Abstract
Mitral annular calcium (MAC) has been shown to be an independent predictor of stroke, but the basis for this association remains incompletely defined. We investigated the extent to which aortogenic embolism may mediate the MAC-stroke relationship. In a cross-sectional study of patients referred for transesophageal echocardiography at our institution for evaluation of cerebral ischemia, we assessed the relationship between MAC and proximal and distal complex aortic atheroma (CAA). Among 419 patients composing the study cohort, MAC was associated with atherosclerosis risk factors, prior cardiovascular disease, atrial fibrillation, ipsilateral large-artery stenosis, left-heart abnormalities and aortic valve calcium. MAC was associated with CAA of the proximal and distal thoracic aorta in unadjusted analyses, and these associations became stronger with increasing MAC severity. After adjustment for clinical and echocardiographic covariates, MAC exhibited a significant association with proximal and distal CAA in the entire cohort. In patients without an alternative potential mechanism for cerebral ischemia, however, the relationship with proximal CAA became more pronounced (adjusted odds ratio=2.74, 95% confidence interval=1.22-6.16), but that for distal CAA changed little and became non-significant (adjusted odds ratio=1.97, 95% confidence interval=0.87-4.45). In this cohort with cerebral ischemia, MAC was significantly associated with proximal and distal CAA independent of clinical and echocardiographic covariates, but among individuals without identifiable alternative mechanisms, the magnitude of the relation increased only for proximal CAA. These findings support the role of proximal CAA as a direct mediator of the increased stroke risk associated with MAC.
Mitral annular calcium (MAC)1 is an independent predictor of stroke,2-4 but the mechanisms underlying the MAC-stroke relationship are not well delineated.4 Protruding, ulcerated or mobile atheromatous plaques of the thoracic aorta also exhibit a strong association with stroke;5-7 the presence of complex plaque of the ascending aorta and aortic arch quadruples the risk of stroke recurrence independent of atrial fibrillation, carotid stenosis and other risk factors.8 This robust association is largely attributable to atheromatous thromboembolism from sites proximal to the cervicocephalic arteries.9 While an association between MAC and aortic atheroma has been reported in cohorts referred for transesophageal echocardiography (TEE) for various indications10, 11 or in patients with atrial fibrillation,12 the relationship between MAC and complex atheroma of the proximal aorta has not been specifically examined in patients with cerebral ischemia. Moreover, most studies reporting an association between MAC and complex aortic atheroma (CAA) have not adjusted for potential clinical or echocardiographic confounders.10, 11, 13 The strength of the association, independent of potential confounders, between MAC and proximal CAA in patients with otherwise unexplained cerebrovascular events would provide a more direct measure of the extent to which thromboembolism from the thoracic aorta might mediate MAC-related cerebrovascular risk. To this end, we assessed the MAC-atheroma relationship in a series of thoroughly evaluated patients with cerebral ischemia.
Methods and Materials
We conducted a retrospective cross-sectional study of patients with cerebral ischemia referred for TEE at Weill-Cornell Medical Center for evaluation of a source of cardioembolism. The Echocardiography Laboratory's electronic database was queried to select consecutive patients referred with cerebrovascular events from January 1998 to September 2004. Subjects with infective endocarditis, congenital heart disease (excepting isolated atrial septal defect), rheumatic valve disease, or valve repair or replacement were excluded. Chart review was performed to confirm the diagnosis of stroke or transient ischemic attack. Clinical characteristics were abstracted from paper and electronic medical records.
