Abstract
The arginine vasopressin (Avp) 1b receptor (Avpr1b) present on anterior pituitary corticotrophs is involved in the stimulation of adrenocorticotrophin (ACTH) secretion, especially during times of stress. Corticotrophin-releasing hormone (Crh) is considered the major ACTH secretagogue during acute stress while Avp appears to be the more dominant mediator of the hypothalamic-pituitary-adrenal (HPA) axis response during chronic stress situations. To investigate the role of the Avpr1b in the HPA axis response to acute stress, we measured ACTH and corticosterone (CORT) plasma levels in Avpr1b knockout (KO) mice and wild-type controls in response to bacterial lipopolysaccharide (LPS) challenge and ethanol (EtOH) administration. Mice deficient in Avpr1b had markedly compromised plasma ACTH and CORT responses to acute (30 min) LPS, but normal ACTH and CORT response to more extended exposure (4 h) to the immune system activator. The plasma ACTH and CORT levels stimulated by intoxicating, sedative doses of EtOH (3.2 and 4g/kg) were significantly decreased in the Avpr1b KO mice, as compared to wild-type littermates. Significantly higher EtOH-induced plasma ACTH and CORT secretion was measured in female than in male Avpr1b wild-type mice. There was no difference in the blood alcohol levels (BALs) following acute EtOH administration in Avpr1b KO or wild-type mice of either gender. Our results clearly suggest that the Avpr1b plays a significant role in the HPA axis response to acute immune stress and EtOH intoxication.
Keywords: Avp 1b receptor, hypothalamic-pituitary-adrenal axis, lipopolysaccharide, ethanol
Introduction
The neurohypophysial hormone arginine vasopressin (Avp) is synthesized predominantly in the magnocellular neurons of the supraoptic nuclei (SON) and paraventricular nuclei (PVN) of the hypothalamus. These vasopressinergic neurones project to the posterior pituitary and upon appropriate stimuli (e.g., an increase in plasma osmolality) the nerve endings are depolarised, releasing Avp into the systemic circulation to promote water resorption from the distal kidney (1,2). Avp is also produced in the parvocellular neurons of the hypothalamic PVN and suprachiasmatic nuclei (SCN), bed nucleus of the stria terminalis and medial amygdala - these neurones send fibres to other brain regions including the lower brainstem, spinal cord, lateral septum and hippocampus, where Avp acts as a neurotransmitter or neuromodulator in processes such as thermoregulation and cardiovascular control, learning and memory, and social behaviour (e.g., aggression)(3-8). In addition to its central role, parvocellular PVN (pPVN) Avp is secreted into the pituitary portal vessels from axons terminating in the external zone of the median eminence, where it functions as a neuroendocrine modulator of adrenocorticotrophin (ACTH) secretion (9). There is evidence that Avp and the related neuropeptide oxytocin (Oxt), of magnocellular origin, can also regulate ACTH release, perhaps by fine-tuning responses in a somato-dendritic fashion to regulate their own and/or each other's release within the PVN and SON (10).
Avp elicits its biological effects by binding to a family of closely-related G protein-coupled receptors, the V1a (Avpr1a), V1b (or V3) (Avpr1b) and V2 (Avpr2) receptors that have been unambiguously characterised by their tissue distribution, structure-activity relationships, second messengers involved in mediating their biological responses, and molecular cloning (11,12). The Avpr1a regulates vascular tone and subserves most of Avp's metabolic roles whereas the Avpr2 controls renal collecting duct water permeability (1). It is generally considered that the Avpr1a and Avpr1b mediate the majority of Avp's central effects (5,8), although Avp also binds to the Oxt receptor (Oxtr) with relatively high affinity (12,13) (and the Oxtr is widely distributed in the brain (12)), and there are other structurally unrelated Avp-binding molecules found in the brain (14). The Avpr1b is highly expressed in the corticotrophs of the anterior pituitary, where it is responsible for the effects of Avp on ACTH release (9,15).
Stress triggers a number of physiological changes that normally allow us to cope with the disturbance. The major endocrine response to stressful events is activation of the hypothalamic-pituitary-adrenal (HPA) axis. This results in rapid increases in circulating ACTH and subsequent rise in glucocorticoids (corticosterone (CORT) in rodents). The principal ACTH secretagogue in response to most acute stressors in rodents appears to be corticotrophin-releasing hormone (Crh), synthesized and secreted by the pPVN neurones into the portal circulation bathing the pituitary (16). Under normal, resting conditions, up to 50% of the pPVN Crh-expressing neurones also express Avp (17). While the prevailing view is that Avp is a minor ACTH secretagogue, there is a large body of evidence that Avp makes a significant contribution to the HPA axis response to acute stress. For example, Avp immunoneutralisation inhibits the increase in plasma ACTH produced by acute stressors such as bacterial lipopolysaccharide (LPS) challenge (18,19), ethanol (EtOH) administration (20,21), restraint (22) and insulin-induced hypoglycaemia (23). In addition, the expression of Avp in the pPVN Crh-expressing cells, external zone of the median eminence, and pituitary portal circulation is increased in response to some acute stressors (24-27), and although Avp acts as a weak ACTH secretagogue on its own, it potentiates Crh-stimulated ACTH release in vitro and in vivo (9). Furthermore, Avp but not Oxt appears to be sufficient to maintain basal ACTH secretion and adequate HPA activity for survival in Crh-type 1 receptor (Crhr1) knockout (KO) mice (28). More recently, a non-peptide Avpr1b antagonist (SSR149415) was shown to block acute restraint-induced ACTH secretion in rats (29). On the other hand, other studies (e.g., recent work on restrained VP-deficient Brattleboro rats (30) conclude that Avp may not subserve an important role in acute restraint-induced ACTH release. The role of Avp in the HPA axis response to acute stress likely reflects the cooperative action of Avp and Crh and depends on the nature and severity of the stimulus. In contrast to Avp's purported (weak) role in acute stress-induced HPA axis activity, there is evidence that Avp may become the main ACTH secretagogue in some chronic (or repeated) stress situations, particularly those that are associated with corticotroph hyperresponsiveness (9,31). For example, repeated restraint stress in rats results in increased expression of Avp in pPVN Crh-containing neurones (32) and the final acute restraint episode following repeated restraint results in a rapid increase in Avp- but not Crh-hnRNA in the pPVN (33).
Recent studies have shown that Avpr1b KO mice have shown a normal HPA axis response to an acute physical-psychological (resident-intruder)(8) or restraint (34) stress but a dramatically reduced ACTH response to acute forced swim (35) and insulin-induced hypoglycaemia (34). The purpose of the current study was to further explore the role of the Avpr1b in the HPA axis response to acute stress. Using Avbr1b wild-type and KO animals, we have investigated the HPA axis responses (measured by plasma ACTH and CORT levels) to acute LPS challenge and EtOH administration, two stressors in which Avp has been implicated to have a role in promoting ACTH secretion in rats.
Materials and Methods
Animals
Adult male and female (8-11 weeks) mice (a mix of the C57BL/6J and 129X1/SvJ strains) lacking the Avpr1b were generated and maintained as described previously (8). Homozygous Avpr1b KO and wild-type littermates of crosses using mice heterozygous for the Avpr1b mutation were identified by PCR analysis of DNA isolated from tail clips. Animals were group housed (two to four per cage) under controlled light and temperature (21 ± 2°C) with food and water available ad libitum and maintained on a 14 h light, 10 h dark cycle (lights on 0500h). Based on the number of animals at our disposal, female and male mice were used for the LPS and EtOH studies, respectively (see below). To explore possible gender differences in the effects of EtOH on the HPA axis female mice were used in addition to males for one EtOH dose (3.3g/kg - see below). All female mice were used at random stages of the oestrous cycle. Approximately 24 h prior to experimentation mice were individually housed. The following day they were removed from their holding room and placed in a nearby experimental room for approximately 2 h to acclimatise to the surroundings. Studies were performed between 0930-1200h, with the exception of the 4h LPS experiment (see below) in which the animals were sacrificed between 1330-1345h. All procedures were conducted in accordance with the Animal Scientific Procedures Act (1986) United Kingdom.
