Abstract
Aged subjects have a poor prognosis after traumatic injury and, regardless of the type of injury, they have slower recoveries and suffer more complications than their younger counterparts. The age-dependent responses may be influenced by the hyper-inflammatory state observed in the aged prior to injury, including elevated levels of interleukin-6 (IL-6). Physiological levels of estrogen are beneficial to the immune system, due, in part, to the hormone's ability to attenuate aberrant production of pro-inflammatory cytokines. Using two independent injury models, we have found increased mortality and elevated serum levels of IL-6 in aged mice, when compared to young animals (p<0.05). In parallel studies, groups of aged mice given estrogen (17β-estradiol) prior to scald burn, had significantly improved survival (p<0.05) and lowered serum IL-6 (p<0.05). Multiple cellular mechanisms may be involved in mediating the beneficial effects of estrogen on inflammatory and immune responses in aged individuals who sustain an injury. These mechanisms are discussed herein.
Keywords: Burn injury, lipopolysaccharide, estrogen, trauma, endotoxin, pro-inflammatory cytokines, IL-6
1. Introduction
Aged individuals who suffer injury have greater mortality than those that are younger. For example, a moderate size burn covering 20% of the total body surface area (TBSA) is associated with a mortality of only 5.5% in healthy young adult patients, while elderly patients with the same burn size have a mortality of 75% (ABA, 2002). Aside from increased mortality after injury, the elderly are also more susceptible to infectious challenges (Linton and Dorshkind, 2004). Pneumonia, influenza, and complications of bacteremia are common in the elderly and are associated with a poorer prognosis (Yoshikawa, 2000).
Of the many factors contributing to the increased lethality of injury in the elderly population, is the diminished function of the immune system. Specific age-related alterations in cells of adaptive immunity have been well-established [reviewed in (DeVeale et al., 2004; Mishto et al., 2003; Sadighi Akha and Miller, 2005)], including age-dependent changes in the number and function of T cells and B cells (Effros, 2001; Grubeck-Loebenstein and Wick, 2002; Johnson et al., 2002). Abnormal functioning in components of innate immunity has also been documented in aged subjects (Gomez et al., 2005; Plackett et al., 2004; Plowden et al., 2004; Sebastian et al., 2005). Moreover, evidence from healthy subjects reveals that advanced age is associated with a hyper-inflammatory state, referred to as “inflamm-aging” (Franceschi et al., 2000), highlighted by elevated circulating levels of pro-inflammatory factors (IL-1β, IL-6, TNFα, and prostaglandin E2) (Franceschi et al., 2000). Increased circulating levels of these mediators is a strong predictor of all-cause mortality risk in longitudinal studies of the elderly (Bruunsgaard et al., 2003; Krabbe et al., 2004).
The post-menopausal decline in the levels of gonadal steroid hormones, including estrogen, could contribute to the observed elevation in pro-inflammatory cytokines seen in elderly females [for a review, see (Kovacs, 2005)]. Physiological levels of estradiol present during the reproductive years boost immune responsiveness in young adults (Verthelyi, 2001), and hormone treatment in postmenopausal women improves many immune parameters when compared to controls (Fahlman et al., 2000).
Low physiological levels of estrogen, like those seen throughout the menstrual cycle in women, suppress the production of pro-inflammatory cytokines. Since lowering the aberrant production of pro-inflammatory mediators improves immunity after injury (Christman et al., 1995; Gregory et al., 2000; Kovacs et al., 2004; Strong et al., 2000), we tested the therapeutic efficacy of estrogen supplementation on the immune response following injury in aged female mice. In this short report, we summarize our main results and discuss plausible cellular mechanisms by which estrogen's beneficial effects could occur in aged injured individuals.
