Abstract
Autoimmune responses were observed in a large proportion of hepatitis C cases and are suspected to be part of viral pathogenesis. The AN6520 antigen (AN-Ag) is a normal cellular protein mainly expressed in liver that was found associated with non-A, non-B hepatitis. To elucidate its pathogenic role in hepatitis C, we developed an IgM capture assay using purified AN-Ag and confirmed that the antibody response to AN-Ag is associated almost exclusively with hepatitis C cases (29%). Screening of a human liver expression library revealed that AN-Ag is mainly the microsomal epoxide hydrolase (mEH), a drug-metabolizing enzyme that plays an important role in the metabolism of some mutagenic and carcinogenic epoxides. Using the purified recombinant human mEH as an antigen, we now found that antibodies against this protein are associated with nearly 82% of hepatitis C virus infections and surprisingly with 46% of patients with hepatitis A. The appearance of AN-Ag/mEH in the incubation period of hepatitis C as previously reported and the antibody responses shown here indicate that this enzyme may be a marker for or even a cause of some of the pathology associated with hepatitis C and A.
Keywords: Baculovirus, Hepatitis A virus, Hepatitis C virus, Purification, Radioimmunoassay
1. Introduction
Hepatitis C virus (HCV), a member of the Flaviviridae family is thought not to be directly cytopathic, rather it triggers an immune-mediated inflammatory response that eliminates the virus and/or slowly damages the hepatocytes [1]. Although humoral and cellular immune responses during HCV infection were extensively studied, their pathogenic roles are still unclear.
Before the isolation of the virus by molecular biological techniques [2], many antigens were claimed to be associated with hepatitis C (non-A, non-B hepatitis; NANBH) using immunological techniques [3–5]. We found an antigen (AN6520 antigen; AN-Ag) in the liver of patients with NANBH which formed a precipitin line with convalescent sera from patients with NANBH. We purified the antigen and developed passive hemagglutination assay (PHA) using antigen-coated erythrocytes to detect antibody in patients’ sera. We found that the antigen is composed of particles with molecular weight of more than 1.5 × 106 Da and diameter of 29–34 nm. The antibody was detected in 37.5% in NANBH cases, but not in control groups [6]. Then we developed monoclonal antibodies (mAbs) and used one of them, 1F12, to develop radioimmunoassays (RIAs) for the antigen and for the patient’s antibody [7]. The antigen and antibody were detected in the acute phase and convalescent phase sera, respectively, of some patients with NANBH. In sera obtained sequentially from chimpanzees infected with NANBH agent (now known as HCV), the AN antigen appears during the incubation time before the elevation of ALT [8]. Based on these results, we initially thought that the AN-Ag is from the viral particles of NANBH agent. However, we later showed that AN-Ag is a normal cellular protein mainly expressed in the microsomal fraction of liver, however, its concentration varies considerably between individuals [8].
Toward isolating and identifying AN-Ag, we initially tried to use the RIA assays developed previously [7]. However, the inhibition RIA is not specific enough in that it cross reacted with an unknown protein present in the serum of many people. Thus, we are reporting herein a novel IgM capture RIA method that is more immunoglobulin-specific than the previous inhibition assay. Using this assay, we investigated the specificity of anti-AN antibody response to HCV infection. Further, we identified and cloned the cDNA of AN-Ag. We also confirmed the antibody response using the purified antigen expressed by cDNA. These results show some insights about the role of the AN-antigen in the pathogenesis of hepatitis.
2. Materials and methods
2.1. Patients
Sera used in the study shown in Table 1 were collected by Jikei University Hospital from 1980 to 1981. During this period, informed consent was not commonly obtained. Hepatitis C cases in this study were from the epidemic in Shimizu city in Japan [9]. They were diagnosed as NANBH and later proven to be hepatitis C by serological diagnosis (Ortho Diagnostic, NY) [10]. Sera from patients with various forms of hepatitis as well as normal blood donors shown in Tables 2 and 3 were obtained from 2000 to 2005 under the appropriate approval guidelines from the following institutions: Tokyo University Hospital, Yamagata University Hospital, Akashi Municipal Hospital, and Kagawa University Hospital, Japan. The diagnosis of viral hepatitis was made on the basis of the results of virological tests with histopathological findings, and drug-induced hepatitis was defined on the basis of the patient’s medical history to identify any possible hepatotoxins with clinical and histopathological findings.
Table 1.
Prevalence of anti-AN6520 IgM in sera from patients with acute hepatitis and normal donors
| Type of hepatitis | No. of cases | Ab positive | % |
|---|---|---|---|
| Type A | 37 (39) | 1 (0) | 2.7 (2.6) |
| Type B | 17 (18) | 0 (0) | 0.0 (0.0) |
| Type C | 31 (38) | 9 (13) | 29.0* (34.2*) |
| Normal donors | 21 (26) | 0 (0) | 0.0 (0.0) |
Results by IgM capture RIA using purified AN-Ag that were obtained from 1980 to 1981 are shown. Combined data of RIA and ELISA are shown in parentheses.
P < 0.01 by Fisher’s exact probability test.
Table 2.
Prevalence of anti-mEH IgM in sera from patients with acute and chronic hepatitis and normal donors
| Type of hepatitis | No. of cases | Ab positive | % |
|---|---|---|---|
| Type A (acute) | 22 | 10 | 45.5* |
| Type B (acute) | 12 | 0 | 0.0 |
| Type B (chronic) | 27 | 2 | 7.4 |
| Type C (acute) | 22 | 18 | 81.8* |
| Type C (chronic) | 63 | 16 | 25.4** |
| Drug-induced | 16 | 0 | 0.0 |
| Normal donors | 13 | 0 | 0.0 |
Results by IgM capture RIA using the membrane-bound form of mEH are shown.
P < 0.01 and
P < 0.05 by Fisher’s exact probability test.
Table 3.
Comparison of anti-mEH and anti-AN IgM in sera from patients with acute and chronic hepatitis and normal donors
| Case | Type of hepatitis | Anti-mEH | anti-AN |
|---|---|---|---|
| 1 | Type A (acute) | 9.69 | 1.18 |
| 2 | Type A (acute) | 1.35 | 1.25 |
| 3 | Type A (acute) | 15.37 | 1.13 |
| 4 | Type B (acute) | 1.68 | 1.12 |
| 5 | Type B (acute) | 1.36 | 1.11 |
| 6 | Type B (acute) | 0.51 | 0.86 |
| 7 | Type B (acute) | 0.69 | 0.98 |
| 8 | Type B (acute) | 0.72 | 0.93 |
| 9 | Type C (acute) | 0.74 | 0.76 |
| 10 | Type C (acute) | 2.48 | 2.63 |
| 11 | Type C (acute) | 1.18 | 0.96 |
| 12 | Type C (acute) | 2.67 | 0.82 |
| 13 | Type C (chronic) | 2.59 | 3.19 |
| 14 | Type C (chronic) | 15.26 | 7.34 |
| 15 | Type C (chronic) | 4.36 | 4.52 |
| 16 | Type C (chronic) | 3.51 | 6.10 |
| 17 | Normal | 1.10 | 1.63 |
| 18 | Normal | 0.76 | 1.00 |
| 19 | Normal | 0.55 | 1.06 |
| 20 | Normal | 1.14 | 0.94 |
S/N ratios are shown. Positive results (≥2.1) are shown in bold.
