Abstract
Objectives
A multicenter prospective study was performed to assess the results and risks associated with radiofrequency ablation in children. This report focuses on recurrences following initially successful ablation.
Methods
Patients recruited for the study were aged 0 to 16 years and had supraventricular tachycardia due to accessory pathways or atrioventricular nodal reentrant tachycardia (AVNRT), excluding patients with more than trivial congenital heart disease. A total of 481 patients were recruited into the prospective cohort and were followed at 2, 6, and 12 months following ablation.
Results
There were 517 successfully ablated substrates out of 540 attempted (95.7%). Loss to follow-up for individual substrates was 3.3%, 10.6%, and 21.2% at 2, 6, and 12 months, respectively. Recurrence was observed in 7.0%, 9.2%, and 10.7% of these substrates at 2, 6, and 12 months, respectively (adjusted for loss to follow-up as an independent source of data censoring). Recurrence rate varied by substrate location (24.6% for right septal, 15.8% for right free wall, 9.3% for left free wall, and 4.8% for left septal), as well as for AVNRT versus all others (4.8% vs 12.9%) at 12 months. The recurrence rate was higher for substrates ablated using power control but was not a function of whether isoproterenol was used for postablation testing.
Conclusions
Recurrence after initially successful ablation occurs commonly in children. It is least common after AVNRT ablation and most common following ablation of right-sided pathways. These results serve as a benchmark for the time course of recurrence following initially successful ablation of supraventricular tachycardia in children.
Keywords: Ablation, Children, Wolff-Parkinson-White syndrome, Atrioventricular nodal reentry, Registry, Recurrence
Introduction
Radiofrequency (RF) ablation represents a major advance in the management of children with arrhythmias and has rapidly become the standard of care for first-line therapy of paroxysmal tachycardia in patients of nearly all ages. There has been concern regarding the safety and long-term effectiveness of the procedure in the growing and developing heart. A high rate of late recurrence of the initially successfully treated arrhythmia has been observed in one large pediatric series.1
A multicenter prospective study was designed and implemented to assess the short- and longer-term outcomes following RF ablation in children. A more complete description of the design of this study has been previously published.2
A total of 2,761 pediatric patients were enrolled in the ongoing study from April 1, 1999 to May 8, 2003. This report focuses on the incidence, time course, and determinants of recurrence of the ablated arrhythmias following initially successful ablation procedures. Initial results, including ablation success and complications, are reported elsewhere.3
Methods
Patients recruited for the study were aged 0 to 16 years and had supraventricular tachycardia due to accessory pathways or atrioventricular nodal reentry. Patients with more than trivial congenital heart disease or with Ebstein’s anomaly of the tricuspid valve were excluded. Patients were enrolled and studied prior to ablation and periodically by clinical evaluation, electrocardiogram, Holter monitor, and echocardiogram. In addition, a national registry was established to which the contributing centers report all pediatric patients (age 0-21 years) undergoing ablation at their center. Clinical endpoints were defined as death, recurrence, proarrhythmia, and echocardiographic abnormality. For display and analysis purposes, patients were divided into three mutually exclusive groups. The first group consists of 481 patients enrolled in the prospective cohort (with final enrollment occurring on November 1, 2001) and are the “cohort participants.” The second group consists of 504 patients who satisfy the eligibility criteria for enrollment in the cohort but were not enrolled because of physician choice, patient choice, or nonparticipation of the clinical center in the cohort study. Members of the second group are the “cohort-eligible registry participants” (CE registry participants). The third group consists of 1,776 patients who did not qualify for the cohort but did qualify for the general registry. These members are the “not cohort-eligible registry participants” (NCE registry participants). The purpose of the division into groups is to assess for possible biases introduced by the recruitment procedures that may limit the ability to generalize the results found in the prospective cohort to the larger population.
For each procedure, one or more substrates may be present. A substrate is defined as a discrete electrophysiologic diagnosis for which RF ablation was attempted.
Baseline data from these groups are obtained from (1) the patient clinical data collection form filled out at enrollment for every patient in the cohort and the registry, and (2) the substrate data collection forms documenting the RF procedures, complications, and outcomes for each substrate at the time of the initial procedure. Recurrence data for the cohort group are obtained from substrate data collection forms at 2, 6, and 12 months. Clinical outcomes data for the registry groups are obtained from patient data collection forms at 12 months. As reported previously,2 quality control procedures included double data entry to eliminate possible keypunch errors and review by an independent reviewer of the electrophysiology tracings to determine the mechanism of arrhythmia in 50% of all cohort patients. This was done to allow confirmation of eligibility for enrollment in the prospective cohort, proper assignment by diagnosis (i.e., AV nodal reentrant tachycardia [AVNRT] vs accessory pathway-mediated tachycardia), and success of ablation. Recurrence is by each individual investigator at the time of follow-up and based on the presence or absence of preexcitation in patients with Wolff-Parkinson-White (WPW) syndrome and/or the recurrence of symptomatic tachycardia in patients with or without WPW syndrome.
The equality of 12-month recurrence rates between sub-groups of cohort patients was tested using the Cochran-Mantel-Haenszel Chi-square test, calculated with the time interval (0-3 months, 3-months, and 6-12 months) as the blocking variable. The equality of 12-month recurrence rates between CE and NCE registry patients was tested using the Chi-square test with continuity correction or, when the expected value in a cell was smaller than 5, Fisher’s exact test.
The protocol was approved by the Institutional Review Board at each contributing center, and the families of the patients gave consent for participation in the study.
Results
The study population for analysis of recurrence includes those patients in the prospective cohort (n = 481) who had an initially successful ablation of one or more substrates (n = 457), as previously reported.3 Overall, there were 517 successfully ablated substrates out of 540 attempted (95.7%), and these 517 substrates were followed. Loss to follow-up by substrate occurred in 17 of 517 at 2 months (3.3%), an additional 38 at 6 months (55/517 [10.6%]), and an additional 60 at 12 months (115/517 [21.2%]).
