Abstract
Explicit cues associated with food consumption when hunger prevails will enhance eating when they are subsequently presented under conditions of satiety. Here we examined whether contextual conditioned stimuli (CSs) paired with consumption of food pellets while rats were food-deprived would enhance consumption of this food in rats that were not food-deprived. The conditioning context enhanced rats' consumption of the training food, but it did not change their consumption of the familiar, lab chow. These results show that the contextual CSs, like discrete cues, could modulate food consumption in a CS-potentiated eating paradigm. Furthermore, the data suggest that CS-potentiation of eating does not induce a general motivation to eat, akin to hunger, but instead more likely produces a more specific motivational state, akin to craving.
Keywords: appetite, conditioning, context, craving, eating, environment, obesity, overeating
Eating is controlled not only by metabolic signals but also by a number of cues that are not related to energy balance [1]. For example, an auditory or visual conditioned stimulus (CS) that is paired with a food unconditioned stimulus (US) when rats are food-deprived will stimulate eating when they are food-sated [2]. A number of recent studies identified components of brain circuitry critical to the occurrence of this cue-potentiated eating [3-7]. However, those studies used a single training protocol, and only evaluated consumption of the food used as the US. In the experiment reported here, we extended the cue-potentiated eating procedure by using contextual cues to signal food availability, and by testing the ability of those cues to potentiate consumption of a familiar food other than the training food. This test procedure allowed us to determine if food-related cues enhance eating by inducing general motivation to consume any food, akin to hunger, or by stimulating consumption of the training food specifically, akin to appetite or craving. We first trained food-deprived rats to consume food pellets in the conditioning context. Rats in a control group were exposed to that context, but received the food pellets in their home cages. All rats were then sated, by ad libitum access to lab chow, and food consumption was tested in the conditioning context. The rats that were previously fed food pellets in the conditioning context when hungry consumed more of those same food pellets during tests compared to the control rats that were never fed in that context. By contrast, when presented with familiar, lab chow, in the conditioning context, all rats consumed similar, small amounts. Thus, our results suggest the mechanism that mediates cue-enhanced eating does not involve induction of general motivation to eat, but rather the selective enhancement of consumption of the food US.
Method
Subjects
The subjects were 24 male Long Evans rats (Charles River Laboratory, Raleigh, NC). After arrival to the laboratory vivarium rats were acclimated for 1 week with ad libitum access to food and water before behavioral experiments began. Throughout the study rats were housed in individual cages with 12hr light/dark cycles (lights on at 7am).
Apparatus
The behavioral training apparatus consisted of four identical, individual chambers (30 × 24 × 30 cm; Colbourn Instruments, Allentown, PA), with aluminum top and sides, and a transparent Plexiglas back and front. Dark blue Plexiglas was placed on top of the grid floor so rats could not see, or feel the grids. The chamber contained a recessed food cup (3.2 × 4.2 cm), and an opaque glass food bowl, 9 cm in diameter and 7 cm high, which was located at the opposite side of the chamber from the food cup. Dim background illumination was provided by two 25 W red bulbs, each placed 1.5 m from the chambers. Masking noise (60 dB) was provided by ventilation fans located outside the conditioning chambers. The experimental chambers were wiped with 1% acetic acid solution before each animal was placed in for training or testing sessions. Video cameras attached to videocassette recorders were placed in the back of the test chambers to record behavior during training and testing.
