Abstract
Myosin binding protein-C (cMyBP-C) is a thick filament accessory protein which in cardiac muscle functions to regulate the kinetics of cross-bridge interaction with actin; however, the underlying mechanism is not yet understood. To explore the structural basis for cMyBP-C function, we used synchrotron low-angle x-ray diffraction to measure interfilament lattice spacing and the equatorial intensity ratio, I11/I10, in skinned myocardial preparations isolated from wild-type (WT) and cMyBP-C null (cMyBP-C-/-). In relaxed myocardium, ablation of cMyBP-C appeared to result in radial displacement of cross-bridges away from the thick filaments, as there was a significant increase (∼30%) in the I11/I10 ratio for cMyBP-C-/- (0.37±0.03) myocardium as compared to WT (0.28±0.01). While lattice spacing tended to be greater in cMyBP-C-/- myocardium (44.18±0.68 nm) when compared to WT (42.95±0.43 nm), the difference was not statistically significant. Furthermore, liquid-like disorder in the myofilament lattice was significantly greater (∼40% greater) in cMyBP-C-/- myocardium as compared to WT. These results are consistent with our working hypothesis that cMyBP-C normally acts to tether myosin cross-bridges nearer to the thick filament backbone, thereby reducing the likelihood of cross-bridge binding to actin and limiting cooperative activation of the thin filament.
INTRODUCTION
Force development in myocardium is a highly regulated process that involves Ca2+ binding to the thin filament regulatory protein troponin and cooperativity in cross-bridge binding to actin. As a consequence of these mechanisms, the amplitude and kinetics of force development vary continuously with the level of thin filament activation1,2. Recent work has implicated myosin binding protein-C (cMyBP-C), an accessory protein that binds tightly to myosin, as a primary regulator of the force and speed of myocardial contraction, but the mechanisms of its effects are not well understood. cMyBP-C is located at 43-nm axial intervals within the central region of each half of the sarcomeric A-band and thus has an axial repeat similar to that of the myosin heads3,4. Recent single-particle analyses of electron micrographs from isolated thick filaments suggest that cMyBP-C may interact with every third crown of heads, thereby perturbing their azimuthal position5. The detailed structural disposition of cMyBP-C in the thick filament, however, has not yet been elucidated. Acute extraction of cMyBP-C was previously shown to increase the Ca2+-sensitivity of force6, and genetic ablation of cMyBP-C significantly increased the rate of force development at submaximal levels of activation1,2 suggesting that cMyBP-C has an inhibitory role in acto-myosin interaction. cMyBP-C has also been shown to modulate contractile function by binding to the S2 sub-fragment of myosin7,8 and to light meromyosin (LMM)9. The mechanisms by which cMyBP-C performs these functions are unknown
The present study was undertaken to examine the structural effects of genetic ablation of cMyBP-C using low angle x-ray diffraction. This approach has long been used in striated muscle to assess the distribution of myofilament mass by examining the equatorial reflections arising from the interdigitating hexagonal arrays of thick and thin filaments10. The most prominent features of the diffraction patterns from myocardium in the present study are two pairs of equatorial reflections, i.e., 1,0 and 1,1. In striated muscles, a shift of mass from the region of the thick filament to the region of the thin filament causes the intensity of the 1,1 reflection to increase and the intensity of the 1,0 reflection to decrease. The ratio of these intensities, I11/I10, may be used, therefore, as a measure of the proximity of myosin cross-bridges to the thick or thin filaments10,11,12,13,14.
Palmer, et al. (2004)15 previously reported that x-ray diffraction patterns obtained from skinned papillary muscles from mice in which the cMyBP-C gene was truncated (cMyBP-Ct/t) had more diffuse 1,0 and 1,1 equatorial reflections than WT, indicating greater filament lattice disorder. Here, we examined the intensity ratios of equatorial x-ray reflections from skinned myocardium from cMyBP-C-/- and WT mice, thereby assessing the effects of cMyBP-C on the radial disposition of myosin heads and using the reflection widths as a quantitative measure of lattice disorder.
Our hypothesis is that ablation of cMyBP-C relieves a physical constraint on myosin, which then allows myosin cross-bridges to move radially from the surface of the thick filament. Supporting this idea, we found a significant increase in the I11/I10 ratio (∼30%), and an increase in liquid-like disorder (∼40% greater), in cMyBP-C-/- myocardium compared to WT.