Cohort recurrences at 2, 6, and 12 months
The first row of Table 1 shows the recurrence rate for substrates that had successful ablations at baseline in the cohort. There were 500 substrates for which the recurrence status was known at 2 months; of these, a recurrence occurred in 35 (7.0%) during the 2-month time interval. There were 427 substrates that did not have a recurrence by 2 months and had a known recurrence status at 6 months; of these, a recurrence occurred in 10 (2.3%) during the time interval of 2 to 6 months. Finally, there were 352 substrates that did not have a recurrence by 6 months and had a known recurrence status at 12 months; of these, a recurrence occurred in 6 (1.7%) during the time interval of 6 to 12 months. Thus, the estimated 2-, 6-, and 12-month recurrence rates were 7.0%, 9.2%, and 10.7%, respectively.
Table 1.
Substrate recurrence rate by patient demographics in the cohort
| 0 to 2 Months | 2 to 6 Months+ | 6 to 12 Months+ | 0 to 6 Months | 0 to 12 Months | |
|---|---|---|---|---|---|
| Total substrates | 7.0% (35/500) | 2.3% (10/427) | 1.7% (6/352) | 9.2% | 10.7% |
| Gender | |||||
| Males | 7.1% (19/268) | 1.8% (4/222) | 1.1% (2/185) | 8.8% | 9.7% |
| Females | 6.9% (16/232) | 2.9% (6/205) | 2.4% (4/167) | 9.6% | 11.8% |
| Age (in years) | |||||
| 0–9 | 7.4% (10/135) | 1.7% (2/118) | 2.0% (2/99) | 9.0% | 10.8% |
| 10–12 | 5.5% (6/109) | 2.1% (2/94) | 2.5% (2/81) | 7.5% | 9.8% |
| 13–14 | 9.7% (10/103) | 4.7% (4/86) | 1.4% (1/73) | 13.9% | 15.1% |
| 15–16 | 6.8% (8/118) | 2.0% (2/99) | 1.4% (1/74) | 8.7% | 9.9% |
| >16 | 2.9% (1/35) | 0.0% (0/30) | 0.0% (0/25) | 2.9% | 2.9% |
| Body mass index | |||||
| <20 | 7.2% (20/279) | 2.8% (7/249) | 1.4% (3/209) | 9.8% | 11.1% |
| 20–29 | 6.4% (13/202) | 1.8% (3/164) | 2.3% (3/132) | 8.1% | 10.2% |
| 30–39 | 11.8% (2/17) | 0.0% (0/13) | 0.0% (0/9) | 11.8% | 11.8% |
| ≥40 | NA (0/0) | NA (0/0) | NA (0/0) | NA | NA |
| Unknown | 0.0% (0/2) | 0.0% (0/1) | 0.0% (0/2) | 0.0% | 0.0% |
| Ethnicity | |||||
| Caucasian, non-Hispanic | 7.4% (32/434) | 2.2% (8/369) | 2.0% (6/305) | 9.4% | 11.2% |
| Black, non-Hispanic | 5.6% (2/36) | 3.0% (1/33) | 0.0% (0/27) | 8.4% | 8.4% |
| Hispanic | 5.3% (1/19) | 0.0% (0/14) | 0.0% (0/13) | 5.3% | 5.3% |
| Asian, non-Hispanic | 0.0% (0/9) | 11.1% (1/9) | 0.0% (0/5) | 11.1% | 11.1% |
| Native American | 0.0% (0/2) | 0.0% (0/2) | 0.0% (0/2) | 0.0% | 0.0% |
| Other | 7.4% (32/434) | 2.2% (8/369) | 2.0% (6/305) | 9.4% | 11.2% |
| Missing | 5.6% (2/36) | 3.0% (1/33) | 0.0% (0/27) | 8.4% | 8.4% |
Substrates with recurrences at end of interval/substrates known not to have prior recurrence at beginning of interval.
The remainder of Table 1 shows the recurrence rate in the cohort by patient demographic characteristics. Substrates in males and females had approximately equal recurrence rates (9.7% and 11.8% respectively, P > .30). There was no discernible trend of recurrence rates with age, body mass index, or ethnicity. Table 2 shows the recurrence rate in the cohort by medical condition prior to ablation procedures. There was no statistically significant difference by presence or absence of structural heart disease, cardiac symptoms before procedure, or indication for ablation.
Table 2.
