Abstract
Background
Little is known about the temporal relationship between an asthma exacerbation and a painful episode in a child with sickle cell disease (SCD). We tested the hypothesis that respiratory symptoms either immediately precede or occur concomitantly with painful episodes more frequently in children with SCD and asthma when compared with children with SCD without asthma.
Methods
A cohort study was conducted. As part of standard care, the primary hematologist referred all children with SCD for evaluation by a pulmonologist. The definition of asthma was based on the National Heart Lung and Blood Institute’s guidelines. All painful episodes during a 25-month sampling frame were reviewed. Events that were diagnosed as asthma exacerbations were excluded from analysis. Respiratory symptoms (cough, wheeze, tachypnea, retractions, or grunting) were included if they occurred up to 96 hours before a painful episode.
Results
A total of 74 children were evaluated for a painful episode. Of these patients, 36 were diagnosed with asthma (mean age 9.8 y; range 2.4 to 19.4) and 38 were determined not to have asthma (mean age, 9.8 y; range 2.4 to 19.5). Among the children with pain and asthma, the odds ratio of having antecedent or concurrent respiratory symptoms was 4.9 (95% confidence intervals, 2.2-10.7) when compared with children with pain and without asthma.
Conclusions
In children with both SCD and asthma, respiratory symptoms are a risk factor for painful episodes within 96 hours.
Keywords: asthma, sickle cell, pain, acute chest syndrome
Painful episodes are the most common sequelae of sickle cell disease (SCD) and individuals with a high incidence rate are at increased risk for premature death.1 Initial studies have established a link between a diagnosis of asthma and an increased rate of painful episodes.2
Airway obstruction and airway lability have been previously described in patients with SCD3-5 and a recent study showed that asthma is more common in children with SCD than age and race-matched controls.6 A retrospective cohort study showed that children with SCD admitted for pain were more likely to develop acute chest syndrome (ACS) if they had asthma, suggesting that asthma may contribute to the complications of SCD.7
In this study, we test the following hypothesis: among children with pain, mild respiratory symptoms either immediately precede or occur concomitantly with painful episodes more frequently in children with SCD and asthma when compared with children with SCD without asthma. The hypothesis was based on our clinical observation that children with SCD and asthma often presented with respiratory symptoms either immediately before or at the same time as the painful episode.
MATERIALS AND METHODS
The Institutional Review Board at Washington University School of Medicine approved the study. Informed consent was not obtained because all information was documented as part of standard medical care and retrieved through medical records.
Composition of the Cohort
All children with SCD, ages 2 to 21 years (Hb SS, Hb SC, and Hb Sβ thalassemia) who were evaluated by a hematologist and pulmonologist at St Louis Children’s Hospital from June 15, 2004 to January 31, 2005 were included in this cohort study. Before June 15, 2004, the hematologists only referred patients with a known history of respiratory disease to the pulmonologist in the combined pulmonary sickle cell clinic. After June 15, all patients were referred to the pulmonologist because of the high rate of previously undetected asthma. All patients were evaluated in the combined pulmonary sickle cell clinic. Children were seen by the pulmonology service within the hematology clinic on a first-come-first-served basis. Up to 12 patients were evaluated in clinic per day. Children under 2 years of age were excluded from the study due to the uncertainty of the diagnosis of asthma before this age.8-11 Patients who were too young to perform spirometry were not excluded if the diagnosis of asthma was clear to the pulmonologist on the basis of their evaluation. Patients who smoke cigarettes were excluded because of the ability of inhaled tobacco to trigger respiratory symptoms (specifically cough). To minimize the number of children who sought medical care at hospitals other than St Louis Children’s Hospital, children whose main residence was not in the greater St Louis metropolitan area were excluded. Patients with greater than 20 hospital visits for pain in the 25-month period (January 1, 2003 to January 31, 2005) were also excluded due to the inability to differentiate between factors other than pain that contributed to their frequent hospitalizations.
Sampling Frame
After an individual was evaluated prospectively, medical records for all visits to the hematology clinic between 1/1/2003 and 1/31/2005 were reviewed retrospectively to determine the presence of pain and their temporal relationship (if any) to respiratory symptoms. The start point of the sampling frame was chosen because 1/1/2003 was the date when pulmonologists began attending SCD clinic. The sampling period for the retrospective chart was chosen because hematologists in the SCD clinic became aware of the increased morbidity associated with asthma SCD after 1/1/2003.7 The end point was chosen because this was when the chart review was performed. All painful episodes that occurred during the sampling frame were reviewed.