Behavioral training procedure
Rats were trained in a conditioned potentiation paradigm in which a behavioral chamber served as a contextual cue, and was paired with food consumption under food-deprivation. The training consisted of eight training sessions. Each training session started at 9am (except session 5, which started at 8:30), and the training was conducted over a 3-hour period, plus additional time lapsed before lab chow was given back. Rats were run in groups of 4 in a counterbalanced order starting with rats in the unpaired group. All rats were food deprived for 20 hours prior to each training session. For each training session rats were transported in their home cages from the animal colony room to the behavioral testing room that contained four identical behavioral chambers (conditioning context). Rats in the paired group were placed in the conditioning context with 7g of training food pellets (formula 5TUL; Test Diets, Richmond, Indiana) in the food cup. After 10 min rats were taken out of the conditioning context, placed into the home cages and transported back to the colony room. Rats in the unpaired group were placed in the same context without food for 10 min, and then taken back to the animal colony room. After at least 30 min had passed from the training, rats in the unpaired group were given the training pellets in their home cages in the animal colony room. The amount of the training pellets given to the rats in the unpaired group was matched to the average amount eaten by the rats in the paired group in the conditioning context. After consumption of the training pellets by the rats in the unpaired group, all rats were given lab chow (18%Protein Rodent Diet; Harlan Teklad Global Diets #2018; Madison, Wisconsin) ad libitum for at least 24 hours before they were deprived for the next training session. The rats in paired and unpaired groups, were matched for the time of the day they were trained, as well as the amount of time that lapsed between the training and the time lab chow was returned to the home cages. The amount of lab chow consumed in the first hour after rats' return to the cages was recorded. Two rats from the paired group failed to increase consumption of the training pellets in the conditioning context during the first half of the training, and were removed from the study.
After training sessions 6 and 8, all rat rats received food consumption tests in the experimental context after 3 days of ad libitum access to lab chow. All food consumption tests started at 10am, and rats were tested in the same order as during training. For each of these tests, rats were placed in the conditioning context for 10 min with 15g of the training pellets in the food bowl, which had been empty during training sessions. We tested food consumption in a receptacle and location other than that to which food had been delivered in training to minimize possible enhancement of food consumption due to conditioned food cup approach behavior, and to instead reveal food consumption due to conditioned motivational properties acquired by the context as a whole.
From the results of these tests, it was clear that a 10-min testing interval was insufficient to reveal differences in food consumption between the two groups. Thus, the rats then received a final set of three food consumption tests that were 20 min in duration, and which provided the primary data of this experiment. The tests occurred on the 5th, 7th and 12th day, after the training, while rats were maintained on ad libitum access to lab chow. For each of those tests, rats were placed in the conditioning context with 15g of food in the food bowl. After 10 min, the rats were taken out of the conditioning context for a brief period of time during which any remaining food was removed quickly (to be weighed later), and replaced with an additional pre-measured 15g of food. Then the rats were placed back into the conditioning context for an additional 10 min. On the first and third of these tests, the rats were tested for consumption of the training pellets. On the second test, consumption of a non-training, but familiar food (the lab chow) was assessed. Immediately after each test, the rats were returned to the animal colony and given access to food chow and water ad libitum. The amount of food chow consumption in the home cages in the first hour after each test was recorded.
Behavioral observations
On the last three training sessions all rats were placed into the conditioning chamber without food for 2 min immediately prior to the training session to allow measurement of conditioned responses. Observations were made from the videotapes by experimenters who were “blind” to group assignments. The observations were paced by auditory signals produced by metronome at 1.25 sec intervals. At each observation, only one behavior was recorded. The primary measure of conditioning [conditioned responses (CRs)] was the percentage of time the rats spent expressing food cup behavior during first 10 sec. Food cup behavior consists of nose pokes into the recessed food cup, standing motionless in front of the food cup, or short, rapid, horizontal, or vertical head jerks in the vicinity of the food cup.
Statistics
Behavioral data were analyzed using ANOVA, followed by post hoc tests when appropriate. In all cases, p < 0.05 was considered significant.
Results
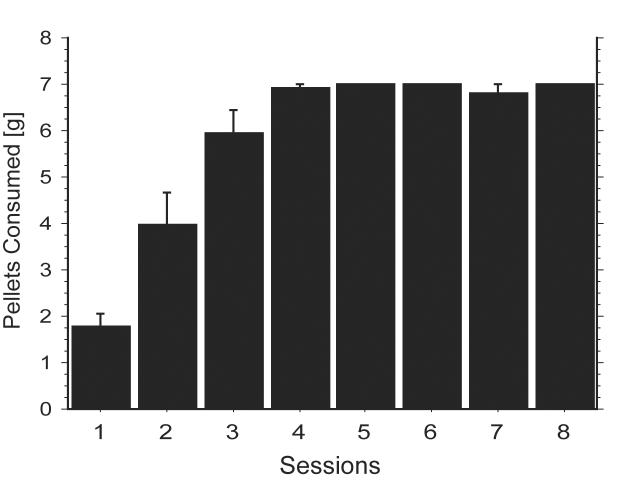
Food pellet consumption of the rats in the paired group increased across the training sessions (Figure 1). The rats in the unpaired group consumed all of the food pellets available in the home cage on each trial. There was no difference in lab chow consumption in the home cages or body weight between rats in the paired and rats in the unpaired groups during training (Table 1). On the last day of training, rats in the paired group spent significantly more time in the food cup during the initial test period before food was placed in the chamber than rats in the unpaired group (paired: 14±3%; unpaired: 4±2%; F(1,20) = 6.703, p < 0.02).