Substrate recurrence rate by medical conditions prior to ablation procedures
| 0 to 2 Months | 2 to 6 Months+ | 6 to 12 Months+ | 0 to 6 Months | 0 to 12 Months | |
|---|---|---|---|---|---|
| Total substrates | 7.0% (35/500) | 2.3% (10/427) | 1.7% (6/352) | 9.2% | 10.7% |
| Structural heart disease | |||||
| Congenital | 7.1% (1/14) | 9.1% (1/11) | 0.0% (0/9) | 15.6% | 15.6% |
| Cardiomyopathy | 0.0% (0/5) | 0.0% (0/5) | 0.0% (0/5) | 0.0% | 0.0% |
| Neither of above | 7.1% (34/481) | 2.2% (9/411) | 1.8% (6/338) | 9.1% | 10.7% |
| Echocardiogram shortening fraction (%) | |||||
| 1–30 | 6.1% (2/33) | 0.0% (0/28) | 0.0% (0/25) | 6.1% | 6.1% |
| 31–35 | 6.8% (10/146) | 3.2% (4/125) | 1.0% (1/98) | 9.8% | 10.8% |
| 36–40 | 7.3% (9/124) | 0.0% (0/104) | 2.3% (2/88) | 7.3% | 9.4% |
| >40 | 1.2% (1/85) | 2.6% (2/77) | 0.0% (0/64) | 3.7% | 3.7% |
| Unknown | 11.6% (13/112) | 4.3% (4/93) | 3.9% (3/77) | 15.4% | 18.7%a |
| Cardiac symptoms before procedure | |||||
| Palpitations | 5.5% (18/325) | 2.5% (7/275) | 1.8% (4/218) | 7.9% | 9.6% |
| Sustained tachycardia | 6.3% (22/349) | 2.3% (7/303) | 1.2% (3/252) | 8.5% | 9.6% |
| Syncope | 10.0% (4/40) | 0.0% (0/31) | 0.0% (0/26) | 10.0% | 10.0% |
| Chest pain | 3.9% (3/77) | 1.5% (1/65) | 0.0% (0/49) | 5.4% | 5.4% |
| Exercise intolerance | 6.7% (2/30) | 0.0% (0/20) | 5.9% (1/17) | 6.7% | 12.2% |
| Shortness of breath | 9.1% (4/44) | 2.9% (1/35) | 0.0% (0/28) | 11.7% | 11.7% |
| Other | 7.5% (5/67) | 3.7% (2/54) | 4.7% (2/43) | 10.9% | 15.0% |
| Indications for ablation | |||||
| Life-threatening arrhythmia | 2.9% (1/34) | 3.2% (1/31) | 0.0% (0/23) | 6.1% | 6.1% |
| Refractory to drug therapy | 7.5% (6/80) | 1.5% (1/66) | 1.8% (1/56) | 8.9% | 10.5% |
| Adverse drug effects | 14.3% (1/7) | 16.7% (1/6) | 0.0% (0/2) | 28.6% | 28.6% |
| Impending congenital heart disease surgery | NA (0/0) | NA (0/0) | NA (0/0) | NA | NA |
| Patient choice | 6.7% (25/374) | 2.2% (7/321) | 1.9% (5/268) | 8.7% | 10.4% |
| Cardiomyopathy | 25.0% (1/4) | 0.0% (0/3) | 0.0% (0/3) | 25.0% | 25.0% |
| Other | 0.0% (0/1) | NA (0/0) | NA (0/0) | NA | NA |
Substrates with recurrences at end of interval/substrates known not to have prior recurrence at beginning of interval.
Unknown echocardiogram shortening fraction vs known fraction, P < .05.
Table 3 shows the recurrence rate in the cohort by patient-specific ablation procedure parameters. There were no statistically significant differences with respect to fluoroscopy time for the patient or maximum achieved temperature for the patient. However, by logistic regression, there is a higher 12-month recurrence rate related to a higher number of lesions of any duration (P = .041). A similar trend, although not quite statistically significant, was found for lesions with duration >20 seconds (P = .074). Table 4 shows the recurrence rate by substrate-specific parameters. Although there were no statistically significant differences in recurrence rate by duration of observation after the last RF application, there was a downward trend in the recurrence rate with increasing duration of observation. There were statistically significant differences in recurrence rate by substrate location, as well as for AVNRT versus all others (4.8% vs 12.9%, P = .011). The recurrence rate by pathway location at 12 months varied substantially (Figure 1): 24.6% for right septal (P < .001 vs all other pathways), 15.8% for right free wall, 9.3% for left free wall (P < .01 vs all other pathways), and 4.8% for left septal. The recurrence rate was higher for substrates ablated using power control mode on the generator only versus other modes (25.7% vs 10.1%, P = .021). Recurrence rates were not statistically different for left-sided accessory pathways ablated by a retrograde route versus the transseptal route, although most (77%) were approached by the transseptalroute. There was no statistically significant difference in the recurrence rate as a function of whether or not isoproterenol was used for post ablation testing for either the AVNRT group or the accessory pathway group.
Table 3.
Substrate recurrence rate by patient-specific ablation procedure parameters
| 0 to 2 Months | 2 to 6 Months+ | 6 to 12 Months+ | 0 to 6 Months | 0 to 12 Months | |
|---|---|---|---|---|---|
| Total substrates | 7.0% (35/500) | 2.3% (10/427) | 1.7% (6/352) | 9.2% | 10.7% |
| Fluoroscopy time for patient (min) | |||||
| 1–20 | 5.5% (8/146) | 0.0% (0/124) | 0.9% (1/111) | 5.5% | 6.3% |
| 21–30 | 2.9% (3/102) | 4.6% (4/87) | 4.1% (3/74) | 7.4% | 11.2% |
| 31–50 | 6.8% (9/132) | 2.5% (3/118) | 0.0% (0/90) | 9.2% | 9.2% |
| >50 | 10.9% (12/110) | 2.2% (2/91) | 0.0% (0/71) | 12.9% | 12.9% |
| Unknown | 30.0% (3/10) | 14.3% (1/7) | 16.7% (1/6) | 40.0% | 50.0% |
| Total procedure time for patient (min) | |||||
| 1–150 | 5.2% (6/116) | 1.0% (1/97) | 1.2% (1/85) | 6.2% | 7.3% |
| 151–200 | 6.1% (8/131) | 3.4% (4/118) | 2.2% (2/91) | 9.3% | 11.3% |