RESULTS
Consistent with earlier results16, Western blots showed that cMyBP-C (∼140 kDa mol wt) was present in WT myocardium but was absent from cMyBP-C-/- preparations. Silver-stained SDS gels of these same preparations showed that there were no other differences in protein content between the two kinds of preparations (data not shown).
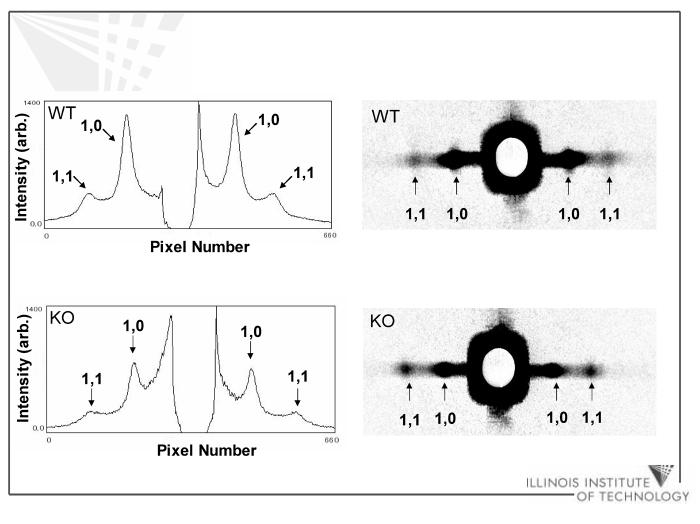
I11/I10 intensity ratios determined from the 1,0 and 1,1 equatorial reflections from skinned trabeculae (Figure 2) were used to determine whether there were differences in mass distribution (presumably due to movement of cross-bridges) between the thick and thin filaments of cMyBP-C-/- and WT myocardium. These measurements were made under resting conditions (pCa 9.0) to avoid possible confounding effects due to attachment of cross-bridges to thin filaments in activated muscle. I11/I ratios in trabeculae from cMyBP-C-/- 10 mice were found to be significantly greater (∼30%, p<0.005) in cMyBP-C-/- myocardium (0.37 ± 0.03) than in WT (0.28 ± 0.01), as summarized in Table 1. This result suggests that there is a net transfer of mass from the thick to thin filaments in cMyBP-C-/- myocardium compared to WT.
Figure 2.
1,0 and 1,1 equatorial reflection intensity peak profiles and patterns from WT and KO skinned myocardium.
Table 1.
Summary data derived from peak intensity analyses of x-ray diffraction patterns from cMyBP-C-/- and WT myocardium. I11/I10 is the ratio of intensities of the 1,1 and 1,0 equatorial x-ray reflections. ΔX/d10 is the paracrystalline disorder parameter. Percent (%) change was in each case based upon the difference between cMyBP-C-/- and WT myocardium expressed as a fraction of the value in WT myocardium. Significant differences (p<0.05) between cMyBP-C-/- and WT myocardium are denoted by asterisks (*).
| Myocardium | I11/I10 (n) | d10 Lattice Spacing (nm) (n) | ΔX/d10 (%) (n) | Sarcomere Length (μm) (n) |
|---|---|---|---|---|
| cMyBP-C-/- | 0.37 ± 0.03 (16)* | 44.18 ± 0.68 (17) | 2.06 ± 0.19 (16)* | 2.15 ± 0.01 (14) |
| Wild-type | 0.28 ± 0.01 (16) | 42.95 ± 0.43 (17) | 1.45 ± 0.12 (16) | 2.14 ± 0.02 (16) |
| Difference upon ablation | 30% | 3% | 42% | 0.3% |
The separations of the 1,0 and 1,1 equatorial reflections in diffraction patterns (Figure 2) were converted to d10 lattice spacings, using Bragg’s Law, as described in the Methods. d10 lattice spacings were not significantly different between cMyBP-C-/- and WT (Table 1), which is consistent with a similar lack of difference between WT and truncated cMyBP-C myocardium observed previously by Palmer, et al. (2004)15.
