Abstract
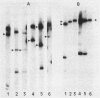
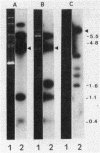
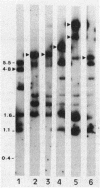
Seventeen oral streptococci and 18 enterococci were tested for the presence of DNA sequences homologous to the conjugative transposon Tn916 encoding tetracycline resistance. All the strains were resistant to tetracyclines, including minocycline, and most of them were resistant to other antibiotics. Tn916-like structures, identified by hybridization of HincII-digested DNA, were found on the chromosomes of 11 oral streptococci and four enterococci and on two plasmids, pIP1549 and pIP1440, one harbored by an Enterococcus hirae strain and the other harbored by an Enterococcus faecalis strain. Sequences homologous to Tn916, only some of which corresponded to its internal HincII structure (Tn916-modified elements), were chromosomally located in three oral streptococci and two enterococci and were plasmid borne in pIP614 harbored by an E. faecalis strain. Nine enterococci and three oral streptococci carried either the Tet M or the Tet O determinant chromosomally, but they carried no other sequences homologous to Tn916.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentorcha F., De Cespédès G., Horaud T. Tetracycline resistance heterogeneity in Enterococcus faecium. Antimicrob Agents Chemother. 1991 May;35(5):808–812. doi: 10.1128/aac.35.5.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge P. D., Sneath P. H. Numerical taxonomy of Streptococcus. J Gen Microbiol. 1983 Mar;129(3):565–597. doi: 10.1099/00221287-129-3-565. [DOI] [PubMed] [Google Scholar]
- Burdett V., Inamine J., Rajagopalan S. Heterogeneity of tetracycline resistance determinants in Streptococcus. J Bacteriol. 1982 Mar;149(3):995–1004. doi: 10.1128/jb.149.3.995-1004.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buu-Hoï A., Le Bouguénec C., Horaud T. Genetic basis of antibiotic resistance in Aerococcus viridans. Antimicrob Agents Chemother. 1989 Apr;33(4):529–534. doi: 10.1128/aac.33.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillaud F., Carlier C., Courvalin P. Physical analysis of the conjugative shuttle transposon Tn1545. Plasmid. 1987 Jan;17(1):58–60. doi: 10.1016/0147-619x(87)90009-6. [DOI] [PubMed] [Google Scholar]
- Christie P. J., Korman R. Z., Zahler S. A., Adsit J. C., Dunny G. M. Two conjugation systems associated with Streptococcus faecalis plasmid pCF10: identification of a conjugative transposon that transfers between S. faecalis and Bacillus subtilis. J Bacteriol. 1987 Jun;169(6):2529–2536. doi: 10.1128/jb.169.6.2529-2536.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clermont D., Horaud T. Identification of chromosomal antibiotic resistance genes in Streptococcus anginosus ("S. milleri"). Antimicrob Agents Chemother. 1990 Sep;34(9):1685–1690. doi: 10.1128/aac.34.9.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., An F. Y., White B. A., Gawron-Burke C. Streptococcus faecalis sex pheromone (cAM373) also produced by Staphylococcus aureus and identification of a conjugative transposon (Tn918). J Bacteriol. 1985 Jun;162(3):1212–1220. doi: 10.1128/jb.162.3.1212-1220.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Gawron-Burke C. Conjugative transposons and the dissemination of antibiotic resistance in streptococci. Annu Rev Microbiol. 1986;40:635–659. doi: 10.1146/annurev.mi.40.100186.003223. [DOI] [PubMed] [Google Scholar]
- Courvalin P. M., Carlier C., Chabbert Y. A. Plasmid-linked tetracycline and erythromycin resistance in group D "streptococcus". Ann Inst Pasteur (Paris) 1972 Dec;123(6):755–759. [PubMed] [Google Scholar]
- Fitzgerald G. F., Clewell D. B. A conjugative transposon (Tn919) in Streptococcus sanguis. Infect Immun. 1985 Feb;47(2):415–420. doi: 10.1128/iai.47.2.415-420.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher H. M., Marri L., Daneo-Moore L. Transposon-916-like elements in clinical isolates of Enterococcus faecium. J Gen Microbiol. 1989 Nov;135(11):3067–3077. doi: 10.1099/00221287-135-11-3067. [DOI] [PubMed] [Google Scholar]