Lipopolysaccharide (LPS) challenge
LPS from Escherichia coli (serotype 055:B55; Sigma, U.K.) was diluted in sterile, apyrogenic 0.9% saline and aliquoted (10mg/ml) at −80°C. The same serotype and lot number was used for each experiment. Frozen aliquots were thawed on ice and further diluted in saline to a concentration of 500μg/ml. Female Avpr1b KO or wild-type mice (approximately 30g) were injected i.p. with 200 μl diluted LPS (100μg) or vehicle (0.9% saline) and sacrificed 30 min or 4 h later. The 30 min time-point as chosen so that we could compare the extent of HPA axis activation to that elicited by other stressors (e.g., restraint - see ref.34) used on mice in our laboratory and those of others (e.g., see ref.36). While the HPA axis response to LPS depends on many factors (e.g., bacterial serotype, LPS purity, dose and route of administration, rodent age and strain), when comparing other reports in the literature we recognize that the peak plasma ACTH and CORT responses to LPS in our mice may be greater than 30 min (e.g., see ref.37). The dose of LPS used in this study is considered high (e.g., doses ranging from 1-300μg i.p. have been reported with 2.5mg/kg, equivalent to approx. 83μg/30g mouse, often employed: e.g., see refs.37-39) and both genotypes exhibited some sickness behaviour (e.g. reduction in locomotor activity) 4 h post-injection. We did not attempt to quantify LPS-induced behaviour or assess whether there was any difference between genotypes in this study.
Ethanol (EtOH) administration
Sterile, absolute EtOH (Martindale Pharmaceuticals, Romford, Essex) was diluted in 0.9% saline to less than 20% (v/v)(15.8% w/v) taking into account the specific gravity of absolute EtOH. Male and female Avpr1b KO or wild-type mice were injected i.p. with 3.2 g/kg EtOH (maximum volume 200μl) or sterile dH2O (diluted to the same extent as absolute EtOH) in 0.9% saline (controls) and sacrificed 30 min later. In one set of experiments male Avpr1b KO and wild-type animals were injected i.p. with 4g/kg EtOH to test whether the dose of EtOH affected the degree of HPA axis activation. In another set of experiments, EtOH was diluted to 3.2g/kg in 0.9% saline containing lidocaine (10mg/kg; Sigma, U.K.) to alleviate gastrointestinal irritation caused by EtOH injection. EtOH doses of approxim. 3-4g/kg are considered an intoxicating dose in rodents (40) and have been previously shown to give peak ACTH responses 30min after administration in Crhr1 KO mice (41). Following EtOH (3.2 and 4g/kg) administration mice were quickly sedated and exhibited loss of righting reflex (LORR) within 2-10 min (data not shown). No attempt was made to quantitify EtOH-induced behavioural phenotypes in Avpr1b mutants or wild-type mice, although no difference in the LORR was observed in a previous study (42).
Plasma ACTH and CORT levels
All experiments were performed at least twice (with the exception of the 4g/kg EtOH experiment in male mice, which was performed only once) and samples measured in duplicate or triplicate (CORT RIA). Mice were sacrificed by decapitation within 10 sec after removal from their home cage. Plasma obtained from trunk blood collected into heparinized tubes was used for all hormonal measurements. Total plasma CORT was measured (10 μl plasma diluted in 500 μl assay buffer) using antiserum kindly supplied by Dr G. Makara (Institute of Experimental Medicine, Budapest, Hungary) as described previously (43). All samples within each experiment were processed in the same assay with an intra-assay variation less than 10%. The tracer was [125I]-corticosterone (ICN Biomedicals, Irvine, CA, USA) with a specific activity of 2-3mCi/μg. The sensitivity of the assay was 10ng/ml. Plasma ACTH was measured as described previously (44) using a rabbit anti-rat ACTH primary antibody (donated by G. Makara) and [125I]ACTH (Amersham Biosciences, Little Chalfont, UK). Plasma ACTH in one (of two) 4 h LPS experiments in female mice was measured using an ELISA kit (Biomerica#DZ-7023 obtained from Immunodiagnostics Systems Ltd, Boldon, Tyne & Waer, U.K.) according to the manufacturer's instructions. The assay has a sensitivity of 0.46 pg/ml and intra-assay CV of 3.1% at 35.7 pg/ml and 4.2% at 255 pg/ml.
Blood alcohol level (BALs) measurement
BALs were determined on 50 μl of plasma from one 3.2g/kg EtOH study in mice of both genotypes and sexes, and from the single 4g/kg EtOH study in male mice. Analysis was performed on a GC8000TOP gas liquid chromatograph with HS850 autosampler (Thermoelectron Corporation) using headspace analysis. Quantitation was achieved using LGC certified standards for EtOH (LGC Promochem. Middlesex, U.K.) and n-propanol (BDH Chemicals Ltd., U.K.) as an internal standard. The limit of detection is 5mg/100ml and the analytical CV of the method is 4.2% at 270mg/100ml and 5.5% at 38mg/100ml.
Data analysis
All results are presented as mean ± SEM. The statistical differences between hormone and BAL measurement groups were determined by two-way ANOVA followed by Bonferroni's post-hoc test using GraphPad Prism (version 4.0b) software. P < 0.05 was considered statistically significant.
Results
Effect of LPS challenge on HPA axis activity in Avpr1b KO mice
LPS evoked a significant increase in plasma ACTH and CORT levels in female Avpr1b wild-type mice 30 min after peripheral (i.p.) challenge (Fig. 1A,B). Plasma ACTH levels increased from 22.3 ± 4.4 pg/ml (saline control, wild-type, mean ± SEM, n = 5) to 114 ± 8.9 pg/ml (LPS, wild-type, mean ± SEM, n = 6) while plasma CORT levels rose approximately 10-fold (98 ± 16.8 and 1026.3 ± 73.5 ng/ml for saline control and LPS in wild-type mice, respectively; mean ± SEM, n = 5-6). In contrast, the LPS-induced ACTH and CORT release in female Avpr1b KO mice was impaired - the plasma ACTH and CORT levels in the KO mice were markedly reduced (approximately 60%; P < 0.001) compared to those found in wild-type mice (Fig. 1A,B) following LPS treatment. However, LPS still significantly increased HPA axis activity (P < 0.05 for plasma ACTH; P < 0.001 for plasma CORT after LPS challenge compared to saline controls) in Avpr1b KO animals.
Figure 1.
Effect of LPS on HPA axis activity in female Avpr1b KO and wild-type mice. Animals were sacrificed 30min following LPS (100μg i.p.) or saline challenge, and plasma levels of ACTH (A) and CORT (B) were measured. Values are mean ± SEM, n = 5-6 mice/treatment group. a, P < 0.0001 LPS wild-type vs. LPS KO; b, P < 0.001 LPS wild-type vs. saline wild-type; c, P < 0.05 LPS KO vs. saline KO. There is no significant difference (P > 0.05) between saline wild-type and saline KO plasma ACTH or CORT levels. There is also no significant difference between handled (mice briefly handled and returned to home cage 30min prior to sacrifice) and saline plasma ACTH and CORT levels in either genotype (e.g., plasma ACTH levels: 17.8 ± 4.9 and 20.4 ± 3.3 pg/ml for handled and saline-treated KO mice, respectively, and 22.4 ± 8.2 and 22.3 ± 4.5 pg/ml for handled and saline-treated wild-type mice, respectively; mean ± SEM, n = 4-5). In (C) plasma CORT levels were measured 4 h after LPS or saline challenge. Values are mean ± SEM, n = 3-4 animals/group. a, P < 0.0001 LPS vs. saline. There is no significant difference (P > 0.05) between saline- or LPS-stimulated CORT levels for both genotypes.