Figure 1.
Food pellets consumption during training. The amount of training pellets consumed (mean ± S.E.M. g) by rats in the paired group during 10 min in the conditioning context is shown for each training session. Rats in the unpaired group were fed (and consumed) the same amount of the training pellets in the home cages.
Table 1.
Body weight and food chow consumption during training
| Paired | Unpaired | |
|---|---|---|
| Body weight (g) | ||
| At start of training | 268±4 | 265±3 |
| At end of training | 360±6 | 351±6 |
| At start of testing | 384±6 | 369±7 |
| At end of testing | 402±8 | 385±8 |
| Food chow consumption (g) | ||
| Post-session 1 | 4.0±1.3 | 5.1±0.8 |
| Post-session 2 | 4.9±0.6 | 4.6±0.5 |
| Post-session 3 | 5.4±0.7 | 4.8±0.5 |
| Post-session 4 | 3.9±0.9 | 4.5±0.8 |
| Post-session 5 | 5.6±0.4 | 5.1±0.5 |
| Post-session 6 | 5.6±0.7 | 4.9±0.4 |
| Post-session 7 | 8.3±1.4 | 7.9±1.0 |
| Post-session 8 | 5.0±0.5 | 5.5±0.6 |
There was no difference in body weight (mean±S.E.M. g) during training and testing between rats in the paired and unpaired groups. Food chow consumption in home cages after each training sessions was similar for rats in paired and unpaired groups.
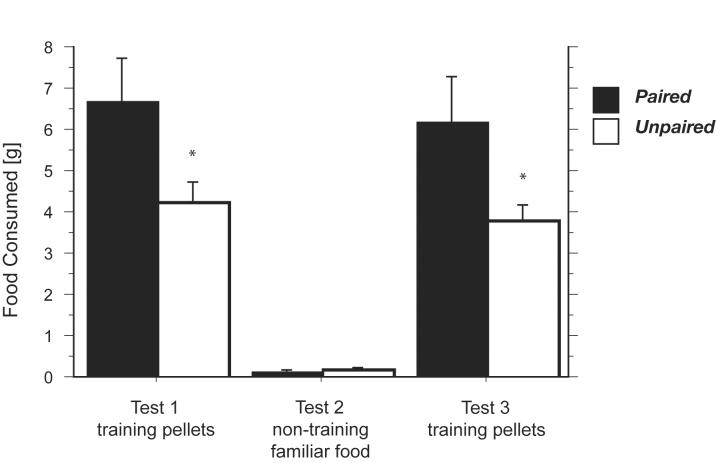
The primary data of this experiment are the results of the final three consumption tests (Figure 2). In each of 3 test sessions, rats were presented with a food substance in the bowls (which had been empty in training). In the first test, rats were presented with the training food pellets. Rats that were previously fed these pellets in the conditioning context under food-deprivation (paired group) consumed significantly more food pellets than the rats that were never fed in the context (the unpaired group) [F(1,20) = 5.013, p < 0.04]). This finding is consistent with previous studies that showed CS-potentiated food consumption with explicit CSs [2-7]. In the second test, rats were presented with a familiar food different from the training pellets, standard lab chow. In contrast to the first test, both groups of rats consumed similar, small, amounts of this familiar food [F(1,20) = 0.114, p = 0.739]. Then, we re-tested rats with the training pellets in the third test to determine whether the lack of potentiation observed in Tests 2 was due to specificity of the potentiation observed in Test 1 or simply extinction of that potentiation. In that test, the pattern of food consumption was very similar to the pattern in the first test. Rats in the paired group consumed significantly more training food pellets compared to the amounts consumed by the rats in the unpaired group [F(1,20) = 4.671, p < 0.05]. These results show that the conditioning context potentiated eating of the training pellets, but did not influence consumption of another, familiar food under the same circumstances. These results suggest that cue-driven enhancement of food consumption is selective and specific to the food US.