2.2. Cell lines
The hepatocellular carcinoma (HCC) cell line, huH-2 [11] was obtained from the Japanese Cancer Research Resources Bank. THLE-5b, a normal liver cell line which was immortalized by transfecting with the plasmid containing SV40 T-Ag [12], was a gift from Dr. C.C. Harris (CCR, NCI, NIH). Both were maintained in RPMI1640 media with 5% heat-inactivated FCS. BHK-21 was obtained from the American Type Culture Collection (Rockville, MD) and maintained in EMEM media with 5% FCS.
2.3. Antibodies
Rabbit anti-AN antibody and mouse anti-AN mAb 1F12 have been described [6,7]. Rabbit anti-mEH antibody was raised by injecting a rabbit (s.c.) with 400 μg of solubilized and purified mEH three times (first with Freund’s complete adjuvant, second and third immunizations with incomplete adjuvant). Ascites of hybridoma producing human IgM (μ-chain)-specific mAb #9 was a kind gift from Dr. M. Arita (National Institute of Infectious Diseases, Japan).
2.4. Transfection, immunofluorescence staining, and immunoblotting
BHK-21 cells were transfected using Lipofectin (Invitrogen, Carlsbad, CA) according to the manufacturer’s instruction. After being transferred to a Lab-Tek chamber slide (NUNC, Naperville, IL), they were double-stained with rabbit antiserum diluted (1:200) in supernatant of 1F12 hybridoma culture, followed by the mixture of TRITC-conjugated goat anti-rabbit IgG (Beckman Coulter, France) (1:50) and FITC-conjugated goat anti-mouse IgG (Sigma, St. Louis, MO) (1:100). SDS-PAGE was performed by applying 400 ng and 50 ng of protein onto a 10% minigel (Mini-Protean; Bio-Rad, Hercules, CA) for silver staining and immunoblotting, respectively. After separation and transfer to an Immobilon-P membrane (Millipore, Bedford, MA), the antigen was detected with rabbit anti-mEH (1:500) followed by alkaline phosphatase-labeled goat anti-rabbit IgG (KPL, Gaithersburg, MA) and BCIP/NBT substrate (KPL).
2.5. IgM capture assay
A flat-bottomed eight-well strip plate (COSTAR, Acton, MA) was used as the solid phase and 50 μl of ascites fluid of anti-human IgM hybridoma #9 diluted (1:10,000) in PBS was added to each well and incubated overnight at 4 °C. After blocking with 200 μl of 5% BSA in PBS for 1 h at 37 °C and washing, 50 μl of serum samples diluted (1:20) in PBS containing 1% BSA and 0.1% NaN3 (RIA buffer) was added and incubated overnight at 4 °C. After washing, 1 × 105 cpm of 125I-labeled AN-Ag or membrane-bound form of mEH in 50 μl of RIA buffer was added and incubated for 2 h at 37 °C. Each well was separated after washing, and the bound radioactivity was counted by a gammacounter. An ELISA was also developed, in which the 125I-AN-Ag was replaced by non-labeled AN-Ag (500 ng/ml in RIA buffer), and the bound antigen was detected by incubation with horseradish peroxidase-labeled 1F12 (for 1 h at 37 °C) followed by OPD substrate (SIGMA St Louis, MO). The reaction was stopped by adding H2SO4 and OD492 was read. Labeling with 125I and peroxidase was performed by the chloramine-T method [13] and P. Nakane’s method [14], respectively. Cut-off values in RIA and ELISA were 2.1 (S/N ratio) and 1.0 (above the mean OD of negative control) using sera from five normal donors as the negative control, respectively. They corresponded to 4 SD above the mean values. All the washing steps were done with PBS containing 0.05% Tween 20. For confirmation of the specificity of the reaction, serum sample was incubated with 50 μg/ml of anti-human IgG (γ-chain) or anti-human IgM (μ-chain) antibody (Pierce, Rockford, IL) overnight at 4 °C in a test tube before being added to the antibody coated plate. Statistical analysis was performed by Scheffé’s F-test (Figs. 1 and 7) and Fisher’s exact probability test (Tables 1 and 2).
Fig. 1.

IgM capture RIA for the detection of anti-AN6520 antibodies. IgM was captured by IgM-specific monoclonal antibody on the solid phase and detected with 125I-AN-Ag. S/N values of sera from patients with acute hepatitis A (type A), hepatitis B (type B), and hepatitis C (type C), and normal donors (normal) are shown. A circle represents a case, and a dotted line represents a cut-off value (S/N = 2.1). The mean value of each group is shown by a horizontal bar. For statistical analysis, Scheffé’s F-test was used.
Fig. 7.
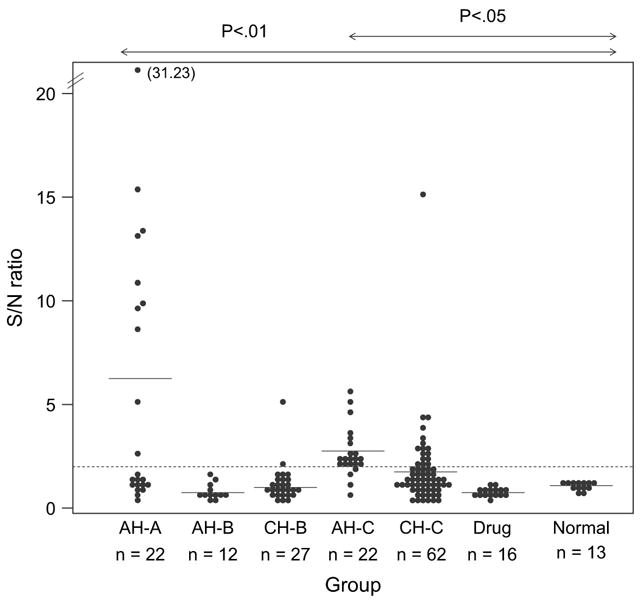
IgM capture RIA with for the detection of anti-mEH antibodies. Capturing of IgM was the same as for Fig. 1 and the bound antibody was detected with 125I-mEH. S/N values of sera from patients with acute hepatitis A (AHA), acute hepatitis B (AH-B), chronic hepatitis B (CH-B), acute hepatitis C (AH-C), chronic hepatitis C (CH-C), drug-induced hepatitis (drug), and normal donors (normal) are shown. A closed circle and an open circle represent a case and two cases, respectively. A dotted line represents a cut-off value (S/N = 2.1). The mean value of each group is shown by a horizontal bar. For statistical analysis, Scheffé’s F-test was used.
2.6. cDNA identification
A λgt11 cDNA library from human liver (Clontech, Pal-oAlto, CA) was screened with rabbit anti-AN antibody [6] (50 μg/ml), by the standard protocol [15]. After four cycles of selection, they were further selected by using their phage lysates for affinity purification [16] of rabbit anti-AN antibody followed by incubation of the eluted antibody on the immunoblot of mEH-containing cell lysate. Briefly, Hybond-C filter (Amersham, Buckinghamshire, UK) (1.0 × 4.0 cm) that bound plaque protein was soaked with 20% FCS in 10 mM Tris–HCl pH 7.4-buffered saline (TBS) containing 0.05% Tween 20 overnight at 4 °C, then incubated with 0.5 ml of antibody overnight at 4 °C. After washing, the bound antibody was eluted by incubating the filter with 160 μl of 0.2 M glycine–HCl (pH 2.5) for 2 min. The eluate was immediately neutralized by adding 80 μl of 1.0 M K2HPO4 (pH 9.0), then 100 μl of 50% FCS in TBS was added. The eluted antibody was incubated on the western blot of the microsomal fraction [17] of HCC line huH-2 which expresses mEH. From the finally selected clone HLC4, the insert DNA was digested with EcoR1 into 3 fragments, and ligated into a pKF3 vector in the Enforcement Cloning System (Takara, Tokyo, Japan). The insert of each plasmid was sequenced with pKF3 sequencing primers F2 and R3 (Takara).
