Abstract
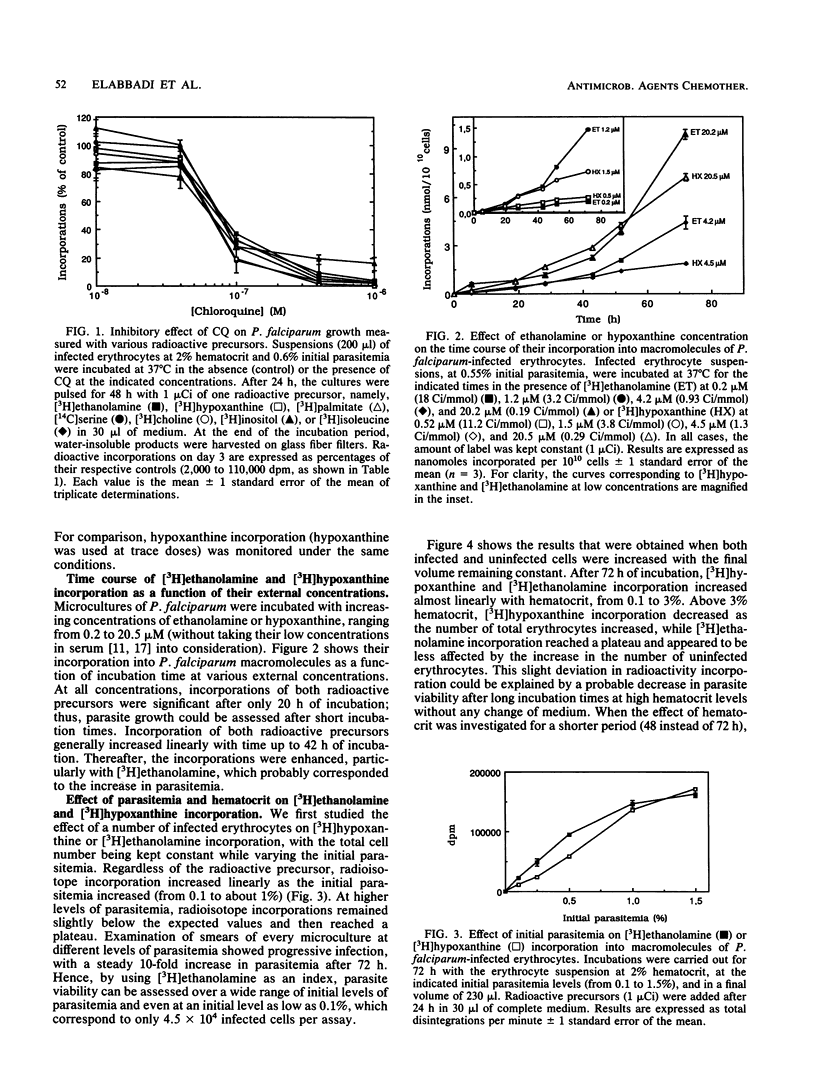
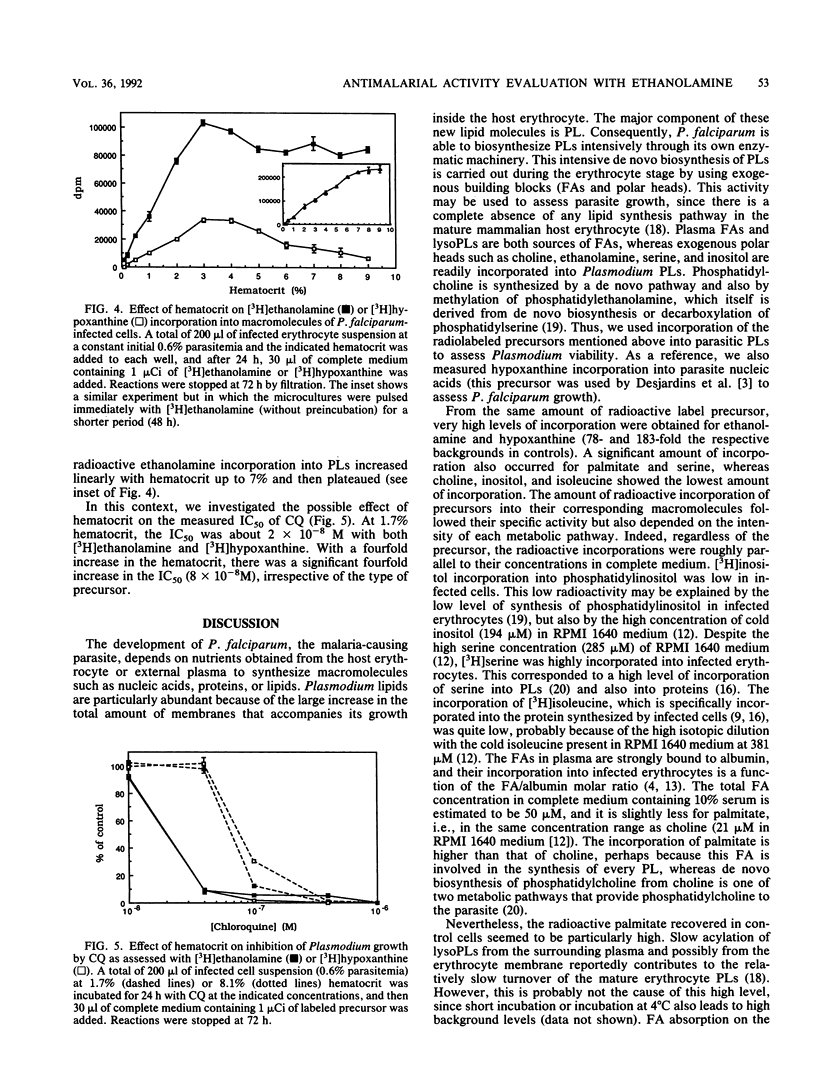
Phospholipid biosynthetic activity is intense in the erythrocytic stage of Plasmodium falciparum because of the parasite's own enzymatic machinery. The incorporation of various labeled phospholipid precursors in comparison with the incorporation of nucleic acid and protein precursors was tested to evaluate P. falciparum growth in vitro. These precursors, namely, [3H]ethanolamine, [3H]hypoxanthine, [3H]palmitate, [14C]serine, [3H]choline, [3H]inositol, and [3H]isoleucine, were all accurate indicators of parasite growth. However, because of its high level of incorporation, [3H]ethanolamine proved to be the best tool for assessing parasite viability. When culture parameters were carefully controlled, [3H]ethanolamine incorporation into phospholipids was proportional to pulse time, precursor concentration, and initial parasitemia and was sensitive to the number of uninfected erythrocytes (hematocrit). It can be used for a wide range of infected erythrocytes, from 2 x 10(4) to 5 x 10(5). The use of [3H]ethanolamine for in vitro antimalarial drug screening is a good alternative to the method of Desjardins et al. (R. E. Desjardins, C. J. Canfield, J. D. Haynes, and J. D. Chulay, Antimicrob. Agents. Chemother. 16:710-718, 1979). The major advantage is that the culture medium can be supplemented with hypoxanthine, which results in better parasite growth. [3H]ethanolamine is also an ideal tool when compounds that interfere with DNA and/or RNA metabolism are to be investigated for their effect on Plasmodium growth.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brockelman C. R., Tan-ariya P. Plasmodium falciparum in continuous culture: a new medium for the in vitro test for sulfadoxine sensitivity. Bull World Health Organ. 1982;60(3):423–426. [PMC free article] [PubMed] [Google Scholar]
- Chulay J. D., Haynes J. D., Diggs C. L. Plasmodium falciparum: assessment of in vitro growth by [3H]hypoxanthine incorporation. Exp Parasitol. 1983 Feb;55(1):138–146. doi: 10.1016/0014-4894(83)90007-3. [DOI] [PubMed] [Google Scholar]