Figure 2.
Food consumption (mean ± S.E.M. g) in the conditioning context during 20-min tests. An asterisk indicates significance in consumption between rats in the paired and rats in the unpaired groups (p < 0.05).
We also measured food chow consumption in the home cages in the first hour immediately after these tests and found no difference between the amounts consumed by rats in the paired and unpaired groups (Table 2).
Table 2.
Food chow consumption after tests
| Paired | Unpaired | |
|---|---|---|
| Food chow consumption (g) | ||
| After test 1 | 0.26±0.19 | 0.47±0.19 |
| After test 2 | 1.58±0.43 | 1.75±0.36 |
| After test 3 | 0.18±0.04 | 0.48±0.22 |
Consumption of food chow (mean±S.E.M. g) in the home cages in the first hour after the tests in the conditioning context.
The cue-potentiated eating effect was more consistent during the second 10-min period of the consumption tests than during the first 10-min period (Table 3). Rats in both the paired and unpaired groups ate large amounts of the training pellets in the first 10 min of the tests, both in the final 20-min tests and in the initial 10-min tests that were interspersed with training sessions (Table 3). The amounts consumed in the first 10-min period did not differ significantly between groups in any of the test sessions. By contrast, for the 20-min test sessions, pellet consumption was significantly greater in the paired rats than in the unpaired rats, both over the entire 20-min test interval (described previously) and in the second 10-min test period alone (Test 1: F(1,20) = 7.564, p < 0.02; Test 3: F(1,20) = 5.816 , p < 0.03). This finding suggests that the exposure to the conditioning context might have a prolonged enhancing effect on food consumption [8].
Table 3.
Consumption during tests with training food
| Paired | Unpaired | |
|---|---|---|
| Food pellets consumption (g) | ||
| Test a | 4.5±0.9 | 3.8±0.6 |
| Test b | 4.1±1.1 | 3.1±0.4 |
| Test 1 (min 1–10) | 4.9±0.9 | 3.7±0.5 |
| Test 3 (min 1–10) | 4.7±1.0 | 3.1±0.4 |
| Test 1 (min 11–20)* | 1.8±0.4 | 0.6±0.2 |
| Test 3 (min 11–20)* | 1.5±0.3 | 0.7±0.2 |
Consumption of training food pellets (mean±S.E.M. g) in the conditioning context during consumption tests. Tests a and b were 10 min in duration and administered after training sessions 6 and 8, respectively. Consumption in Tests 1 and 3, which were 20-min in duration and were administered 5 and 12 days after training, respectively, is shown separately for the first and second 10-min periods. An asterisk indicates statistical significance of the difference between paired and unpaired rats (p<0.03).
Discussion
There are two main findings in this study. First, we showed that contextual cues could serve as conditioning cues to powerfully stimulate eating in a cue-potentiated eating paradigm. As in previous studies with discrete CSs, the enhancement of food consumption observed in the paired rats in this experiment was likely due to associative learning, rather than some non-specific effects of exposure to the context or food pellets. Rats in the unpaired control group had similar exposure to both the conditioned context and pellets, but those exposures were not paired. Moreover, because the testing was conducted at a time of day more comparable to the time when the unpaired rats had received food pellets during training, the greater consumption in the paired rats is not easily attributable to circadian entrainment of feeding. Nor was the learned context-induced enhancement of eating simply a consequence of animals' learning to approach the food cup, because during food consumption tests the foods were presented in a food receptacle that was different in appearance and location from the food cup used in training. Thus, cue-driven enhancement of food consumption is a consequence of motivational properties acquired through Pavlovian conditioning [4].