Clinical Definitions
Asthma
Cases were identified as children diagnosed with asthma. In all cases, asthma was based on a clinical diagnosis by a pulmonologist. Pulmonary function tests (PFT) were used to clarify difficult diagnoses and to follow patients’ progress. PFT were not the sole criteria used to make the diagnosis of asthma. Three criteria on the basis of the National Heart Lung and Blood Institute guidelines12,13 were used to diagnose asthma: (1) episodic symptoms of airflow obstruction are present; (2) airflow obstruction is at least partially reversible; (3) alternative diagnoses are excluded. Symptoms of airflow obstruction were defined as any one of the following: (1) recurrent episodic respiratory symptoms of cough, wheeze, and chest tightness induced by precipitating factors characteristic of asthma; or (2) PFT indicating airway obstruction (forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) ratio below the lower 95% confidence intervals (CIs) for age, race, gender, and height)14 or air trapping present on body plethysmography (RV/TLC ratio greater than 30%).15 Reversibility of airflow obstruction was defined as either: (1) respiratory symptoms that improve with administration of a bronchodilator; or (2) a 12% or greater increase in FEV1 after bronchodilator administration. A primary diagnosis of asthma was supported by a normal chest radiograph during a time when the patient was asymptomatic and no evidence of other causes of airway obstruction on clinical evaluation. In all children, the diagnosis of asthma was made clinically.
Painful Episodes
All painful episodes during the sampling frame were examined. The primary exposure was respiratory symptoms occurring up to 96 hours before a painful episode. Pain was defined as the complaint of body pain (excluding the head) requiring the administration of opioids in the hospital, clinic, or emergency department. Only episodes in which the primary complaint was pain were included. In such cases, respiratory symptoms when present were often mild and did not concern the child or parent enough to present to the hospital. All events where the clinician diagnosed the child with an asthma exacerbation or ACS were excluded from the analyses. Episodes where pain was not the primary complaint were excluded. For example, an episode of a child presenting with an asthma exacerbation that secondarily complained of abdominal pain was excluded.
Respiratory Symptoms
Respiratory symptoms were defined as any of the following: (1) cough, (2) wheeze, (3) tachypnea, (4) retractions, or (5) grunting. Tachypnea was defined as a respiratory rate greater than 30 or a clinician’s comment that the patient was tachypneic. Tachypnea, in the absence of other respiratory symptoms, which resolved with the administration of opioids was not included as a respiratory symptom. These symptoms, especially in a known asthmatic patient, are concerning for an asthma exacerbation. Although none of the included episodes were diagnosed as asthma exacerbations, some of them may have been undiagnosed asthma exacerbations. When respiratory symptoms were noted in the chart, no attempt was made to diagnose the etiology of those symptoms (upper respiratory tract infection vs. unrecognized asthma exacerbation vs. other etiologies) once ACS was excluded. Secondary outcomes included ACS that was diagnosed after admission to the hospital and did not include ACS that was diagnosed at the time of admission, pulse oximetry reading, and hemoglobin concentration. ACS was defined as the presence of new radiodensity on chest radiograph with chest pain and respiratory symptoms.
Respiratory symptoms preceding pain were defined as symptoms that began up to 96 hours before and continued through the onset of pain. Assessment of whether respiratory symptoms either preceded or occurred simultaneously with the painful episode was only assessed in visits to the combined pulmonary sickle cell clinic. To account for the fact that airways remain hyperreactive for up to 5 weeks after an asthma exacerbation, the study period was divided into 60-day intervals. Only the first episode of pain within a 60-day period was considered an event.
Documentation of Respiratory Symptoms
Adequate documentation of respiratory symptoms was defined as documentation of respiratory symptoms in the history of present illness or review of systems. For quality assurance, 10% of the medical records were then reviewed by a pulmonologist, and there was 100% concordance between reviewers for the presence and absence of respiratory symptoms. The pulmonologist who reviewed the data was one of the doctors who assessed the patients. At the time he reviewed the data he was unaware of the conclusions of the manuscript. Both reviewers went through the medical records without prior knowledge of the asthma status; however, complete masking of the diagnosis of asthma was not possible. Subsequently, in the hematology clinic respiratory symptoms were elicited more carefully in patients with pain.