Paracrystaline (liquid-like) disorder (σs, %1,0 spacing) was also assessed and was found to be significantly greater in cMyBP-C-/- myocardium compared to WT. Upon conversion to ΔX/d10, i.e., the standard deviation of the lattice spacing divided by lattice spacing17, paracrystalline disorder was seen to be ∼40% greater (p<0.02) in cMyBP-C-/- myocardium as compared to WT. Also consistent with myofilament lattice disorder, the background signal was qualitatively greater in cMyBP-C-/- myocardium (Figure 2). Lattice spacing heterogeneity between the myofibrils did not differ significantly between cMyBP-C-/- (Δd10/d10 = 12.7 ± 0.3%) and WT (11.8 ± 0.4%) myocardium.
DISCUSSION
The primary result of this study is that in cardiac muscle where myosin binding protein-C (cMyBP-C) is ablated, cross-bridges are, on average, located further from the surface of the thick filament backbone and closer to the thin filament. This conclusion follows from the observation that the I11/I10 ratio was significantly greater in the absence of cMyBP-C in cMyBP-C-/- null myocardium. This finding suggests that cMyBP-C normally acts as a structural restraint on some fraction of the myosin heads, keeping them near to the thick filament backbone and, thereby reducing the probability of their interaction with actin. Such a mechanism is consistent with earlier suggestions that cMyBP-C serves as a mechanical tether on myosin6,18. Similarly, the closer proximity of myosin heads to thin filaments in cMyBP-C null myocardium could account for the greater submaximal force and the faster kinetics of force development compared to control myocardium, as reported previously1,2. Changes in the availability of cross-bridges to actin would also modulate the degree of cooperative activation of the thin filament due to strong binding of myosin cross-bridges19.
Consistent with these conclusions, acute partial extraction of cMyBP-C using biochemical methods resulted in a reversible increase in the Ca2+-sensitivity of force6. Furthermore, the absence of cMyBP-C may also increase the flexibility of cross-bridges, as reduced cross-bridge stiffness has been observed in myocardium expressing truncated cMyBP-C15.
Thick filament lattice spacings were measured to determine whether the increased Ca2+-sensitivity of force and the acceleration of cooperative activation of the thin filament in cMyBP-C-/- mutant myocardium1,2 were simply a result of increased myosin-actin interaction due to closer proximity of the thick filaments in the absence of cMyBP-C. We found no significant difference in lattice spacing between cMyBP-C-/- and WT myocardium, which is consistent with results of a previous study15 in which lattice spacings in cMyBP-Ct/t and WT myocardium were also not different. Thus, the accelerated kinetics of stretch activation due to ablation of cMyBP-C20 are not an artifact due to distortion of lattice spacing but instead result primarily from myosin heads that are radially displaced further from the surface of the thick filament backbone and closer to the thin filament.
We also measured the width of the distribution of lattice spacings amongst different myofibrils in the trabeculae. The value we obtained, ∼12%, is much larger than previously reported for vertebrate muscle17,22, but does not differ between WT and cMyBP-C-/-, indicating that the degree of variance in lattice spacing inhomogeneity was similar in the presence and absence of cMyBP-C.
Paracrystalline (liquid-like) disorder is defined as a reduction in the distance over which individual myofilaments are correlated with their neighbors within the myofibrils17,21,22. Paracrystalline disorder is significantly increased in activated skeletal muscle17 and has been speculated to arise from distortions of the hexagonal myofilament lattice due to random attachment of cross-bridges to the thin filaments causing displacement of myofilament segments away from their equilibrium positions under resting conditions17. In our analysis of diffraction peak widths, we found that cMyBP-C-/- myocardium exhibits a significant increase in paracrystalline disorder as compared to WT myocardium, although the overall magnitude of the disorder was quite small. Myocardium has a higher resting stiffness than skeletal muscle23, indicating a higher degree of weakly acting crossbridges under resting conditions. Also in contrast to skeletal muscle, cyclically contracting cardiac muscle have a significant number of myosin heads that appear to remain attached to actin with producing force during the diastolic phase24. Furthermore, a significant number of cardiac myosin heads appear to be attached to (or in the immediate vicinity of) actin without producing tension at low Ca2+ activation25. It could be that the increase in paracrystalline disorder in the absence of cMyBP-C is due to increased weak acto-myosin interactions, consistent with our hypothesis that cMyBP-C acts to inhibit such interactions. We cannot exclude, however, that these relative changes in myofilament disorder are a consequence of a direct effect of cMyBP-C (when present) to stabilize the lattice, or that the disorder could be the result of assembly abnormalities in the transgenic mice during development. In this regard, Squire and colleagues (2003, 2004)26,27 have suggested, on the basis of X-ray and modeling studies, that there are direct interactions between the C0-subunit of cMyBP-C and actin, which, when present, would presumably stabilize the filament lattice. In either case, Palmer, et al. (2004)15 also found greater disorder in diffraction patterns from myocardium expressing cMyBP-Ct/t as compared to wild-type.