Echocardiographic data were abstracted from electronic reports generated clinically by one of five experienced echocardiographers in our Laboratory. As a rule, comprehensive studies are performed for all patients referred for TEE at our institution, but particular focus on detection of cardiac sources of embolism is given to patients with cerebrovascular events. This includes assessment of all aortic segments and evaluation of the interatrial septum with color Doppler and agitated-saline injections at rest and with Valsalva/coughing. During the study period, TEEs were performed with multiplane probes using commercially available ultrasound machines. MAC was identified by the presence of bright echoes at the base of the mitral leaflets by two-dimensional TEE imaging, and severity graded along a semi-quantitative scale (mild, moderate, or severe) based on the extent of annular calcific deposits. Proximal CAA was defined as atheromatous plaque located before the origin of the left subclavian artery, and distal CAA as plaque detected beyond this point. For the purposes of our analyses, we defined CAA a priori as sessile plaque protruding ≥4mm into the lumen or with ulcerated or mobile components.14 This accords with the definition used clinically for the majority of studies after 2001. In earlier studies, however, the practice of the Echocardiography Laboratory was to report aortic atheroma severity according to a previous classification scheme.15 In such instances, during extraction of data from echocardiogram reports, plaques that were reported to be Grades 1 or 2 (no plaque or intimal thickening) were given a “no atheroma” designation, while those reported as Grades 4 or 5 (≥5 mm in thickness or with mobile components) were classified as “complex atheroma”. In the case of 36 patients, where Grade 3 (simple plaque <5 mm in thickness) was assigned in the reports, one echocardiographer (J.R.K.) re-assessed the videotape images of the thoracic aorta blinded to MAC, aortic valve calcium, or other cardiac features – through appropriate queuing of the videotape and cover-up of the monitor screen by another investigator (M.G.K. or S.F.). LV hypertrophy was defined qualitatively based on LV wall thickness and LV internal dimension when accurate measurements could not be obtained. Alternatively, when adequate determinations of LV dimensions were possible from transgastric TEE images, LV mass was calculated based on a validated formula,16 and LV hypertrophy was assigned using partition values of LV mass indexed to body-surface area.17 Aortic valve calcium was identified by visualization of bright echoes on the leaflets, irrespective of restricted leaflet motion. Left atrial enlargement was defined qualitatively. Definite cardiac sources of embolism other than proximal CAA included interatrial shunt (patent foramen ovale or atrial septal defect) with documented deep-vein thrombosis or pulmonary embolism, left atrial thrombus, left-sided cardiac myxoma, LV thrombus, or non-infective vegetation of the mitral or aortic valve.18 A probable cause of cerebral ischemia other than proximal CAA was then defined as presence of a definite cardiac source of embolism; atrial fibrillation irrespective of left atrial thrombus detection; cervicocranial artery stenosis ≥50% ipsilateral to the ischemic brain territory; recent (<4 weeks) arterial endovascular intervention; or hypercoagulable state, including anti-phospholipid syndrome, malignancy, and recent surgery (<4 weeks).
Hypertension was defined by history, antihypertensive therapy, or blood pressure >160/100 mmHg during hospitalization for the index cerebrovascular event. Diabetes mellitus was defined by history, hypoglycemic therapy, fasting blood glucose ≥140 mg/dl, or random blood glucose ≥200 mg/dl. Hypercholesterolemia was defined by history, cholesterol-lowering therapy, or fasting plasma cholesterol ≥240 mg/dl during the index hospitalization. Current smokers were defined by cigarette use at the time of index event. Renal insufficiency was defined as serum creatinine ≥2.0 mg/dl.
Transient ischemic attack was defined as an acute neurologic deficit of presumed vascular origin lasting <24 hours, based on the diagnosis assigned by an attending neurologist or upon clinical features documented in the medical chart. Stroke was defined as an acute neurologic deficit of presumed vascular origin lasting ≥24 hours, or the presence of brain infarction on neuroimaging. Carotid, intracerebral (anterior cerebral, middle cerebral and posterior cerebral artery) and vertebrobasilar artery stenosis was defined as a ≥50% narrowing of the vessel lumen by Duplex sonography, transcranial Doppler, or magnetic resonance or computed tomography angiography. Subjects with a Modified Rankin Scale >2 were considered to have disabling strokes.19
Comparisons of categorical and continuous variables applied the chi-square or Fisher's exact test, or the Wilcoxon rank-sum test, as appropriate. Logistic regression was used to calculate unadjusted and adjusted odds ratios (ORs). Seven clinical variables were considered as potential confounders, namely, age (continuous), and sex, hypertension, diabetes, hypercholesterolemia, current smoking, and renal insufficiency (all dichotomous); so too were the echocardiographic variables LV hypertrophy, left atrial enlargement, and aortic valve calcium (all dichotomous). For assembly of multivariable models in the entire cohort, only covariates exhibiting a relationship with proximal, distal, or any CAA at a significance level of 0.20 in univariable analyses were included. Because of the smaller number of instances of CAA in the subgroup without an alternative explanation for cerebral ischemia, more parsimonious multivariable models were used in these analyses. SPSS version 12, SPSS, Inc., Chicago, IL, was used in all analyses.