2.7. Expression and purification of mEH
Total RNA of human liver cell line THLE-5b was extracted with ISOGEN (Nippon Gene, Tokyo, Japan), and mRNA was isolated with Dynabeads with oligo dT25 (Dynal, Oslo, Norway). The first strand cDNA was synthesized by reverse transcriptase (Takara), and the mEH cDNA was amplified by PCR with KOD-Plus polymerase (Toyobo, Tokyo, Japan) and 5′- and 3′-end primers of mEH cDNA accompanying HindIII and BamHI sequences, respectively; 5′-AAGCTTATGTGGCTAGAAATCCTCCTCA-3′ and 5′-GGATCCTCATTGCCGCTCCAGCACCGACAGG-3′. The PCR product was digested with HindIII and BamHI, and ligated into the same cloning sites of pcDNA3(+) (Invitrogen). The sequence of the insert mEH cDNA in the ligated plasmid was confirmed with the appropriate primers synthesized from the determined sequence. Expression of human mEH in a recombinant baculovirus system has been described [18]. The purification procedure of the solubilized form of mEH was based on the method of Lacourciere [19] with some modifications: Sf9 cells (1 L culture) were infected with recombinant baculovirus (m.o.i. = 10), and after 96 h incubation, the cells were suspended in 0.1 M phosphate buffer pH 7.4 containing 1 mM PMSF, 1 mM EDTA and 1 mM DTT. The cells were homogenized with Polytron homogenizer and centrifuged at 100,000 × g for 60 min. The pellet was suspended with buffer A (10 mM Tris–HCl, pH 7.6) containing 1% Tween 20 by sonication, and centrifuged at 100,000 × g for 60 min. The pellet was suspended with buffer A containing 1% Triton X-100 by sonication, and centrifuged at 100,000 × g for 60 min. The supernatant was dialyzed overnight against buffer B (10 mM Tris–HCl, pH 9.0 containing 0.05% Triton X-100) and applied onto a 10 × 2.5 cm Q-Sepharose (Amersham, Uppsala, Sweden) column equilibrated with buffer B. The column was washed with buffer B containing 100 mM NaCl, and then the enzyme was eluted with buffer B containing 200 mM NaCl. Active fractions were pooled, concentrated using Centriplus YM-10 (Millipore, Bedford, MA), and then dialyzed against buffer A containing 0.05% Triton X-100. Membrane-bound form of mEH was purified according to the method for AN-Ag [6]. The homogenate from 1.2 g of infected SF9 cell pellet was applied onto a Sephacryl S-300 (Amersham, Uppsala, Sweden) column (2.5 × 90 cm) and eluted with TBS containing 1 mM EDTA (buffer C). Void volume fractions were centrifuged at 100,000 × g for 120 min. The pellet was suspended by sonication with 1.5 ml of buffer C containing 1% Tween 20 and then 200 μl of 1 M Tris pH 9.0. was added. It was layered onto the gradient of 5–60% (w/v) sucrose in buffer C (30 ml) and after centrifugation at 63,000 × g for 16 h, 1.2 ml fractions were collected. Antigen-positive fractions were pooled, dialyzed against buffer C overnight, and then concentrated with Centriplus YM-100. It was next layered on CsCl gradients of 7% to 42% (w/v) in buffer C (4.5 ml) and centrifuged at 175,000 × g for 20 h. One hundred and seventy microliter fractions were collected and antigen-positive peak was dialyzed against buffer C overnight, and concentrated with Centriplus YM-100. All the purification procedures were performed at 4 °C. Protein concentration was measured by micro BCA assay (Pierce), and the antigen was titrated by antibody sandwich ELISA using horseradish peroxidase-labeled 1F12 using purified AN-Ag as the standard.
2.8. Analysis of enzyme activity of mEH
The mEH enzyme activity was determined by measuring the formation rate of dihydrobenzoin produced from hydrolysis of cis-stilbene oxide by the method of Maekawa et al. [20]. HPLC analysis was performed using a Tosoh 8000 system (Tosoh, Tokyo, Japan).
2.9. Chimpanzee experiments
The housing, maintenance, and care of the chimpanzees used in this study met requirements for the humane use of animals in scientific research as defined by the National Institutes of Health. Two chimpanzees (Ch1360 and Ch1365) were inoculated i.v. with ~103 infectious doses of the HM175 strain of HAV [21]. Twenty-three weeks after HAV infection, Ch1365 was inoculated i.v. with ~103 infectious doses of the H strain of HCV along with the third chimpanzee Ch1397 as described [22]. Serum samples were taken weekly for up to 32 weeks.
3. Results
3.1. IgM capture assay using purified AN antigen
Results of the IgM capture assay for antibody to the purified AN-Ag are presented in Table 1. This assay eliminated the false positive results observed in the older sandwich type RIA in which a non-immunoglobulin factor that bound to AN-Ag was detected. Using this method, we found that 29% of the hepatitis C sera had antibody to the AN-Ag compared to 2.7% of hepatitis A patients while hepatitis B and normal donors did not have the antibody. The S/N ratios were also significantly higher in hepatitis C than any other groups (Fig. 1). Specificity of the reaction was confirmed by the blocking experiments in which antibody-positive sera were incubated with anti-μ chain or anti-γ chain antibody before being added onto the solid phase (not shown). We also developed an IgM capture ELISA which is more specific than RIA because the bound Ag was detected by the enzyme-labeled anti-AN-Ag mAb. By combining the results of the two assays, the prevalence of the antibody in the hepatitis C group rose to 34.2% (Table 1). Sequential serum samples from three patients with hepatitis C were tested by ELISA (Fig. 2). It shows that the antibody appeared while ALT was still elevated and declined along with resolving of hepatitis.
Fig. 2.

IgM capture ELISA of serial serum samples from three patients with acute hepatitis C (A–C). Capturing of IgM was the same as for Fig. 1 but the bound antibody was detected by non-labeled AN-Ag followed by horseradish peroxidase-labeled monoclonal antibody 1F12. ALT (-●-) is expressed by international units per liter. ELISA data (-○-) are expressed by OD492.
3.2. Isolation of cDNA of AN antigen
To identify the cDNA of AN antigen, a λgt11 expression library from human liver was used because we previously found that this antigen is a normal cellular protein mainly expressed in liver [8]. mAbs to AN-Ag are known to recognize only conformational epitopes that are easily disrupted by detergents. Therefore, polyclonal rabbit antibody that revealed a band in western blotting was chosen for immunoscreening of the library [8]. Out of about one million plaques, we obtained 46 positive plaques in the first screening. After repeating the screening four times, we selected 12 plaques. Their phage lysates were used for affinity purification [16] of the rabbit antiserum (Fig. 3). Although the antiserum showed additional bands on the immunoblot of the huH-2 cell extract, the antibody eluted from two clones (HLC3 and HLC4) out of 12 gave a single band (~49 kDa) corresponding to AN-Ag. The inserted cDNA of HLC4 was subcloned and sequenced. GenBank BLAST search revealed that the sequence matches perfectly human mEH cDNA [23] encompassing nucleotide 74 to 1460 of the reported transcript.