To examine the role the Avpr1b in the HPA axis response to more prolonged immune activation, Avpr1b KO and wild-type were sacrificed 4 h following LPS challenge. Plasma CORT levels increased approximately 13-fold (71 ± 21.9 and 907 ± 43 ng/ml for saline control and LPS, respectively; mean ± SEM, n = 4-5) in wild-type mice (Fig. 1C). While both the plasma ACTH and CORT levels obtained after LPS treatment for 30 min differed between genotypes, there was no significant difference (P > 0.05) between plasma ACTH and CORT levels in Avpr1b KO and wild-type mice 4h after LPS injection (Fig.1C; plasma ACTH levels 4 h after saline or LPS challenge measured by ELISA: 7.4 ± 1.9 and 7.8 ± 3.6 pg/ml for 4 h saline controls in the wild-type and Avpr1b KO, respectively; mean ± SEM, n = 6, P > 0.05, not significantly different; 139.2 ± 11.9 and 129.4 ± 10 pg/ml for 4 h LPS in wild-type and Avpr1b KO, respectively; mean ± SEM, n = 8, P > 0.05, not statistically different). In addition, there was no significant difference (P > 0.05) between the plasma ACTH or CORT levels obtained 30 min or 4 h following LPS injection in wild-type mice (Fig.1A,B).
Effect of EtOH administration on HPA axis activity in Avpr1b KO mice
The effect of acute (30 min) EtOH administration (3.2g/kg i.p.) on plasma ACTH and CORT levels in male Avpr1b KO and wild-type mice is shown in Fig.2. Plasma ACTH and CORT were elevated approximately 4-fold and 6-fold, respectively, in wild-type mice 30 min following EtOH injection (Fig.2A, B). EtOH (3.2g/kg) also significantly increased plasma ACTH and CORT levels in Avpr1b KO animals (P < 0.05 and P < 0.01 for ACTH and CORT levels, respectively), but this increase was approximately 33-50% less than that observed in Avpr1b wild-type mice (Fig.2A,B). Addition of lidocaine (10mg/kg injected ip with EtOH) reduced the amount of flinching observed upon EtOH injection into Avpr1b KO and wild-type mice, and had no effect on the levels of ACTH or CORT obtained (e.g., 74.4 ± 4.24 vs. 72.1 ± 4.5 pg/ml for EtOH-induced plasma ACTH levels in male Avpr1b wild-type mice with and without lidocaine, respectively; mean ± SEM, n = 4-5, P > 0.05; 461 ± 30.4 vs. 391.7 ± 19.31 ng/ml for EtOH-induced plasma CORT levels in male Avpr1b wild-type mice with and without lidocaine, respectively, mean ± SEM, n = 4-5, P > 0.05). BALs (about 300mg/100ml) were not significantly different (P > 0.05) between the two genotypes (Fig.2C).
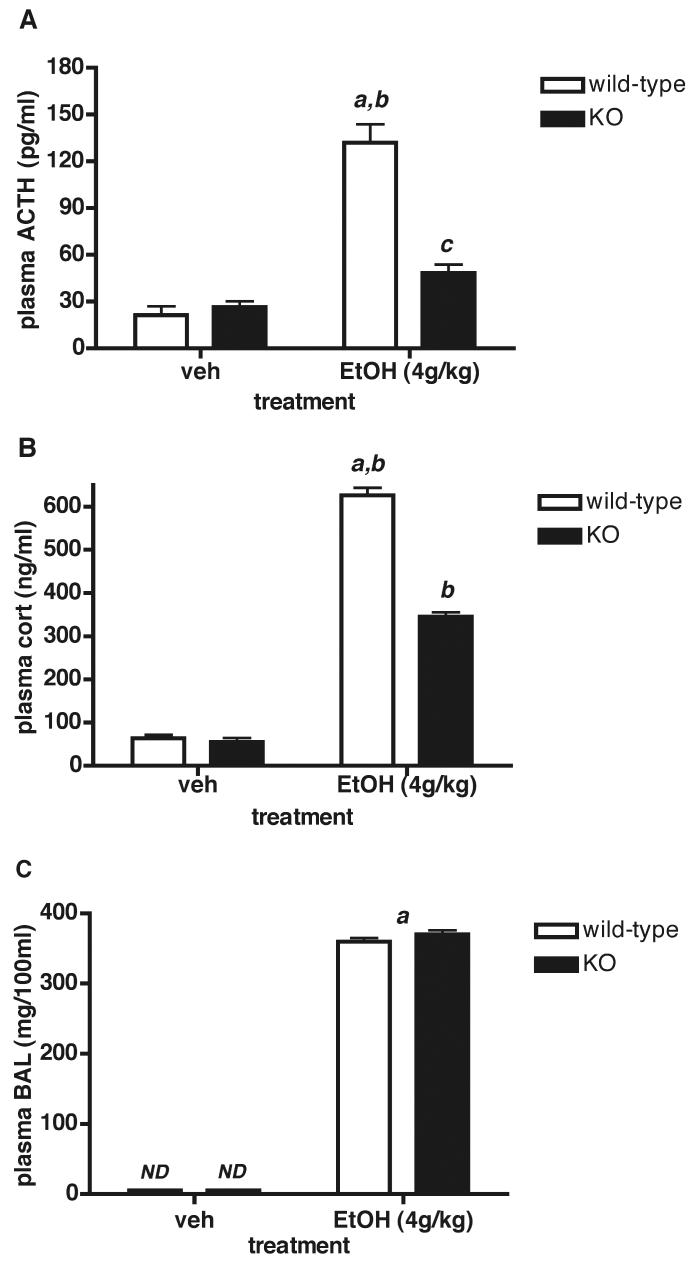
Figure 2.
HPA axis activity in male Avpr1b KO and wild-type mice 30 min after administration of EtOH (3.2g/kg i.p.). Values are mean ± SEM, n = 4-5 mice/group. A = ACTH; B = CORT. a, P < 0.001 EtOH wild-type vs. vehicle (veh) wild-type; b, P < 0.01 EtOH wild-type vs. EtOH KO; c, P < 0.05 EtOH KO vs. vehicle KO. There is no significant difference (P > 0.05) between vehicle wild-type and vehicle KO plasma ACTH or CORT levels. There is also no significant difference between handled and saline plasma ACTH and CORT levels in either genotype (e.g., plasma ACTH levels: 18.3 ± 8.3 and 23.7 ± 6.0 pg/ml for handled and saline-treated KO mice, respectively, and 24.4 ± 7.3 and 17.5 ± 3.4 pg/ml for handled and saline-treated wild-type mice, respectively; mean ± SEM, n = 4-5). Blood alcohol measurements (BALs) are shown in (C). a, P < 0.0001 EtOH vs. vehicle; ND, not detectable. There is no significant difference between EtOH wild-type and EtOH KO plasma BALs.
When the concentration of EtOH was increased from 3.2 to 4g/kg (a strong, sedative dose), BALs in male mice were also significantly increased (P < 0.0001) from an average of 303 ± 6.6 mg/100ml for 3.2g/kg (mean ± SEM, data from the two genotypes was pooled because the individual genotype means did not differ) to 365 ± 4.2mg/100ml for 4g/kg (mean ± SEM of pooled genotypes)(Fig.3C). The elevated BALs in response to the increase in EtOH dose were accompanied by a further increase in the plasma ACTH and CORT response in wild-type mice - i.e., ACTH values were 72 ± 4.5 vs. 132 ± 11.8pg/ml (mean ± SEM; significantly increased, P < 0.0001), while CORT values were 412 ± 26.8 vs. 626 ± 17.8 ng/ml (mean ± SEM; significantly increased, P < 0.001) for male mice treated with 3.2 and 4g/kg EtOH, respectively. The ACTH and CORT responses to 4g/kg EtOH were significantly reduced (approximately 64% and 45% for ACTH and CORT, respectively) in male Avpr1b KOs compared to wild-type controls (Fig.3A,B).
Figure 3.