2. Materials and Methods
2.1. Animals
Young (3–4 month) and aged (18–22 month) female BALB/c mice from the National Institute on Aging's breeding colonies at Charles River (Portage, MI) and Harlan Sprague Dawley, Inc (Indianapolis, IN) were maintained on a 12 hour light/dark cycle with food and water available ad libitum. The animal studies described herein were performed in strict accordance with the guidelines set forth by the Loyola University Chicago Institutional Animal Care and Use Committee. At the time of sacrifice, all mice were dissected and the organs screened for visible tumors and/or gross abnormalities. Animals with visible tumors or abnormalities were excluded from these studies.
2.2. Induction of injury and exposure to lipopolysaccharide
Mice were subjected to a 15% TBSA dorsal scald or sham injury as previously described (Faunce et al., 1997), with modification (Kovacs et al., 2002). In brief, after induction of anesthesia (Nembutal 40 mg/kg of animals weight, intraperitoneally (i.p.)), clippers were used to remove the hair from the dorsum of each animal. Anesthetized mice receiving scald injuries were placed on their dorsum in a plastic template that exposed 15% TBSA and immersed in a 100°C water bath for 8 seconds. Following burn injury, mice were immediately dried off to prevent any further scalding. Sham-injured animals were anesthetized, shaved, held in a plastic template and placed into a room temperature water bath for the same time period. Both burn and sham injured animals were resuscitated with 1.0 ml of 0.9% normal saline (i.p.). After recovery from anesthesia, the mice were kept warm by placing them under heating lamps. Mice were returned to their cages and maintained under barrier conditions in the animal facility.
In other studies, young and aged mice were given a single i.p. injection of 1.5 μg LPS/g of weight derived from Pseudomonas aeruginosa, serotype 10 (Sigma Chemical Co., St. Louis, MO) dissolved in sterile saline, in a volume of injection averaging 170 μl. This dose has been demonstrated to produce mortality close to 25% in aged mice and no mortality in young animals (Gomez et al., 2006). Control mice received saline alone. In order to avoid confounding factors related to circadian rhythms, all animal studies were performed between 8–10 AM.
2.3. Determination of circulating levels of IL-6
Animals were either sacrificed 24 hours after treatment or were monitored over a 10 days period. Mice were sacrificed by CO2 inhalation and subsequent cervical dislocation. For measurement of circulating IL-6, blood was obtained by cardiac puncture at the time of sacrifice and serum was stored at 80°C prior to assay, as described previously (Faunce et al., 1998). The IL-6 content in serum was determined by ELISA according to the manufacturers' specifications (Endogen Inc., Cambridge, MA, for burn injury studies, and Pharmingen, San Diego, CA for LPS administration studies), the minimal level of detection of the Endogen kit was 25 pg/ml, and that of Pharmingen was 15 pg/ml.
2.4. Hormone replacement
In some studies, aged mice were given 17β-estadiol in the form of slow release cholesterol based polymer pellets implanted subcutaneously (IRA, Tampa, FL), as previously described (Gregory et al., 2000). The pellet delivers 30 pg/ml of 17β-estadiol (or placebo) for 21 days. Hormone or placebo pellets were given 7 days prior to injury.
2.5. Statistical analyses
Data are expressed as mean ± SEM unless otherwise noted. Differences between groups were determined by ANOVA followed by Newman-Keuls post hoc test, using GB-Stat School Pack software (Dynamic Microsystems, Silver Spring, MD). For mortality studies, the Mann-Whitney U-test was used. A difference of p<0.05 was considered significant.
3. Results
3.1. Effect of aging on survival after insult
Survival was examined in aged animals at 24 hours after burn injury or LPS administration (Table 1). No mortality was observed in either of the control groups (sham injury or saline treatment), regardless of age. In contrast, burn-injured aged mice had 58 % mortality. In a separate series of experiments, the aged LPS treated mice had 23% morality. These results indicate higher mortality in aged mice undergoing two different models of injury.
TABLE 1.