Although their test consumption of food pellets was markedly less than that of the paired rats, compared to rats in our previous studies of potentiated feeding with discrete auditory cues for food, the unpaired rats ate surprisingly large amounts of pellets in the test session, despite their free access to lab chow in their home cages. This moderately high level of consumption might reflect a number of influences independent of context-food pairings, including for example, circadian effects (mentioned earlier), the relatively high palatability of the food pellets overcoming the satiety produced by free access to lab chow in the home cage, and learning effects produced by simple exposure to the food pellets while food-deprived in training. Sensory properties of the pellets could have become learned cues for positive post-ingestive consequences during the course of the training when rats consumed these pellets in their home cages. Thus, the consumption of the rats in the unpaired group might reflect a CS-potentiation of eating, solely depending on sensory characteristics of the food pellets themselves. Nevertheless, as shown in Figure 2, in Group paired the conditioning context enhanced food consumption beyond the consumption driven by any of these other factors in Group unpaired.
Second, we demonstrated that cue-enhanced eating is relatively specific to the food US. Sated rats showed enhanced food consumption in the conditioning context only when presented with the training pellets, but not when presented with another, familiar food. This finding suggests that the mechanism by which the CS enhances eating does not involve solely the induction of general motivation to eat, akin to hunger. Instead, the cue-induced conditioned motivation to eat was highly specific to the food US, perhaps similar to appetite or craving.
However, other factors may also have contributed to this specificity. First, the low levels of consumption of the familiar lab chow in testing might also reflect inhibition of eating due to sensory-specific satiety [9, 10]. Rats were allowed ad libitum access to lab chow in their home cages prior to all tests, which in turn could have masked a possible contextual-driven potentiation in tests with lab chow. This possibility is consistent with the low levels of lab chow consumption observed in both paired and unpaired rats. However, in our previous studies we showed that 1-hour ad libitum access to the training pellets does not eliminate CS-driven potentiation of eating [e.g., 5], although the overall eating baseline was much lower in those studies than in the current experiment. Furthermore, in unpublished experiments similar to the current study, we found that rats in paired and unpaired group also consumed similar, small amounts of another food that differed from both the training and home-cage foods. Thus, although sensory-specific satiety may have contributed to the overall low consumption of lab chow in consumption tests by all rats, it is unlikely that it selectively masked context-driven enhancement of consumption of the rats in the paired group.
Second, it might be argued that the selective cue-driven enhancement of consumption observed here reflects an inhibition of the consumption of the alternate food due to novelty or surprise. Although the lab chow was highly familiar to the rats, its presence in the conditioning context (in a single test) could be considered a novel or surprising event. If the consumption of food chow was indeed modulated by surprise/novelty one would expect differential consumption by rats in the paired and unpaired groups due to their different acquired expectancies. During the training rats in the paired group were fed training pellets, while rats in the unpaired groups were never fed any food in the conditioning context. However, rats in the paired and unpaired groups consumed similar amounts of lab chow during the test in the conditioning context. These results suggest that the consumption of the familiar food during tests in the conditioning context reflected mainly the rats' deprivation state, rather than some combination of cue- and surprise-induced processes.
The current study is the first to use a context as a cue in a cue-potentiated eating paradigm. It is valuable to contrast the present results with the findings of another study that also employed contextual cues and learning to stimulate food consumption in sated rats. Roitman et al [11] removed sated rats from their home cages and placed them in one of two distinctive contexts for 12-hour trial periods. In one context, food was freely available and in the other, no food was available. After five trials of each type, food was placed in both contexts, and consumption was evaluated in either the context in which the rats had previously received food or the context in which they had not received food. Rats consumed significantly more food if they were tested in the context in which they had previously not received food than if they were tested in the previously food-paired context. Roitman et al [11] attributed this outcome to enhancement of food consumption by surprise, the violation of a no-food expectancy.