Results
Four hundred nineteen patients met inclusion criteria. Their clinical and demographic characteristics are listed in Table 1. Slightly over one-fifth of the cohort had MAC of at least mild severity. Patients with MAC were older, and generally had higher prevalences of traditional atherosclerosis risk factors than those without MAC. The presence of MAC was also more frequently associated with prior coronary or cerebrovascular disease, and with previous or newly diagnosed atrial fibrillation. Subjects with MAC had a higher frequency of significant carotid or intracranial artery stenosis, as well as large-artery stenosis ipsilateral to the ischemic cerebral territory. Their infarcts were more often cortical and disabling than those of patients without MAC.
Table 1.
Clinical and Demographic Characteristics
| Mitral Annular Calcium | ||||
|---|---|---|---|---|
| Variable | Entire Cohort (N=419) |
Yes (N=94) |
No (N=325) |
P Value |
| Age* (years) | 59(17-92) | 72(35-92) | 55(17-88) | <0.001 |
| Female | 47.3% | 48.9% | 46.8% | 0.711 |
| Hypertension | 54.5% | 83.3% | 45.8% | <0.001 |
| Diabetes mellitus | 16.4% | 23.6% | 14.2% | 0.035 |
| Hypercholesterolemia | 40.7% | 60.0% | 34.7% | <0.001 |
| Body mass index* (kg/m2) | 25.3(14.1-52.5) | 25.4(17.2-52.5) | 25.2(14.1-52.5) | 0.695 |
| Current smoker | 18.0% | 20.5% | 17.3% | 0.503 |
| Renal insufficiency | 4.1% | 7.4% | 3.1% | 0.074 |
| Previous myocardial infarction | 7.3% | 12.5% | 5.8% | 0.034 |
| Heart failure | 3.9% | 4.5% | 3.7% | 0.756 |
| Prior stroke or transient ischemic attack |
23.2% | 31.5% | 20.7% | 0.036 |
| Previous or new atrial fibrillation | 8.8% | 18.1% | 6.2% | <0.001 |
| History of venous thromboembolism | 2.6% | 3.2% | 2.5% | 0.716 |
| Hypercoagulable state | 6.0% | 9.6% | 4.9% | 0.094 |
| Recent endovascular intervention | 0.2% | 1.1% | 0% | 0.224 |
| Cerebrovascular Events | ||||
| Transient ischemic attack | 24.1% | 19.5% | 25.4% | 0.260 |
| Anterior circulation stroke | 55.7% | 59.1% | 54.7% | 0.469 |
| Cortical infarct | 46.1% | 55.8% | 43.2% | 0.040 |
| Hemorrhagic transformation | 7.2% | 6.9% | 7.3% | 0.894 |
| Multiple vascular territories | 26.7% | 25.5% | 27.1% | 0.766 |
| Carotid stenosis | 12.8% | 28.8% | 8.4% | <0.001 |
| Intracranial stenosis | 8.5% | 17.0% | 6.3% | 0.024 |
| Vertebrobasilar stenosis | 5.9% | 7.3% | 5.6% | 0.748 |
| Ipsilateral stenosis | 4.3% | 9.6% | 2.8% | 0.008 |
| Disabling (Modified Rankin Scale>2) | 37.0% | 52.3% | 32.4% | 0.001 |
Median(range)
The echocardiographic characteristics of the cohort are presented in Table 2. Patients with MAC more frequently had aortic valve calcium and stenosis, as well as left atrial enlargement and LV hypertrophy, than those without MAC. The presence of MAC was associated both with CAA and atheroma with mobile components, whether of the proximal or distal thoracic aorta, or both. Moreover, the relationship between MAC and CAA was graded, irrespective of aortic location. As shown (Figure), increasing MAC severity was associated with rising prevalence of CAA of the proximal or distal thoracic aorta, or of either segment. Furthermore, largely through its association with large-artery stenosis ipsilateral to the ischemic brain territory, MAC was more commonly related to a probable cause of the index cerebrovascular event other than proximal CAA (Table 2).