Mortality after burn injury or LPS
| % Mortalitya of young and aged mice given burn injury or intraperitoneal LPS | ||||
|---|---|---|---|---|
| Burn Model | LPS Model | |||
| Sham | Burn | Saline | LPS | |
| Young | 0 | 0 | 0 | 0 |
| Aged | 0 | 58b | 0 | 23c |
Values are expressed as percent of mortality (%) for young and aged BALB/C mice at 24 hours after either a 15% total body surface area scald, sham injury, an intraperitoneal injection of LPS (1.5 μg/g body weight), or saline. N = 12-18 mice per group for burn injury studies. N = 8-12 mice per group for LPS administration studies.
<0.05 from all other groups,
<0.05 from all other groups, as determined by Mann-Whitney U-test.
3.2. Effect of advanced age on serum levels of IL-6 after insult
We next evaluated serum levels of IL-6, a cytokine previously reported to be elevated in healthy aged subjects (Franceschi et al., 2000), and elevated more markedly after an inflammatory challenge in aged individuals (Bruunsgaard and Pedersen, 2003; Tateda et al., 1996). In the burn model, serum levels of IL-6 were undetectable in sham injured young mice (Table 2). After burn injury, young mice had an increase in the level of serum IL-6, when compared to young sham-injured animals. In contrast to young sham-injured animals, aged sham-injured mice had elevated levels of IL-6. Moreover, the serum concentration of IL-6 was almost doubled in aged burn-injured mice, when compared to young burn-injured and aged sham-injured mice.
TABLE 2.
Serum IL-6 in young and aged mice given burn injury or LPS
| Serum IL-6a in young and aged mice given burn injury or intraperitoneal LPS | ||||
|---|---|---|---|---|
| Burn Model | LPS Model | |||
| Sham | Burn | Saline | LPS | |
| Young | n.d.c | 151 ± 25 | n.d. | 37 ± 27 |
| Aged | 116 ± 37 | 298 ± 73b | 32 ± 9 | 5151 ± 3000b |
Values are expressed as mean IL-6 pg/ml ± standard error. Twenty-four hours after young and aged mice were given a 15% total body surface area scald, a sham injury, an intraperitoneal injection of LPS (1.5 μg/g body weight), or saline. blood was collected by cardiac puncture and serum assayed for IL-6 by ELISA. Data are shown as mean IL-6 pg/ml ± SEM. N = 8-12 mice per group.
p< 0.05 from all other groups.
n.d. = not detectable
In the endotoxin model, IL-6 was not detectable in the serum of young mice given saline, but was present after LPS treatment. Even in the absence of LPS injection, aged mice had detectable IL-6 in their serum. Finally, aged mice given LPS showed a dramatic elevation in the serum IL-6 levels, which was over 100 times that of young mice given LPS (p<0.05). Overall, these results show that aged mice have greater elevation in the serum levels of the pro-inflammatory cytokine, IL-6, after either inflammatory insult (burn injury or LPS exposure).
3.3. Effects of estrogen treatment on survival of aged mice following burn injury
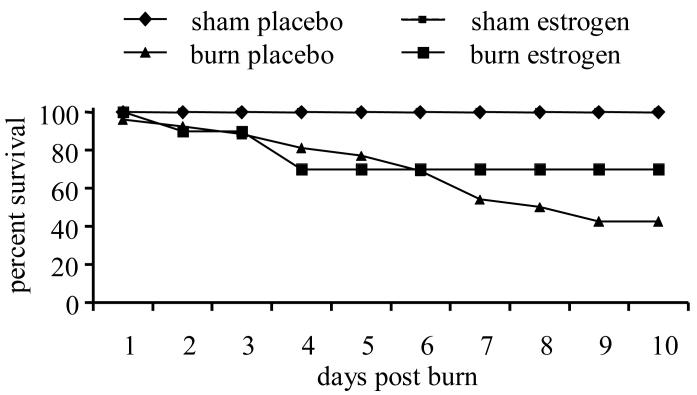
Since physiological levels of estrogen boost immune responses, further studies examined the effects of estrogen treatment on cellular immunity after burn injury in aged mice. Survival was followed for 10 days after burn injury in aged mice (Figure 1). No mortality was observed in the sham-injured placebo-treated or sham-injured estrogen-treated groups over the 10 day period. Mortality in the burn-injured placebo-treated group was observed as soon as 1 day after burn and reached 58% mortality (42% survival) after 6 days after injury. Mortality in the burn-injured estrogen-treated group was similar to the observed for aged mice administered placebo over the first 6 days, but increased survival was observed for the rest of the study, reaching a 70% survival at day 10. Thus, there was a 28% increase in survival of estrogen-treated mice, when compared to the aged burn group given the placebo control (p<0.05).