At first glance, this result seems opposed to ours: we found rats ate more in the conditioning context if it had been paired with food than if it had been associated with the absence of food. However, it is notable that in our study, food-deprived rats were exposed to the conditioning context for brief periods (10 min) in training, whereas in theirs, food-sated rats were exposed to the experimental contexts for long periods (12 hr) in training. Thus, in our study, for the paired rats, the conditioning context was associated with the consumption of relatively large amounts of food amounts in brief time periods, and a reduction in the food deprivation state, and for the unpaired rats, the context was not associated with either of these consequences. By contrast, in Roitman et al's study [11], the food-paired context was likely associated with maintenance of food satiety and slow rates of consumption over long time periods, and the no-food context with substantial increases in food deprivation. Thus, it is possible that these food-paired and food-unpaired contexts influenced eating in the consumption tests in part by inducing conditioned states of satiety and hunger, respectively [12-15]. It is interesting to speculate that eating stimulated by violations of food expectancy and/or conditioned motivational states in procedures like Roitman et al's [11] might involve induction of a general motivational state, similar to hunger, whereas the cue-enhanced consumption we observed is specific to the conditioning food, and involves a conditioned specific appetite or craving.
Similarly, cue-induced conditioned motivation and the consequent enhancement of eating might involve brain circuitry distinct from the circuitry recruited by more general (hunger-like) conditioned motivation. Within this context, Roitman and colleagues showed that dopamine plays a critical role in food consumption stimulated by an unexpected cue [11], in agreement with previous evidence for the role of dopamine in signaling errors in reward prediction [16]. Likewise, numerous studies link dopaminergic brain systems, including the nucleus accumbens (ACB), to motivation and food reward [ref reviews see 17-21]. Currently there is no evidence for whether dopamine is critical in cue-potentiated feeding. Interestingly, the ACB does not seem to be recruited by the circuitry that mediates cue-potentiated feeding [22, 23]. On the other hand the BLA-lateral hypothalamic (LHA) system, which is critical for cue-induced consumption [5] is also needed in ACB-dependent, μ-opiod induced consumption of fat [24]. Thus, distinct sub-circuitries might be recruited within a larger common system, depending on whether processes that underlie general motivation to eat, motivation for highly palatable foods, or selective cue-driven consumption are activated. Clearly more work is needed to further elucidate the exact circuitry and mechanisms that mediate control of food consumption by different aspects of learning.
The current study provides evidence that mechanisms by which Pavlovian CSs stimulate eating might involve specific motivational states, similar to craving. Unfortunately, there is no consensus definition of food craving, especially in animal models [25]. Nevertheless, in addition to the selectivity of cue-enhanced eating, there are other parallels between cue-induced consumption and food craving. For example, craving for food can be elicited by exposure to food cues [25, 26], and cue-elicited craving is associated with binge eating [for reviews see 27, 28]. Indeed, in our preparation, cue-induced enhancement of eating could be considered binging—sated rats consume large amounts of food pellets in a very short period of time. Most notably, a recent human study showed that in restrained eaters food cues elicited specific appetite/craving for the cued food, rather than general desire to eat any food [29]. And importantly, restrained eaters' craving for the cued food was correlated with intake, as they consumed more of the cued food [29].
There is also commonality in the brain systems that mediate cue-enhanced feeding and brain areas activated in humans by cues for preferred and/or craved food. Most notably, the amygdala, which is critical for cue-enhanced eating, is also activated in sated humans while viewing names of preferred versus neutral foods [30], and while thinking of the sensory properties of food that is liked or craved [31]. Finally, parallels have recently been drawn between brain systems and mechanisms that mediate food reward learning and those that mediate drug addiction [32-34]. Contextual cues, which profoundly stimulated eating in the present study, are also very powerful cues in drug addiction. Contextual cues associated with drug exposure play critical roles in drug craving and relapse [35, 36].
Future studies are needed to answer a number of questions in regard to the mechanisms by which food cues stimulate eating in sated states. For example, cues might retrieve the affective component (emotional and/or visceral) that the animals experienced previously when they alleviated hunger with the same food in the conditioning context, or they might retrieve and enhance the sensory properties of the food, or they might enhance arousal and the speed by which food is consumed.