Fig. 3.
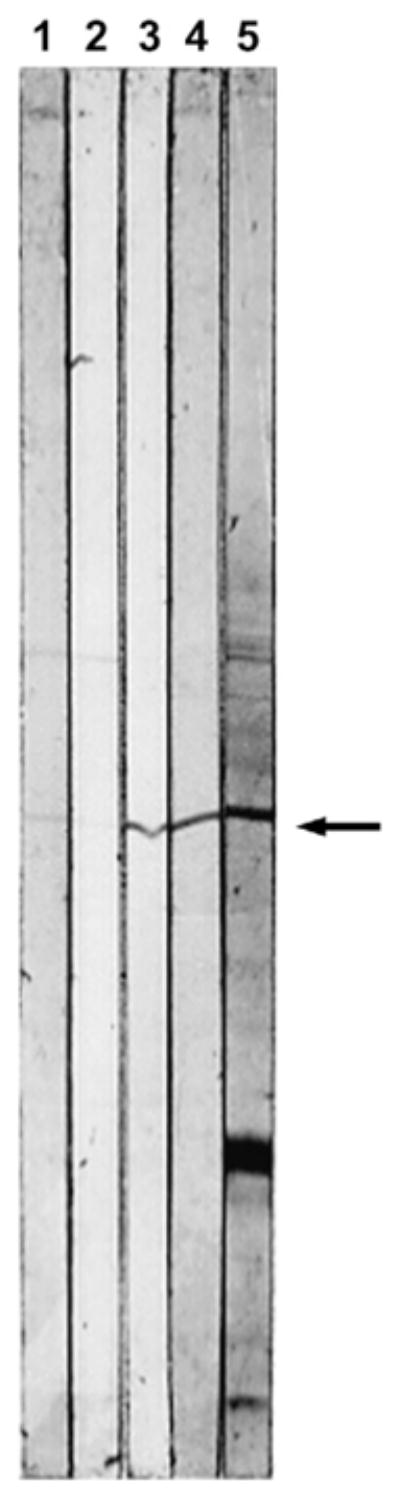
Second step selection of λgt11 cDNA library from human liver by affinity purification of the antibody. The filter that bound the phage lysate of the candidate clone was incubated with rabbit anti-AN antibody. After washing, the bound antibody was reacted with western blot of microsomal fraction of huH-2 cells that expresses AN-Ag. The eluates from the phage lysates of HLC1–HLC4 (lanes 1–4), rabbit anti-AN antibody before purification (lane 5). The arrow denotes the AN-Ag (~49 kDa).
3.3. Confirmation of identity of AN antigen as mEH
The entire coding region of mEH cDNA was cloned from a normal human liver cell line, inserted in an expression vector pcDNA3(+) and then transfected into BHK-21 cells. mEH was also expressed in insect cells by recombinant baculovirus and used for purification as described [18,19]. The purified mEH was then used to raise rabbit antiserum. When BHK cell transfectants were double-stained with rabbit anti-mEH and mouse anti-AN mAb 1F12, both antibodies exhibited similar staining patterns with the former being a little broader than the other (Fig. 4A–C). In addition, the rabbit polyclonal anti-AN antibody and 1F12 also gave almost identical staining (Fig. 4D–F). This indicates that the human mEH is responsible for most of the antigenicity of AN-Ag. Purified forms of mEH and AN-Ag were compared by SDS-PAGE and western blotting. Both showed a single band of more than 95% purity as judged by densitometer scanning (Fig. 5A). These bands had similar size, and were also detected by both the rabbit anti-mEH (Fig. 5B), and the rabbit anti-AN antibody (not shown).
Fig. 4.
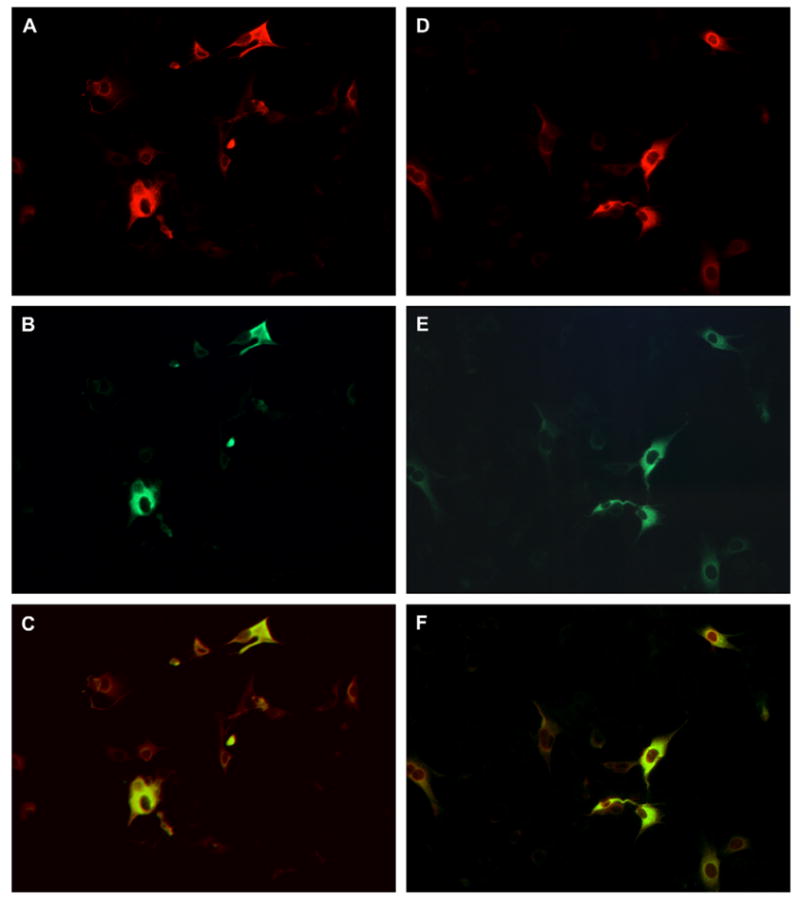
Double-staining of BHK transfectant expressing mEH. After transfection, the cells were transferred to a chamber slide and cultured. Then they were fixed with acetone and double-stained by rabbit anti-mEH and mouse anti-AN mAb 1F12 (A and B), or by rabbit anti-AN antibody and 1F12 (D and E). Rabbit and mouse antibodies were detected by TRITC- and FITC-labeled second antibodies, respectively. The merged image of A and B and that of D and E are shown in C and F, respectively.
Fig. 5.

Comparison of purified mEH and AN-Ag. Ten percent of SDS-gel was loaded with molecular weight marker (M), AN-Ag (1), and purified mEH (2), and underwent silver staining (A), or western blotting (B). The immunoblot was detected with rabbit anti-mEH antibody followed by alkaline phosphatase-labeled second antibody.
