Abstract
The integrase protein of bacteriophage λ (Int) catalyzes site-specific recombination between λ phage and Escherichia coli genomes. Int is a tyrosine recombinase that binds to DNA core sites via a C-terminal catalytic domain and to a collection of arm DNA sites, distant from the site of recombination, via its N-terminal domain. The arm sites, in conjunction with accessory DNA-bending proteins, provide a means of regulating the efficiency and directionality of Int-catalyzed recombination. Recent crystal structures of λ Int tetramers bound to synaptic and Holliday junction intermediates, together with new biochemical data, suggest a mechanism for the allosteric control of the recombination reaction through arm DNA binding interactions.
Introduction
The temperate coliphage λ has long served as a model system for studies of regulated site-specific recombination. In conditions favorable for bacterial growth, the phage genome is inserted into the Escherichia coli genome by an ‘integrative’ recombination reaction, which takes place between DNA attachment sites called attP and attB in the phage and bacterial genomes, respectively. As a result, the integrated λ DNA is bounded by hybrid attachment sites, termed attL and attR. In response to the physiological state of the bacterial host or to DNA damage, λ phage DNA excises itself from the host chromosome. This excision reaction recombines attL with attR to precisely restore the attP and attB sites on the circular λ and E. coli DNAs [1] (Figure 1).
Figure 1.

Integrative and excisive λ site-specific recombination. Integrative recombination between the phage attP and the bacterial attB sites (top) requires supercoiled attP DNA, Int and IHF. The Int pair bound to C and B core sites (red and pink arrows, respectively) nicks, exchanges and ligates the top strands (continuous lines) to form a HJ intermediate. The Int pair bound to C′ and B′ core sites then nicks, exchanges and ligates the bottom strands (dashed lines), thereby resolving the HJ to recombinant products attR and attL. attR and attL are shown here as the substrates of excisive recombination (bottom), a reaction that additionally requires Xis (and Fis, in vivo). Core-type Int-binding sites (C, C′, B and B′) and the seven base pair overlap region (sequence between cleavage sites, marked by black dots) comprise the ‘core region’. The arm regions contain the five arm-type Int-binding sites (P1, P2, P′1, P′2 and P′3, shown as green arrows) and the binding sites for the DNA-bending proteins IHF (H1, H2 and H′, blue triangles), Xis (X1 and X2, pink hexagons) and Fis (F, purple oval). The overlapping subsets of sites in the arm region that are used for either integrative or excisive recombination are shown as filled symbols.
The phage-encoded λ integrase protein (Int) splices together bacterial and phage attachment sites by a mechanism that is common to a large family of tyrosine recombinases with diverse biological functions (for reviews, see [2,3]). Recombination initiates with the pairing of two specific DNA segments by a tetramer of recombinase molecules. A four-way DNA junction (Holliday junction) is formed by the cleavage, exchange and ligation of one pair of strands, and is resolved to helical DNA products by the exchange of the second pair of strands (Figure 2) [4–7]. The DNA cleavage activity is strictly regulated within the tetramer, with only one pair of molecules active at a time. This control ensures the ordered pairwise exchange of DNA strands and avoids potentially harmful double-strand breaks. The switch in DNA cleavage activity from one pair of DNA strands to the other results from isomerization of the recombinase tetramer, as illustrated by recently published structures of λ recombination complexes [8••].
Figure 2.
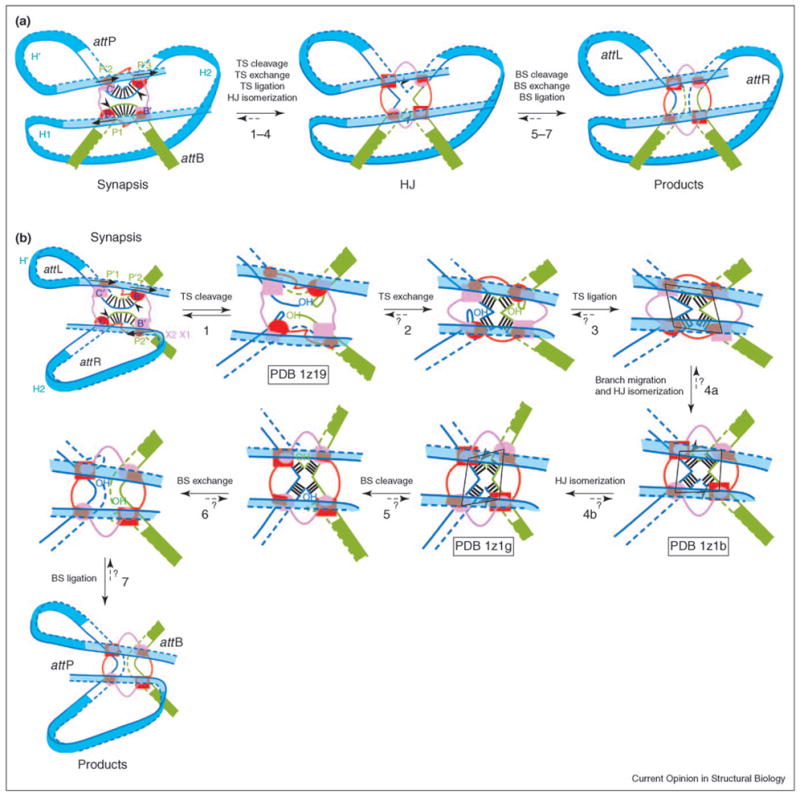
Reaction steps for (a) integration and (b) excision. Reaction steps are illustrated using the current model of the complete higher order recombination complex. Top strands (TS, continuous lines) are cleaved first and bottom strands (BS, dashed lines) are cleaved second to resolve the HJ. Cleavage sites (black arrowheads) are located on either side of the seven base pairs of the overlap region [vertical black lines in the cartoons for synapsis (a,b) and steps 3–6]. Active and inactive Int monomers are colored red and pink, respectively. Top-strand-cleaving Ints bound to core sites C and B, and bottom-strand-cleaving Ints bound to C′ and B′ are drawn as ovals and rectangles, respectively. The smaller ovals and rectangles represent the N-domains, which are connected to the C-terminal domains (bigger rectangles and ovals) by a flexible linker (red and pink lines). Note that the shapes of the arm DNA loops are not drawn for the reaction