In a previous study16, we showed that, in histological examination, cMyBP-C-/- knockout hearts showed morphological changes consistent with hypertrophic cardiomyopathy, including some myocyte disarray and fibrosis, and increase in the number of mitochondria. This disarray appeared as an overall decrease in apparent tissue organization. The individual myocytes, however, were not misshapen or distorted. Clear sarcomere striation patterns were seen in cMyBP-C-/- hearts. Ultrastructural examination of sections by transmission electron microscopy also showed sarcomeres with prominent Z lines, M lines, and A bands in cMyBP-C-/- hearts. It was also reported in this study that the close lateral alignment of myofibrils was often not maintained in cMyBP-C-/- hearts such that Z lines of adjacent myofibrils were frequently out of register. This would not be expected to have an appreciable affect on the equatorial pattern. Thus it appears that while the tissue in cMyBP-C-/- myocardium is less well-organized, which can explain the appreciable increase in diffuse background in the x-ray patterns, the differences at the myofibrillar and myofilament level are likely to be subtle. That these differences are, in fact, subtle, is indicated by the lack of a difference in lattice spacing heterogeneity between WT and cMyBP-C-/-. Similarly, while the changes in ΔX/d10 between WT and cMyBP-C-/- were statistically significant, the overall magnitude of the disorder in both cases is quite small (2.57 vs. 1.82 %).
Biochemical results led Moolman-Smook, et al. (2002)28 to propose that cMyBP-C trimerizes to form a collar around the thick filament that acts to restrain cross-bridges. Specifically, adjacent cMyBP-C molecules in the trimeric collar appear to interact within two overlapping domains, C5-C7 and C8-C1028, and may also bind to cMyBP-C binding sites on myosin, i.e., light meromyosin (LMM)9 and subfragment-2 (S2)7,8,29. Though the precise disposition of cMyBP-C on the thick filament remains to be determined, structural tethering of myosin heads nearer to the thick filament backbone30 could explain the slower kinetics of force redevelopment during submaximal activation of myocardium that contains cMyBP-C1,2.
Similar to the effect of ablation, PKA-mediated phosphorylation of cMyBP-C has been postulated to relieve the collar-like constraint of myosin heads31. In this regard, recent studies reported similar accelerating effects of cross-bridge cycling kinetics due to phosphorylation or ablation of cMyBP-C32. X-ray diffraction studies of the effects of cMyBP-C phosphorylation on cross-bridge disposition are needed to determine whether the structural mechanisms underlying the accelerated kinetics are the same in the two cases.
MATERIALS AND METHODS
Experimental animals
Homozygous cMyBP-C null mice (cMyBP-C-/-) were generated as previously described16 and maintained on a SV/129 background. Wild-type (WT) mice (3-6 months old; either sex) were obtained from Taconic Farms (Germantown, NY). Animal usage was conducted in accordance with institutional guidelines using protocols approved by the Animal Care and Use Committees of the University of Wisconsin-Madison.
Experimental solutions
Experimental solutions were prepared using the computer program developed by Fabiato (1988)33 and stability constants listed by Godt and Lindley (1982)34 corrected to pH 7.0 and 22°C, as described previously6. Relaxing solution for final dissections of skinned myocardial preparations contained (mM) 100 KCl, 20 imidazole, 2 EGTA, 4 ATP, and 7 MgCl2. Relaxing solution (pCa 9.0) used for x-ray diffraction measurements contained (mM) 100 N,N-bis[2-hydroxyethyl]-2aminoethanesulfonic acid (BES), 15 creatine phosphate, 5 dithiotheitol (DDT), 7 EGTA, 0.02 CaCl2, 5.49 MgCl2, and 4.66 ATP. Ionic strength of solutions was adjusted to 180 mM with potassium propionate.