3.4. Purification of membrane-bound form of mEH
We then tested if anti-AN antibodies in the sera of hepatitis C patients recognize mEH. Purified mEH for the experiments described above was obtained following a conventional methodology: solubilization with detergent and ion-exchange chromatography. However, we previously showed that AN-Ag is composed of particles 29–34 nm in diameter containing ~49 kDa subunits. If treated with detergents, the particles lose reactivity with human antibodies as well as mouse monoclonal antibodies [8]. Therefore, we decided to purify mEH without disrupting the membrane-bound form, and followed the method used for purification of AN-Ag from liver [6]. A homogenate of recombinant baculovirus-infected Sf9 cells was first passed through a gel-filtration column. The void volume fraction was then subjected to ultracentrifugation in a sucrose-gradient. As shown in Fig. 6A, we obtained two antigen-positive peaks. As observed in SDS-PAGE, the first peak (fractions 3–7) had the highest specific antigenic activity and contained mostly the ~49 kDa band with few, very light additional protein bands. The first peak fractions were pooled and applied onto a CsCl gradient (Fig. 6B). The main antigen-positive fractions (12–22) were pooled and concentrated. This method yielded 32 μg of purified protein from 1.2 g of cell pellet. This membrane-bound form and the detergent-solubilized form of human mEH are both at least 95% pure as judged by SDS-PAGE (Fig. 6C). Enzyme activity was measured on both preparations using cis-stilbene oxide as substrate; the membrane-bound form displayed almost three times higher activity than the solubilized form (1550 vs. 571 nmol min−1 mg−1). The antigen/protein ratio of the membrane-bound form was comparable to AN-Ag (99.5% of AN-Ag) whereas the detergent-solubilized form had no reactivity with mAb 1F12 as expected (not shown).
Fig. 6.
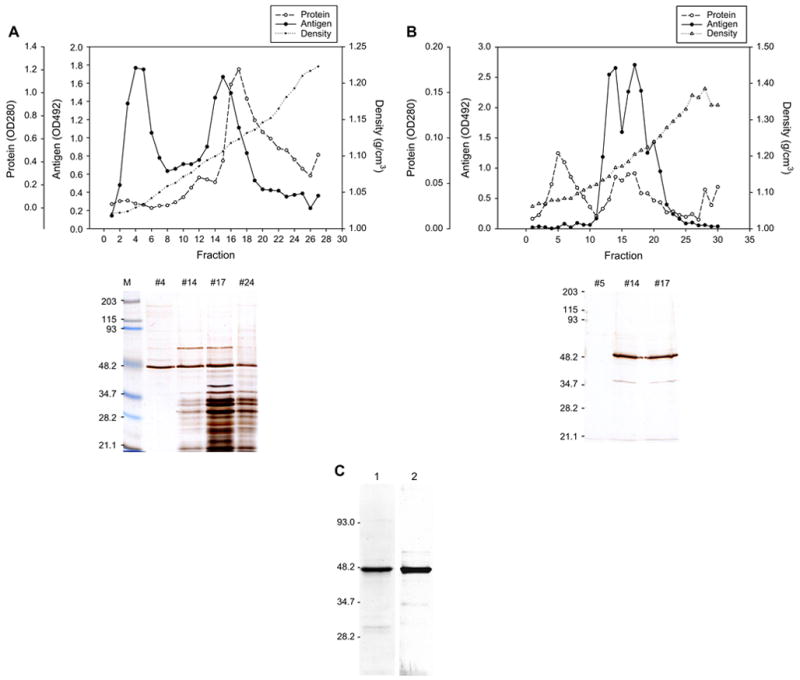
Purification of membrane-bound form of mEH by sucrose-gradient ultracentrifugation. After gel-filtration of the homogenate of baculovirus-infected Sf9 cells, void volume fractions were pooled and underwent sucrose-gradient ultracentrifugation (A). Each fraction was assayed for AN-Ag by antibody sandwich ELISA and the data are shown by OD492 (-●-). OD280 (-○-) and density (-●-) are also shown. Aliquots of four fractions (#4, #14, #17 and #24) were applied on a 10% SDS-gel, separated, and detected by silver staining. The first peak fractions (#2 to #6) were pooled, and after dialysis and concentration, underwent CsCl gradient ultracentrifugation (B). SDS-PAGE and silver staining of the fraction #5, #14, and #17 are shown. Antigen-positive fractions (#12 to #21) were pooled, dialyzed and concentrated. The membrane-bound form of mEH (2) was compared with the solubilized form (1) by SDS-PAGE and silver staining (C).
3.5. IgM capture assay using mEH as antigen
Antibody to the purified membrane-bound form of mEH was detected by the IgM capture RIA in serum samples from patients with various forms of hepatitis (Table 2). The antibody was detected in ~82% of patients with acute hepatitis C and also in ~25% of chronic hepatitis C cases. Unexpectedly, it was also detected in ~46% of patients with acute hepatitis A. The incidence in other forms of hepatitis such as type B and drug-induced hepatitis was very low. The S/N ratios were also significantly higher in hepatitis C and hepatitis A than any other groups (Fig. 7). When the radio-labeled mEH was treated with NP40 or SDS, it lost the reactivity with antibodies in both hepatitis A and C sera (not shown) suggesting that the antibodies recognize conformational epitope of membrane-bound mEH similar to what we previously observed for AN-Ag [8].
Because the results with sera of hepatitis A patients (Table 2) were very different from the results previously obtained with AN-Ag (Table 1), the remaining AN-Ag was labeled and applied to some of the sera used for the experiments of Table 2. Table 3 shows that AN-Ag did not react with the antibodies in hepatitis A, but did react positively with some of anti-mEH positive hepatitis C sera. Therefore, AN-Ag seems to have more selectivity for hepatitis C sera than mEH.
In order to better define the relationship of anti-mEH to disease, we tested serial serum samples from three chimpanzees experimentally infected with HAV and/or HCV (Ch1360, HAV; Ch1365, HAV and HCV; Ch1397, HCV). Sera were collected first between four and two weeks before inoculation, then regularly for up to at least six weeks after the peak of enzyme elevation. Although chimpanzee’s IgM was bound to the capturing antibody with the same efficiency as human IgM, we did not see any positive signals at any time point (S/N = 1.06 (mean) ± 0.06 (SD)).
4. Discussion
Using IgM capture RIA and ELISA methods, the antibody to AN-Ag was detected almost exclusively in sera from patients with hepatitis C (34.2%) with only one exception out of 39 cases of hepatitis A (2.6%) (Table 1). Because hepatitis C virus (HCV) is thought to not be directly cytopathic as are hepatitis A and B viruses, we hypothesized that AN-Ag that is particular to HCV infection, plays a role in the pathogenesis of hepatitis C. As a first step, we identified the antigen to which these hepatitis C-specific antibodies are directed. For this purpose, we screened a human liver expression library, and we isolated a cDNA which codes for a protein that reacts strongly with AN antibodies. The sequence of this cDNA matches almost perfectly the sequence of the human microsomal epoxide hydrolase (mEH) cDNA encompassing 1288 bp of the coding region and 98 bp of 3′-noncoding region. The recombinant purified human mEH had the same size as the purified AN-Ag when analyzed by SDS-PAGE, and we observed that they have similar antigenicity in immunofluorescence staining (Fig. 4) and western blotting (Fig. 5). These findings indicate that the human mEH is responsible for most of the antigenicity of AN-Ag.