| 201–250 | 9.7% (11/113) | 3.4% (3/88) | 1.3% (1/76) | 12.8% | 14.0% |
| >250 | 5.8% (7/120) | 1.0% (1/105) | 1.2% (1/84) | 6.7% | 7.8% |
| Unknown | 15.0% (3/20) | 5.3% (1/19) | 6.3% (1/16) | 19.5% | 24.5%a |
| Number of RF applications >20 s for patient | |||||
| 1–3 | 4.2% (11/265) | 2.1% (5/235) | 1.0% (2/198) | 6.2% | 7.1% |
| 4–6 | 10.4% (11/106) | 2.4% (2/84) | 1.5% (1/67) | 12.5% | 13.8% |
| 7–10 | 12.5% (9/72) | 3.5% (2/57) | 2.2% (1/45) | 15.6% | 17.4% |
| >10 | 6.3% (2/32)c | 0.0% (0/30) | 4.3% (1/23) | 6.3% | 10.3% |
| Unknown | 8.0% (2/25) | 4.8% (1/21) | 5.3% (1/19) | 12.4% | 17.0% |
| Total number of RF applications for patient* | |||||
| 1–3 | 3.9% (6/154) | 1.5% (2/137) | 0.8% (1/121) | 5.3% | 6.1% |
| 4–6 | 6.5% (8/123) | 2.0% (2/101) | 1.2% (1/83) | 8.4% | 9.5% |
| 7–10 | 4.4% (4/91) | 5.1% (4/78) | 1.6% (1/63) | 9.3% | 10.7% |
| >10 | 11.8% (15/127)c | 0.9% (1/107) | 2.5% (2/81) | 12.6% | 14.8% |
| Unknown | 40.0% (2/5) | 25.0% (1/4) | 25.0% (1/4) | 55.0% | 66.3%b |
| Maximum temperature achieved for patient (°C) | |||||
| 1–50 | 15.2% (5/33) | 4.0% (1/25) | 0.0% (0/23) | 1–50 | 15.2% (5/33) |
| 51–60 | 4.3% (9/208) | 2.2% (4/186) | 0.7% (1/50) | 51–60 | 4.3% (9/208) |
| 61–70 | 8.7% (16/183) | 1.3% (2/156) | 1.5% (2/130) | 61–70 | 8.7% (16/183) |
| >70 | 5.0% (3/60) | 2.2% (1/45) | 2.9% (1/35) | >70 | 5.0% (3/60) |
| Unknown | 12.5% (2/16) | 13.3% (2/15) | 14.3% (2/14) | Unknown | 12.5% (2/16) |
Substrates with recurrences at end of interval/substrates known not to have prior recurrence at beginning of interval.
Statistical significance at
P < .05.
Unknown total procedure time vs known total procedure time, P < .05.
Unknown number of applications >20 s vs known number of applications >20 s, P < .01.
There were 127 substrates for which 2-month follow-up data were available for patients who had >10 radiofrequency (RF) applications at baseline. There were 32 substrates for which 2-month follow-up data were available for patients who had >10 RF applications at baseline with >20 s duration. Because the duration of RF application was measured at the patient level rather than the substrate level, some of those 32 substrates may have no lesions that required an RF application >20 s.
Table 4.
Substrate recurrence rate by substrate-specific parameters
| 0 to 2 Months | 2 to 6 Months+ | 6 to 12 Months+ | 0 to 6 Months | 0 to 12 Months | |
|---|---|---|---|---|---|
| Total substrates | 7.0% (35/500) | 2.3% (10/427) | 1.7% (6/352) | 9.2% | 10.7% |
| Substrate/pathway location | |||||
| Manifest accessory pathway | 9.7% (21/216) | 1.1% (2/183) | 0.7% (1/149) | 10.7% | 11.3% |
| Concealed accessory pathway | 6.6% (9/137) | 5.2% (6/116) | 4.2% (4/96) | 11.4% | 15.1% |
| PJRT | 20.0% (2/10) | 0.0% (0/6) | 0.0% (0/5) | 20.0% | 20.0% |
| AVNRT | 2.2% (3/134) | 1.7% (2/120) | 1.0% (1/100) | 3.9% | 4.8%*a |
| Atrial fibrillation | NA (0/0) | NA (0/0) | NA (0/0) | NA | NA |
| Atrial flutter/intraatrial reentry | 0.0% (0/1) | 0.0% (0/1) | 0.0% (0/1) | 0.0% | 0.0% |
| Ectopic atrial tachycardia | 0.0% (0/1) | NA (0/0) | NA (0/0) | NA | 0.0% |
| Junctional ectopic tachycardia | NA (0/0) | NA (0/0) | NA (0/0) | NA | NA |
| Ventricular tachycardia | NA (0/0) | NA (0/0) | NA (0/0) | NA | NA |
| Other (Mahaim) | 0.0% (0/1) | 0.0% (0/1) | 0.0% (0/1) | 0.0% | 0.0% |
| Power mode vs temperature mode | |||||
| Temperature control only | 7.0% (29/413) | 2.3% (8/354) | 1.0% (3/290) | 9.1% | 10.1% |
| Power control only | 20.0% (4/20) | 7.1% (1/14) | 0.0% (0/12) | 25.7% | 25.7%*b |
| Both temperature and power control | 0.0% (0/1) | 0.0% (0/1) | 0.0% (0/1) | 0.0% | 0.0% |
| Missing | 3.0% (2/66) | 1.7% (1/58) | 6.1% (3/49) | 4.7% | 10.5% |
| Duration of observation after last RF application (min) | |||||
| 0–30 | 8.0% (16/199) | 1.7% (3/173) | 2.1% (3/146) | 9.6% | 11.5% |
| 31–60 | 5.7% (12/209) | 3.3% (6/180) | 2.0% (3/149) | 8.9% | 10.7% |
| 61–90 | 5.4% (2/37) | 0.0% (0/30) | 0.0% (0/23) | 5.4% | 5.4% |
| >90 | 3.2% (1/31) | 0.0% (0/26) | 0.0% (0/22) | 3.2% | 3.2% |
| Missing | 16.7% (4/24) | 5.6% (1/18) | 0.0% (0/12) | 21.3% | 21.3% |
| Use of isoproterenol in postablation testing | |||||
| AVNRT w/isoproterenol | 1.8% (2/110) | 2.0% (2/99) | 1.2% (1/83) | 3.8% | 5.0% |
| AVNRT w/o isoproterenol | 4.2% (1/24) | 0.0% (0/21) | 0.0% (0/17) | 4.2% | 4.2% |
| Non-AVNRT w/isoproterenol | 8.3% (21/252) | 2.8% (6/218) | 2.2% (4/184) | 10.9% | 12.8% |
| Non-AVNRT w/o isoproterenol | 9.6% (11/114) | 2.2% (2/89) | 1.5% (1/68) | 11.7% | 13.0% |
| Pathway | |||||
| Right free wall | 15.8% (9/57) | 0.0% (0/46) | 0.0% (0/39) | 15.8% | 15.8% |
| Right septal | 14.1% (10/71) | 5.3% (3/57) | 7.3% (3/41) | 18.6% | 24.6%***c |
| Left septal | 4.8% (1/21) | 0.0% (0/18) | 0.0% (0/14) | 4.8% | 4.8% |
| Left free wall | 5.6% (12/215) | 2.7% (5/185) | 1.3% (2/157) | 8.1% | 9.3%**c |
Substrates with recurrences at end of interval/substrates known not to have prior recurrence at beginning of interval.