Figure 1.
Estrogen treatment in aged mice improves survival after burn injury. Aged mice given estrogen or placebo hormone treatment were subjected to a 15% total body surface area burn or sham injury. Survival was monitored over a 10 day period of time. Data are expressed as % survival. N=12 mice per group, p<0.01.
4. Discussion
Aged patients have higher mortality after injury than younger subjects (ABA, 2002; Yoshikawa, 2000). Using a murine model, we found that a higher number of aged mice succumb after being subjected to burn injury or endotoxemia. The diminished function of the immune system and the overproduction of pro-inflammatory cytokines, which are associated with the natural aging process, are likely to play a role in the increased mortality after injury or infection.
In our experiments, the treatment of aged mice with low proestrous levels of estrogen resulted in a marked improvement in survival over a 10 day period after burn injury. This improved survival was accompanied by a diminution in the serum levels of IL-6 (Kovacs et al., 2004). Additionally, we have reported that estrogen treatment partially restored the delayed-type hypersensitivity response (Kovacs et al., 2004), a systemic cellular immune response previously described to be markedly attenuated in aged mice after burn injury (Kovacs et al., 2002; Plackett et al., 2003).
Multiple molecular mechanisms have been proposed to explain the regulatory effects of estrogen on the production of pro-inflammatory cytokines (Pfeilschifter et al., 2002). Among these interactions are direct effects on cytokine production due to alterations in NF-κB function, and indirect effects due to reactive oxygen species production, alteration of the hypothalamic-pituitary-adrenal axis, and changes in adipose tissue. Estrogen, at pro-estrous levels, inhibits expression and release of pro-inflammatory cytokines, such as IL-6 and TNFα, in a variety of cell types, including peripheral blood mononuclear cells (Bernard-Poenaru et al., 2001), Kupffer cells (Yokoyama et al., 2003), bone marrow-derived stromal cells and osteoclasts (Girasole et al., 1992), and endothelial cells (Keck et al., 1998; Sukovich et al., 1998). Additionally, the hormone regulates lymphocyte functions (Olsen and Kovacs, 1996), such as the distribution of T cell subsets (Fujita et al., 1984) and B cell lymphopoiesis in bone marrow (Masuzawa et al., 1994). Hence, the effects of estrogen on immune cells are broad ranging.
Since pro-inflammatory cytokine genes lack the classical estrogen response elements (ERE) in their promoters (Pottratz et al., 1994), a mechanism other than the binding of the estrogen-estrogen receptor (ER) complex to ERE must be responsible for the transcriptional regulation of those genes. Studies have shown that transcription factors, including nuclear factor-κB (NF-κB) (Stein and Yang, 1995), fos/jun homo-or heterodimers (Sanchez et al., 2002), and CCAAT / enhancer-binding protein (C/EBP)β (Stein and Yang, 1995), interact with the hormone-receptor complex to mediate transcriptional regulation of IL-6 and TNFα in cell cultures. Of these, the best described mechanism involves ER and NF-κB interaction (Kalaitzidis and Gilmore, 2005), in which dimmers of estrogen-ER bind to NF-κB and prevent its activation and interaction with their DNA binding sites (Kalaitzidis and Gilmore, 2005). Additionally, studies have revealed that in vivo low proestrus levels of estrogen attenuate the elevated activation of NF-κB in ex vivo macrophages obtained from young adult mice in a model of acute ethanol exposure followed by burn injury (Messingham et al., 2001). These results indicate that systemic exposure to the hormone can reduce activation of this transcription factor.