To confirm these results, we then wished to test that the anti-AN antibody activity in hepatitis C sera was against mEH. However, human anti-AN antibody, like the mouse monoclonal antibody 1F12, is known to recognize only the three-dimensional structure of AN-Ag, and lost its recognition when AN-Ag is treated with some detergents [8]. Therefore, to overcome this shortcoming, we expressed the mEH in large amounts in insect cells using recombinant baculovirus system, and then purified the protein without disrupting its membrane conformation by not using any detergent. The purified membrane-bound form of mEH had similar homogeneity to the solubilized form of mEH purified by the conventional method as well as AN-Ag (Figs. 5A and 6C). Its enzymatic activity with cis-stilbene oxide as substrate was about three times higher than the solubilized form. Preservation of mEH in membrane in its right orientation may be beneficial for its enzymatic function. This form of mEH had very similar antigenic activity as AN-Ag as determined by RIA using the mAb 1F12. It was found to react well with the human IgM antibodies in sera of hepatitis C, but unexpectedly, also with many sera from patients with acute hepatitis A. On the other hand, the solubilized form of mEH did not react with either 1F12 or human antibodies. When the membrane-bound form of mEH was treated with detergents, it lost the reactivity with 1F12 and human antibodies. These findings suggest that human antibodies recognize the three-dimensional structure of mEH on membrane.
The unexpected positive results with hepatitis A sera prompted us to use all the remaining AN-Ag to compare its antigenicity with mEH against the same serum samples. The results (Table 3) confirmed our previous data which were obtained 20 years ago (Table 1) showing that AN-Ag preferentially reacts with the antibody in hepatitis C sera. We do not know why it does not react with the antibody in hepatitis A sera. One could hypothesize that the epitope for the antibody is lacking in hepatitis A sera. Alternatively, the epitope for the antibody in hepatitis A is usually masked inside a complex structure of mEH in the membrane of hepatocytes. Several findings support this latter possibility. It was postulated that mEH binds to Na+-independent organic anion transport protein forming a multiple transport system [24]. Furthermore, mEH was reported to be associated with various cytochrome P450s [25,26], and also with phospholipids [27]. Moreover, there is a possibility that mEH is complexed with HCV proteins in AN-Ag because it was purified from the liver with NANBH. To address these possibilities, we have incubated 125I-labeled AN-Ag in wells coated with several antibodies including anti-HCV structural protein (C, E1, E2) mAbs. Although HCV proteins were not detected, anti-HLA class I mAb (W6/32) gave a positive S/N value of 3.50, while the value of 1F12 was 14.97. Although silver staining of AN-Ag on SDS-gel showed high purity (Fig. 5A), coexistence of such substance including HCV nonstructural proteins cannot be ruled out.
To our knowledge, this is the first report of anti-mEH antibody formation during viral hepatitis. It was previously reported that anti-mEH antibody was induced by germander tea that caused hepatitis [28]. The mEH is a drug-metabolizing enzyme that plays an important role in the metabolism of some mutagenic and carcinogenic epoxides [29]. It is mainly expressed on the ER membrane in the liver [29] and constitutes about 2% of microsomal fraction [30]. It is also expressed on the surface of hepatocytes [31] and may act as a sodium-dependent bile acid transporter [32]. In humans, mEH is the product of single locus (EPHX1) on chromosome 1. Several single nucleotide polymorphism sequences were found in association with the onset of several diseases and cancers including aflatoxin B1-associated hepatocellular carcinoma [33], chronic hepatitis C, and HCV-associated hepatocellular carcinoma [34]. Taken these into account, it is possible to speculate that the events that occur during virus infection may change the environment of the mEH to exhibit its antigenicity and reduce its ability to metabolize potential alkylating agents. This may partially explain the association between hepatitis and cancer and such an association may depend on the genotype of EPHX1.
Mechanisms of the autoantibody response are still unknown, but several possibilities can be enumerated. The first one is molecular mimicry. Several other drug-metabolizing enzymes such as CYP2A6/2A7 and UGT have been recognized as the targets of autoantibodies in type 2 autoimmune hepatitis (AIH-2) and in a proportion of chronic viral hepatitis [35]. As the mechanisms of initiation of those autoimmune response, molecular mimicry between CYP2D6 or CYP2A6 and HCV proteins at B-cell [36] and T-cell [37] levels, respectively, was proposed. We did not find significant sequence homology between mEH, HCV and HAV; however, HCV shares properties with picornaviruses which include a similar genome organization of 5′-noncoding region and possibly translation regulation through an internal ribosome entry site [38]. As the second possibility, the mEH that leaked into the blood circulation during hepatocyte damage can sensitize the immune system. However, this is fairly improbable because: (1) anti-mEH antibodies were not found in drug-induced hepatitis or hepatitis B (Table 2); (2) after inducing severe hepatitis in rats and rabbits by injecting D-galactosamine and carbon tetra-chloride, respectively, we detected the AN-Ag in parallel with ALT elevation in the sera of those animals but anti-AN/mEH antibodies were never detected (not shown); and (3) the anti-mEH antibodies in patients were found when ALT was still high (Fig. 2), which indicates antibody production must have happened before the elevation of ALT. Otherwise, in the experimental HCV infection of chimpanzee, the antigen was detected during the incubation period before the elevation of ALT [8]. These findings raise the third possibility that replication of such RNA viruses could lead to changes in the environment surrounding mEH or of its structure. Viral replication may loosen the association of mEH with the membrane by some unknown mechanisms. However, these mechanisms need to be specific to HCV replication because the replication of other viruses such as hepatitis B virus does not cause the autoimmune response. The released mEH might have a new antigenicity that is different from that of the mEH that leaked during hepatocyte damage, and has never been recognized by the immune system. This seems to be somewhat related to the preneoplastic antigen (PNA). Okita and Farber [39] demonstrated that there is an antigen in preneoplastic foci in livers that is released into the blood. Following extensive studies by the Farber lab, Levin et al. [40] demonstrated that the PNA is similar if not identical to the microsomal epoxide hydrolase. Later a rapid radiochemical assay for the PNA was developed for human blood and shown to be associated with liver cancer, but it was also found to be associated with several other types of liver damage [41]. Interestingly, another alpha/beta hydrolase fold enzyme –carboxylesterase –also has been shown to be released from hepatic endoplasmic reticulum and appears in the blood following liver injury [42]. These early data caution of course that the mEH antigen may not be a perfectly diagnostic marker for hepatitis, but they also bring up that a small battery of proteins including possible alpha-fetoprotein [43] could be very useful diagnostic tools and possibly give hints to the mechanisms by which these viruses cause severe liver damage. This report and previous studies indicate as well that this antigen as well as the previously discussed PNA have subtle differences from the microsomal epoxide hydrolase. For HCV infection, we showed that the mEH needs to be attached to the membrane to generate the autoimmune response, indicating that AN-Ag and PNA are different, but both generated from mEH.
The profile of the antibody response obtained from serial serum samples (Fig. 2) suggests that anti-mEH response has pathogenic role, but larger number of samples are need. It is not easy to test the effects of anti-mEH antibody on hepatocytes in vitro. Although mEH is expressed on the surface of hepatocytes [31], it is rapidly down-regulated in culture. mEH is not expressed on the surface of established cell lines or our BHK transfectants.
Interestingly, the anti-AN antibody was not detected in sera from three chimpanzees infected with HAV or HCV at any time points of experiments even at several weeks after the peak of enzyme elevation. We cloned the chimpanzee’s mEH cDNA and found that its protein sequence differs from the human one by three amino acids which are unique to this species (unpublished). We do not know if the antibody response of infected chimpanzees will react against the purified chimpanzee mEH. If this protein still does not react with the sera, it may explain why chimpanzees do not develop severe hepatitis in HCV and HAV infection.