In summary, the present study shows that the environment in which food was consumed under food-deprivation can modulate subsequent feeding under sated conditions. After a few pairings with food consumption the feeding environment acquired conditioned motivational properties that allowed it to stimulate eating in a highly selective and specific manner. The important role of feeding environment in control of food intake shown here in an animal model might be relevant to human eating. Indeed, the environment in which food is consumed has been changing over the last 30 years in the USA. Increasingly larger proportions of the total daily intake are consumed in distinct environments such as restaurants and fast food places [37]. Thus, it is enticing to extrapolate the current findings from an animal model, and suggest that such distinct environments could acquire properties, through simple pairings with food consumption, that could allow them to control subsequent intake, similar to what we observed here in an animal model. This might be particularly relevant in the case of fast food and other chain restaurants that are designed to look uniform, and provide limited menu choices, and as such provide a good opportunity for specific food-context pairings. Nevertheless, future studies will determine whether cue-driven enhancement of food consumption that could lead to overeating, is indeed similar to craving, and whether this model is applicable to human eating.
Footnotes
Supported by National Institute of Mental Health Grants MH67252 (G.D.P.) and MH60179 (M.G.,P.C.H.).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Woods SC. Signals that influence food intake and body weight. Physiol Behav. 2005;86:709–16. doi: 10.1016/j.physbeh.2005.08.060. [DOI] [PubMed] [Google Scholar]
- 2.Weingarten HP. Conditioned cues elicit feeding in sated rats: A role for learning in meal initiation. Science. 1983;220:431–433. doi: 10.1126/science.6836286. [DOI] [PubMed] [Google Scholar]
- 3.Holland PC, Hatfield T, Gallagher M. Rats with basolateral amygdala lesions show normal increases in conditioned stimulus processing but reduced conditioned potentiation of eating. Behav Neurosci. 2001;115:945–950. [PubMed] [Google Scholar]
- 4.Holland PC, Petrovich GD, Gallagher M. The effects of amygdala lesions on conditioned stimulus-potentiated eating in rats. Physiol Behav. 2002;76:117–29. doi: 10.1016/s0031-9384(02)00688-1. [DOI] [PubMed] [Google Scholar]
- 5.Petrovich GD, Setlow B, Holland PC, Gallagher M. Amygdalo-hypothalamic circuit allows learned cues to override satiety and promote eating. J Neurosci. 2002;22:8748–8753. doi: 10.1523/JNEUROSCI.22-19-08748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holland PC, Gallagher M. Double dissociation of the effects of lesions of basolateral and central amygdala on CS-potentiated feeding and Pavlovian-instrumental transfer. E J Neurosci. 2003;17:1680–1694. doi: 10.1046/j.1460-9568.2003.02585.x. [DOI] [PubMed] [Google Scholar]
- 7.McDannald MA, Saddoris MP, Gallagher M, Holland PC. Lesions of orbitofrontal cortex impair rats' differential outcome expectancy learning but not CSpotentiated feeding. J Neurosci. 2005;25:4626–2632. doi: 10.1523/JNEUROSCI.5301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zamble E. Augmentation of eating following a signal for feeding in rats. Learn Motiv. 1973;4:138–147. [Google Scholar]
- 9.Rolls BJ, Rolls ET, Rowe EA, Sweeney K. Sensory specific satiety in man. Physiol Behav. 1981;27:137–42. doi: 10.1016/0031-9384(81)90310-3. [DOI] [PubMed] [Google Scholar]
- 10.Ahn S, Phillips AG. Dopaminergic correlates of sensory-specific satiety in the medial prefrontal cortex and nucleus accumbens of the rat. J Neurosci. 1999;19:RC29. doi: 10.1523/JNEUROSCI.19-19-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roitman MF, van Dijk G, Thiele TE, Bernstein IL. Dopamine mediation of the feeding response to violations of spatial and temporal expectancies. Behav Brain Res. 2001;122:193–9. doi: 10.1016/s0166-4328(01)00189-9. [DOI] [PubMed] [Google Scholar]