In addition to its direct effect on cytokine gene expression, estrogen also exerts effects of inflammation via the free radial production. Estrogen has direct anti-oxidant effects related to scavenging of reactive oxygen species (Bruce-Keller et al., 2000). The decline in estrogen concentrations with advanced age may increase the amount of free radicals, which, in turn, can act as potent stimulants of NF-κB-mediated pro-inflammatory cytokine expression (Pfeilschifter et al., 2002). Additional studies are needed to further examine, the role of the anti-oxidative effects of estrogen on cytokine production and the effects of hormonal treatment on the restoration of a favorable redox state in the elderly after injury.
Estrogen alters the hypothalamic-pituitary-adrenal axis by modulation of the release of growth hormone and prolactin, which in turn may modify immune responses and cytokine secretion (Chowen et al., 2004; Peeva et al., 2000). Additionally, the hormone enhancement of corticotropin-releasing hormone gene expression (Chrousos et al., 1998) may stimulate the central noradrenergic pathways and thereby suppress the inflammatory responses (Pfeilschifter et al., 2002). Hence, there are also indirect actions of estrogens on pro-inflammatory cytokine release due to effects on the hypothalamic-pituitary-adrenal axis.
Aside from immune cells, adipose tissue is also a source of increased pro-inflammatory cytokines (Ahima and Flier, 2000). In particular, adipocytes obtained from visceral fat can release 2-3 more IL-6 than those from the subcutaneous compartment (Fried et al., 1998). Since aged animals and humans have more visceral fat than the young, (Barzilai and Rossetti, 1995; Beaufrere and Morio, 2000) the increased amount of visceral fat could be partially responsible for the higher levels of production of IL-6 seen with advanced age. In humans, estrogen replacement can prevent the age associated increase in visceral fat and reduce serum IL-6 (Straub et al., 2000). Preliminary studies by our laboratory suggest that estrogen treatment in aged burn-injured animals decreases the production of fat-derived IL-6 production. Further investigation is needed to confirm these results.
5. Summary
The poor outcome in the aged who sustain injury is associated with a greater level of inflammation, when compared to young adults. Estrogen treatment lowers inflammatory responses and, hence, has beneficial effects in aged injured subjects. Although the cellular mechanisms responsible for the attenuation of inflammatory response and improvement in cellular immunity have yet to be fully elucidated, progress in the field is being made. From the current work, it is clear that age-specific treatments should be considered.
Acknowledgements
The authors thank Dr. Pamela Witte, Director of the Immunology and Aging Program at Loyola University Medical Center for her thoughtful discussions. This work was supported by the National Institutes of Health R01 AG18859 and AG18859-S1, an Illinois Excellence in Academic Medicine Grant, and the Dr. Ralph and Marion C. Falk Medical Research Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Burn Association (ABA National Burn Repository: 2002 Report. 2002 [Google Scholar]
- Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends Endocrinol Metab. 2000;11:327–32. doi: 10.1016/s1043-2760(00)00301-5. [DOI] [PubMed] [Google Scholar]
- Barzilai N, Rossetti L. Relationship between changes in body composition and insulin responsiveness in models of the aging rat. Am J Physiol. 1995;269:E591–7. doi: 10.1152/ajpendo.1995.269.3.E591. [DOI] [PubMed] [Google Scholar]