Isolation of skinned trabeculae
Adult mice were injected intraperitoneally with 5,000 U heparin/kg body weight, and after 5 minutes were anesthetized by inhalation of isofluorane. After deep anesthesia was established, mice were euthanized by creating a pneumothorax. Hearts were rapidly excised and placed in a petri dish containing modified Tyrodes solution [(mM) 120 NaCl, 19 NaHCO3, 10 Glucose, 5 KCl, 1.2 CaCl2, 25 BDM, pH was adjusted to 7.0 by bubbling with 95% O2/5% CO2 for 30 minutes]. Trabeculae were dissected from the right ventricle and secured to wooden dowels using two loops of 8-0 suture. Trabeculae were bathed for 12-16 hrs in ice-cold relaxing solution containing 1% Triton X-100, and washed in fresh ice-cold relaxing solution for one hour. Preparations were then stored at -20°C for up to four days in fresh relaxing solution containing 50% glycerol.
Mounting of trabeculae in experimental chamber
Trabeculae in 50% glycerol/relaxing solution were warmed and then transferred to a petri dish containing ice-cold relaxing solution. An aluminum T-clip (Kem-Mil Co., Hayward, CA) was secured to each end of a trabecula. The trabecula was then transferred to a simple x-ray chamber35 with 0.001” thick mylar™ windows and was mounted in relaxing solution between two styluses for x-radiation. Sarcomere length was set to ∼2.1 μm by adjusting overall muscle length via a micrometer attached to one of the styluses. SL was measured from a FFT of digitized striation images using established methods36,37.
X-ray diffraction and analysis
X-ray experiments were performed at 22°C using the small-angle instrument on the BioCAT undulator-based beamline 18-D at the Advanced Photon Source, Argonne National Lab38. The high x-ray flux density and low beam divergence of this instrument are highly advantageous for small-angle x-ray studies of small specimens such as mouse trabeculae. The x-ray camera was adjusted to an ∼2.8 m distance between sample and detector, and beam. Energy was set to 12 keV (1.03 Å). All flight paths were evacuated except for a small gap around the sample chamber. The beam was collimated to ∼0.4 × 0.8 mm at the sample and to ∼0.040 × 0.14 mm at the detector, with the incident flux limited to ∼1 × 1012 photon/s by use of aluminum attenuators. No more than 3 exposures of 1 s each were obtained from each specimen (at least 16 exposures can be obtained under these conditions before changes due to radiation damage are seen in the diffraction pattern38).
Diffraction patterns were collected on a CCD-based x-ray detector (PCCD 168080, Aviex L.L.C., Napierville, IL) with a 160 mm × 80 mm active area and 39 μm pixels and corrected for dark current and for flat field, and spatial distortions38. Spacings on images were measured using the distance-measuring tool of the software package FIT2D101 on a UNIX workstation38. Spacings of the 1,0 and 1,1 equatorial reflections were converted to d10 lattice spacings using Bragg’s Law. (d10 lattice spacing can be converted to inter-thick filament spacing by multiplying d10 by 2/√322). Intensities of the 1,0 and 1,1 equatorial reflections were determined from one-dimensional projections along the equator and analyzed as described previously22. I11/I10 intensity ratios can be used to estimate shifts of mass (presumably cross-bridges) from the region of the thick filament to region of the thin filament.
In the fitting program, the width of the Gaussian representing a given peak σh,k is expressed as √(σc2+σd2Shk2+σs2Shk2), where Shk=√(h2+k2+hk). σc is the width of the X-ray beam, σd is related to amount of heterogeneity in inter-filament spacing among the myofibrils, and σs is related to the amount of paracrystalline (liquid-like) disorder of the myofilaments in the hexagonal lattice. Both σd and σs may be used as free parameters for the fits17,22. σd can be expressed relative to Δd10/d10 as a measure of the width of the distribution of lattice spacings between the different myofibrils in the sample. σs can be expressed as ΔX/d10 where ΔX is a measure of the width of distribution of interfilament spacings within a given myofibril. Both are conveniently expressed as a percent of the d10 lattice spacing. σ increases substantially during contraction in skeletal muscle17 .
Statistics
All data are expressed as means ± SEM. A two-tailed t test for unpaired samples or a paired t test was used as a post hoc test of significance (p<0.05), as appropriate.