Table 2.
Echocardiographic Characteristics
| Mitral Annular Calcium |
||||
|---|---|---|---|---|
| Variable | Entire Cohort (N=419) |
Yes (N=94) |
No (N=325) |
P Value |
| Mitral regurgitation, ≥ moderate | 4.8% | 8.5% | 3.7% | 0.094 |
| Mitral stenosis, any | 0.2% | 1.1% | 0% | 0.224 |
| Aortic valve calcium | 42.6% | 74.5% | 32.7% | <0.001 |
| Aortic regurgitation, ≥ moderate | 2.6% | 5.3% | 1.8% | 0.075 |
| Aortic stenosis, any | 6.9% | 22.3% | 2.5% | <0.001 |
| Left atrial enlargement | 18.6% | 43.6% | 11.4% | <0.001 |
| Left ventricular hypertrophy | 14.6% | 29.8% | 10.2% | <0.001 |
| Reduced left ventricular function | 9.3% | 11.7% | 8.6% | 0.369 |
| Mild/moderate (LV ejection fraction 35-54%) | 7.9% | 10.6% | 7.1% | 0.268 |
| Severe (LV ejection fraction <35%) | 1.4% | 1.1% | 1.5% | 1.00 |
| Akinetic/dyskinetic LV segment | 3.3% | 6.4% | 2.5% | 0.096 |
| Proximal complex aortic atheroma | 22.1% | 52.2% | 13.6% | <0.001 |
| Mobile | 4.1% | 9.8% | 2.5% | 0.004 |
| Distal complex aortic atheroma | 17.1% | 42.9% | 9.9% | <0.001 |
| Mobile | 2.9% | 6.6% | 1.9% | 0.028 |
| Any complex aortic atheroma | 27.0% | 59.8% | 17.6% | <0.001 |
| Mobile | 5.3% | 13.3% | 3.1% | 0.001 |
| Definite cardiac source of embolism | 7.2% | 10.6% | 6.2% | 0.137 |
| Probable alternative cause of cerebrovascular event* |
14.8% | 25.5% | 11.7% | 0.001 |
Definite cardiac source of embolism, ipsilateral large artery stenosis, recent endovascular intervention, or hypercoagulable state. (Excludes proximal CAA.)
Figure.
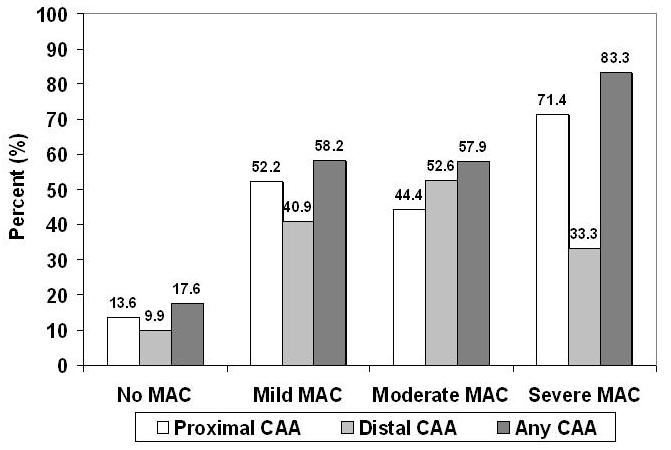
Relationship between Severity of Mitral Annular Calcium and Complex Aortic Atheroma in the Entire Cohort
Unadjusted and adjusted odds ratios for the relationship between MAC, treated as a binary variable, and CAA are shown in Table 3. While MAC was significantly associated in the entire cohort with CAA of the proximal or distal thoracic aorta, or both, these relationships weakened considerably after adjustment for age, reflecting important confounding. Additional adjustment for potential clinical and echocardiographic confounders modestly reduced the magnitude of these associations, but all three remained statistically significant in comprehensive models that adjusted for the aggregate of confounding. In the latter models, inclusion of left atrial enlargement and aortic valve calcium did not appreciably modify the magnitude of the odds ratios obtained by adjusting for clinical variables and LV hypertrophy, and the adjusted odds ratios for the smaller models are presented.
