003;25:1787–1794. doi: 10.1023/a:1026224101580. [DOI] [PubMed] [Google Scholar]
- Mullaney EJ, Daly CB, Kim T, Porres JM, Lei XG, Sethumadhavan K, Ullah AHJ. Site-directed mutagenesis of Aspergillus niger NRRL 3135 phytase at residue 300 to enhance catalysis at pH 4.0. Biochem Biophys Res Commun. 2002;297:1016–1020. doi: 10.1016/s0006-291x(02)02325-2. [DOI] [PubMed] [Google Scholar]
- Mullaney EJ, Daly CB, Ullah AHJ. Advances in phytase research. Adv Appl Microbiol. 2000;47:157–199. doi: 10.1016/s0065-2164(00)47004-8. [DOI] [PubMed] [Google Scholar]
- Mullaney EJ, Ullah AH. The term phytase comprises several different classes of enzymes. Biochem Biophys Res Commun. 2003;312:179–184. doi: 10.1016/j.bbrc.2003.09.176. [DOI] [PubMed] [Google Scholar]
- Mullaney EJ, Ullah AH. Conservation of cysteine residues in fungal histidine acid phytases. Biochem Biophys Res Commun. 2005;328:404–408. doi: 10.1016/j.bbrc.2004.12.181. [DOI] [PubMed] [Google Scholar]
- Nogimori K, Hughes PJ, Glennon MC, Hodgson ME, Putney JW, Jr, Shears SB. Purification of an inositol (1,3,4,5)-tetrakisphosphate 3-phosphatase activity from rat liver and its substrate specificity. J Biol Chem. 1991;266:16499–16506. [PubMed] [Google Scholar]
- Oh BC, Choi WC, Park S, Kim YO, Oh TK. Biochemical properties and substrate specificities of alkaline and histidine acid phosphatases. Appl Microbiol Biotechnol. 2004;63:362–372. doi: 10.1007/s00253-003-1345-0. [DOI] [PubMed] [Google Scholar]
- Romano P, Wang J, O’Keefe RJ, Puzas JE, Rosier RN, Reynolds PR. HiPER1, a phosphatase of the endoplasmic reticulum with a role in chondrocyte maturation. J Cell Sci. 1998;111:803–813. doi: 10.1242/jcs.111.6.803. [DOI] [PubMed] [Google Scholar]
- Sánchez R, Sali A. Large-scale protein structure modeling of the Saccharomyces cerevisiae genome. Proc Nat Acad Sci USA. 1998;95:13597–13602. doi: 10.1073/pnas.95.23.13597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shears SB. Measurement of inositol phosphate turnover in intact cells and cell-free systems. In: Shears SB, editor. Signalling by Inositides: A Practical Approach. Oxford University Press; Oxford, UK: 1997. pp. 33–52. [Google Scholar]
- Shears SB. Assessing the omnipotence of inositol hexakisphosphate. Cell Signal. 2001;13:151–158. doi: 10.1016/s0898-6568(01)00129-2. [DOI] [PubMed] [Google Scholar]
- Simon O, Igbasan F. In vitro properties of phytases from various microbial sources. Int J Food Sci Technol. 2002;37:813–822. [Google Scholar]
- Torres J, Domínguez S, Cerdá FM, Obal G, Mederos A, Irvine RF, Dìaz A, Kremer C. Solution behaviour of myo-inositol hexakisphosphate in the presence of multivalent cations. Prediction of a neutral pentamagnesium species under cytosolic/nuclear conditions. J Inorg Biochem. 2005;99:828–840. doi: 10.1016/j.jinorgbio.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Ullah AH, Sethumadhavan K, Lei XG, Mullaney EJ. Biochemical characterization of cloned Aspergillus fumigatus phytase (phyA) Biochem Biophys Res Commun. 2000;275:279–285. doi: 10.1006/bbrc.2000.3271. [DOI] [PubMed] [Google Scholar]
- Ward KA. Phosphorus-friendly transgenics. Nat Biotechnol. 2001;19:415–416. doi: 10.1038/88064. [DOI] [PubMed] [Google Scholar]
- Wilson DW, Lewis MJ, Pelham HRB. pH-dependent binding of KDEL to its receptor in vitro. J Biol Chem. 1993;268:7465–7468. [PubMed] [Google Scholar]
- Winget CM, Ashton GC, Cawley AJ. Changes in gastrointestinal pH associated with fasting in the laying hen. Poult Sci. 1962;41:1115–1120. [Google Scholar]
- Wodzinski RJ, Ullah AH. Phytase. Adv Appl Microbiol. 1996;42:263–302. doi: 10.1016/s0065-2164(08)70375-7. [DOI] [PubMed] [Google Scholar]
- Wyss M, Brugger R, Kronenberger A, Remy R, Fimbel R, Oesterhelt G, Lehmann M, van Loon AP. Biochemical characterization of fungal phytases (myo-inositol hexakisphosphate phosphohydrolases): catalytic properties. Appl Environ Microbiol. 1999a;65:367–373. doi: 10.1128/aem.65.2.367-373.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss M, Pasamontes L, Friedlein A, Remy R, Tessier M, Kronenberger A, Middendorf A, Lehmann M, Schnoebelen L, Rothlisberger U, Kusznir E, Wahl G, Muller F, Lahm HW, Vogel K, van Loon AP. Biophysical characterization of fungal phytases (myo-inositol hexakisphosphate phosphohydrolases): molecular size, glycosylation pattern, and engineering of proteolytic resistance. Appl Environ Microbiol. 1999b;65:359–366. doi: 10.1128/aem.65.2.359-366.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