Plasma ACTH, CORT and BAL responses after i.p. EtOH (4g/kg) injection in male Avpr1b KO and wild-type mice. Values are mean ± SEM, n = 4-6 mice/group. A = plasma ACTH levels. a, P < 0.0001 EtOH wild-type vs. vehicle (veh) wild-type; b, P < 0.001 EtOH wild-type vs. EtOH KO; c, P < 0.05 EtOH KO vs. vehicle KO. B = plasma CORT levels. a, P < 0.0001 EtOH wild-type vs. vehicle wild-type; b, P < 0.001 EtOH wild-type vs. EtOH KO; EtOH KO vs. vehicle KO. There is no significant difference between vehicle wild-type and vehicle KO plasma ACTH or CORT levels. C = blood alcohol levels (BALs). a, P < 0.0001 EtOH vs. vehicle of both genotypes.; ND, not detectable. There is no significant difference between EtOH wild-type and EtOH KO plasma BALs.
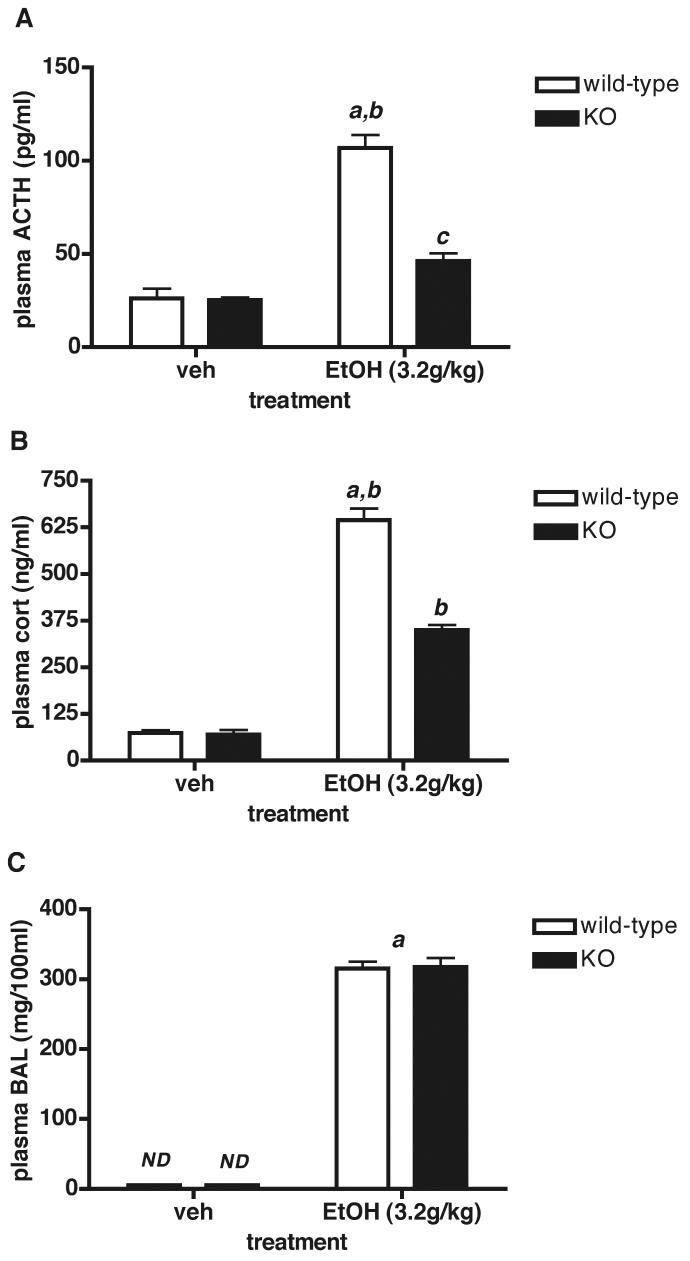
Since the HPA axis response to EtOH is gender-specific (females have greater ACTH/CORT but not BAL responses than males (45)), we determined the ACTH and CORT responses to EtOH in female Avpr1b KO and wild-type mice. EtOH (3.2g/kg) administered acutely elevated plasma ACTH and CORT levels by approximately 4- and 9-fold, respectively in female Avpr1b wild-type animals (Fig.4A,B). As shown in male Avpr1b KO mice, the HPA axis response to EtOH in female Avpr1b KO animals was significantly impaired, rising only approximately 1.8- and 5-fold for plasma ACTH and CORT levels, respectively. The ACTH and CORT plasma levels in female Avpr1b wild-type mice were significantly greater (P < 0.001) than those obtained for the same dose of EtOH (3.2g/kg) and duration of treatment (30 min) used in male Avpr1b wild-type mice (e.g., 107 ± 6.8 and 72.1 ± 4.5 pg/ml for EtOH-induced plasma ACTH; mean ± SEM; see Fig.2A). In contrast, BALs in female mice administered EtOH were not significantly different between the two genotypes (Fig.4C), and reached a similar level to that obtained in male mice.
Figure 4.
HPA axis activity in female Avpr1b KO and wild-type mice 30 min after administration of EtOH (3.2g/kg i.p.). Values are mean ± SEM, n = 5 mice/group. A = plasma ACTH levels. a, P < 0.001 EtOH wild-type vs. vehicle (veh) wild-type; b, P < 0.01 EtOH wild-type vs. EtOH KO; c, P < 0.05 EtOH KO vs. vehicle KO. B = plasma CORT levels. a, P < 0.0001 EtOH wild-type vs. vehicle wild-type; b, P < 0.001 EtOH wild-type vs. EtOH KO; EtOH KO vs. vehicle KO. There is no significant difference between vehicle wild-type and vehicle KO plasma ACTH or CORT levels. Blood alcohol measurements (BALs) are shown in (C). a, P < 0.0001 EtOH vs. vehicle; ND, not detectable. There is no significant difference between EtOH wild-type and EtOH KO plasma BALs
Discussion
It is clear from our results that an intact Avpr1b is required for a normal, acute HPA axis response to LPS challenge and EtOH administration. Female Avpr1b KO mice have a markedly diminished (but not absent) ACTH and CORT response to peripheral LPS challenge, and male and female Avpr1b KO animals have impaired ACTH /CORT responses to i.p. administration of EtOH. Our data also suggest that the extent of EtOH-induced HPA axis activation is dose-dependent, and that there is a sexual dichotomy in the HPA axis response to EtOH that does not appear to depend on the absolute levels of alcohol in the blood. Our findings in mice mirror those obtained by Rivier and coworkers (e.g., see refs. 18-21,40,45,46) on the acute effects of LPS and EtOH on the HPA axis in rats, and confirm and extend the results reported by others showing that LPS and EtOH can stimulate PVN Avp neurones (e.g., see ref. 47). We cannot exclude that LPS and/or EtOH-induced Oxt secretion may also have an impact on the overall HPA axis activity in Avpr1b wild-type mice - reduced Oxt stimulation of corticotroph function could account for the impaired LPS/EtOH-induced HPA axis activity in Avpr1b KO mice because the direct action of Oxt on stimulating corticotroph ACTH release is mediated by the Avpr1b (48). It should be noted, however, that Oxt is generally considered to inhibit stress-induced HPA axis activity (49).