HAV can replicate in cell culture. The recent development of an in vitro replication system in which the HCV replicon functions [44–46] will also enable us to analyze the influence of virus replication to mEH in vitro. We have preliminary data suggesting that HAV and HCV replication causes translocation of mEH to cell surface and culture fluid in infected hepatocyte cell lines and also alterations in enzyme activity (manuscript in preparation). Taken together, the virus infection seems to influence the association of mEH with the membrane, and possibly affect its physiological function. This might be related to pathogenesis in HAV and HCV infections.
Acknowledgments
The authors thank Hiroe Akatsuka and Akira Takagi for technical assistance. We also thank Dr Curt C. Harris (NCI, NIH) for providing us with THLE-5b and Dr Stephen M. Feinstone (CBER, FDA) for helpful discussions and preparing the manuscript. This work was supported by the grant from the Ministry of Science, Culture, Technology, and Sports of Japan to the Research Center for Genomic Medicine, Saitama Medical University, and partly supported by NIEHS Grant R37 ES02710, NIEHS Superfund Grant P42 ES04699, and NIEHS Center for Environmental Health Sciences P30 ES05707.
Abbreviations
- ELISA
enzyme-linked immunosorbent assay
- S/N ratio
signal/noise ratio
- ALT
alanine aminotransferase
- CsCl
cesium chloride
- OPD
o-phenylenediamine dihydrochloride
- FCS
fetal calf serum
- BSA
bovine serum albumin
- FITC
fluorescein isothiocyanate
- TRITC
tetramethyl rhodamine isothiocyanate
References
- 1.Chisari FV. Unscrambling hepatitis C virus-host interactions. Nature. 2005;436:930–2. doi: 10.1038/nature04076. [DOI] [PubMed] [Google Scholar]
- 2.Choo Q-L, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–62. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 3.Shirachi R, Shiraishi H, Tateda A, Kikuchi K, Ishida N. Hepatitis “C” antigen in non-A, non-B post-transfusion hepatitis. Lancet. 1978;2:853–6. doi: 10.1016/s0140-6736(78)91567-2. [DOI] [PubMed] [Google Scholar]
- 4.Vitvitski L, Trepo C, Prince AM, Brotman B. Detection of virus-associated antigen in serum and liver of patients with non-A non-B hepatitis. Lancet. 1979;2:1263–7. doi: 10.1016/s0140-6736(79)92280-3. [DOI] [PubMed] [Google Scholar]
- 5.Tabor E, Mitchell FD, Goudeau AM, Gerety RJ. Detection of an antigen-antibody system in serum associated with human non-A, non-B hepatitis. J Med Virol. 1979;4:161–9. doi: 10.1002/jmv.1890040302. [DOI] [PubMed] [Google Scholar]
- 6.Tohmatsu J, Morimoto T, Katsuhara N, Abe K, Shikata T. AN6520 Ag: an antigen purified from liver with non-A, non-B Hepatitis. J Med Virol. 1985;15:357–71. doi: 10.1002/jmv.1890150406. [DOI] [PubMed] [Google Scholar]
- 7.Akatsuka T, Tohmatsu J, Yoshihara N, Katsuhara N, Okamoto T, Shikata T, et al. Detection of an antigen (AN6520) possibly related to non-A, non-B hepatitis, by monoclonal antibodies. I J Med Virol. 1986;20:33–42. doi: 10.1002/jmv.1890200106. [DOI] [PubMed] [Google Scholar]
- 8.Akatsuka T, Tohmatsu J, Abe K, Shikata T, Ishikawa T, Nakajima K, et al. Non-A, non-B hepatitis related AN6520 Ag is a normal cellular protein mainly expressed in liver. II J Med Virol. 1986;20:43–56. doi: 10.1002/jmv.1890200107. [DOI] [PubMed] [Google Scholar]
- 9.Yamauchi M, Nakajima H, Kimura K, Fujisawa K, Kameda H, Nakahara M, et al. An epidemic of non-A, non-B hepatitis in Japan. Am J Gastroenterol. 1983;78:652–5. [PubMed] [Google Scholar]
- 10.Fujisawa K, Yamauchi M, Kameda H, Shikata T, Nishioka K. Detection of antibodies to hepatitis C virus in patients involved in an outbreak of non-A, non-B hepatitis in the Okitsu area of Japan. In: Hollinger FB, Lemon SM, Margolis H, editors. Viral hepatitis and liver disease. Baltimore: Williams and Wilkins; 1991. pp. 415–7. [Google Scholar]
- 11.Huh N, Utakoji T. Production of HBs-antigen by two new human hepatoma cell lines and its enhancement by dexamethasone. Gann. 1981;72:178–9. [PubMed] [Google Scholar]
- 12.Lechner JF, Smoot DT, Pfeifer AMA, Cole KH, Weston A, Groopman JD, et al. A non-tumorigenic human liver epithelial cell culture model for chemical and biological carcinogenesis investigations. In: Rhim JS, Dritschilo A, editors. Neoplastic transformation in human cell systems. New Jersey: Humana Press; 1991. pp. 307–21. [Google Scholar]
- 13.McConahey PJ, Dixon FJ. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29:185–9. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- 14.Nakane PK, Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974;22:1084–91. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- 15.Mierendorf RC, Percy C, Young RA. Gene isolation by screening λgt11 libraries with antibodies. In: Berger SL, Kimmel AR, editors. Guide to molecular cloning techniques. San Diego; Academic Press: 1987. pp. 458–69. [Google Scholar]
- 16.Stanley JR, Tanaka T, Mueller S, Klauskovtun V, Roop D. Isolation of complementary DNA for bullous pemphigoid antigen by use of patients’ autoantibodies. J Clin Invest. 1988;82:1864–70. doi: 10.1172/JCI113803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ekins S, Maenpaa J, Wrighton A. In vitro metabolism: subcellular fractions. In: Woolf TF, editor. Handbook of drug metabolism. New York: Marcel Dekker; 1999. pp. 363–99. [Google Scholar]
- 18.Hinton AC, Hammock BD. Juvenile hormone esterase (JHE) from Tenebrio molitor: full-length cDNA sequence, in vitro expression, and characterization of the recombinant protein. Insect Biochem Molec Biol. 2003;33:477–87. doi: 10.1016/s0965-1748(03)00010-9. [DOI] [PubMed] [Google Scholar]
- 19.Lacourciere GM, Vakharia VN, Tan CP, Morris DI, Edwards GH, Moos M, et al. Interaction of hepatic microsomal epoxide hydrolase derived from a recombinant baculovirus expression system with an azarene oxide and an aziridine substrate analogue. Biochemistry. 1993;32:2610–6. doi: 10.1021/bi00061a019. [DOI] [PubMed] [Google Scholar]
- 20.Maekawa K, Itoda M, Hanioka N, Saito Y, Murayama N, Nakajima O, et al. Non-synonymous single nucleotide alterations in the microsomal epoxide hydrolase gene and their functional effects. Xenobiotica. 2003;33:277–87. doi: 10.1080/0049825021000061615. [DOI] [PubMed] [Google Scholar]