intermediates in (b). We do not know which of the steps (maybe all of them) are kinetically irreversible, as illustrated by short dashed arrows and question marks. The shape of the Int tetramer (shown as a black parallelogram in steps 3, 4a and 4b) changes during HJ isomerization, and Int activity switches from top strand to bottom strand cleavage. Steps 1, 4a and 4b are represented by crystal structure PDB codes 1z19, 1z1b and 1z1g [C75 Int (truncated Int 75–356) tetramer bound to core DNA; a post-strand-exchange complex containing an Int tetramer, two core DNA segments and two short arm DNAs; and an Int tetramer bound to a HJ and two short arm DNA segments], respectively [8••]. The more pronounced twofold symmetry of the second structure (step 4b) suggests that it has undergone further HJ isomerization than the structure in step 4a. During HJ isomerization, the HJ also performs branch migration of one base pair (step 4a). Consequently, the two bottom-strand-cleaving Ints end up being separated by six base pairs, as opposed to the eight base pair separation between the top-strand-cleaving Ints. This movement is thought to rearrange catalytic domain interactions in the Int tetramer, thus activating the bottom-strand-cleaving Ints. The structures suggest that arm DNA binding and the conformation of the N-domain tetramer favor the HJ conformation that activates the bottom-strand-cleaving Ints.
Simple tyrosine recombinases, such as Cre and Flp, catalyze a reversible recombination reaction between identical DNA sites [9,10], whereas λ recombination has a strong directional bias in response to environmental conditions. Accessory factors, whose expression levels change in response to host physiology, control the action of Int and determine whether the phage genome will remain integrated or be excised. Int has two DNA-binding domains: a C-terminal domain, consisting of a catalytic domain and a core-binding (CB) domain, that interacts with the core recombining sites and an N-terminal domain (N-domain) that recognizes the regulatory arm DNA sites [11] (Figure 3a). The heterobivalent Int molecules bridge distant core and arm sites with the help of accessory proteins, such as integration host factor (IHF), which bend the DNA at intervening sites, and appose arm and core sequences for interaction with the Int recombinase. Five arm DNA sites in the regions flanking the core of attP are differentially occupied during integration and excision reactions. The integration products attL and attR cannot revert back to attP and attB without assistance from the phage-encoded factor Xis, which bends DNA on its own or in combination with the host-encoded factor Fis [12–17]. Xis also inhibits integration, and prevents the attP and attB products of excision from reverting to attL and attR [13,18]. Excision is inhibited by high concentrations of IHF [19,20]. Because the cellular levels of IHF and Fis proteins respond to growth conditions, these host-encoded factors have been proposed as the master signals for integration and excision [15,19–23].
Figure 3.

Twofold symmetric catalytic domain interactions of Int, Cre and Flp. (a) Domain structure of a single active monomer overlaid on the Int, Cre and Flp tetramers bound to their respective HJs (shown in the background). The active site tyrosine nucleophiles of Int, Cre and Flp are Y342, Y324 and Y343, respectively. The CB-domain of Int is the equivalent of the N-domains of Cre and Flp. Int has an additional domain, the N-domain (black), which binds to arm DNA sites. The C-terminal β9 sheet and the N-helix of Int and Cre, respectively, interact with neighboring subunits within the tetramer. The Y343 nucleophile of Flp is supplied in trans by a neighboring subunit of the tetramer (shown in blue). The linker regions between the N-domains and catalytic domains of Cre and Flp (orange) have an additional helix involved in intermolecular interaction that is not present in the equivalent linker region of Int (orange). (b) Cartoons illustrating how the twofold symmetric intermolecular interactions of the catalytic domains of Int, Cre and Flp tetramers bound to DNA affect the position of the active site tyrosine with respect to the active (red circles) and inactive (white circles) scissile phosphates. The view is from the top (i.e. the N-domains would be above the drawn tetramers). These twofold symmetric interactions probably also influence the positions of other active site residues and thus further contribute to the activation of two out of four monomers, as has been most clearly seen in the Cre crystal structures [10]. The cis interaction of β9 and β6 in the unliganded Int monomer removes Y342 from the active site pocket and inactivates Int (small diagram on the left [41]).