Data Analysis
All data were double entered into a database. Data were analyzed using SPSS 11.0. After data extraction, patients were randomly assigned an identification number, and no other identifiers were included. A χ2 test was used to determine whether a diagnosis of asthma was associated with respiratory symptoms during a painful episode. An odds ratio for respiratory symptoms was calculated and was considered to be significantly increased if it was >1.0 and if the 95% CIs did not include 1.0. To compare the rate of pain crises in the asthma group versus the control group, a Breslow test for nonproportionality was calculated using a separate program designed for comparing person-time incidence rates.
RESULTS
Demographics
A total of 275 children were seen in clinic and 100 children were evaluated by a hematologist and pulmonologist over a 7-month period. For this analysis, 26 children were excluded by the pulmonologist because the diagnosis of asthma was unclear (n = 6), too young for a spirometry evaluation to provide confirmation of a diagnosis of asthma (n = 8), excessive number of admissions (n = 4), lived outside of St Louis Metropolitan area (n = 7), or cigarette smoker (n = 1). Of the 175 children who were not evaluated by the pulmonologists, 63 were below the age of 2 and 112 were not evaluated due to time constraints, Figure 1. Of the eligible patients, 36 were diagnosed with asthma (mean age 9.8 y; median 9.6 y; range 2.4 to 19.4) and 38 were determined not to have asthma (mean age, 9.8 y; median 9.7 y; range 2.4 to 19.5); Table 1. No significant differences in age, gender, or phenotype existed between the case and control groups.
FIGURE 1.
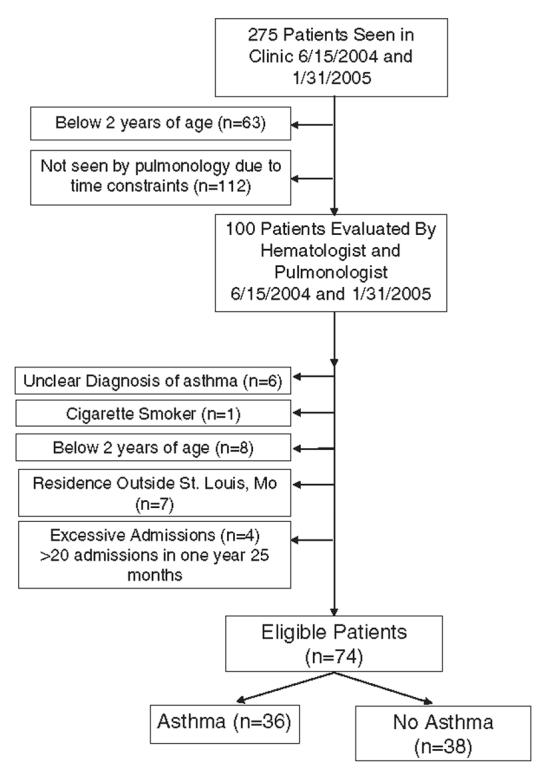
Patient selection for cohort in the combined Sickle Cell Disease and Pulmonary Clinic at St Louis Children’s Hospital.
TABLE 1.
Demographic Characteristics for Children Evaluated in the Combined Sickle Cell Disease and Pulmonary Clinic Over a 25-mo Period
| Cases (N = 36) | Controls (N = 38) | P | |
|---|---|---|---|
| Sex | |||
| Male | 55.6% | 50.0% | 0.650 |
| Phenotype | |||
| HbSS | 55.6% | 63.2% | 0.156 |
| HbSC | 36.1% | 18.4% | — |
| HbSβ-thalassemia | 8.3% | 18.4% | — |
| Age (y) | |||
| Mean | 9.78 | 9.77 | 0.990 |
| Median | 9.59 | 9.65 | — |
| Range | 2.41-19.36 | 2.41-19.52 | — |
Respiratory Symptoms Precede Pain in Patients With Asthma
A total of 124 painful episodes occurred in 74 individuals with SCD in the combined pulmonary sickle cell clinic during the sampling frame. Among the 124 painful episodes, 76% (94 of 124) had explicit documentation of the presence or absence of the patient’s respiratory history. Of the 94 painful episodes with proper documentation, 54% (51 of 94) and 46% (43 of 94) occurred in children with and without asthma, respectively. There was no statistical difference in the rate of adequate documentation between the 2 groups, (P>0.05). Of the respiratory symptoms that were cataloged, no child was noted to have grunting. Tachypnea did occur; however, tachynpea always occurred in the presence of at least one other respiratory symptom. Retractions did occur, however, they were always concurrent with cough.