- Beaufrere B, Morio B. Fat and protein redistribution with aging: metabolic considerations. Eur J Clin Nutr. 2000;54(Suppl 3):S48–53. doi: 10.1038/sj.ejcn.1601025. [DOI] [PubMed] [Google Scholar]
- Bernard-Poenaru O, Roux C, Blanque R, Gardner C, de Vemejoul MC, Cohen-Solal ME. Bone-resorbing cytokines from peripheral blood mononuclear cells after hormone replacement therapy: a longitudinal study. Osteoporos Int. 2001;12:769–76. doi: 10.1007/s001980170054. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Keeling JL, Keller JN, Huang FF, Camondola S, Mattson MP. Antiinflammatory effects of estrogen on microglial activation. Endocrinology. 2000;141:3646–56. doi: 10.1210/endo.141.10.7693. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H, Ladelund S, Pedersen AN, Schroll M, Jorgensen T, Pedersen BK. Predicting death from tumour necrosis factor-alpha and interleukin-6 in 80-year-old people. Clin Exp Immunol. 2003;132:24–31. doi: 10.1046/j.1365-2249.2003.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruunsgaard H, Pedersen BK. Age-related inflammatory cytokines and disease. Immunol Allergy Clin North Am. 2003;23:15–39. doi: 10.1016/s0889-8561(02)00056-5. [DOI] [PubMed] [Google Scholar]
- Chowen JA, Frago LM, Argente J. The regulation of GH secretion by sex steroids. Eur J Endocrinol. 2004;151(Suppl 3):U95–100. doi: 10.1530/eje.0.151u095. [DOI] [PubMed] [Google Scholar]
- Christman JW, Holden EP, Blackwell TS. Strategies for blocking the systemic effects of cytokines in the sepsis syndrome. Crit Care Med. 1995;23:955–63. doi: 10.1097/00003246-199505000-00027. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Torpy DJ, Gold PW. Interactions between the hypothalamic-pituitary-adrenal axis and the female reproductive system: clinical implications. Ann Intern Med. 1998;129:229–40. doi: 10.7326/0003-4819-129-3-199808010-00012. [DOI] [PubMed] [Google Scholar]
- DeVeale B, Brummel T, Seroude L. Immunity and aging: the enemy within? Aging Cell. 2004;3:195–208. doi: 10.1111/j.1474-9728.2004.00106.x. [DOI] [PubMed] [Google Scholar]
- Effros RB. Ageing and the immune system. Novartis Found Symp. 2001;235:130–9. doi: 10.1002/0470868694.ch12. discussion 139-45, 146-9. [DOI] [PubMed] [Google Scholar]
- Fahlman MM, Boardley D, Flynn MG, Bouillon LE, Lambert CP, Braun WA. Effects of hormone replacement therapy on selected indices of immune function in postmenopausal women. Gynecol Obstet Invest. 2000;50:189–93. doi: 10.1159/000010308. [DOI] [PubMed] [Google Scholar]
- Faunce DE, Gregory MS, Kovacs EJ. Effects of acute ethanol exposure on cellular immune responses in a murine model of thermal injury. J Leukoc Biol. 1997;62:733–40. doi: 10.1002/jlb.62.6.733. [DOI] [PubMed] [Google Scholar]
- Faunce DE, Gregory MS, Kovacs EJ. Acute ethanol exposure prior to thermal injury results in decreased T-cell responses mediated in part by increased production of IL-6. Shock. 1998;10:135–40. doi: 10.1097/00024382-199808000-00009. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–54. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–50. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- Fujita T, Matsui T, Nakao Y, Watanabe S. T lymphocyte subsets in osteoporosis. Effect of 1-alpha hydroxyvitamin D3. Miner Electrolyte Metab. 1984;10:375–8. [PubMed] [Google Scholar]