Statistical significance at
P < .05
P < .01
P < .001
AVNRT vs no AVNRT, P < .05.
Power control vs. no power control, P < .05.
Each pathway vs all other pathways.
AVNRT = atrioventricular nodal reentrant tachycardia; PJRT = permanent junctional reciprocating tachycardia.
Figure 1.
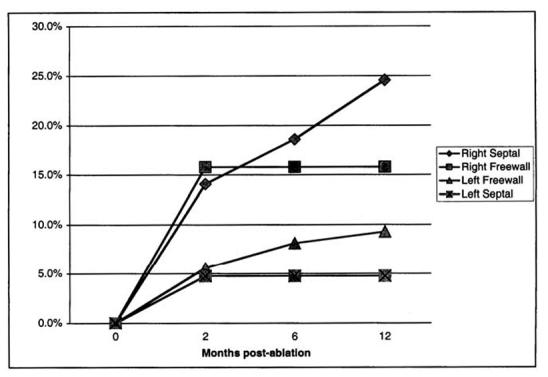
Recurrence rate by pathway location at 2, 6, and 12 months after initially successful ablation in the prospectively enrolled cohort.
Status of CE and NCE registry participants at 12 months
Among the 504 patients in the CE group, there were 537 ablated substrates, of which 508 (94.6%) were successful. Among the 1,776 patients in the NCE group, there were 1,906 ablated substrates, of which 1,761 (92.4%) were successful.3 Table 5 provides information about the status of the registry patients at 12 months. There were 229 patients with 12-month follow-up among the CE registry participants (45.4%) and 860 among the NCE registry participants (48.4%). Of these, 13.1% of the CE registry had one or more of the listed events (cardiac catheterization, electrophysiologic study, RF ablation, cardiac surgery, other hospitalization, or other serious event) since the last contact versus 18.6% of the NCE registry participants (P < .05). Repeat RF ablations since the last contact were relatively rare (2.8% of all registry participants). A total of 33% were experiencing at least one of the listed symptoms (palpitations, sustained rapid heart beat, fainting, chest pain, exercise intolerance, shortness of breath, and other), with the most common symptom being palpitations (12.1%). About one sixth (14.3%) of registry patients were taking medication at 12 months, with a statistically significant difference between the proportions of CE and NCE registry participants (7.0% vs 16.3%, P < .001).
Table 5.
Twelve-month patient outcomes in the registry
| Cohort-eligible (CE) registry | Not cohort-eligible (NCE) registry | Total registry | |
|---|---|---|---|
| Total patients with 12-month follow-up | 229 | 860 | 1,089 |
| Since last contact, have any of the following events occurred? | |||
| Cardiac catheterization | 0.9% (2/229) | 1.7% (15/860) | 1.6% (17/1,089) |
| Electrophysiologic study | 1.7% (4/229) | 3.4% (29/860) | 3.0% (33/1,089) |
| Radiofrequency ablation | 1.7% (4/229) | 3.0% (26/860) | 2.8% (30/1,089) |
| Cardiac surgery | 0.0% (0/229) | 2.4% (21/860) | 1.9% (21/1,089) |
| Other hospitalization | 4.4% (10/229) | 3.7% (32/860) | 3.9% (42/1,089) |
| Other serious event | 4.4% (10/229) | 4.3% (37/860) | 4.3% (47/1,089) |
| Any of above | 13.1% (30/229)*a | 18.6% (160/860) | 17.4% (190/1,089) |
| None of above | 86.9% (199/229)a | 81.4% (700/860) | 82.6% (899/1,089) |
| Symptoms that the patient is currently experiencing: | |||
| Palpitations | 14.0% (32/229) | 11.6% (100/860) | 12.1% (132/1,089) |
| Sustained rapid heart beat | 6.6% (15/229) | 5.9% (51/860) | 6.1% (66/1,089) |
| Fainting | 0.9% (2/229) | 1.3% (11/860) | 1.2% (13/1,089) |
| Chest pain | 4.4% (10/229) | 5.8% (50/860) | 5.5% (60/1,089) |
| Exercise intolerance | 0.4% (1/229) | 1.7% (15/860) | 1.5% (16/1,089) |
| Shortness of breath | 2.6% (6/229) | 2.9% (25/860) | 2.8% (31/1,089) |
| Other | 2.6% (6/229) | 4.1% (35/860) | 3.8% (41/1,089) |
| Any of above | 31.4% (72/229) | 33.4% (287/860) | 33.0% (359/1,089) |
| None of above | 68.6% (157/229) | 66.6% (573/860) | 67.0% (730/1,089) |
| Is the patient currently taking antiarrhythmic medication? | |||
| Yes | 7.0% (16/229)**a | 16.3% (140/860) | 14.3% (156/1,089) |
Statistical significance at
P < .05
P < .001
CE registry vs NCE registry.