- 21.Cohen JI, Ticehurst JR, Purcell RH, Buckler-White A, Baroudy BM. Complete nucleotide sequence of wild-type hepatitis A virus: comparison with different strains of hepatitis A virus and other picornaviruses. J Virol. 1987;61:50–9. doi: 10.1128/jvi.61.1.50-59.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shindo M, Di Bisceglie AM, Biswas R, Mihalik K, Feinstone SM. Hepatitis C virus replication during acute infection in the chimpanzee. J Infect Dis. 1992;166:424–7. doi: 10.1093/infdis/166.2.424. [DOI] [PubMed] [Google Scholar]
- 23.Jackson MR, Craft JA, Burchell B. Nucleotide and deduced amino acid sequence of human liver microsomal epoxide hydrolase. Nucleic Acids Res. 1987;15:7188. doi: 10.1093/nar/15.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Dippe P, Levy D. Reconstitution of the immunopurified 49-kDa sodium-dependent bile acid transport protein derived from hepatocyte sinusoidal plasma membranes. J Biol Chem. 1990;265:14812–6. [PubMed] [Google Scholar]
- 25.Holder J, Yagi H, Dansette P, Jerina DM, Levin W, Lu AYH, et al. Effects of inducers and epoxide hydrolase on the metabolism of benzo[a]pyrene by liver microsomes and a reconstituted system: analysis by high pressure liquid chromatography. Proc Natl Acad Sci U S A. 1974;71:4356–60. doi: 10.1073/pnas.71.11.4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishii Y, Takeda S, Yamada H, Oguri K. Functional protein–protein interaction of drug metabolizing enzymes. Front Biosci. 2005;10:887–95. doi: 10.2741/1583. [DOI] [PubMed] [Google Scholar]
- 27.Griffin MJ. Regulation of rat liver epoxide hydrolase by tightly bound phosphoinositides. Proc Okla Acad Sci. 1999;79:1–6. [Google Scholar]
- 28.De Berardinis V, Moulis C, Maurice M, Beaune P, Pessayre D, Pompon D, et al. Human microsomal epoxide hydrolase is the target of germander-induced autoantibodies on the surface of human hepatocytes. Mol Pharmacol. 2000;58:542–51. doi: 10.1124/mol.58.3.542. [DOI] [PubMed] [Google Scholar]
- 29.Newman JW, Morisseau C, Hammock BD. Epoxide hydrolases: their roles and interactions with lipid metabolism. Prog Lipid Res. 2005;44:1–51. doi: 10.1016/j.plipres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Gill SS, Wie SI, Guenthner TM, Oesch F, Hammock BD. Rapid and sensitive enzyme-linked immunosorbent assay for the microsomal epoxide hydrolase. Carcinogenesis. 1982;3:1307–10. doi: 10.1093/carcin/3.11.1307. [DOI] [PubMed] [Google Scholar]
- 31.Zhu QS, von Dippe P, Xing W, Levy D. Membrane topology and cell surface targeting of microsomal epoxide hydrolase. J Biol Chem. 1999;274:27898–904. doi: 10.1074/jbc.274.39.27898. [DOI] [PubMed] [Google Scholar]
- 32.von Dippe P, Zhu QS, Levy D. Cell surface expression and bile acid transport function of one topological form of m-epoxide hydrolase. Biochem Biophys Res Commun. 2003;309:804–9. doi: 10.1016/j.bbrc.2003.08.074. [DOI] [PubMed] [Google Scholar]
- 33.McGlynn KA, Rosvold EA, Lustbader ED, Hu Y, Clapper ML, Zhou T, et al. Susceptibility to hepatocellular carcinoma is associated with genetic variation in the enzyme detoxication of aflatoxin B1. Proc Natl Acad Sci U S A. 1995;92:2384–7. doi: 10.1073/pnas.92.6.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sonzogni L, Silvestri L, De Silvestri A, Gritti C, Foti L, Zavaglia C, et al. Polymorphisms of microsomal epoxide hydrolase gene and severity of HCV-related liver disease. Hepatology. 2002;36:195–201. doi: 10.1053/jhep.2002.33898. [DOI] [PubMed] [Google Scholar]
- 35.Bogdanos DP, Mcfarlane IG. Cytochrome P450 2A6 meets P450 2D6: an enigma of viral infections and autoimmunity. J Hepatol. 2003;39:860–3. doi: 10.1016/s0168-8278(03)00417-3. [DOI] [PubMed] [Google Scholar]
- 36.Kerkar N, Choudhuri K, Ma Y, Mahmoud A, Bogdanos DP, Muratori L, et al. Cytochrome P4502D6193–212: a new immunodominant epitope and target of virus/self cross-reactivity in liver kidney microsomal autoantibody type 1-positive liver disease. J Immunol. 2003;170:1481–9. doi: 10.4049/jimmunol.170.3.1481. [DOI] [PubMed] [Google Scholar]
- 37.Kammer AR, van der Burg SH, Grabscheid B, Hunziker IP, Kwappenberg KMC, Reichen J, et al. Molecular mimicry of human cytochrome P450 by hepatitis C virus at the level of cytotoxic T cell recognition. J Exp Med. 1999;190:169–76. doi: 10.1084/jem.190.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoo BJ, Spaete RR, Geballe AP, Selby M, Houghton M, Han JH. 5′ end-dependent translation initiation of hepatitis-C viral RNA and the presence of putative positive and negative translational control elements within the 5′ untranslated region. Virology. 1992;191:889–99. doi: 10.1016/0042-6822(92)90264-p. [DOI] [PubMed] [Google Scholar]
- 39.Okita K, Farber E. An antigen common to preneoplastic hepatocyte populations and to liver cancer induced by N-2-fluorenyl-acetamide, ethionine, or other hepatocarcinogens. Gann Monogr Canc Res. 1975;17:283–99. [Google Scholar]
- 40.Levin W, Lu AYH, Thomas PE, Ryan D, Kizer DE, Griffin MJ. Identification of epoxide hydrolase as the preneoplastic antigen in rat liver hyperplastic nodules. Proc Natl Acad Sci U S A. 1978;75:3240–3. doi: 10.1073/pnas.75.7.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hammock BD, Loury DN, Moody DE, Ruebner B, Baselt R, Milam KM, et al. A methodology for the analysis of the preneoplastic antigen. Carcinogenesis. 1984;5:1467–73. doi: 10.1093/carcin/5.11.1467. [DOI] [PubMed] [Google Scholar]
- 42.Talcott RE, Pond SM, Ketterman A, Becker CE. Ethylesterases as indicators of liver damage. I. Studies on malathion carboxylesterases. Toxicol Appl Pharmacol. 1982;65:69–74. doi: 10.1016/0041-008x(82)90363-5. [DOI] [PubMed] [Google Scholar]
- 43.Ruoslahti E, Seppala M. Alpha-fetoprotein in cancer and fetal development. Adv Cancer Res. 1979;29:275–346. doi: 10.1016/s0065-230x(08)60849-0. [DOI] [PubMed] [Google Scholar]
- 44.Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, Zhao Z, et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nature Med. 2005;11:791–6. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lindenbach BD, Evans MJ, Syder AJ, Wolk B, Tellinghuisen TL, Liu CC, et al. Complete replication of hepatitis C virus in cell culture. Science. 2005;309:623–6. doi: 10.1126/science.1114016. [DOI] [PubMed] [Google Scholar]
- 46.Zhong J, Gastaminza P, Cheng G, Kapadia S, Kato T, Burton DR, et al. Robust hepatitis C virus infection in vitro. Proc Natl Acad Sci U S A. 2005;102:9294–9. doi: 10.1073/pnas.0503596102. [DOI] [PMC free article] [PubMed] [Google Scholar]