- Gawron-Burke C., Clewell D. B. Regeneration of insertionally inactivated streptococcal DNA fragments after excision of transposon Tn916 in Escherichia coli: strategy for targeting and cloning of genes from gram-positive bacteria. J Bacteriol. 1984 Jul;159(1):214–221. doi: 10.1128/jb.159.1.214-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horaud T., Delbos F., de Cespédès G. Tn3702, a conjugative transposon in Enterococcus faecalis. FEMS Microbiol Lett. 1990 Oct;60(1-2):189–194. doi: 10.1016/0378-1097(90)90370-6. [DOI] [PubMed] [Google Scholar]
- Horodniceanu T., Delbos F. Les streptocoques non groupables dans les infections humaines: identification et sensibilité aux antibiotiques. Ann Microbiol (Paris) 1982 Sep-Oct;133(2):255–269. [PubMed] [Google Scholar]
- Hächler H., Kayser F. H., Berger-Bächi B. Homology of a transferable tetracycline resistance determinant of Clostridium difficile with Streptococcus (Enterococcus) faecalis transposon Tn916. Antimicrob Agents Chemother. 1987 Jul;31(7):1033–1038. doi: 10.1128/aac.31.7.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A. E., Hobbs S. J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974 Feb;117(2):360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bouguénec C., de Cespédès G., Horaud T. Molecular analysis of a composite chromosomal conjugative element (Tn3701) of Streptococcus pyogenes. J Bacteriol. 1988 Sep;170(9):3930–3936. doi: 10.1128/jb.170.9.3930-3936.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bouguénec C., de Cespédès G., Horaud T. Presence of chromosomal elements resembling the composite structure Tn3701 in streptococci. J Bacteriol. 1990 Feb;172(2):727–734. doi: 10.1128/jb.172.2.727-734.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S. B., McMurry L. M., Burdett V., Courvalin P., Hillen W., Roberts M. C., Taylor D. E. Nomenclature for tetracycline resistance determinants. Antimicrob Agents Chemother. 1989 Aug;33(8):1373–1374. doi: 10.1128/aac.33.8.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P., Trieu-Cuot P., Courvalin P. Nucleotide sequence of the tetM tetracycline resistance determinant of the streptococcal conjugative shuttle transposon Tn1545. Nucleic Acids Res. 1986 Sep 11;14(17):7047–7058. doi: 10.1093/nar/14.17.7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., An F. Y., Clewell D. B. Plasmids and pheromone response of the beta-lactamase producer Streptococcus (Enterococcus) faecalis HH22. Antimicrob Agents Chemother. 1988 Apr;32(4):547–551. doi: 10.1128/aac.32.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper K., Horaud T., Le Bouguénec C., de Cespédès G. Location of antibiotic resistance markers in clinical isolates of Enterococcus faecalis with similar antibiotypes. Antimicrob Agents Chemother. 1987 Sep;31(9):1394–1402. doi: 10.1128/aac.31.9.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senghas E., Jones J. M., Yamamoto M., Gawron-Burke C., Clewell D. B. Genetic organization of the bacterial conjugative transposon Tn916. J Bacteriol. 1988 Jan;170(1):245–249. doi: 10.1128/jb.170.1.245-249.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. E., Hiratsuka K., Ray H., Manavathu E. K. Characterization and expression of a cloned tetracycline resistance determinant from Campylobacter jejuni plasmid pUA466. J Bacteriol. 1987 Jul;169(7):2984–2989. doi: 10.1128/jb.169.7.2984-2989.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson R., Gutmann L., Horaud T., Delbos F., Acar J. F. Use of penicillin-binding proteins for the identification of enterococci. J Gen Microbiol. 1986 Jul;132(7):1929–1937. doi: 10.1099/00221287-132-7-1929. [DOI] [PubMed] [Google Scholar]