Table 3.
Relation between Mitral Annular Calcium and Complex Aortic Atheroma
| Proximal CAA | Distal CAA | Any CAA | ||||
|---|---|---|---|---|---|---|
| Odds Ratio 95% CI |
P Value |
Odds Ratio 95% CI |
P Value |
Odds Ratio 95% CI |
P Value |
|
| Entire Cohort (n=419) | ||||||
| Crude | 6.94 (4.14-11.65) |
<0.001 | 6.82 (3.92-11.86) |
<0.001 | 6.94 (4.18-11.50) |
<0.001 |
| Age-adjusted | 2.44 (1.35-4.43) |
0.003 | 2.67 (1.43-4.93) |
0.002 | 2.38 (1.32-4.30) |
0.004 |
| Multivariable-adjusted Clinical covariates* |
2.23 (1.15-4.31) |
0.017 | 2.59 (1.28-5.24) |
0.008 | 2.19 (1.09-4.39) |
0.027 |
| Multivariable-adjusted Age + echo covariates† |
2.45 (1.30-4.60) |
0.005 | 2.16 (1.13-4.14) |
0.021 | 2.11 (1.13-3.93) |
0.019 |
| Multivariable-adjusted Clinical* + LV hypertrophy† |
2.10‡ (1.09-4.18) |
0.030 | 2.48‡ (1.22-5.04) |
0.012 | 2.08‡ (1.03-4.18) |
0.041 |
| Subgroup with Otherwise Unexplained Cerebral Ischemia (n=331)§ | ||||||
| Crude | 8.51 (4.54-15.96) |
<0.001 | 6.71 (3.42-13.15) |
<0.001 | 8.23 (4.44-15.24) |
<0.001 |
| Age-adjusted | 3.26 (1.61-6.58) |
0.007 | 2.56 (1.21-5.40) |
0.014 | 2.97 (1.48-6.00) |
0.002 |
| Multivariable-adjusted Clinical covariates* |
3.01 (1.02-6.69) |
0.007 | 2.34 (0.97-5.65) |
0.058 | 2.34 (1.02-5.38) |
0.045 |
| Multivariable-adjusted Age + echo covariates† |
3.29 (1.53-7.07) |
0.002 | 1.97 (0.87-4.45) |
0.103 | 2.77 (1.29-5.93) |
0.009 |
| Multivariable-adjusted Clinical* + LV hypertrophy† |
2.74 (1.22-6.16) |
0.015 | -- | -- | 2.15 (0.93-4.99) |
0.075 |
Age, hypertension, diabetes (for proximal and any CAA only), hypercholesterolemia, smoking (for distal and any CAA only), renal insufficiency
LV hypertrophy, aortic valve calcium, left atrial enlargement
Inclusion of aortic valve calcium and left atrial enlargement in the model did not meaningfully influence the magnitude of the odds ratio
No atrial fibrillation, ipsilateral large-artery stenosis ≥50%, definite cardiac source of embolism, or hypercoagulable state
Restricting the analyses to patients without another probable cause of cerebral ischemia, however, resulted in substantial strengthening of the effect estimate for proximal CAA after adjustment for clinical and echocardiographic variables, but did not meaningfully influence that for any CAA, which became non-significant. This reflected the absence of an independent relationship between MAC and distal CAA; more limited adjustment – mandated by the smaller proportion of cases with distal CAA in the subgroup with otherwise unexplained cerebral ischemia – whether for clinical covariates, or for age plus echocardiographic variables, did not yield significant associations between MAC and distal CAA (Table 3).