Recently reported structural and biochemical studies have revealed the mechanisms by which the regulatory arm DNA sites and the N-domain of Int are likely to direct the λ site-specific recombination reaction. In this review, we will contrast the features of λ recombination with those of reactions catalyzed by monovalent recombinases such as Cre and Flp.
λ Integrase multimer assembly
Int’s biological functions are intrinsically linked to the formation of specific multimeric assemblies on DNA called intasomes [24], whose proper formation is essential for context-sensitive regulation of λ recombination (Figures 1–3) [25–29]. Even though an Int monomer can cleave DNA, recombination occurs only in the context of a multimeric complex. Three new crystal structures of Int multimers bound to DNA reveal the physical interactions that contribute to the regulation of DNA cleavage and ligation activities [8••]. The first structure shows N-terminally truncated (residues 75–356) Int dimers bound to core DNA, with one Int covalently linked to the scissile phosphate of the cleaved DNA through a phosphotyrosine bond. Two Int dimers pack as a twofold symmetric tetramer in the crystal. The second crystal structure, with full-length Int dimers bound to core and arm DNA segments, represents the step immediately following the first exchange of DNA strands. Once again, the Int dimers pack as a twofold symmetric tetramer. In the third structure, four full-length Int proteins are bound to two arm DNA segments and a Holliday junction (HJ) biased towards the exchange of the second pair of strands (Figure 2). One striking feature of the λ recombination complexes is the cyclically permutated arrangement of N-domains in the Int tetramer, with the N-domain of each Int monomer stacked against the CB-domain of a neighboring monomer. A similar arrangement of swapped N-domains was also seen in a FRET-based analysis of λ recombination complexes [30••].
Interactions between catalytic domains modulate DNA cleavage activity
Tyrosine recombinases catalyze DNA strand exchange, with only two molecules of the tetrameric complex active for DNA cleavage and ligation at any point during recombination. A conformational change termed isomerization interconverts active and inactive pairs of subunits after exchange of the first pair of DNA strands and before exchange of the second pair of strands. Two types of protein–protein interfaces within the twofold symmetric tetramer determine which pair of recombinases is active. However, different mechanisms for interconverting these interfaces, and thereby defining the active and inactive recombinase pairs, have been revealed for the tyrosine recombinases studied thus far. Crystal structures of the Cre tetramer bound to DNA substrates show a cyclically permuted packing arrangement of C-terminal α helices that enables several active site residues to be repositioned during isomerization of the protein–DNA complex, thereby switching activity from one pair of subunits to the other pair [10] (Figure 3). This elegant conformationally sensitive switch accounts for ‘half-the-sites’ reactivity, which ensures a productive outcome of the recombination reaction. Flp recombinase employs a variation on this theme, wherein the tyrosine nucleophile that initiates DNA cleavage is supplied in trans by a neighboring recombinase molecule [31,32] (Figure 3). Thus, the proper positioning of the DNA-cleaving tyrosine is critically dependent upon subunit packing interactions and the changes brought about by isomerization. Int employs a hybrid mechanism, whereby the trans interaction of a C-terminal β strand (β9) displaces the Y342 nucleophile [33] from the active site pocket (containing the absolutely conserved RHRK tetrad [34]) in two subunits to prevent uncoordinated DNA cleavage [8••,35••] (Figures 3 and 4). Moreover, the arrangement of catalytic domains in the Int tetramer is constrained by the arm DNA binding interactions discussed in the following section.
Figure 4.
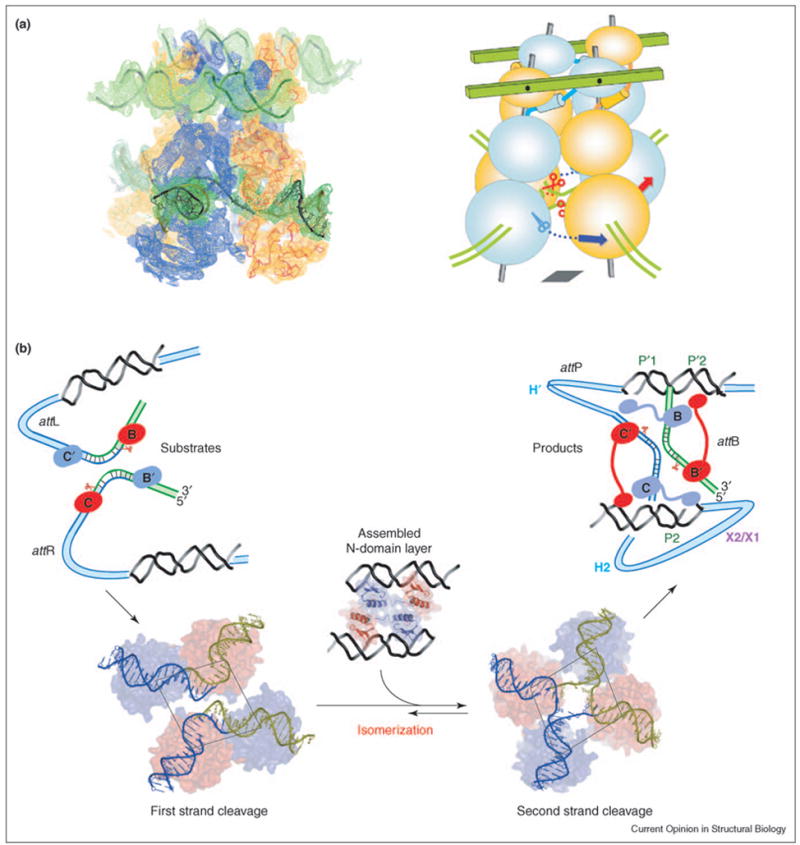
Illustration of the proposed role of N-domains in the coordination of Int activity within the tetramer. (a) The electron density calculated from the experimental phases is compared with the model to highlight features of the assembly of four Int molecules. The N, CB and catalytic domains of each protomer are stacked on an imaginary central rod, which is connected through the N-domain to arm DNAs, represented as green planks. Note that an N-domain is stacked on the same rod as the CB and catalytic domains of the neighboring Int. The relative positions of the N-domains are determined by their interactions with the arm DNAs and with each other. Through a poorly understood allosteric mechanism, this assembly positions the catalytic domains at