- Desjardins R. E., Canfield C. J., Haynes J. D., Chulay J. D. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979 Dec;16(6):710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donabedian R. K., Karmen A. Fatty acid transport and incorporation into human erythrocytes in vitro. J Clin Invest. 1967 Jun;46(6):1017–1027. doi: 10.1172/JCI105591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch C. D. Plasmodium falciparum in owl monkeys: drug resistance and chloroquine binding capacity. Science. 1970 Jul 17;169(3942):289–290. doi: 10.1126/science.169.3942.289. [DOI] [PubMed] [Google Scholar]
- Franklin R. M., Brun R., Grieder A. Microscopic and flow cytophotometric analysis of parasitemia in cultures of Plasmodium falciparum vitally stained with Hoechst 33342--application to studies of antimalarial agents. Z Parasitenkd. 1986;72(2):201–212. doi: 10.1007/BF00931147. [DOI] [PubMed] [Google Scholar]
- Geary T. G., Divo A. A., Jensen J. B. An in vitro assay system for the identification of potential antimalarial drugs. J Parasitol. 1983 Jun;69(3):577–583. [PubMed] [Google Scholar]
- Geary T. G., Jensen J. B., Ginsburg H. Uptake of [3H]chloroquine by drug-sensitive and -resistant strains of the human malaria parasite Plasmodium falciparum. Biochem Pharmacol. 1986 Nov 1;35(21):3805–3812. doi: 10.1016/0006-2952(86)90668-4. [DOI] [PubMed] [Google Scholar]
- Iber P. K., Pavanand K., Wilks N. E., Colwell E. J. Evaluation of in vitro drug sensitivity of human Plasmodium falciparum by incorporation of radioactive isoleucine. J Med Assoc Thai. 1975 Nov;58(11):559–566. [PubMed] [Google Scholar]
- Jensen J. B., Trager W. Plasmodium falciparum in culture: use of outdated erthrocytes and description of the candle jar method. J Parasitol. 1977 Oct;63(5):883–886. [PubMed] [Google Scholar]
- Lartigue-Mattéi C., Chabard J. L., Bargnoux H., Petit J., Berger J. A., Ristori J. M., Bussière J. L., Rampon S. Dosage de la xanthine et de l'hypoxanthine plasmatiques par couplage chromatographie en phase gazeuse-spectrométrie de masse et problèmes d'échantillonnage. Ann Biol Clin (Paris) 1984;42(5):355–361. [PubMed] [Google Scholar]
- Müller W. E., Wollert U. Human serum albumin as a 'silent receptor' for drugs and endogenous substances. Pharmacology. 1979;19(2):59–67. doi: 10.1159/000137289. [DOI] [PubMed] [Google Scholar]