Discussion
The data arising from this study are unique in that they were developed in a prospective, multicenter clinical study that was funded by the National Institutes of Health. Prior studies of the outcomes of RF ablation with comparable numbers of patients have been either single-center retrospective studies4-13 or multicenter studies sponsored by industry.14,15 Although single centers may obtain excellent long-term follow-up (96% at a mean of 21.5 months in one pediatric cohort),16 such studies report the results from a limited number of operators and one laboratory, and they often suffer from observation bias due to a lack of external data review. Consequently, retrospective single-center studies are known to overestimate the success and safety of a procedure while underestimating the risk of recurrence both at the individual center and particularly of the average center. The only independently reviewed multicenter study of RF ablation for supraventricular tachycardia reported in the literature was prospectively performed with an industry sponsor and did include pediatric patients.15 However, the applicability of that study is limited by its use of a single ablation system and the fact that most of the pediatric patients came from only two centers. For one large multicenter prospective study sponsored by the North American Society of Pacing and Electrophysiology,17 data submission was entirely voluntary, and the study lacked the resources for extensive quality control. Furthermore, loss to follow-up was high, occurring in 42% of patients at 6 months.
The current results extend and confirm the prior results of the Pediatric Radiofrequency Ablation Registry, a voluntary registry that first reported results in 1994.1 That study included data from the very earliest days of catheter ablation in children. As such, it reported a lower success rate than later studies and single-center studies. Furthermore, a very high clinical recurrence rate was reported, as high as 49% at 24 months for right free-wall pathways. There was concern that the high rate of loss to follow-up in this study might have artificially inflated the recurrence rate. Although we are not yet reporting results at 24 months, in our study the rate of loss to follow-up was 10.6% at 6 months, which is substantially better than the prior study by Scheinman et al17 and artificially inflated the recurrence rate. Although we are not yet reporting results at 24 months, in our study the rate of loss to follow-up was 10.6% at 6 months, substantially better than the prior study by Scheinman et al17 and comparable to the prior Pediatric Registry (12% at 6 months).1 In the study reported here, pathway location was an important predictor of recurrence, with right-sided accessory pathways having the highest recurrence risk. For the Atakr study noted above,14 Calkins et al reported only a 6% recurrence rate in a population that included mainly adults and which apparently achieved a high follow-up rate. However, this study noted, as does ours, the higher incidence of recurrence after ablation of right freewall pathways and septal pathways. This consistent observation that right-sided pathways recur more frequently suggests that differences may exist in either the anatomy of these pathways or in the technical factors related to lesion formation. This report attempts to define these technical factors in a limited way, finding that maximum achieved temperature and the number of lesions with >20 seconds’ duration were not predictive of recurrence, similar to the findings in other studies.14 The observation that there was a higher rate of recurrence with a higher number of applications of any duration, but not with applications of >20 seconds’ duration (Table 3), is interesting and suggests that either shorter applications produce more ineffective heating or that scouting for successful locations with RF applications (“burn mapping”) may not be an effective strategy for long-term success. This might be particularly important if there is variability in pathway anatomy, such as a more epicardial location for the accessory pathway. Our observation that the use of power control rather than temperature control is associated with recurrence, in the absence of a clear relationship between recurrence and achieved temperature, suggests that the interaction among substrate, tip temperature, and delivered energy is complex. Although power control no longer is commonly used in ablation procedures, the observation is less important from the point of view of determining the best method of performing RF ablation and more for what it might tell us about the underlying causes of recurrence after initially successful ablation. Clearly, evaluation of lesion depth would be ideal but currently is not feasible.
A potentially useful observation from this analysis is the trend toward lower recurrence rate with longer duration of acute observation following successful ablation (Table 4). Based on these results, practitioners may prefer to extend their standard observation time following successful ablation to beyond 30 to 60 minutes. On the other hand, the use of isoproterenol in retesting, although common, does not seem to contribute to identification of recurrence potential.
The data collected in the registry patients (CE and NCE groups) in many ways confirm the findings in the cohort patients. Because there is no requirement for registry patients to have follow-up at set intervals with a cardiologist, the incidence of recurrence cannot be determined in the same way as for the cohort group; therefore, a direct comparison cannot be made. However, one may consider outcome variables, such as the occurrence of cardiac symptoms and the prescription of antiarrhythmic medication, as reasonable indicators. In this respect, the use of antiarrhythmic medications in 7.0% of CE patients is consistent with our reported incidence of recurrence of 10.7% in the cohort patients, considering that not all patients with recurrence will necessarily require or choose to take antiarrhythmic medication. The higher incidence of the use of medications, and of the occurrence of procedures and operations in the NCE group clearly reflects the fact that this group includes patients with other, less easily treated diagnoses (e.g., intraatrial reentry, ventricular tachycardia) and patients with significant congenital heart disease.
There are clear implications of these data for clinical practice and health policy. Any determination of the cost effectiveness of this technology versus other modalities must take into account the success, complication, and recurrence rate of ablation. One attempt at such an analysis for pediatric WPW syndrome used a 90% success rate but did not account for recurrences and the possibility of repeat ablation procedures.18 Clearly, there are significant costs associated with recurrence from medical management with or without repeat ablation, as well as the increased morbidity. Although single-center studies may well report higher success rates and lower rates of recurrence, the current study is the best available benchmark for recurrence in a broad pediatric population. As new technologies such as trans-catheter cryoablation emerge,19-22 they inevitably will be compared with RF ablation, and it will be important to track the outcomes of procedures, both in the short and long term. The dataset established by this study will be invaluable in assessing the place of such new technologies in modern cardiology practice.
Acknowledgments
The authors thank Gaye Courtney, Clinical Study Coordinator, and Ruth Krasnow, Data Manager/Statistical Programmer, both of SRI International, for their diligent work in the implementation of this clinical study.