their respective corners of the imaginary parallelogram [bottom right panel of (b)]. The plane of the tetrameric catalytic domains illustrated within the parallelograms in (b) is denoted by a gray parallelogram in the cartoon. (b) In the left panel, the N-domains are shown before the formation of the tetrameric complex with their respective arm DNAs and the catalytic domains are shown positioned for the first pair of DNA cleavages (at sites B and C). The pair of Ints lying on the short diagonal of the parallelogram is active, whereas those lying on the long diagonal are inactive. Assembly of the N-domains in complex with the arm DNAs reorients the parallelogram so that the alternate pair of catalytic domains lies on the short diagonal of the parallelogram, thus positioned for the second pair of DNA cleavages and strand exchanges at the C′ and B′ core sites (right panel).
Assembly of the Int tetramer increases the overall efficiency of DNA strand cleavage and additionally provides a conditional switch to activate enzymatic activity in only one pair of subunits at a time. Int’s DNA cleavage activity increases in multimeric complexes, perhaps by increasing avidity for DNA and/or correctly positioning the active site residues on the DNA substrate [36•]. The enhanced DNA cleavage of multimeric complexes is abrogated by a single residue substitution, R169D, which has no effect on DNA cleavage by Int monomers. The results of biochemical activity complementation studies suggested that R169 may interact with E153 of a neighboring subunit in the Int tetramer [36•]. This proposed interaction is consistent with the ~3.2 Å distance between these side-chains in the crystallized Int tetramers [8••]. Moreover, the R169D mutant is more effective than wild-type Int at exchanging DNA strands with illegitimate base pairings [37]. Int’s increased DNA cleavage activity upon forming multimeric complexes was shown to also result from trans interactions of its C-terminal β strand (β9), which appears to have the dual role of suppressing cleavage by an Int monomer and stimulating Int activity in the tetramer [36•,38,39]. Furthermore, the receipt of the β9 strand from a neighboring Int protomer allosterically stimulates HJ resolution [40•]. This finding is consistent with the crystal structure of the unliganded catalytic domain, in which the cis interaction of the C-terminal tail tethers the Y342 [33] nucleophile more than 20 Å away from the active site pocket (the RHRK tetrad [34]) and suppresses Int activity [41] (Figure 3b). The fact that the β9 strand may be positioned in cis or trans in monomers or multimers, respectively, may explain the observation that, in the absence of proper interactions with neighboring molecules, the C-terminal tail of Int is found to be flexible [42].
Influence of arm DNAs on λ recombination complexes
All steps of λ recombination, including DNA binding, synapsis, catalysis and isomerization, are influenced by binding to arm DNA sites. Int binds weakly to core DNA and, consequently, relies on binding to high-affinity arm DNA sites for its delivery to core sites (Figure 2). However, arm DNA binding offers an additional pathway for activating recombination. Truncated Int mutants lacking the N-domain bind to core DNA much better than the full-length protein. The intrinsic inhibition of core DNA binding activity caused by the N-domain is alleviated upon binding to arm DNA [43]. This regulatory function of the N-domain may have evolved to prevent aberrant DNA cleavage by Int monomers outside the context of a properly assembled intasome.
Accessory proteins bend the att DNA at sites interposed between the core and arm binding sites; these DNA-bending proteins are essential for λ recombination (Figure 1). In addition to delivering Int to the core sites, these accessory factors shape the paths of the recombining DNA substrates differently in intasomes configured for integration or excision (Figures 1 and 2). The assembly of the intasome is required for synapsis of the att sites and for subsequent progression through the entire recombination reaction. It is therefore striking that pairs of Int molecules bound to opposing att core DNAs (e.g. B′ and C sites during integration, Figure 2) are anchored via their N-domains to the same arm DNA molecule. This bridging interaction with the arm DNA potentially stabilizes the synapse of two att sites. The crystal structures of λ recombination complexes further suggest that close packing interactions between N-domains influence the shape of the Int tetramer and promote isomerization (Figure 4). This allosteric effect of arm binding could be mediated by the interaction of several residues located in the CB and catalytic domains that are important for cooperative binding to arm DNA [44].