Peripheral administration of endotoxin is a commonly used model to study the HPA axis response to physiologically relevant immune stimuli such as bacterial infection and sepsis. Endotoxins are LPS constituents of the outermost part of a Gram-negative bacterial cell membrane that are released upon bacterial lysis. LPS potently stimulates cytokine (primarily interleukin (IL)-1, IL-6 and tumour necrosis factor (TNF)-α) production by binding to toll-like receptors (TLR-4) present on circulating monocytes/neutrophils and tissue macrophages (50). Immune mediators such as LPS/cytokines can activate the HPA axis, depending on the nature, route of administration and the intensity of the immune signal generated. These mechanisms have been extensively reviewed (e.g., see refs.39,51,52) and include: (1) Crh/Avp neurones in the PVN and/or Crh/Avp pPVN nerve terminals in the external zone of the median eminence may be activated directly or indirectly by the cytokines or by other secretagogues (e.g., prostaglandins, nitric oxide); (2) recruitment of hierarchical pathways (e.g., aminergic, GABAergic) that impinge on and alter the activity of Crh/Avp neurones; (3) during prolonged immune stimulation, cytokines or other mediators may act directly or indirectly on the pituitary and adrenal to facilitate ACTH and CORT secretion and (4) stimulation of primary nociceptive afferents (e.g., activation of vagal afferents by i.p. LPS injection) can trigger Crh/Avp release (39,51,52). Notwithstanding all of the aforementioned possibilities, a number of physiological responses (e.g. acute phase responses such as sickness behaviour, heart rate elevation, sympathetic nervous system activation) to LPS may precede the detection of cytokines in the systemic circulation, and these may directly or indirectly activate the HPA axis (39,50-52). Overall, the consensus view is that the systemic production of cytokines may not be an essential step in the early changes provoked by LPS and that the marked rises in ACTH and CORT secretion initiated by acute LPS or cytokine administration are triggered mainly by activation of vagal inputs to the CNS and a consequent increase in the hypothalamic drive to the pituitary corticotrophs (39,50-52). Cytokine production in the periphery and/or within brain parenchyma may contribute more to the prolonged and sustained HPA axis responses to systemic endotoxemia (51,52).
In rats Crh appears to be much more important in driving the ACTH response to stressors such as restraint than to LPS or cytokine challenge (53). However, LPS and cytokines (i.v. or i.p.) increase Crh hnRNA and/or mRNA expression in the pPVN, and Crh-immunoneutralisation and Crh antagonists partially inhibit the rise in plasma ACTH or CORT produced by i.v. LPS, IL-1β, IL-6 or TNF-α (18,19,50, 53). Studies in mice are less clear - Crh KO animals have impaired LPS-induced ACTH and CORT secretion (54), but the absence of Crhr1 only marginally compromises the ACTH response to LPS (55). Other studies conclude that Crh only participates in the early ACTH response to low doses of LPS (37).
The residual ACTH secretion remaining after LPS stimulation in rodents treated with Crh antagonists or antibodies has been attributed to the action of Avp on the pituitary Avpr1b (18,53). A number of studies have shown that LPS and cytokines activate Avp- pPVN neurones and alter Avp release after immune challenge. For example, LPS induces c-fos expression (47) and Avp hnRNA and/or mRNA in pPVN cells (56). In contrast, others found no change in Avp mRNA after LPS challenge in rats (57) or Crh KO, leukemia inhibitory factor (Lif) KO, double Crh/Lif KO or wild-type mice (58). Perhaps the most direct evidence that Avp is involved in LPS/cytokine action on the HPA axis is that Avp immunoneutralisation inhibits the rise in plasma ACTH produced by LPS (18,19). However, it is important to note that although Avp/Crh immunoneutralisation significantly reduces ACTH secretion during LPS treatment, plasma CORT levels still remain high (19). It has been proposed that PVN activation initiated by low doses of LPS is mainly associated with increased HPA axis activity whereas the higher doses of LPS, as used in the current study, more widely activate autonomic centres including the PVN and also stimulates changes in sympathetic output and hypothalamic Avp synthesis (56). The present results demonstrating an impaired LPS-induced, acute ACTH and CORT response in Avpr1b KO mice supports this concept. Furthermore, the equivalence of ACTH and CORT levels in Avpr1b KO and wild-type mice 4 h after LPS challenge indicates that the Avpr1b is not a critical mediator of the pituitary-adrenal response to extended immune stimulation, and suggests that factors other than Avp (and its cognate Avpr1b) and probably Crh (e.g., cytokines impinging directly on the pituitary and adrenal - see above) are largely responsible for driving the HPA axis response to sustained immune activity. While we did not investigate the origin of LPS- or EtOH-induced Avp (or Oxt) in the current study, findings on direct sampling of hypophysial portal, and peripheral blood in sheep (59) render it likely that LPS stimulates both the acute release of both Crh and Avp from the pPVN terminals into the pituitary portal circulation, and also the release of Avp into the systemic circulation from stores in the posterior pituitary.
It is well established that acute administration of EtOH stimulates the HPA axis (46). In rodents this effect appears to require a threshold BAL of at least 100mg/100ml or greater (40). In the present study, EtOH-induced ACTH and CORT responses were dose- (although only two, relatively high doses of EtOH were used) and gender- (females responded with a greater secretion of both ACTH and CORT than males to one dose of EtOH) dependent, and obtained with BALs (approximately 300-400mg/100ml) well over those reported necessary for HPA axis activation (40). Gender differences in HPA axis activation by EtOH appear to be attributable in part to the influence of circulating oestrogen (45). One caveat concerning studies investigating the effects of EtOH on the HPA axis is that the drug administered i.p. is a gastro-intestinal irritant, and this stress may contribute significantly to the overall impact on the HPA axis. It is worth noting that in the present study we did not observe any difference in the ACTH/CORT responses to EtOH administered with or without a dose of local anaesthetic that minimized discomfort (e.g., prevented any abdominal ‘flinching’ and vocalization during injection).
Whether EtOH acts directly on the PVN, via PVN afferents or at the level of the median eminence is not known, and it is possible that all three levels are activated in concert. EtOH affects a variety of central neurotransmitter/neuromodulator pathways including the neurotransmitter-gated ion channels, aminergic processes, the endogenous opioid and cannabinoid systems, and neuro-peptides/hormones and/or their receptors (46,60-62), all of which are capable of interacting directly or indirectly with the Crh and/or Avp elements within the hypothalamus. A number of studies have shown that EtOH stimulates PVN Crh gene expression in vivo and in vitro, and Crh immunoneutralisation or pituitary Crhr1 blockade clearly inhibits (though does not completely suppress) acute ethanol-induced ACTH secretion (46,63). Acute EtOH administration also increases Avp mRNA in the magnocellular PVN and SON, and Avp immunoneutralisation reduces EtOH-induced ACTH secretion by 40-50% (20,46). The level of inhibition achieved with Avp immunoneutralisation is comparable to the extent of inhibition of LPS- or EtOH-induced ACTH and CORT release in the Avpr1b KO found in the present study. Rivier and coworkers have shown that the HPA response to EtOH does not require the presence of circulating levels of the drug, i.e., increased pituitary proopiomelanocortin (Pomc) synthesis and ACTH release can be elicited by icv injection of EtOH, presumably primarily through central activation of Crh and/or Avp PVN neurones (21). In addition, these workers found that EtOH-induced Pomc synthesis could be completely blocked by immunoneutralisation of both Crh and Avp, but only partially inhibited if only one peptide or the other was removed. The hypothalamic synthesis/release of Avp and/or Crh may also be altered by the action of EtOH on Crhr1 present on pPVN neurones (64). Interestingly, a non-PVN source contributing to the stimulatory effect of EtOH on ACTH release is supported by studies showing that lesioning of the PVN only inhibits the ACTH response to EtOH by approximately 60% (20). Whether or not the magnocellular Avp-expressing neurones of the SON participate in the HPA axis response to EtOH awaits to be determined.
It is possible that EtOH causes a fast, transient release of Avp into the systemic circulation (65,66). Some studies have reported rapid changes in plasma Avp levels, presumably of magnocellular neurone origin, in response to stress (67,68) - in particular, forced swim induces a rapid, transient elevation in plasma Avp with a return to basal levels within one minute (10). Notably, the HPA axis response to acute forced swim is substantially reduced in Avpr1b KO mice (35; SJL, LQS, JAR; unpublished data). These studies do not obviate a role for pPVN Avp - it is possible that there is an increase in pituitary portal circulation Avp levels independent of peripheral levels of Avp. It is equally plausible that the increase in Avp mRNA in the magnocellular PVN and SON following acute EtOH administration reflects somato-dendritic Avp release within the magnocellular cells of the PVN and SON, and thereby possibly alters the neuropeptide drive to the corticotroph. Whatever the source of Avp, it is apparent from immunoneutralisation studies (20,46) that there are sufficient amounts of hypothalamic Avp to augment Crh-stimulated ACTH release following EtOH (and LPS) administration.