Western blots and silver stained gels
To confirm the molecular phenotypes of cMyBP-C-/- and WT myocardium, Western blot analysis was performed on samples stored in SDS-buffer following exposure to the x-ray beam. 5μl aliquots of trabeculae dissolved in sample buffer were separated by 12% SDS-PAGE and transferred to a PVDF membrane. A poly-clonal antibody16 was used to probe for cMyBP-C. PVDF membranes were exposed on x-ray film. To ensure that there were no additional differences in protein content, gels with samples from the same preparations were run and then silver stained39.
Figure 1.
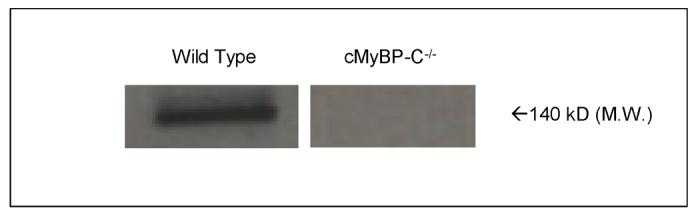
Western blot displaying cMyBP-C band region (140 kD M.W.) present in wild-type and absent in cMyBP-C-/- skinned myocardium following x-ray experiment.
Acknowledgements
We would like to thank Heather King and Krystyna Shioura for technical assistance with x-ray data analysis. This work was supported by funding from the National Institutes of Health (R3782900) to R. L. Moss. Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Basic Energy Sciences, and Office of Science, under contract No. W-31-109-ENG-38. BioCAT is a National Institutes of Health-supported Research Center (RR-08630).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Korte FS, McDonald KS, Harris SP, Moss RL. Loaded Shortening, Power Output, and Rate of Force Redevelopment Are Increased With Knockout of Cardiac Myosin Binding Protein-C. Circ. Res. 2003;93(8):752–758. doi: 10.1161/01.RES.0000096363.85588.9A. [DOI] [PubMed] [Google Scholar]
- 2.Stelzer JE, Fitzsimons DP, Moss RL. Ablation of myosin binding protein-C accelerates force development in mouse myocardium. Biophys. J. 2006;90(11):4119–4127. doi: 10.1529/biophysj.105.078147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Offer G, Moos C, Starr R. A new protein of the thick filaments of vertebrae skeletal myofibrils. J. Mol. Biol. 1973;74:653–676. doi: 10.1016/0022-2836(73)90055-7. [DOI] [PubMed] [Google Scholar]
- 4.Craig R, Offer G. The location of C-protein in rabbit skeletal muscle. Proc. R. Soc. Lond. B. Biol. Sci. 1976;192:451–461. doi: 10.1098/rspb.1976.0023. [DOI] [PubMed] [Google Scholar]
- 5.Al-Khayat HA, Morris EP, Kensler RW, Squire JM. 3D structure of relaxed fish muscle myosin filaments by single particle analysis. J Struct Biol. 2006;155(2):217. doi: 10.1016/j.jsb.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 6.Hofmann PA, Hartzell HC, Moss RL. Alterations in Ca2+ Sensitive Tension Due to Partial Extraction of C-Protein from Rat Skinned Cardiac Myocytes and Rabbit Skeletal Muscle Fibers. J. Gen. Physiol. 1991;97:1141–1163. doi: 10.1085/jgp.97.6.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris SP, Rostkova E, Gautel M, Moss RL. Binding of myosin binding protein-C to myosin subfragment S2 affects contractility independent of a tether mechanism. Circ. Res. 2004;95(9):930–6. doi: 10.1161/01.RES.0000147312.02673.56. [DOI] [PubMed] [Google Scholar]
- 8.Starr R, Offer G. The interaction of C-protein with heavy meromyosin and subfragment-2. Biochem J. 1978;171(3):813–816. doi: 10.1042/bj1710813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moos C, Offer G, Starr R, Bennett P. Interaction of C-protein with myosin, myosin rod and light meromyosin. J. Mol. Biol. 1975;97:1–9. doi: 10.1016/s0022-2836(75)80017-9. [DOI] [PubMed] [Google Scholar]
- 10.Squire JM, Knupp C. X-ray diffraction studies of muscle and the cross-bridge cycle. In: Squire JM, Parry DD, editors. Advances in Protein Chemistry - Fibrous Proteins: Muscle and Molecular Motors. Vol. 71. Elsevier; London: 2005. pp. 195–255. [DOI] [PubMed] [Google Scholar]