Recombination initiates with the exchange of a designated pair of DNA strands, thought to be selected by the direction of bending of att DNAs in the synaptic complex. Following exchange of the first pair of strands, the tetramer isomerizes to a form that is ready for cleavage and exchange of the second pair of strands. Isomerization involves the repositioning of Int monomers and the reshaping of the DNA substrate. As a result, the angles between branches of the HJ change and base-pairing interactions at the center of the HJ are altered. The crystal structures of Int tetramers complexed to arm and core DNA sites suggest that the twofold symmetry of the N-domains bound to arm DNAs may bias the HJ towards an isomeric form favoring the second (resolving) exchange of DNA strands, as assigned by the topological connectivity of core and arm sites [8••] (Figure 2). The suggestion that arm binding imposes asymmetrical constraints on the recombination complex is supported by an earlier finding that resolution of a fixed HJ isomer is stimulated by arm DNA only when it is bound to the Int tetramer in a particular orientation [30••]. Additionally, Lee et al. [45•] have identified twofold symmetric interactions between residues R30 and D71 of adjacent N-domains that bias the outcome of HJ resolution. Thus, the biochemical data support the new model drawn from the crystal structures: a stable assembly of the N-domains allosterically regulates the catalytic activity at a distance. However, this proposed mechanism of arm-DNA-driven HJ isomerization, although congruent with the results of biochemical and genetic studies, is based on an assumed temporal relationship/order between three static crystal structures that were determined for three different tetrameric Int complexes [a truncated Int (C75) without the N-domain and without arm sites, an Int with a suicide substrate and arms sites, and a cleavage-defective Int bound to a preformed HJ and arm sites].
The oblong assembly of N-domains in complex with arm DNAs generates two different types of interactions between adjacent N-domains of the Int tetramer. Mutational analyses are consistent with the observed twofold symmetric packing of N-domains and further highlight the importance of several N-domain residues. In two independent studies, E47 was shown to be important for the cooperative binding of Int to tandemly repeated arm DNA sites, and for the interaction between Int and Xis bound to adjacent P2 and X1 sites (Figure 1) [46•,47•]. The crystal structures of λ recombination complexes show that E47 of one Int monomer can form a salt bridge with K60 of another monomer bound to an adjacent arm DNA site. This salt bridge would form across the synaptic interface and involve pairs of subunits bound to the same arm DNA segment. Consequently, the unutilized E47 residue of the Int monomer bound to the P2 arm site would be available to interact with Xis bound to the X1 site in the excisive complex (Figure 2). Alanine scanning mutagenesis identified L64A as a mutation that decreases the cooperativity of binding to P′1 and P′2 arm sites, and more strongly attenuates the cooperativity of binding to P′2 and P′3 arm sites [48]. The structures suggest that K60 of the monomer bound to P′2 could interact with L64 of the monomer bound to P′1 [8••]. Given that P′2 and P′3 are required for integration, it is notable that the L64A mutation has a stronger negative effect on integration than excision (for the location of the DNA sites discussed above, see Figures 1 and 2).
It has long been known that interactions with arm DNA sites direct the assembly of different intasome structures that strongly influence the directionality of recombination. However, the molecular architectures of integrative and excisive intasomes and other higher order reaction intermediates were not known (Figure 2). Recent biochemical evidence, in conjunction with the new crystal structures, has finally enabled us to more confidently draw models of the complete integrative and excisive HJ intermediates [8••,49]. These three-dimensional models suggest how two arm DNAs can stabilize twofold symmetric interactions between N-domains of the recombinogenic tetramer in a way that could coordinate Int activity and bias the outcome of the strand-exchange reactions [8••,30••,45•,50•]. Biochemical analyses of HJ intermediates that were trapped with synthetic peptides are suggestive of structural differences between the excisive and integrative HJ complexes [51••]. These differences are not accounted for by the current models of integrative and excisive complexes (Figure 2), as both models are based on the same crystal structures of core λ recombination complexes (Figures 2 and 4).