Last, a subset of patients (N=214) underwent transthoracic echocardiography (TTE) within 3 months of TEE, which permitted assessment of the relation between TTE-detected MAC and CAA identified by TEE. After adjustment for age, LV hypertrophy, left atrial enlargement and aortic valve calcium, MAC identified by TTE exhibited a directionally similar, but non-significant, association with proximal CAA in the broader cohort (OR=1.74, 95% CI=0.74−4.06), which became stronger in the subgroup without an alternative mechanism for cerebral ischemia (OR=2.26, 95% CI=0.81−6.30).
Discussion
In this study of consecutive patients referred for TEE evaluation of cerebral ischemia, MAC was significantly associated with complex atheroma of the proximal thoracic aorta after adjustment for clinical and echocardiographic predictors, and the magnitude of this relation increased among individuals in whom no alternative stroke mechanism was identified. Moreover, MAC and CAA exhibited a dose-response relationship, such that increasing levels of MAC severity were associated with greater prevalence of proximal or distal CAA, or both, as well as of mobile atheroma. While a similar independent association was detected for distal CAA, alone or together with proximal CAA, in the entire cohort, corresponding significant relations were not observed in the subgroup with otherwise unexplained cerebral ischemia.
This is the first study to examine the relation between MAC and CAA exclusively in patients with cerebrovascular events. An association between MAC and CAA has been previously reported in patients referred for TEE for different indications10, 11 or with high-risk atrial fibrillation.12 By focusing strictly upon patients with cerebral ischemia, in whom systematic neurovascular imaging and laboratory evaluation was undertaken, and adjusting for potential clinical and echocardiographic confounders, the current study affords the opportunity to assess the potential pathogenetic role of proximal CAA in mediating brain ischemia associated with MAC. In this regard, the observation that the association between MAC and proximal CAA, but not that between MAC and distal CAA, was bolstered by exclusion of patients with alternative stroke etiologies supports the notion that a proportion of otherwise unexplained strokes associated with MAC are related to atheroma of proximal location.
Although the relationship between MAC and subclinical vascular disease has long been invoked to explain the association between MAC and cerebral ischemia,2, 4 the current findings buttress this contention, especially as it relates to the proximal aorta. Proximal CAA is a highly emboligenic finding, as attested by its association with frequent high-intensity transient signals by transcranial Doppler,20 and by the pronounced increase in associated risk of clinical vascular events.8, 21 It bears noting that MAC-associated thrombotic,22 vegetative23 or mobile calcific debris,24 which might incriminate MAC as a direct emboligenic substrate in our cohort, was not detected by direct TEE visualization. Hence, the more frequent identification of proximal CAA in patients in sinus rhythm with MAC and otherwise unexplained events points to aortogenic embolism or embolism from a downstream subclinical vascular source as the most likely mediator of the MAC-associated cerebrovascular events in these individuals. The marginally non-significant association observed for distal CAA in patients with otherwise unexplained ischemia suggests, however, that it is aortogenic embolism that accounts for the stronger link between MAC and proximal CAA in this subgroup, providing evidence that aortic atherothromboembolism represents an important mechanism underlying MAC-associated cerebral ischemia.
Several limitations merit consideration. The present retrospective study rests on physicians' impressions of echocardiographic findings in the context of clinical practice. Because adjudication of CAA was not blinded to MAC status during conduct and clinical interpretation of TEE studies, the possibility of biased ascertainment, though unlikely, cannot be excluded. Nevertheless, in the subset of studies in which the aorta was evaluated without knowledge of MAC status, the relation between MAC and proximal CAA was consistent with the main finding (P=0.133 for interaction). Furthermore, MAC and LV hypertrophy were not systematically assessed through criteria developed and validated for TTE. TEE does provide clear visualization of the mitral annulus and LV wall thickness, however, which lends face validity to qualitative and semi-quantitative ascertainment applied clinically. The latter concept is supported by the directionally similar relations observed for TTE-detected MAC in the subset with precordial imaging as for MAC identified by TEE. In addition, the prevalence of distal CAA in the subgroup with otherwise unexplained events prevented full adjustment for covariates, but partial adjustment and consideration of proximal and distal CAA combined permitted adequate investigation of the relationships in question. Last, these findings apply to patients at a single center and require independent confirmation, preferably in a prospective fashion.