- 11.Eliott GF, Worthington CR. A small-angle opically focusing x-ray diffraction camera in biological research (I) J. Ultrastruct. Res. 1963;49:166–170. doi: 10.1016/s0022-5320(63)80044-1. [DOI] [PubMed] [Google Scholar]
- 12.Matsurbara I, Millman BM. X-ray diffraction patterns from mammalian heart muscle. J. Mol. Biol. 1974;82(4):527–536. doi: 10.1016/0022-2836(74)90246-0. [DOI] [PubMed] [Google Scholar]
- 13.Haselgrove JC, Huxley HE. X-ray Evidence for Radial Cross-bridge Movement and for the Sliding Filament Model in Actively Contracting Skeletal Muscle. J. Mol. Biol. 1973;77:549–568. doi: 10.1016/0022-2836(73)90222-2. [DOI] [PubMed] [Google Scholar]
- 14.Yu LC. Analysis of equatorial x-ray diffraction patterns from skeletal muscles. Biophys. J. 1989;55:433–440. doi: 10.1016/S0006-3495(89)82837-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmer BM, et al. Reduced cross-bridge dependent stiffness of skinned myocardium from mice lacking cardiac myosin binding protein-C. Mol. Cell. Biochem. 2004;263:73–80. doi: 10.1023/B:MCBI.0000041849.60591.45. [DOI] [PubMed] [Google Scholar]
- 16.Harris SP, Bartley CR, Hacker TA, McDonald KS, Douglas PS, Greaser ML, Powers PA, Moss RL. Hypertrophic Cardiomyopathy in Cardiac Myosin Binding Protein-C Knockout Mice. Circ. Res. 2002;90(5):594–601. doi: 10.1161/01.res.0000012222.70819.64. [DOI] [PubMed] [Google Scholar]
- 17.Yu LC, et al. Distribution of mass in relaxed frog skeletal muscle and its redistribution upon activation. Biophys. J. 1985;47(3):311–321. doi: 10.1016/S0006-3495(85)83921-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calaghan SC, Trinick J, Knight PJ, White E. A role for C-protein in the regulation of contraction and intracellular Ca2+ in intact rat ventricular myocytes. J. Physiol. 2000;528:151–6. doi: 10.1111/j.1469-7793.2000.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moss RL, Razumova M, Fitzsimons DP. Myosin cross-bridge activation of cardiac thin filaments: implications for myocardial function in health and disease. Circ. Res. 2004;94(10):1290–1300. doi: 10.1161/01.RES.0000127125.61647.4F. [DOI] [PubMed] [Google Scholar]
- 20.Stelzer JE, Dunning SB, Moss RL. Ablation of cardiac myosin-binding protein-C accelerates stretch activation in murine skinned myocardium. Circ. Res. 2006;98(9):1212–8. doi: 10.1161/01.RES.0000219863.94390.ce. [DOI] [PubMed] [Google Scholar]
- 21.Vainshtein BK. Diffraction of X-rays by chain molecules. Elsevier; Amsterdam: 1966. [Google Scholar]
- 22.Irving TC, Millman B. Changes in thick filament structure during compression of the filament lattice in relaxed frog sartorius muscle. J. Muscle Res. Cell Motility. 1989;10:385–396. doi: 10.1007/BF01758435. [DOI] [PubMed] [Google Scholar]
- 23.Colomo F, Piroddi N, Poggesi C, te Kronnie G, Tesi C. Active and passive forces of isolated myofibrils from cardiac and fast skeletal muscle of the frog. J Physiol. 1997;500(2):535–48. doi: 10.1113/jphysiol.1997.sp022039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsubara I, Kamiyama A, Suga H. X-ray diffraction study of contracting heart muscle. J Mol Biol. 1977;111(2):121–8. doi: 10.1016/s0022-2836(77)80118-6. [DOI] [PubMed] [Google Scholar]
- 25.Matsubara I, Maughan DW, Saeki Y, Yagi N. Cross-bridge movement in rat cardiac muscle as a function of calcium concentration. J Physiol. 1989;417:555–65. doi: 10.1113/jphysiol.1989.sp017818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Squire JM, Luther PK, Knupp C. Structural evidence for the interaction of C-protein (MyBP-C) with actin and sequence identification of a possible actin-binding domain. J Mol Biol. 2003;331:713–724. doi: 10.1016/s0022-2836(03)00781-2. [DOI] [PubMed] [Google Scholar]