Conclusions
Crystal structures of the core components of λ recombination complexes provide a foundation for addressing several fundamental questions about the dynamic regulation of this complex machinery. It should now be possible to carry out detailed biochemical analyses of synapsis and isomerization, and to identify specific paths for the transmission of the allosteric effects of arm DNA binding. The answers to questions regarding the role of supercoiling, and the role of accessory factors in regulating the temporal order of binding and catalysis, the order of DNA strand exchange and the mechanism of irreversibility now seem closer at hand, albeit at arm’s length.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Campbell AM. Episomes. In: Caspari EW, Thoday JM, editors. Advances in Genetics. 1. New York: Academic Press; 1962. pp. 101–145. [Google Scholar]
- 2.Hallet B, Sherratt DJ. Transposition and site-specific recombination: adapting DNA cut-and-paste mechanisms to a variety of genetic rearrangements. FEMS Microbiol Rev. 1997;21:157–178. doi: 10.1111/j.1574-6976.1997.tb00349.x. [DOI] [PubMed] [Google Scholar]
- 3.Azaro MA, Landy A. λ Int and the λ Int family. In: Craig NL, Craigie R, Gellert M, Lambowitz A, editors. Mobile DNA II. Washington, DC: ASM Press; 2002. pp. 118–148. [Google Scholar]
- 4.Kitts PA, Nash HA. An intermediate in the phage λ site-specific recombination reaction is revealed by phosphorothioate substitution in DNA. Nucleic Acids Res. 1988;16:6839–6856. doi: 10.1093/nar/16.14.6839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitts PA, Nash HA. Bacteriophage λ site-specific recombination proceeds with a defined order of strand-exchanges. J Mol Biol. 1988;204:95–107. doi: 10.1016/0022-2836(88)90602-x. [DOI] [PubMed] [Google Scholar]
- 6.Nunes-Dü by SE, Matsumoto L, Landy A. Site-specific recombination intermediates trapped with suicide substrates. Cell. 1987;50:779–788. doi: 10.1016/0092-8674(87)90336-9. [DOI] [PubMed] [Google Scholar]
- 7.Holliday R. A mechanism for gene conversion in fungi. Genet Res. 1964;5:282–304. doi: 10.1017/S0016672308009476. [DOI] [PubMed] [Google Scholar]
- 8.••.Biswas T, Aihara H, Radman-Livaja M, Filman D, Landy A, Ellenberger T. A structural basis for allosteric control of DNA recombination by λ integrase. Nature. 2005;435:1059–1066. doi: 10.1038/nature03657. Crystal structures of Int tetramers complexed to both arm and core DNAs are likely to represent different steps of the recombination reaction. They suggest how N-domain interactions could promote the isomerization of a HJ intermediate to favor its resolution as recombinant products.
- 9.Chen Y, Rice PA. New insight into site-specific recombination from Flp recombinase-DNA structures. Annu Rev Biophys Biomol Struct. 2003;32:135–139. doi: 10.1146/annurev.biophys.32.110601.141732. [DOI] [PubMed] [Google Scholar]
- 10.Van Duyne GD. A structural view of cre-loxp site-specific recombination. Annu Rev Biophys Biomol Struct. 2001;30:87–104. doi: 10.1146/annurev.biophys.30.1.87. [DOI] [PubMed] [Google Scholar]
- 11.Wojciak JM, Sarkar D, Landy A, Clubb RT. Arm-site binding by the lambda integrase protein: solution structure and functional characterization of its amino-terminal domain. Proc Natl Acad Sci USA. 2002;99:3434–3439. doi: 10.1073/pnas.052017999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abremski K, Gottesman S. Purification of the bacteriophage λ xis gene product required for λ excisive recombination. J Biol Chem. 1982;257:9658–9662. [PubMed] [Google Scholar]
- 13.Abremski K, Gottesman S. Xis-independent excisive recombination of bacteriophage lambda. J Mol Biol. 1981;153:67–78. doi: 10.1016/0022-2836(81)90527-1. [DOI] [PubMed] [Google Scholar]
- 14.Hoess RH, Foeller C, Bidwell K, Landy A. Site-specific recombination functions of bacteriophage λ: DNA sequence of regulatory regions and overlapping structural genes for Int and Xis. Proc Natl Acad Sci USA. 1980;77:2482–2486. doi: 10.1073/pnas.77.5.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson JF, Moitoso de Vargas L, Koch C, Kahmann R, Landy A. Cellular factors couple recombination with growth phase: characterization of a new component in the λ site-specific recombination pathway. Cell. 1987;50:901–908. doi: 10.1016/0092-8674(87)90516-2. [DOI] [PubMed] [Google Scholar]
- 16.Thompson JF, Moitoso de Vargas L, Skinner SE, Landy A. Protein-protein interactions in a higher-order structure direct lambda site-specific recombination. J Mol Biol. 1987;195:481–493. doi: 10.1016/0022-2836(87)90177-x. [DOI] [PubMed] [Google Scholar]