- Perry T. L., Hansen S., Christie R. G. Amino compounds and organic acids in CSF, plasma, and urine of autistic children. Biol Psychiatry. 1978 Oct;13(5):575–586. [PubMed] [Google Scholar]
- Reber-Liske R. A labour-saving method for the in vitro culture of Plasmodium falciparum. Acta Trop. 1983 Mar;40(1):39–43. [PubMed] [Google Scholar]
- Richards W. H., Maples B. K. Studies on Plasmodium falciparum in continuous cultivation. I. The effect of chloroquine and pyrimethamine on parasite growth and viability. Ann Trop Med Parasitol. 1979 Apr;73(2):99–108. [PubMed] [Google Scholar]
- Sherman I. W. Amino acid metabolism and protein synthesis in malarial parasites. Bull World Health Organ. 1977;55(2-3):265–276. [PMC free article] [PubMed] [Google Scholar]
- Vial H. J., Ancelin M. L., Philippot J. R., Thuet M. J. Biosynthesis and dynamics of lipids in Plasmodium-infected mature mammalian erythrocytes. Blood Cells. 1990;16(2-3):531–561. [PubMed] [Google Scholar]
- Vial H. J., Thuet M. J., Philippot J. R. Phospholipid biosynthesis in synchronous Plasmodium falciparum cultures. J Protozool. 1982 May;29(2):258–263. doi: 10.1111/j.1550-7408.1982.tb04023.x. [DOI] [PubMed] [Google Scholar]
- Waki S., Tamura J., Jingu M., Adachi M., Suzuki M. A new technique for drug susceptibility tests for Plasmodium falciparum by ethidium bromide fluoroassay. Trans R Soc Trop Med Hyg. 1986;80(1):47–49. doi: 10.1016/0035-9203(86)90192-6. [DOI] [PubMed] [Google Scholar]
- Ye Z. G., Van Dyke K., Wimmer M. Effect of artemisinin (qinghaosu) and chloroquine on drug-sensitive and drug-resistant strains of Plasmodium falciparum malaria: use of [2,8-3H]adenosine as an alternative to [G-3H]hypoxanthine in the assessment of in vitro antimalarial activity. Exp Parasitol. 1987 Dec;64(3):418–423. doi: 10.1016/0014-4894(87)90055-5. [DOI] [PubMed] [Google Scholar]
- Zolg J. W., MacLeod A. J., Dickson I. H., Scaife J. G. Plasmodium falciparum: modifications of the in vitro culture conditions improving parasitic yields. J Parasitol. 1982 Dec;68(6):1072–1080. [PubMed] [Google Scholar]
- Zolg J. W., Macleod A. J., Scaife J. G., Beaudoin R. L. The accumulation of lactic acid and its influence on the growth of Plasmodium falciparum in synchronized cultures. In Vitro. 1984 Mar;20(3 Pt 1):205–215. doi: 10.1007/BF02618189. [DOI] [PubMed] [Google Scholar]