Painful episodes with adequate documentation of respiratory symptoms were more frequently preceded by respiratory symptoms in the group with asthma when compared with those without asthma. In children with asthma, 35% (18 out of 51) of the painful episodes were clearly preceded by respiratory symptoms versus 12% (5 out of 43) for children without asthma (P = 0.016). The difference between the 2 rates was 23% (95% CI 5.3%-42.1%).
Patients with asthma were also more likely to have respiratory symptoms concurrent with their painful episodes. None of these patients were diagnosed as having an asthma exacerbation. The odds ratio of having a pain episode associated with antecedent or concurrent respiratory symptoms was 4.9 (95% CI 2.2-10.7) in children with asthma compared with those without asthma.
Patients With Asthma Have a Higher Rate of Painful Episodes
The incidence rate of pain was higher in children with asthma when compared with those without asthma. During the study period the patients with asthma experienced a rate of 1.7 episodes per patient year (125 pain episodes in 72 patient years) versus 1.2 per patient year (89 episodes in 76 patient years) for asthma and nonasthma groups, respectively, an absolute difference of 0.56 episodes per patient year (P = 0.005, 95% CI 0.52, 0.89).
Children with asthma did not have a higher rate of ACS when compared with children without asthma. There were a total of 20 episodes of ACS that occurred during the sampling frame. The rate of ACS was 0.17 episodes per patient year (12 episodes in 72 patient years) versus 0.11 episodes per patient year (8 episodes in 76 patient years) for asthma and no asthma, respectively (P = 0.31).
Pulse Oximetry Measurements are Decreased During Painful Episodes
The mean pulse oximetry measurement was lower during acute painful episodes than at baseline. The mean pulse oximetry measurement was 95.43% (range 85% to 100%) versus 97.08% (range 88% to 100%) for painful episodes and baseline measurements, respectively (P = 0.01). There was no significant correlation between the change in pulse oximetry reading during a painful episode and the change in hemoglobin concentration. There was no significant difference in pulse oximetry values when comparing children with asthma versus no asthma.
DISCUSSION
Painful episodes are the most common complication of SCD and individuals with frequent painful episodes are at risk for early death when compared with those with fewer episodes of pain.16 In a prospective infant cohort study from the Cooperative Study for Sickle Cell Disease, Boyd et al2 showed that asthma is associated with a higher rate of pain episodes. However, Boyd et al2 were unable to establish whether an asthma exacerbation was temporally associated with a painful episode.
The most significant finding in the current study is that among children with pain, mild respiratory symptoms often precede or occur simultaneously with painful episodes in children with SCD and asthma when compared to children with SCD and no asthma. In the presence of a painful episode, children with asthma are 5 times more likely than children without asthma to have mild respiratory symptoms associated with the painful episode. Only the medical records of patients that presented with painful episodes were reviewed. None of the episodes were diagnosed as asthma exacerbations (diagnosed asthma exacerbations were excluded). We suspect that many of the respiratory symptoms noted in this study may have been associated with undiagnosed or early asthma exacerbations. The temporal relationship between respiratory symptoms and painful episodes provides additional evidence that asthma exacerbations increase the incidence of painful episodes in patients with asthma and SCD.2
Children with asthma did have a higher rate of ACS when compared with children without asthma; however, this difference was not statistically significant. This finding is unexpected, but strongly suggests that our findings of an increase in frequency of respiratory symptoms among children with asthma were not related to a higher rate of ACS episodes. It is possible that with a larger sample size, a significant difference would have been detected.
Several potential limitations exist in our study design. Small sample size made it impossible to evaluate the impact of asthma treatment (steroids and bronchodilators) on clinical course. Despite the fact that patients were identified prospectively, data were collected retrospectively and in many cases documentation was incomplete. Care providers were not specifically alerted to document respiratory history and they did not in 24% of cases. Patients with asthma are more likely to have respiratory symptoms in general; and most likely there were more cases of missed respiratory symptoms in the asthma group. This would bias the results toward the null hypothesis. It is possible that clinicians and parents would be more likely to recognize respiratory symptoms in children with asthma. This would bias the results away from the null hypothesis. To evaluate this we compared the rate of adequate documentation of respiratory symptoms between the 2 groups and there was no difference. Ascertainment bias also exists in this study. Approximately, 50% of our children with SCD evaluated had asthma. Children with asthma and SCD have more pain2; therefore, are more likely to be evaluated by a physician for hospital follow up or painful episode in our combined pulmonary SCD clinic. Our sampling frame for individuals identified in the study included the fall and winter months, the period when you would expect to havemore visits to the clinic. These ascertainment bias resulted in a much higher than expected proportion of children with asthma (cases). However, we do not believe that this bias significantly altered the association between respiratory symptoms and pain episodes because approximately 76% of all painful episodes included a medical history regarding the presence of preceding and/or concurrent respiratory symptoms.