- 12.Booth DA. Food-conditioned eating preferences and aversions with interoceptive elements: conditioned appetites and satieties. Ann N Y Acad Sci. 1985;443:22–41. doi: 10.1111/j.1749-6632.1985.tb27061.x. [DOI] [PubMed] [Google Scholar]
- 13.Hull CL. 1943 Principles of Behavior. Appleton-century-crofts; New York: 1943. [Google Scholar]
- 14.Konorski J. Integrative activity of the brain. University of Chicago Press; Chicago: 1967. [Google Scholar]
- 15.Mineka S. Some new perspectives on conditioned hunger. J Exp Psychol Anim Behav Process. 1975;12:134–48. doi: 10.1037//0097-7403.1.2.134. [DOI] [PubMed] [Google Scholar]
- 16.Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 18.Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 19.Phillips AG, Ahn S, Howland JG. Amygdalar control of the mesocorticolimbic dopamine system: parallel pathways to motivated behavior. Neurosci Biobehav Rev. 2003;27:543–554. doi: 10.1016/j.neubiorev.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Kelley AE. Ventral striatal control of appetitive motivation: role of ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–94. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 22.Petrovich GD, Holland PC, Gallagher M. Amygdalar and prefrontal pathways to the lateral hypothalamus are activated by a learned cue that stimulates eating. J Neurosci. 2005;25:8295–302. doi: 10.1523/JNEUROSCI.2480-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holland PC, Petrovich GD. A neural systems analysis of the potentiation of feeding by conditioned stimuli. Physiol Behav. 2005;86:747–61. doi: 10.1016/j.physbeh.2005.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Will MJ, Kelley AE. Intra – accumbens μ-opioid - induced fat intake depends on activation of a basolateral amygdala - lateral hypothalamic pathway. Soc Neurosci. 2005;31:532.21. (Abst.) [Google Scholar]
- 25.Weingarten HP, Elston D. The phenomenology of food cravings. Appetite. 1990;15:231–46. doi: 10.1016/0195-6663(90)90023-2. [DOI] [PubMed] [Google Scholar]
- 26.Tiggemann M, Kemps E. The phenomenology of food cravings: the role of mental imagery. Appetite. 2005;45:305–13. doi: 10.1016/j.appet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Jansen A. A learning model of binge eating: cue reactivity and cue exposure. Behav Res Ther. 1998;36:257–72. doi: 10.1016/s0005-7967(98)00055-2. [DOI] [PubMed] [Google Scholar]
- 28.Sobik L, Hutchison K, Craighead L. Cue-elicited craving for food: a fresh approach to the study of binge eating. Appetite. 2005;44:253–61. doi: 10.1016/j.appet.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Fedoroff I, Polivy J, Herman CP. The specificity of restrained versus unrestrained eaters' responses to food cues: general desire to eat, or craving for the cued food? Appetite. 2003;41:7–13. doi: 10.1016/s0195-6663(03)00026-6. [DOI] [PubMed] [Google Scholar]
- 30.Arana FS, Parkinson JA, Hinton E, Holland AJ, Owen AM, Roberts AC. Dissociable contributions of the human amygdala and orbitofrontal cortex to incentive motivation and goal selection. J Neurosci. 2003;23:9632–8. doi: 10.1523/JNEUROSCI.23-29-09632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. Images of desire: food-craving activation during fMRI. Neuroimage. 2004;23:1486–93. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 32.Cardinal RN, Everitt BJ. Neural and psychological mechanisms underlying appetitive learning: links to drug addiction. Curr Opin Neurobiol. 2004;14:156–62. doi: 10.1016/j.conb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–79. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 34.Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- 35.Childress AR, Ehrman R, Rohsenow DJ, Robbins SJ, O'Brien CP. Classically conditioned factors in drug dependence. In: Lowinson JW, Luiz P, Millman RB, Langard G, editors. Substance abuse: A comprehensive textbook. 2nd ed. Williams & Wilkins; Baltimore: 1992. pp. 56–69. [Google Scholar]
- 36.Crombag HS, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci. 2002;116:169–73. doi: 10.1037//0735-7044.116.1.169. [DOI] [PubMed] [Google Scholar]
- 37.Nielsen SJ, Siega-Riz AM, Popkin BM. Trends in food locations and sources among adolescents and young adults. Prev Med. 2002;35:107–13. doi: 10.1006/pmed.2002.1037. [DOI] [PubMed] [Google Scholar]