Appendix Participants and contributing centers
Macdonald Dick II, MD, University of Michigan-CS Mott Children’s Hospital, Ann Arbor, MI; Robert Campbell, MD, Eggleston Children’s Hospital, Atlanta, GA; Yung R. Lau, MD, University of Alabama, Birmingham, AL; Edward P. Walsh, MD, Children’s Hospital, Boston, MA; J. Philip Saul, MD, Medical University of South Carolina, Charleston, SC; Timothy Knilans, MD, Children’s Hospital Medical Center, Cincinnati, OH; William Scott, MD, Children’s Medical Center, Dallas, TX; Jeanny Park, MD, University of California Davis, Davis, CA; Michael S. Schaffer, MD, Children’s Hospital, Denver, Denver, CO; Peter Karpawich, MD, Children’s Hospital of Michigan, Detroit, MI; Ronald Kanter, MD, Duke University, Durham, NC; Margaret Bell, MD, Arrhythmia Associates, Fairfax, VA; Richard Friedman, MD, Texas Children’s Hospital, Houston, TX; Steven Weindling, MD, Dartmouth-Hitchcock Medical Center, Lebanon, NH; Christopher Erickson, MD, Arkansas Children’s Hospital, Little Rock, AR; Ruchir Sehra, MD, Loma Linda University Medical Center, Loma Linda, CA; Kevin M. Shannon, MD, UCLA Children’s Hospital, Los Angeles, CA; Ming-Lon Young, MD, University of Miami, Miami, FL; Ann Dunnigan, MD, Minneapolis Heart Institute, Minneapolis, MN; Frank Fish, MD, Vanderbilt University, Nashville, TN; Steven Fishberger, MD, Children’s Hospital of New York, New York, NY; Bertrand Ross, MD, Children’s Hospital of the King’s Daughters, Norfolk, VA; John Kugler, MD, University of Nebraska, Omaha, NE; Anne M. Dubin, MD, Lucile Packard Children’s Hospital at Stanford, Palo Alto, CA; Ronn Tanel, MD, Children’s Hospital of Philadelphia, Philadelphia, PA; Mary Sokoloski, MD, St. Christopher’s Hospital for Children, Philadelphia, PA; Lee Beerman, MD, Children’s Hospital of Pittsburgh, Pittsburgh, PA; Marc LeGras, MD, Emanuel Children’s Hospital, Portland, OR; Seshadri Balaji, MD, University of Oregon Health Sciences Center, Portland, OR; Coburn Porter, MD, Mayo Clinic, Rochester, MN; Jeanny Park, MD, University of California Davis, Sacramento, CA; Susan Etheridge, MD, University of Utah Primary Children’s Hospital, Salt Lake City, UT; James C. Perry, MD, Children’s Hospital San Diego, San Diego, CA; George F. Van Hare, MD, University of California San Francisco, San Francisco, CA; Frank Cecchin, MD, Children’s Hospital Seattle, Seattle, WA; Frank Zimmerman, MD, St. Louis Children’s Hospital, St. Louis, MO; Burt Bromberg, MD, Children’s Heart Center, St. Louis, St. Louis, MO; Craig Byrum, MD, SUNY-Syracuse, Syracuse, NY; Ricardo Samson, MD, University of Arizona, Tucson, AZ; Robert Hamilton, MD, Hospital for Sick Children, Toronto, Toronto, Ontario, Canada; Jeff Moak, MD, Children’s Hospital National Medical Center, Washington, DC.
Footnotes
This study was supported by grant R01 HL58620 from the National Heart, Lung, and Blood Institute, National Institutes of Health.
Presented in part at the 52nd Annual Scientific Session, American College of Cardiology, Chicago, Illinois, March 31, 2003.
References
- 1.Kugler JD, Danford DA, Deal BJ, Gillette PC, Perry JC, Silka MJ, Van Hare GF, Walsh EP. Radiofrequency catheter ablation for tachyarrhythmias in children and adolescents. The Pediatric Electrophysiology Society. N Engl J Med. 1994;330:1481–1487. doi: 10.1056/NEJM199405263302103. [DOI] [PubMed] [Google Scholar]
- 2.Van Hare GF, Carmelli D, Smith WM, Kugler J, Silka M, Friedman R, Atkins D, Saul P, Schaffer M, Byrum C, Dunnigan A, Colan S, Serwer G. Prospective assessment after pediatric cardiac ablation: design and implementation of the multicenter study. Pacing Clin Electrophysiol. 2002;25:332–341. doi: 10.1046/j.1460-9592.2002.00332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Hare GF, Javitz H, Carmelli D, Saul JP, Tanel RE, Fischbach PS, Kanter RJ, Schaffer MS, Dunnigan A, Colan S, Serwer G. Prospective assessment after pediatric cardiac ablation: demographics, medical profiles and initial outcomes. J Cardiovasc Electrophysiol. doi: 10.1046/j.1540-8167.2004.03645.x. (in press) [DOI] [PubMed] [Google Scholar]
- 4.Baker JH, 2nd, Plumb VJ, Epstein AE, Kay GN. Predictors of recurrent atrioventricular nodal reentry after selective slow pathway ablation. Am J Cardiol. 1994;73:765–769. doi: 10.1016/0002-9149(94)90878-8. [DOI] [PubMed] [Google Scholar]
- 5.Brugada J, Matas M, Mont L, Petit M, Navarro-Lopez F. One thousand consecutive radiofrequency ablation procedures: indications, results, and complications. Rev Esp Cardiol. 1996;49:810–814. [PubMed] [Google Scholar]
- 6.Fenelon G, Elvas L, D’Avila A, Tsakonas K, Malacky T, Manios E, Geelen P, Declerck L, Ramchurn H, De Vusser P, et al. Radiofrequency ablation of atrioventricular node reentrant tachycardia: experience in 302 patients. Acta Cardiol. 1995;50:397–410. [PubMed] [Google Scholar]