In summary, we provide further evidence that asthma is a potentially modifiable risk factor associated with painful events in children with SCD. Future prospective studies may answer the question of whether early treatment of asthma exacerbations can prevent painful episodes.
Footnotes
Supported in part by the Doris Duke Charitable Foundation and by the National Institutes of Health, National Heart, Lung, and Blood Institute, NO1-HB47099, NO1-HB47110, and RO1-HL79937.
REFERENCES
- 1.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 2.Boyd JH, Macklin EA, Strunk RC, et al. Asthma is associated with acute chest syndrome and painful episodes in children with sickle cell anemia. Blood. 2006 doi: 10.1182/blood-2006-01-011072. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santoli F, Zerah F, Vasile N, et al. Pulmonary function in sickle cell disease with or without acute chest syndrome. Eur Respir J. 1998;12:1124–1129. doi: 10.1183/09031936.98.12051124. [DOI] [PubMed] [Google Scholar]
- 4.Koumbourlis A, Zar H, Hurlet-Jensen A, et al. Prevalence and reversibility of lower airway obstruction in children with sickle cell disease. J Pediatr. 2001;132:188–192. doi: 10.1067/mpd.2001.111824. [DOI] [PubMed] [Google Scholar]
- 5.Leong MA, Dampier C, Varlotta L, et al. Airway hyperreactivity in children with sickle cell disease. J Pediatr. 1997;131:278–283. doi: 10.1016/s0022-3476(97)70166-5. [DOI] [PubMed] [Google Scholar]
- 6.Knight-Madden JM, Forrester TS, Lewis NA, et al. Asthma in children with sickle cell disease and its association with acute chest syndrome. Thorax. 2005;60:206–210. doi: 10.1136/thx.2004.029165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyd JH, Moinuddin A, Strunk RC, et al. Asthma and acute chest in sickle-cell disease. Pediatr Pulmonol. 2004;38:229–232. doi: 10.1002/ppul.20066. [DOI] [PubMed] [Google Scholar]
- 8.Martinez FD, Wright AL, Taussig LM, et al. Asthma and wheezing in the first six years of life. N Engl J Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 9.Martinez FD. In: Postma DS, Gerritsen J, editors. The origins of asthma in early life; Proceedings of the Bronchitis V International Symposium, Groningen, the Netherlands; Assen, the Netherlands: Van Gorcum. 1994.pp. 161–169. [Google Scholar]
- 10.Wright AL, Taussig LM, Ray CG, et al. The Tucson Children's Respiratory Study. II. Lower respiratory tract illness in the first year of life. Am J Epidemiol. 1989;129:1232–1246. doi: 10.1093/oxfordjournals.aje.a115243. [DOI] [PubMed] [Google Scholar]
- 11.Samet JM, Tager IB, Speizer FE. The relationship between respiratory illness in childhood and chronic air-flow obstruction in adulthood. Am Rev Respir Dis. 1983;127:508–523. doi: 10.1164/arrd.1983.127.4.508. [DOI] [PubMed] [Google Scholar]
- 12.National Asthma Education and Prevention Program Expert Panel . Guidelines for the Diagnosis and Management of Asthma—Update on Selected Topics 2002. US Department of Health and Human Services National Heart, Lung, and Blood Institute; Bethesda, MD: 2002. [Google Scholar]
- 13.National Asthma Education Program Expert Panel Report 2:Guidelines for the Diagnosis and Management of Asthma. NIH; Bethesda, MD: 1997. National Institutes of Health publication no 97-4051. [PubMed] [Google Scholar]
- 14.Wang X, Dockery DW, Wypij D, et al. Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15:75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 15.Zapletal A, Samanek M, Paul T. Progress in Respiration Research. Vol. 22. Karger; 1987. Lung Function in Children and Adolescents. [Google Scholar]
- 16.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 330:1639–644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]