Footnotes
This work was supported in part by grant K23 HL070854 from the National Heart, Lung, and Blood Institute, Bethesda, MD (Dr. Kizer).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sell S, Scully RE. Aging changes in the aortic and mitral valves. Histologic and histochemical studies, with observations on the pathogenesis of calcific aortic stenosis and calcification of the mitral annulus. Am J Pathol. 1965;46:345–365. [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Plehn JF, D'Agostino RB, Belanger AJ, Comai K, Fuller DL, Wolf PA, Levy D. Mitral annular calcification and the risk of stroke in an elderly cohort. N Engl J Med. 1992;327:374–379. doi: 10.1056/NEJM199208063270602. [DOI] [PubMed] [Google Scholar]
- 3.Aronow WS, Ahn C, Kronzon I, Gutstein H. Association of mitral annular calcium with new thromboembolic stroke at 44-month follow-up of 2,148 persons, mean age 81 years. Am J Cardiol. 1998;81:105–106. doi: 10.1016/s0002-9149(97)00854-0. [DOI] [PubMed] [Google Scholar]
- 4.Kizer JR, Wiebers DO, Whisnant JP, Galloway JM, Welty TK, Lee ET, Best LG, Resnick HE, Roman MJ, Devereux RB. Mitral annular calcification, aortic valve sclerosis, and incident stroke in adults free of clinical cardiovascular disease: The strong heart study. Stroke. 2005;36:2533–2537. doi: 10.1161/01.STR.0000190005.09442.ad. [DOI] [PubMed] [Google Scholar]
- 5.Tunick PA, Perez JL, Kronzon I. Protruding atheromas in the thoracic aorta and systemic embolization. Ann Intern Med. 1991;115:423–427. doi: 10.7326/0003-4819-115-6-423. [DOI] [PubMed] [Google Scholar]
- 6.Amarenco P, Duyckaerts C, Tzourio C, Henin D, Bousser MG, Hauw JJ. The prevalence of ulcerated plaques in the aortic arch in patients with stroke. N Engl J Med. 1992;326:221–225. doi: 10.1056/NEJM199201233260402. [DOI] [PubMed] [Google Scholar]
- 7.Amarenco P, Cohen A, Tzourio C, Bertrand B, Hommel M, Besson G, Chauvel C, Touboul PJ, Bousser MG. Atherosclerotic disease of the aortic arch and the risk of ischemic stroke. N Engl J Med. 1994;331:1474–1479. doi: 10.1056/NEJM199412013312202. [DOI] [PubMed] [Google Scholar]
- 8.Atherosclerotic disease of the aortic arch as a risk factor for recurrent ischemic stroke. The french study of aortic plaques in stroke group. N Engl J Med. 1996;334:1216–1221. doi: 10.1056/NEJM199605093341902. [DOI] [PubMed] [Google Scholar]
- 9.Kronzon I, Tunick PA. Aortic atherosclerotic disease and stroke. Circulation. 2006;114:63–75. doi: 10.1161/CIRCULATIONAHA.105.593418. [DOI] [PubMed] [Google Scholar]
- 10.Adler Y, Shohat-Zabarski R, Vaturi M, Shapira Y, Ehrlich S, Jortner R, Assali A, Zonshin A, Sagie A. Association between mitral annular calcium and aortic atheroma as detected by transesophageal echocardiographic study. Am J Cardiol. 1998;81:784–786. doi: 10.1016/s0002-9149(97)01014-x. [DOI] [PubMed] [Google Scholar]
- 11.Adler Y, Vaturi M, Fink N, Tanne D, Shapira Y, Weisenberg D, Sela N, Sagie A. Association between mitral annulus calcification and aortic atheroma: A prospective transesophageal echocardiographic study. Atherosclerosis. 2000;152:451–456. doi: 10.1016/s0021-9150(99)00497-9. [DOI] [PubMed] [Google Scholar]
- 12.Blackshear JL, Pearce LA, Hart RG, Zabalgoitia M, Labovitz A, Asinger RW, Halperin JL. Aortic plaque in atrial fibrillation: Prevalence, predictors, and thromboembolic implications. Stroke. 1999;30:834–840. doi: 10.1161/01.str.30.4.834. [DOI] [PubMed] [Google Scholar]