Taken together, the results from this study and many previous studies, mainly on rats, strongly suggest that a normal ACTH and CORT response to many acute stressors requires the co-ordinated participation of both Crh and Crhr1, and Avp and Avpr1b. With respect to the Avpr1b KO model, it appears that other hypothalamic peptides such as Crh (and/or cytokines in the case of immune, and perhaps other stressors) cannot compensate for the loss of the Avpr1b in maintaining the normal HPA axis response to these stressors. Importantly, the Avpr1b KO does not seem to be “just” another Crh-type KO - it appears to faithfully reflect Avp's role as the dynamic variable as opposed to the permissive role of Crh in regulating ACTH release (24).
In conclusion, our studies support the hypothesis that Avp is an important mediator in the acute HPA axis response to LPS and EtOH. Given the postulated roles for Avp and the Avpr1b in the adaptation to chronic stress (9,31-33), and the adaptation of the CORT response to repeated EtOH administration in rats (69), it would be of interest to investigate the HPA axis response to chronic immune stimulation, or chronic/repeated EtOH administration in the Avpr1b KO animals.
Acknowledgements
The work was supported by Wellcome Trust (U.K.) grants to SJL and A-MO'C. WSY is supported by the NIMH Intramural Research Program (Z01-MH-002498-16). The authors thank Ms. Evangelia Papanikolaou for her contribution with some of the animal experiments.
References
- 1.Knepper MA. Molecular physiology of urinary concentrating mechanism: regulation of aquaporin water channels by vasopressin. Am J Physiol Renal Physiol. 1997;272:F3–F12. doi: 10.1152/ajprenal.1997.272.1.F3. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham ET, Jr, Sawchenko PE. Reflex control of magnocellular vasopressin and oxytocin secretion. Trends Neurosci. 1991;14:406–411. doi: 10.1016/0166-2236(91)90032-p. [DOI] [PubMed] [Google Scholar]
- 3.Sawchenko PE, Swanson LW. Immunohistochemical identification of neurones in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol. 1982;205:260–272. doi: 10.1002/cne.902050306. [DOI] [PubMed] [Google Scholar]
- 4.Sofroniew MV. Morphology of vasopressin and oxytocin neurones and their central and vascular projections. Prog Brain Res. 1983;60:101–114. doi: 10.1016/S0079-6123(08)64378-2. [DOI] [PubMed] [Google Scholar]
- 5.De Wied D, Diamont M, Fodor M. Central nervous system effects of the neurohypophseal hormones and related peptides. Front Neuroendocrinol. 1993;14:251–302. doi: 10.1006/frne.1993.1009. [DOI] [PubMed] [Google Scholar]
- 6.Young LJ, Nilsen R, Waymire KG, MacGregor GR, Insel TR. Increased affiliative response to vasopressin in mice expressing the V1a receptor from a monogamous vole. Nature. 1999;400:766–768. doi: 10.1038/23475. [DOI] [PubMed] [Google Scholar]
- 7.Koshimizu T, Nasa Y, Tanoue A, Oikawa R, Kawahara Y, Kiyono Y, Adachi T, Tanaka T, Kuwaki T, Mori T, Takeo S, Okamura H, Tsujimoto G. V1a vasopressin receptors maintain normal blood pressure by regulating circulating blood volume and baroreflex sensitivity. Proc Natl Acad Sci USA. 2006;103:7807–7812. doi: 10.1073/pnas.0600875103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wersinger SR, Ginns EI, O'Carroll A-M, Lolait SJ, Young WS., 3rd Vasopressin V1b receptor knockout reduces aggressive behaviour in male mice. Molecular Psychiatry. 2002;7:975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- 9.Antoni F. Vasopressinergic control of pituitary adrenocorticotropin secretion comes of age. Front Neuroendocrinol. 1993;14:76–122. doi: 10.1006/frne.1993.1004. [DOI] [PubMed] [Google Scholar]
- 10.Engelmann M, Landgraf R, Wotjak CT. The hypothalamic- neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: an old concept revisited. Front Neuroendocrinol. 2004;25:132–139. doi: 10.1016/j.yfrne.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Lolait SJ, O'Carroll A-M, Brownstein MJ. Molecular biology of vasopressin receptors. Ann NY Acad Sci. 1995;771:273–292. doi: 10.1111/j.1749-6632.1995.tb44688.x. [DOI] [PubMed] [Google Scholar]
- 12.Barberis C, Tribollet E. Vasopressin and oxytocin receptors in the central nervous system. Crit Rev Neurobiol. 1996;10:119–154. doi: 10.1615/critrevneurobiol.v10.i1.60. [DOI] [PubMed] [Google Scholar]
- 13.Jeng YF, Lolait SJ, Strakova Z, Chen C, Copland JA, Mellman D, Hellmich MR, Soloff MS. Molecular cloning and functional characterization of the oxytocin receptor from a rat pancreatic cell line (RINm5F) Neuropeptides. 1996;30:557–565. doi: 10.1016/s0143-4179(96)90039-6. [DOI] [PubMed] [Google Scholar]
- 14.Hurbin A, Orcel H, Ferraz C, Moos FC, Rabie A. Expression of the genes encoding the vasopressin-activated calcium mobilizing receptor and the dual angiotensin II/vasopressin receptor in the rat central nervous system. J Neuroendocrinol. 2000;12:677–684. doi: 10.1046/j.1365-2826.2000.00499.x. [DOI] [PubMed] [Google Scholar]
- 15.Lolait SJ, O'Carroll A-M, Mahan LC, Felder CC, Button DC, Young WS, 3rd, Mezey E, Brownstein MJ. Extrapituitary expression of the rat V1b vasopressin receptor gene. Proc Natl Acad Sci USA. 1995;92:6783–6787. doi: 10.1073/pnas.92.15.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sawchenko PE, Imaki T, Potter E, Kovacs K, Imaki J, Vale W. The functional neuroanatomy of corticotropin-releasing factor. Ciba Found Symp. 1993;172:5–21. doi: 10.1002/9780470514368.ch2. [DOI] [PubMed] [Google Scholar]
- 17.Whitnall MH. Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog Neurobiol. 1993;40:573–629. doi: 10.1016/0301-0082(93)90035-q. [DOI] [PubMed] [Google Scholar]
- 18.Turnbull AV, Lee S, Rivier C. Mechanisms of hypothalamic-pituitary-adrenal axis stimulation by immune signals in the adult rat. Ann NY Acad Sci. 1998;840:434–443. doi: 10.1111/j.1749-6632.1998.tb09582.x. [DOI] [PubMed] [Google Scholar]
- 19.Aubry J-M, Turnbull AV, Pozzoli G, Rivier C, Vale W. Endotoxin decreases corticotropin-releasing factor receptor 1 messenger ribonucleic acid levels in the rat pituitary. Endocrinology. 1997;138:1621–1626. doi: 10.1210/endo.138.4.5050. [DOI] [PubMed] [Google Scholar]
- 20.Ogilvie KM, Lee S, Rivier C. Role of vasopressin and corticotropin-releasing factor in mediating alcohol-induced adrenocorticotropin and vasopressin secretion in male rats bearing lesions of the paraventricular nuclei. Brain Res. 1997;744:83–95. doi: 10.1016/s0006-8993(96)01082-7. [DOI] [PubMed] [Google Scholar]