- 27.Squire JM, Roessle M, Knupp C. New X-ray diffraction observations on vertebrate muscle: organization of C-protein (MyBP-C) and troponin and evidence for unknown structures in the vertebrate A-band. J Mol Biol. 2004;343:1345–1363. doi: 10.1016/j.jmb.2004.08.084. [DOI] [PubMed] [Google Scholar]
- 28.Moolman-Smook J., Flashman, E., de Lange W, et al. Identification of novel interactions between domains of Myosin binding protein-C that are modulated by hypertrophic cardiomyopathy missense mutations. Circ. Res. 2002;91(8):704–711. doi: 10.1161/01.res.0000036750.81083.83. [DOI] [PubMed] [Google Scholar]
- 29.Okagaki T, Weber FE, Fischman DA, Vaughan KT, Mikawa T, Reinach FC. The major myosin-binding domain of skeletal muscle MyBP-C (C protein) resides in the COOH-terminal, immunoglobulin C2 motif. J. Cell Biol. 1993;123:619–626. doi: 10.1083/jcb.123.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flashman E, Redwood C, Moolman-Smook J, Watkins H. Cardiac myosin binding protein C: its role in physiology and disease. Circ. Res. 2004;94:1279–1289. doi: 10.1161/01.RES.0000127175.21818.C2. [DOI] [PubMed] [Google Scholar]
- 31.Weisberg A, Winegrad S. Alteration of myosin cross bridges by phosphorylation of myosin-binding protein C in cardiac muscle. Proc. Natl. Acad. Sci. 1996;93:8999–9003. doi: 10.1073/pnas.93.17.8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stelzer JE, Patel JR, Moss RL. Protein Kinase A-Mediated Acceleration of the Stretch Activation Response in Murine Skinned Myocardium Is Eliminated by Ablation of cMyBP-C. Circ. Res. 2006 Sep 14; doi: 10.1161/01.RES.0000245191.34690.66. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Fabiato A. Computer programs for calculating total from specified free and free from specified total ionic concentrations in aqueous solutions containing multiple metals or ligands. Meth. Enzymol. 1998;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- 34.Godt RE, Lindley BD. Influence of temperature upon contractile activation and isometric force production in mechanically skinned muscle fibers of the frog. J. Gen. Physiol. 1982;80:279–297. doi: 10.1085/jgp.80.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farman GP, Walker JS, de Tombe PP, Irving TC. Impact of osmotic compression on sarcomer structure and myofilament calcium sensitivity of isolated rat myocardium. Am. J. Phyiol. Heart Circ. Physiol. 2006;291:H1847–55. doi: 10.1152/ajpheart.01237.2005. [DOI] [PubMed] [Google Scholar]
- 36.Fan DS, Wannenburg T, de Tombe PP. Decreased myocyte tension development and calcium responsiveness in rat right ventricular pressure overload. Circulation. 1997;95:2312–2317. doi: 10.1161/01.cir.95.9.2312. [DOI] [PubMed] [Google Scholar]
- 37.Irving TC, Konhilas J, Perry D, Fischetti R, de Tombe PP. Lattice spacings in skinned rat trabeculae as a function of sarcomere length in rat myocardium. Am. J. Physiol. 2000;279:H2568–H2573. doi: 10.1152/ajpheart.2000.279.5.H2568. [DOI] [PubMed] [Google Scholar]
- 38.Irving TC, Fischetti R, Rosenbaum G, Bunker GB. Fiber diffraction using the BioCAT undulator beamline at the Advanced Photon Source. Nuclear Instr. Methods. 2000;448:250–254. [Google Scholar]
- 39.Sweitzer NK, Moss RL. Determinants of loaded shortening velocity in single cardiac myocytes permeabilized with alpha-hemolysin. Circ. Res. 1993;73:1150–1162. doi: 10.1161/01.res.73.6.1150. [DOI] [PubMed] [Google Scholar]