- 13.Hueb JC, Zanati SG, Okoshi K, Raffin CN, Silveira LV, Matsubara BB. Association between atherosclerotic aortic plaques and left ventricular hypertrophy in patients with cerebrovascular events. Stroke. 2006;37:958–962. doi: 10.1161/01.STR.0000208112.18484.e6. [DOI] [PubMed] [Google Scholar]
- 14.Kizer JR, Silvestry FE, Kimmel SE, Kasner SE, Wiegers SE, Erwin MB, Schwalm SA, Viswanathan MN, Pollard JR, Keane MG, Sutton MG. Racial differences in the prevalence of cardiac sources of embolism in subjects with unexplained stroke or transient ischemic attack evaluated by transesophageal echocardiography. Am J Cardiol. 2002;90:395–400. doi: 10.1016/s0002-9149(02)02496-7. [DOI] [PubMed] [Google Scholar]
- 15.Katz ES, Tunick PA, Rusinek H, Ribakove G, Spencer FC, Kronzon I. Protruding aortic atheromas predict stroke in elderly patients undergoing cardiopulmonary bypass: Experience with intraoperative transesophageal echocardiography. J Am Coll Cardiol. 1992;20:70–77. doi: 10.1016/0735-1097(92)90139-e. [DOI] [PubMed] [Google Scholar]
- 16.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 17.Palmieri V, de Simone G, Arnett DK, Bella JN, Kitzman DW, Oberman A, Hopkins PN, Province MA, Devereux RB. Relation of various degrees of body mass index in patients with systemic hypertension to left ventricular mass, cardiac output, and peripheral resistance (the hypertension genetic epidemiology network study) Am J Cardiol. 2001;88:1163–1168. doi: 10.1016/s0002-9149(01)02054-9. [DOI] [PubMed] [Google Scholar]
- 18.Dutta T, Karas MG, Segal AZ, Kizer JR. Yield of transesophageal echocardiography for nonbacterial thrombotic endocarditis and other cardiac sources of embolism in cancer patients with cerebral ischemia. Am J Cardiol. 2006;97:894–898. doi: 10.1016/j.amjcard.2005.09.140. [DOI] [PubMed] [Google Scholar]
- 19.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 20.Rundek T, Di Tullio MR, Sciacca RR, Titova IV, Mohr JP, Homma S, Sacco RL. Association between large aortic arch atheromas and high-intensity transient signals in elderly stroke patients. Stroke. 1999;30:2683–2686. doi: 10.1161/01.str.30.12.2683. [DOI] [PubMed] [Google Scholar]
- 21.Khatibzadeh M, Mitusch R, Stierle U, Gromoll B, Sheikhzadeh A. Aortic atherosclerotic plaques as a source of systemic embolism. J Am Coll Cardiol. 1996;27:664–669. doi: 10.1016/0735-1097(95)00526-9. [DOI] [PubMed] [Google Scholar]
- 22.Eicher JC, Soto FX, DeNadai L, Ressencourt O, Falcon-Eicher S, Giroud M, Louis P, Wolf JE. Possible association of thrombotic, nonbacterial vegetations of the mitral ring-mitral annular calcium and stroke. Am J Cardiol. 1997;79:1712–1715. doi: 10.1016/s0002-9149(97)00233-6. [DOI] [PubMed] [Google Scholar]
- 23.Burnside JW, Desanctis RW. Bacterial endocarditis on calcification of the mitral anulus fibrosus. Ann Intern Med. 1972;76:615–618. doi: 10.7326/0003-4819-76-4-615. [DOI] [PubMed] [Google Scholar]
- 24.Shohat-Zabarski R, Paz R, Adler Y, Vaturi M, Jortner R, Sagie A. Mitral annulus calcification with a mobile component as a possible source of embolism. Am J Geriatr Cardiol. 2001;10:196–198. doi: 10.1111/j.1076-7460.2001.00018.x. [DOI] [PubMed] [Google Scholar]