- 21.Lee S, Selvage D, Hansen K, Rivier C. Site of action of acute alcohol administration in stimulating the rat hypothalamic-pituitary-adrenal axis: comparison between the effect of systemic and intracerebroventricular injection of this drug on pituitary and hypothalamic responses. Endocrinology. 2004;145:4470–4479. doi: 10.1210/en.2004-0110. [DOI] [PubMed] [Google Scholar]
- 22.Kjaer A, Knigge U, Bach FW, Warberg J. Histamine- and stress-induced secretion of ACTH and beta-endorphin: involvement of corticotropin-releasing hormone and vasopressin. Neuroendocrinology. 1992;56:419–28. doi: 10.1159/000126258. [DOI] [PubMed] [Google Scholar]
- 23.Suda T, Nakano Y, Tozawa F, Sumitomo T, Sata Y, Yamada M, Demura H. The role of corticotropin-releasing factor and vasopressin in hypoglycemia-induced proopiomelanocortin gene expression in the rat anterior pituitary gland. Brain Res. 1992;579:303–308. doi: 10.1016/0006-8993(92)90065-h. [DOI] [PubMed] [Google Scholar]
- 24.Plotsky PM, Bruhn TO, Vale W. Hypophysiotropic regulation of adrenocorticotropin secretion in response to insulin-induced hypoglycemia. Endocrinology. 1985;117:323–329. doi: 10.1210/endo-117-1-323. [DOI] [PubMed] [Google Scholar]
- 25.Berkenbosch F, De Goeij DCE, Tilders FJH. Hypoglycemia enhances turnover of corticotropin-releasing factor and of vasopressin in the zona externa of the rat median eminence. Endocrinology. 1989;125:28–34. doi: 10.1210/endo-125-1-28. [DOI] [PubMed] [Google Scholar]
- 26.Paulmyer-Lacroix O, Anglade G, Grino M. Insulin-induced hypoglycaemia increases colocalization of corticotrophin-releasing factor and arginine vasopressin mRNAs in the rat hypothalamic paraventricular nucleus. J Mol Endocrinol. 1994;13:313–320. doi: 10.1677/jme.0.0130313. [DOI] [PubMed] [Google Scholar]
- 27.Engler D, Pham T, Fullerton MJ, Ooi G, Funder JW, Clarke IJ. Studies of the secretion of corticotropin-releasing factor and arginine vasopressin into the hypophysial-portal circulation of the conscious sheep. I. Effect of an audiovisual stimulus and insulin-induced hypoglycemia. Neuroendocrinology. 1989;49:367–381. doi: 10.1159/000125141. [DOI] [PubMed] [Google Scholar]
- 28.Muller MB, Landgraf R, Preil J, Sillaber I, Kresse AE, Keck ME, Zimmerman S, Holsboer F, Wurst W. Selective activation of the hypothalamic vasopressinergic system in mice deficient for the corticotropin-releasing hormone receptor 1 is dependent on glucocorticoids. Endocrinology. 2000;141:4262–4269. doi: 10.1210/endo.141.11.7767. [DOI] [PubMed] [Google Scholar]
- 29.Serradeil-Le Gal C, Wagnon J, Simiand J, Griebel G, Lacour C, Guillon G, Barberis C, Brossard G, Soubrie P, Nisato D, Pascal M, Pruss R, Scatton B, Maffrand J-P, Le Fur G. Characterization of (2S,4R)-1-[5-Chloro-1[(2,4-dimethoxyphenyl)sulfonyl]-3-(2-methoxyphenyl)-2-oxo-2.3-dihydro-1H-indol-3-yl]-4-hydroxy-N,N-dimethyl-2-pyrrolidine carboxamide (SSR149415), a selective and orally active vasopressin V1b receptor antagonist. J Pharmacol Exp Therap. 2002;300:1122–1130. doi: 10.1124/jpet.300.3.1122. [DOI] [PubMed] [Google Scholar]
- 30.Zelena D, Foldes A, Mergl Z, Barna I, Kovacs KJ, Makara GB. Effects of repeated restraint stress on hypothalamo-pituitary-adrenocortical function in vasopressin-deficient Brattleboro rats. Brain Res Bull. 2004;15:21–530. doi: 10.1016/j.brainresbull.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Volpi S, Rabadan-Diehl C, Aguilera G. Vasopressinergic regulation of the hypothalamic pituitary adrenal axis and stress adaptation. Stress. 2004;7:75–83. doi: 10.1080/10253890410001733535. [DOI] [PubMed] [Google Scholar]
- 32.De Goeij DCE, Jezova D, Tilders FJ. Repeated stress enhances vasopressin synthesis in corticotropin releasing factor neurons in the paraventricular nucleus. Brain Res. 1992;577:165–168. doi: 10.1016/0006-8993(92)90552-k. [DOI] [PubMed] [Google Scholar]
- 33.Ma X-M, Levy A, Lightman SL. Emergence of an isolated arginine vasopressin (AVP) response to stress after repeated restraint: A study of both AVP and corticotropin releasing hormone messenger ribonucleic acid (RNA) and heteronuclear RNA. Endocrinology. 1997;138:4351–4357. doi: 10.1210/endo.138.10.5446. [DOI] [PubMed] [Google Scholar]
- 34.Lolait SJ, Stewart LQ, Jessop DS, Young WS, 3rd, O'Carroll A-M. The hypothalamic-pituitary-adrenal axis response to stress in mice lacking functional vasopressin V1b receptors. Endocrinology. 2007;148:849–856. doi: 10.1210/en.2006-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanoue A, Ito S, Honda K, Oshikawa S, Kitagawa Y, Koshimizu T, Mori T, Tsujimoto G. The vasopressin V1b receptor critically regulates hypothalamic-pituitary-adrenal axis activity under both stress and resting conditions. J Clin Invest. 2004;113:302–309. doi: 10.1172/JCI19656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keeney A, Jessop DS, Harbuz MS, Marsden CA, Hogg S, Blackburn-Munro RE. Differential effects of acute and chronic social defeat stress on HPA axis function and hippocampal serotonin release in mice. J. Neuroendocrinol. 2006;18:330–338. doi: 10.1111/j.1365-2826.2006.01422.x. [DOI] [PubMed] [Google Scholar]
- 37.Schotanus K, Makara GB, Tilders FJH, Berkenbosch F. ACTH response to a low dose but not to a high dose of bacterial endotoxin in rats is completely mediated by corticotropin-releasing hormone. Neuroimmunomodulation. 1994;1:300–307. doi: 10.1159/000097180. [DOI] [PubMed] [Google Scholar]
- 38.Ginevich V, Ma X-M, Verbalis J, Aguilera G. Hypothalamicpituitary adrenal axis and hypothalamic-neurohyphyseal responsiveness in water-deprived rats. Exp Neurol. 2001;171:329–341. doi: 10.1006/exnr.2001.7784. [DOI] [PubMed] [Google Scholar]
- 39.Beishuizen A, Thijs LG. Endotoxin and the hypothalamo-pituitary-adrenal (HPA) axis. J Endotoxin Research. 2003;9:3–24. doi: 10.1179/096805103125001298. [DOI] [PubMed] [Google Scholar]
- 40.Ogilvie K, Lee S, Rivier C. Effect of three different modes of alcohol administration on the activity of the rat hypothalamic-pituitary-adrenal axis. Alcohol Clin Exp Res. 1997;21:467–476. doi: 10.1111/j.1530-0277.1997.tb03792.x. [DOI] [PubMed] [Google Scholar]
- 41.Lee S, Smith GW, Vale W, Lee K-F, Rivier C. Mice that lack corticotropin-releasing factor (CRF) receptors type 1 show a blunted ACTH response to acute ethanol despite up-regulated constitutive hypothalamic CRF gene expression. Alcohol Clin Exp Res. 2001;25:427–433. [PubMed] [Google Scholar]
- 42.Caldwell HK, Stewart J, Wiedholz LM, Millstein RA, Iacangelo A, Holmes A, Young WS, 3rd, Wersinger SR. The acute intoxicating effects of ethanol are not dependent on the vasopressin 1a or 1b receptors. Neuropeptides. 2007;40:325–337. doi: 10.1016/j.npep.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Harbuz MS, Rees RG, Eckland DJA, Jessop DS, Brewerton D, Lightman SL. Paradoxical responses of hypothalamic CRF mRNA and CRF-41 peptide and adenohypophyseal POMC mRNA during chronic inflammatory stress. Endocrinology. 1992;130:1394–1400. doi: 10.1210/endo.130.3.1537299. [DOI] [PubMed] [Google Scholar]