- 17.Ball CA, Johnson RC. Efficient excision of phage λ from the Escherichia coli chromosome requires the Fis protein. J Bacteriol. 1991;173:4027–4031. doi: 10.1128/jb.173.13.4027-4031.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nash HA. Integrative recombination of bacteriophage lambda DNA in vitro. Proc Natl Acad Sci USA. 1975;72:1072–1076. doi: 10.1073/pnas.72.3.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bushman W, Thompson JF, Vargas L, Landy A. Control of directionality in lambda site-specific recombination. Science. 1985;230:906–911. doi: 10.1126/science.2932798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson JF, Waechter-Brulla D, Gumport RI, Gardner JF, Moitoso de Vargas L, Landy A. Mutations in an integration host factor-binding site: effect on lambda site-specific recombination and regulatory implications. J Bacteriol. 1986;168:1343–1351. doi: 10.1128/jb.168.3.1343-1351.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nilsson L, Verbeek H, Vijgenboom E, van Drunen C, Vanet A, Bosch L. FIS-dependent trans activation of stable RNA operons of Escherichia coli under various growth conditions. J Bacteriol. 1992;174:921–929. doi: 10.1128/jb.174.3.921-929.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ball CA, Osuna R, Ferguson KC, Johnson RC. Dramatic changes in Fis levels upon nutrient upshift in Escherichia coli. J Bacteriol. 1992;174:8043–8056. doi: 10.1128/jb.174.24.8043-8056.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ball CA, Johnson RC. Multiple effects of Fis on integration and the control of lysogeny in phage λ. J Bacteriol. 1991;173:4032–4038. doi: 10.1128/jb.173.13.4032-4038.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Better M, Wickner S, Auerbach J, Echols H. Role of the Xis protein of bacteriophage λ in a specific reactive complex at the attR prophage attachment site. Cell. 1983;32:161–168. doi: 10.1016/0092-8674(83)90506-8. [DOI] [PubMed] [Google Scholar]
- 25.Ross W, Landy A. Bacteriophage λ int protein recognizes two classes of sequence in the phage att site: characterization of arm-type sites. Proc Natl Acad Sci USA. 1982;79:7724–7728. doi: 10.1073/pnas.79.24.7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross W, Landy A. Patterns of λ Int recognition in the regions of strand exchange. Cell. 1983;33:261–272. doi: 10.1016/0092-8674(83)90355-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moitoso de Vargas L, Pargellis CA, Hasan NM, Bushman EW, Landy A. Autonomous DNA binding domains of λ integrase recognize different sequence families. Cell. 1988;54:923–929. doi: 10.1016/0092-8674(88)90107-9. [DOI] [PubMed] [Google Scholar]
- 28.Moitoso de Vargas L, Kim S, Landy A. DNA looping generated by the DNA-bending protein IHF and the two domains of lambda integrase. Science. 1989;244:1457–1461. doi: 10.1126/science.2544029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richet E, Abcarian P, Nash HA. Synapsis of attachment sites during lambda integrative recombination involves capture of a naked DNA by a protein-DNA complex. Cell. 1988;52 :9–17. doi: 10.1016/0092-8674(88)90526-0. [DOI] [PubMed] [Google Scholar]
- 30.••.Radman-Livaja M, Biswas T, Mierke D, Landy A. Architecture of recombination intermediates visualized by In-gel FRET of λ integrase-Holliday junction-arm-DNA complexes. Proc Natl Acad Sci USA. 2005;102:3913–3920. doi: 10.1073/pnas.0500844102. The relative positions of arm DNA and core DNA sites in two Int higher order complexes were calculated using in-gel FRET measurements and a distance geometry algorithm. The results indicate how the position of arm DNAs may determine the directional bias of HJ resolution and suggest models of the complete HJ recombination intermediates.
- 31.Chen J-W, Lee J, Jayaram M. DNA cleavage in trans by the active site tyrosine during Flp recombination: switching protein partners before exchanging strands. Cell. 1992;69:647–658. doi: 10.1016/0092-8674(92)90228-5. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Narendra U, Iype LE, Cox MM, Rice PA. Crystal structure of a Flp recombinase-Holliday junction complex: assembly of an active oligomer by helix swapping. Mol Cell. 2000;6:885–897. [PubMed] [Google Scholar]
- 33.Pargellis CA, Nunes-Dü by SE, Moitoso de Vargas L, Landy A. Suicide recombination substrates yield covalent λ integrase-DNA complexes and lead to identification of the active site tyrosine. J Biol Chem. 1988;263:7678–7685. [PubMed] [Google Scholar]
- 34.Nunes-Dü by S, Tirumalai RS, Kwon HJ, Ellenberger T, Landy A. Similarities and differences among 105 members of the Int family of site-specific recombinases. Nucleic Acids Res. 1998;26:391–406. doi: 10.1093/nar/26.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.••.Aihara H, Kwon HJ, Nunes-Dü by SE, Landy A, Ellenberger T. A conformational switch controls the DNA cleavage activity of lambda integrase. Mol Cell. 2003;12:187–198. doi: 10.1016/s1097-2765(03)00268-5. The crystal structure of λ Int (residues 75-356) in complex with a cleaved core DNA shows the organization of the active site and the trans interactions of the C-terminal β strand that contribute to the regulation of DNA cleavage activity.
- 36.•.Lee SY, Aihara H, Ellenberger T, Landy A. Two structural features of λ integrase that are critical for DNA cleavage by multimers but not by monomers. Proc Natl Acad Sci USA. 2004;101:2770–2775. doi: 10.1073/pnas.0400135101. Using allele-specific suppressor mutations and an Int mutant lacking the six C-terminal residues, two regions necessary for the multimeric enhancement of Int cleavage activity were identified: an intermolecular salt bridge between R169 and E153, and the C-terminal β strand, respectively.