- 7.Yu WC, Chen SA, Tai CT, Chiang CE, Lee SH, Chiou CW, Ueng KC, Wen ZC, Chen YJ, Huang JL, Feng AN, Chang MS. Radiofrequency catheter ablation of slow pathway in 760 patients with atrioventricular nodal reentrant tachycardia: long-term results. Zhonghua Yi Xue Za Zhi (Taipei) 1997;59:71–77. [PubMed] [Google Scholar]
- 8.Schluter M, Cappato R, Ouyang F, Antz M, Schluter CA, Kuck KH. Clinical recurrences after successful accessory pathway ablation: the role of “dormant” accessory pathways. J Cardiovasc Electrophysiol. 1997;8:1366–1372. doi: 10.1111/j.1540-8167.1997.tb01033.x. [DOI] [PubMed] [Google Scholar]
- 9.Chen YJ, Chen SA, Tai CT, Chiang CE, Lee SH, Chiou CW, Ueng KC, Wen ZC, Yu WC, Huang JL, Feng AN, Chang MS. Long-term results of radiofrequency catheter ablation in patients with Wolff-Parkinson-White syndrome. Zhonghua Yi Xue Za Zhi (Taipei) 1997;59:78–87. [PubMed] [Google Scholar]
- 10.Pfeiffer D, Neugebauer A, Tebbenjohanns J, Schumacher B, Niehaus M, Rother T, Luderitz B. Radiofrequency ablation of atrioventricular nodal reentrant tachycardia: mechanisms and recurrence rate. Z Kardiol. 89(Suppl 3):103–109. doi: 10.1007/s003920070066. [DOI] [PubMed] [Google Scholar]
- 11.Schlapfer J, Fromer M. Late clinical outcome after successful radiofrequency catheter ablation of accessory pathways. Eur Heart J. 2001;22:605–609. doi: 10.1053/euhj.2000.2409. [DOI] [PubMed] [Google Scholar]
- 12.Clague JR, Dagres N, Kottkamp H, Breithardt G, Borggrefe M. Targeting the slow pathway for atrioventricular nodal reentrant tachycardia: initial results and long-term follow-up in 379 consecutive patients. Eur Heart J. 2001;22:82–88. doi: 10.1053/euhj.2000.2124. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Yao R. Radiofrequency catheter ablation of accessory path-way-mediated tachycardia is a safe and effective long-term therapy. Arch Med Res. 2003;34:394–398. doi: 10.1016/j.arcmed.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 14.The Atakr Multicenter Investigators Group. Calkins H, Prystowsky E, Berger RD, Saul JP, Klein LS, Liem LB, Huang SK, Gillette P, Yong P, Carlson M. Recurrence of conduction following radiofrequency catheter ablation procedures: relationship to ablation target and electrode temperature. J Cardiovasc Electrophysiol. 1996;7:704–712. doi: 10.1111/j.1540-8167.1996.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 15.Calkins H, Yong P, Miller JM, Olshansky B, Carlson M, Saul JP, Huang SK, Liem LB, Klein LS, Moser SA, Bloch DA, Gillette P, Prystowsky E. Catheter ablation of accessory pathways, atrioventricular nodal reentrant tachycardia, and the atrioventricular junction: final results of a prospective, multicenter clinical trial. The Atakr Multicenter Investigators Group. Circulation. 1999;99:262–270. doi: 10.1161/01.cir.99.2.262. [DOI] [PubMed] [Google Scholar]
- 16.Van Hare GF, Witherell CL, Lesh MD. Follow-up of radiofrequency catheter ablation in children: results in 100 consecutive patients. J Am Coll Cardiol. 1994;23:1651–1659. doi: 10.1016/0735-1097(94)90670-x. [DOI] [PubMed] [Google Scholar]
- 17.Scheinman MM, Huang S. The 1998 NASPE prospective catheter ablation registry. Pacing Clin Electrophysiol. 23:1020–1028. doi: 10.1111/j.1540-8159.2000.tb00891.x. [DOI] [PubMed] [Google Scholar]
- 18.Garson A, Jr, Kanter RJ. Management of the child with Wolff-Parkin-son-White syndrome and supraventricular tachycardia: model for cost effectiveness. J Cardiovasc Electrophysiol. 1997;8:1320–1326. doi: 10.1111/j.1540-8167.1997.tb01024.x. [DOI] [PubMed] [Google Scholar]
- 19.Riccardi R, Gaita F, Caponi D, Grossi S, Scaglione M, Caruzzo E, Di Donna P, Pistis G, Richiardi E, Giustetto C, Bocchiardo M. Percutaneous catheter cryothermal ablation of atrioventricular nodal reentrant tachycardia: efficacy and safety of a new ablation technique. Ital Heart J. 2003;4:35–43. [PubMed] [Google Scholar]
- 20.Timmermans C, Ayers GM, Crijns HJ, Rodriguez LM. Randomized study comparing radiofrequency ablation with cryoablation for the treatment of atrial flutter with emphasis on pain perception. Circulation. 2003;107:1250–1252. doi: 10.1161/01.cir.0000061915.06069.93. [DOI] [PubMed] [Google Scholar]
- 21.Carlson MD. Transvenous cryoablation of supraventricular tachycardias: it works but is it better. J Cardiovasc Electrophysiol. 2002;13:1090–1091. doi: 10.1046/j.1540-8167.2002.01090.x. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez LM, Geller JC, Tse HF, Timmermans C, Reek S, Lee KL, Ayers GM, Lau CP, Klein HU, Crijns HJ. Acute results of transvenous cryoablation of supraventricular tachycardia (atrial fibrillation, atrial flutter, Wolff-Parkinson-White syndrome, atrioventricular nodal reentry tachycardia) J Cardiovasc Electrophysiol. 2002;13:1082–1089. doi: 10.1046/j.1540-8167.2002.01082.x. [DOI] [PubMed] [Google Scholar]