- Girasole G, Jilka RL, Passeri G, Boswell S, Boder G, Williams DC, Manolagas SC. 17 beta-estradiol inhibits interleukin-6 production by bone marrow-derived stromal cells and osteoblasts in vitro: a potential mechanism for the antiosteoporotic effect of estrogens. J Clin Invest. 1992;89:883–91. doi: 10.1172/JCI115668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez CR, Boehmer ED, Kovacs EJ. The aging innate immune system. Curr Opin Immunol. 2005;17:457–462. doi: 10.1016/j.coi.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Gomez CR, Goral J, Ramirez L, Kopf M, Kovacs EJ. Aberrant Acute-Phase Response In Aged Interleukin-6 Knockout Mice. Shock. 2006;25:581–585. doi: 10.1097/01.shk.000029553.39081.ec. [DOI] [PubMed] [Google Scholar]
- Gregory MS, Duffner LA, Faunce DE, Kovacs EJ. Estrogen mediates the sex difference in post-burn immunosuppression. J Endocrinol. 2000;164:129–38. doi: 10.1677/joe.0.1640129. [DOI] [PubMed] [Google Scholar]
- Gregory MS, Faunce DE, Duffner LA, Kovacs EJ. Gender difference in cell-mediated immunity after thermal injury is mediated, in part, by elevated levels of interleukin-6. J Leukoc Biol. 2000;67:319–26. [PubMed] [Google Scholar]
- Grubeck-Loebenstein B, Wick G. The aging of the immune system. Adv Immunol. 2002;80:243–84. doi: 10.1016/s0065-2776(02)80017-7. [DOI] [PubMed] [Google Scholar]
- Johnson KM, Owen K, Witte PL. Aging and developmental transitions in the B cell lineage. Int Immunol. 2002;14:1313–23. doi: 10.1093/intimm/dxf092. [DOI] [PubMed] [Google Scholar]
- Kalaitzidis D, Gilmore TD. Transcription factor cross-talk: the estrogen receptor and NF-kappaB. Trends Endocrinol Metab. 2005;16:46–52. doi: 10.1016/j.tem.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Keck C, Herchenbach D, Pfisterer J, Breckwoldt M. Effects of 17beta-estradiol and progesterone on interleukin-6 production and proliferation of human umbilical vein endothelial cells. Exp Clin Endocrinol Diabetes. 1998;106:334–9. doi: 10.1055/s-0029-1211994. [DOI] [PubMed] [Google Scholar]
- Kovacs EJ. Aging, traumatic injury, and estrogen treatment. Exp Gerontol. 2005;40:549–55. doi: 10.1016/j.exger.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Kovacs EJ, Grabowski KA, Duffner LA, Plackett TP, Gregory MS. Survival and cell mediated immunity after burn injury in aged mice. J. Amer. Aging Assoc. 2002;25:3–10. doi: 10.1007/s11357-002-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs EJ, Plackett TP, Witte PL. Estrogen replacement, aging, and cell-mediated immunity after injury. J Leukoc Biol. 2004;76:36–41. doi: 10.1189/jlb.1103538. [DOI] [PubMed] [Google Scholar]
- Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004;39:687–99. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–9. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- Masuzawa T, Miyaura C, Onoe Y, Kusano K, Ohta H, Nozawa S, Suda T. Estrogen deficiency stimulates B lymphopoiesis in mouse bone marrow. J Clin Invest. 1994;94:1090–7. doi: 10.1172/JCI117424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messingham KA, Heinrich SA, Kovacs EJ. Estrogen restores cellular immunity in injured male mice via suppression of interleukin-6 production. J Leukoc Biol. 2001;70:887–95. [PubMed] [Google Scholar]
- Mishto M, Santoro A, Bellavista E, Bonafe M, Monti D, Franceschi C. Immunoproteasomes and immunosenescence. Ageing Res Rev. 2003;2:419–32. doi: 10.1016/s1568-1637(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Olsen NJ, Kovacs WJ. Gonadal steroids and immunity. Endocr Rev. 1996;17:369–84. doi: 10.1210/edrv-17-4-369. [DOI] [PubMed] [Google Scholar]
- Peeva E, Grimaldi C, Spatz L, Diamond B. Bromocriptine restores tolerance in estrogen-treated mice. J Clin Invest. 2000;106:1373–9. doi: 10.1172/JCI10420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeilschifter J, Koditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002;23:90–119. doi: 10.1210/edrv.23.1.0456. [DOI] [PubMed] [Google Scholar]