- 44.Jessop DS, Eckland DJA, Todd K, Lightman SL. Osmotic regulation of hypothalamo-neurointermediate lobe corticotrophin-releasing factor-41 in the rat. J Endocrinol. 1989;120:119–124. doi: 10.1677/joe.0.1200119. [DOI] [PubMed] [Google Scholar]
- 45.Ogilvie KM, Rivier C. Gender differences in hypothalamic-pituitary-adrenal axis response to alcohol in the rat: activational role of gonadal steroids. Brain Res. 1997;766:19–28. doi: 10.1016/s0006-8993(97)00525-8. [DOI] [PubMed] [Google Scholar]
- 46.Rivier C. Alcohol stimulates ACTH secretion in the rat: mechanism of action and interactions with other stimuli. Alcohol Clin Exp Res. 1996;20:240–254. doi: 10.1111/j.1530-0277.1996.tb01636.x. [DOI] [PubMed] [Google Scholar]
- 47.Matsunaga W, Miyata S, Takamata A, Bun H, Nakashima T, Kiyohara T. LPS-I induced Fos expression in oxytocin and vasopressin neurons of the rat hypothalamus. Brain Res. 2000;858:9–18. doi: 10.1016/s0006-8993(99)02418-x. [DOI] [PubMed] [Google Scholar]
- 48.Schlosser SF, Almeida OFX, Patchev VK, Yassouridis A, Elands J. Oxytocin-stimulated release of adrenocorticotropin from the rat pituitary is mediated by arginine vasopressin receptors of the V1b type. Endocrinology. 1994;135:2058–2063. doi: 10.1210/endo.135.5.7956927. [DOI] [PubMed] [Google Scholar]
- 49.Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behaviour in rats. Endocrinology. 1997;138:2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- 50.Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002;25:154–159. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- 51.Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- 52.John CD, Buckingham JC. Cytokines: regulation of the hypothalamo-pituitary-adrenocortical axis. Current Opinion Pharmacol. 2003;3:78–84. doi: 10.1016/s1471-4892(02)00009-7. [DOI] [PubMed] [Google Scholar]
- 53.Rivier CL, Grigoriadis DE, Rivier J. Role of corticotropin-releasin factor receptors type 1 and 2 in modulating the rat adrenocorticotropin response to stressors. Endocrinology. 2003;144:2396–2403. doi: 10.1210/en.2002-0117. [DOI] [PubMed] [Google Scholar]
- 54.Pournajafi Nazarloo H, Takao T, Taguchi T, Ito H, Hashimoto K. Modulation of type I IL-1 receptor and IL-1β mRNA expression followed by endotoxin treatment in the corticotropin-releasing hormone-deficient mouse. J Neuroimmunol. 2003;140:102–108. doi: 10.1016/s0165-5728(03)00176-0. [DOI] [PubMed] [Google Scholar]
- 55.Turnbull AV, Smith GW, Lee S, Vale WW, Lee K-F, Rivier C. CRF type I receptor-deficient mice exhibit a pronounced pituitary-adrenal response to local inflammation. Endocrinology. 1999;140:1013–1017. doi: 10.1210/endo.140.2.6675. [DOI] [PubMed] [Google Scholar]
- 56.Xia Y, Krukoff TL. Differential neuronal activation in the hypothalamic paraventricular nucleus and autonomic/neuroendocrine responses to i.c.v. endotoxin. Neuroscience. 2003;121:219–231. doi: 10.1016/s0306-4522(03)00290-2. [DOI] [PubMed] [Google Scholar]
- 57.Juaneda C, Lafon-Dubourg P, Ciofi P, Sarrieau A, Corio M, Tramu G. Immune challenge-stimulated hypophysiotropic corticotropin-releasing hormone messenger RNA expression is associated with an induction of neurotensin messenger RNAs without alterationof vasopressin messenger RNAs. Neuroscience. 1999;93:393–400. doi: 10.1016/s0306-4522(99)00133-5. [DOI] [PubMed] [Google Scholar]
- 58.Kariagina A, Romanenko D, Ren SG, Chesnokova V. Hypothalamic-pituitary cytokine network. Endocrinology. 2004;145:104–112. doi: 10.1210/en.2003-0669. [DOI] [PubMed] [Google Scholar]
- 59.Battaglia DF, Brown EB, Krasa HB, Thrun LA, Viguie C, Karsch FJ. Systemic challenge with endotoxin stimulates corticotropin-releasing hormone and arginine vasopressin secretion into hypophyseal portal blood: coincidence with gonadotropin-releasing hormone suppression. Endocrinology. 1998;139:4175–4181. doi: 10.1210/endo.139.10.6226. [DOI] [PubMed] [Google Scholar]
- 60.Madeira MD, Paula-Barbosa MM. Effects of alcohol on the synthesis and expression of hypothalamic peptides. Brain Res Bull. 1999;1:3–22. doi: 10.1016/s0361-9230(98)00131-2. [DOI] [PubMed] [Google Scholar]
- 61.Larsson A, Engel JA. Neurochemical and behavioural studies on ethanol and nicotine interactions. Neurosci Biobehav Rev. 2004;27:713–720. doi: 10.1016/j.neubiorev.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 62.Mechoulam R, Parker L. Cannabis and alcohol - a close relationship. Trends Neurosci. 2003;24:266–268. doi: 10.1016/S0165-6147(03)00107-X. [DOI] [PubMed] [Google Scholar]
- 63.Li Z, Kang SS, Lee S, Rivier C. Effect of ethanol on the regulation of corticotropin-releasing factor (CRF) gene expression. Mol Cell Neurosci. 2005;29:345–354. doi: 10.1016/j.mcn.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 64.Lee S, Rivier C. Alcohol increases the expression of type 1, but not type 2α corticotropin-releasing factor (CRF) receptor messenger ribonucleic acid in the rat hypothalamus. Mol Brain Res. 1997;52:78–89. doi: 10.1016/s0169-328x(97)00226-x. [DOI] [PubMed] [Google Scholar]
- 65.Colbern DL, ten Haaf J, Tabakoff B, van Wimersma Greidanus TB. Ethanol increases plasma vasopressin shortly after intraperitoneal injection in rats. Life Sci. 1985;37:1029–1032. doi: 10.1016/0024-3205(85)90592-2. [DOI] [PubMed] [Google Scholar]
- 66.Laszlo FA, Varga C, Pavo I, Gardi J, Vecsernyes M, Galfi M, Morschl E, Laszlo F, Makara GB. Vasopressin pressor-receptor-mediated activation of HPA axis by acute ethanol stress in rats. Am J Physiol Reg Integ Comp Physiol. 2001;280:R458–R465. doi: 10.1152/ajpregu.2001.280.2.R458. [DOI] [PubMed] [Google Scholar]
- 67.Hashimoto K, Murakami K, Takao T, Makino S, Sugawara M, Ota Z. Effects of acute ether or restraint stress on plasma corticotropin-releasing hormone, vasopressin and oxytocin levels in the rat. Acta Med Okayama. 1989;43:161–167. doi: 10.18926/AMO/30888. [DOI] [PubMed] [Google Scholar]
- 68.Onaka T, Yaki K. Effect of novelty stress on vasopressin and oxytocin secretion by the pituitary in the rat. J Neuroendocrinol. 1993;5:365–369. doi: 10.1111/j.1365-2826.1993.tb00496.x. [DOI] [PubMed] [Google Scholar]
- 69.Spencer RL, McEwen BS. Adaptation of the hypothalamic-pituitary-adrenal axis to chronic ethanol stress. Neuroendocrinology. 1990;52:481–489. doi: 10.1159/000125632. [DOI] [PubMed] [Google Scholar]