- 37.Lee SY, Landy A. The efficiency of mispaired ligations by λ integrase is extremely sensitive to context. J Mol Biol. 2004;342:1647–1658. doi: 10.1016/j.jmb.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 38.Tekle M, Warren DJ, Biswas T, Ellenberger T, Landy A, Nunes-Dü by SE. Attenuating functions of the C-terminus of λ integrase. J Mol Biol. 2002;324:649–665. doi: 10.1016/s0022-2836(02)01108-7. [DOI] [PubMed] [Google Scholar]
- 39.Kazmierczak RA, Swalla BM, Burgin AB, Gumport RI, Gardner JF. Regulation of site-specific recombination by the carboxyl terminus of λ integrase. Nucleic Acids Res. 2002;30:5193–5204. doi: 10.1093/nar/gkf652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.•.Hazelbaker D, Radman-Livaja M, Landy A. Receipt of the C-terminal tail from a neighboring Int protomer allosterically stimulates Holliday junction resolution. J Mol Biol. 2005;351:948–955. doi: 10.1016/j.jmb.2005.06.077. The HJ resolution defect of an Int mutant lacking six C-terminal residues (β9) can be complemented by the addition of the corresponding hexapeptide free in solution. This indicates that the intermolecular interaction between β9 and β6 detected in [35••] is important for DNA cleavage by an Int tetramer, in addition to the previously documented intramolecular role of β9 in positioning the Y342 nucleophile.
- 41.Kwon HJ, Tirumalai RS, Landy A, Ellenberger T. Flexibility in DNA recombination: structure of the λ integrase catalytic core. Science. 1997;276:126–131. doi: 10.1126/science.276.5309.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Subramaniam S, Tewari AK, Nunes-Dü by SE, Foster MP. Dynamics and DNA substrate recognition by the catalytic domain of lambda integrase. J Mol Biol. 2003;329:423–439. doi: 10.1016/s0022-2836(03)00469-8. [DOI] [PubMed] [Google Scholar]
- 43.Sarkar D, Radman-Livaja M, Landy A. The small DNA binding domain of λ Int is a context-sensitive modulator of recombinase functions. EMBO J. 2001;20:1203–1212. doi: 10.1093/emboj/20.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han YW, Gumport RI, Gardner JF. Mapping the functional domains of bacteriophage lambda integrase protein. J Mol Biol. 1994;235:908–925. doi: 10.1006/jmbi.1994.1048. [DOI] [PubMed] [Google Scholar]
- 45.•.Lee SY, Radman-Livaja M, Warren D, Aihara H, Ellenberger T, Landy A. Nonequivalent interactions between amino-terminal domains of neighboring λ integrase protomers direct Holliday junction resolution. J Mol Biol. 2005;345:475–485. doi: 10.1016/j.jmb.2004.10.068. Complementation assays showed that twofold symmetric intermolecular interactions between residues R30 and D71 in the Int tetramer determine the direction of HJ resolution.
- 46.•.Warren D, Sam M, Manley K, Sarkar D, Lee SY, Abbani M, Clubb RT, Landy A. Identification of the λ integrase surface that interacts with the Xis accessory protein reveals a residue that is also critical for homomeric dimer formation. Proc Natl Acad Sci USA. 2003;100:8176–8181. doi: 10.1073/pnas.1033041100. Alanine scanning mutagenesis of α1 of the Int N-domain revealed that E47 is important for cooperative Int binding to tandemly repeated arm sites, as well as Int and Xis cooperative DNA binding. This suggests that the same protein surface of Int can form interfaces either with another Int or with Xis.
- 47.•.Swalla BM, Cho EH, Gumport RI, Gardner JF. The molecular basis of cooperative DNA binding between lambda integrase and excisionase. Mol Microbiol. 2003;50:89–99. doi: 10.1046/j.1365-2958.2003.03687.x. The Int E47K mutant shows a striking defect in excision compared to integration, which can be explained by the disruption of an interaction with Xis that is dependent upon residue E47 of Int. This study and [46•] identified the same residue critical for intermolecular Int interaction using different experimental approaches.
- 48.Warren D, Lee SY, Landy A. Mutations in the amino-terminal domain of lambda integrase have differential effects on integrative and excisive recombination. Mol Microbiol. 2005;55:1104–1112. doi: 10.1111/j.1365-2958.2004.04447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cassell G, Klemm M, Pinilla C, Segall A. Dissection of bacteriophage λ site-specific recombination using synthetic peptide combinatorial libraries. J Mol Biol. 2000;299:1193–1202. doi: 10.1006/jmbi.2000.3828. [DOI] [PubMed] [Google Scholar]
- 50.•.Radman-Livaja M, Shaw C, Azaro M, Biswas T, Ellenberger T, Landy A. Arm sequences contribute to the architecture and catalytic function of a λ integrase-Holliday junction complex. Mol Cell. 2003;11:783–794. doi: 10.1016/s1097-2765(03)00111-4. Arm DNA reduces the formation of aberrant resolution products that arise from uncoordinated DNA cleavage within the Int tetramer, suggesting that arm DNA may play a role in the coordination of Int activity. The ternary Int complexes characterized in this study were used to make the crystals described in [8••].
- 51.••.Boldt JL, Pinilla C, Segall AM. Reversible inhibitors of λ Int-mediated recombination efficiently trap Holliday junction intermediates and form the basis of a novel assay for junction resolution. J Biol Chem. 2004;279:3472–3483. doi: 10.1074/jbc.M309361200. Synthetic peptides have been optimized by the authors to specifically trap HJ intermediates of λ Int-dependent recombination. Several classes of peptides that trap various recombination intermediates had been previously identified by screening a synthetic peptide library [48,51••]. These peptides specifically recognize structural features of four-way DNA junctions, and have proven to be great tools for trapping unstable HJ intermediates of the λ and Cre systems, thus facilitating their biochemical and structural characterization.