- Plackett TP, Boehmer ED, Faunce DE, Kovacs EJ. Aging and innate immune cells. J Leukoc Biol. 2004;76:291–9. doi: 10.1189/jlb.1103592. [DOI] [PubMed] [Google Scholar]
- Plackett TP, Schilling EM, Faunce DE, Choudhry MA, Witte PL, Kovacs EJ. Aging enhances lymphocyte cytokine defects after injury. Faseb J. 2003;17:688–9. doi: 10.1096/fj.02-0452fje. [DOI] [PubMed] [Google Scholar]
- Plowden J, Renshaw-Hoelscher M, Engleman C, Katz J, Sambhara S. Innate immunity in aging: impact on macrophage function. Aging Cell. 2004;3:161–7. doi: 10.1111/j.1474-9728.2004.00102.x. [DOI] [PubMed] [Google Scholar]
- Pottratz ST, Bellido T, Mocharla H, Crabb D, Manolagas SC. 17 beta-Estradiol inhibits expression of human interleukin-6 promoter-reporter constructs by a receptor-dependent mechanism. J Clin Invest. 1994;93:944–50. doi: 10.1172/JCI117100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadighi Akha AA, Miller RA. Signal transduction in the aging immune system. Curr Opin Immunol. 2005;17:486–91. doi: 10.1016/j.coi.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Sanchez R, Nguyen D, Rocha W, White JH, Mader S. Diversity in the mechanisms of gene regulation by estrogen receptors. Bioessays. 2002;24:244–54. doi: 10.1002/bies.10066. [DOI] [PubMed] [Google Scholar]
- Sebastian C, Espia M, Serra M, Celada A, Lloberas J. MacrophAging: a cellular and molecular review. Immunobiology. 2005;210:121–6. doi: 10.1016/j.imbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Stein B, Yang MX. Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-kappa B and C/EBP beta. Mol Cell Biol. 1995;15:4971–9. doi: 10.1128/mcb.15.9.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RH, Hense HW, Andus T, Scholmerich J, Riegger GA, Schunkert H. Hormone replacement therapy and interrelation between serum interleukin-6 and body mass index in postmenopausal women: a population-based study. J Clin Endocrinol Metab. 2000;85:1340–4. doi: 10.1210/jcem.85.3.6355. [DOI] [PubMed] [Google Scholar]
- Strong VE, Mackrell PJ, Concannon EM, Naama HA, Schaefer PA, Shaftan GW, Stapleton PP, Daly JM. Blocking prostaglandin E2 after trauma attenuates pro-inflammatory cytokines and improves survival. Shock. 2000;14:374–9. doi: 10.1097/00024382-200014030-00023. [DOI] [PubMed] [Google Scholar]
- Sukovich DA, Kauser K, Shirley FD, DelVecchio V, Halks-Miller M, Rubanyi GM. Expression of interleukin-6 in atherosclerotic lesions of male ApoE-knockout mice: inhibition by 17beta-estradiol. Arterioscler Thromb Vasc Biol. 1998;18:1498–505. doi: 10.1161/01.atv.18.9.1498. [DOI] [PubMed] [Google Scholar]
- Tateda K, Matsumoto T, Miyazaki S, Yamaguchi K. Lipopolysaccharide-induced lethality and cytokine production in aged mice. Infect Immun. 1996;64:769–74. doi: 10.1128/iai.64.3.769-774.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verthelyi D. Sex hormones as immunomodulators in health and disease. Int Immunopharmacol. 2001;1:983–93. doi: 10.1016/s1567-5769(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Yokoyama Y, Kuebler JF, Matsutani T, Schwacha MG, Bland KI, Chaudry IH. Mechanism of the salutary effects of 17beta-estradiol following trauma-hemorrhage: direct downregulation of Kupffer cell proinflammatory cytokine production. Cytokine. 2003;21:91–7. doi: 10.1016/s1043-4666(03)00014-0. [DOI] [PubMed] [Google Scholar]
- Yoshikawa TT. Epidemiology and unique aspects of aging and infectious diseases. Clin Infect Dis. 2000;30:931–3. doi: 10.1086/313792. [DOI] [PubMed] [Google Scholar]