Abstract
Objective—To investigate whether localised skeletal muscle training, which does not have a great influence on the heart, improves abnormalities of calf muscle metabolism in patients with chronic heart failure. Methods—Seven cardiac patients in New York Heart Association class II and III undertook a random order crossover trial. Training consisted of unilateral calf plantar flexion exercise. Before and after training, the patients' metabolic responses were examined during the calf exercise test with phosphorus-31 nuclear magnetic resonance spectroscopy (31P-MRS) and calf blood flow with plethysmography. The new Borg scale was employed as a subjective fatigue scale. Results—In a constant load exercise test (70% of maximum load achieved during the incremental exercise), standardised phosphocreatine and intracellular pH decreased less after training (p < 0.05, repeated measures analysis of variance). The new Borg scale improved significantly after training (p < 0.05). Blood flow did not change significantly in either test. Conclusions—In patients with chronic heart failure, localised calf skeletal muscle training improved oxidative capacity without changes in calf blood flow. This training also improved the subjective fatigue scale. This training method may therefore alleviate leg fatigue experienced in daily activities. Keywords: heart failure; magnetic resonance spectroscopy; skeletal muscle; localised training
Full Text
The Full Text of this article is available as a PDF (147.6 KB).
Figure 1 .
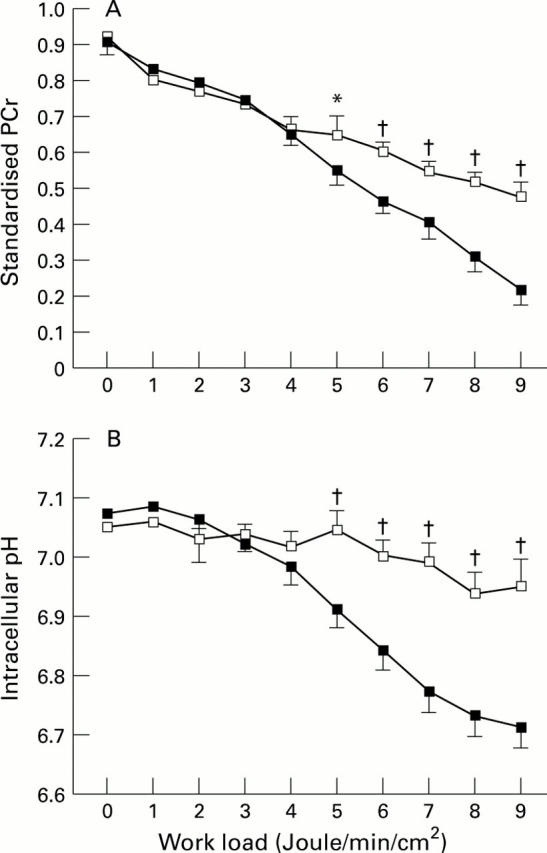
Comparison of standardised phosphocreatine (PCr) utilisation (A) and intracellular pH (B) in incremental exercise between patients with chronic heart failure and normal control subjects. There was no significant difference between the two groups in either standardised PCr or intracellular pH by repeated measures analysis of variance (ANOVA). Data on exercise ranging from 0 to 9 J/min/cm2 are presented. Since all patients could complete at least 6 J/min/cm2, data for 0 to 6 J/min/cm2 were used to compare these two groups by repeated measures ANOVA. When matched points were compared, both standardised PCr values and intracellular pH were significantly different at 5 to 9 J/min/cm2. *p < 0.05, †p < 0.01, compared by unpaired t test. Empty squares, normal control subjects; filled squares, patients with chronic heart failure.
Figure 2 .

Comparison of standardised phosphocreatine (PCr) utilisation (A) and intracellular pH (B) in constant load exercise test between patients with chronic heart failure and normal control subjects. Patients showed more depletion than normal control subjects by repeated measures analysis of variance (ANOVA). Comparing matched points, standardised PCr values were significantly different at 2 to 6 minutes, and intracellular pH at 1 to 6 minutes. ‡p = 0.01, ¶p < 0.01 by repeated measures ANOVA; *p < 0.05, †p < 0.01, compared by unpaired t test. Empty squares, normal control subjects; filled squares, patients with chronic heart failure.
Figure 3 .

Effects of localised training on standardised phosphocreatine (PCr) utilisation (A) and intracellular pH (B) in constant load exercise test. There was less standardised PCr depletion as well as less intracellular pH reduction after training. ‡p < 0.05, compared by repeated measures analysis of variance. *p < 0.05, †p < 0.01, comparing matched points by paired t tests. Empty circles, training phase; filled circles, detraining phase.
Figure 4 .
Effects of localised training on calf blood flow in constant load exercise test. There was no difference between the training phase and the detraining phase by repeated measures analysis of variance (p = NS). Comparing matched points, there were no significant changes. Empty circles, training phase; filled circles, detraining phase.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamopoulos S., Coats A. J., Brunotte F., Arnolda L., Meyer T., Thompson C. H., Dunn J. F., Stratton J., Kemp G. J., Radda G. K. Physical training improves skeletal muscle metabolism in patients with chronic heart failure. J Am Coll Cardiol. 1993 Apr;21(5):1101–1106. doi: 10.1016/0735-1097(93)90231-o. [DOI] [PubMed] [Google Scholar]
- Ahlborg G., Jensen-Urstad M. Metabolism in exercising arm vs. leg muscle. Clin Physiol. 1991 Sep;11(5):459–468. doi: 10.1111/j.1475-097x.1991.tb00818.x. [DOI] [PubMed] [Google Scholar]
- Arvan S. Exercise performance of the high risk acute myocardial infarction patient after cardiac rehabilitation. Am J Cardiol. 1988 Aug 1;62(4):197–201. doi: 10.1016/0002-9149(88)90211-1. [DOI] [PubMed] [Google Scholar]
- Beaver W. L., Wasserman K., Whipp B. J. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol (1985) 1986 Jun;60(6):2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- Borg G. A. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. [PubMed] [Google Scholar]
- Coats A. J., Adamopoulos S., Meyer T. E., Conway J., Sleight P. Effects of physical training in chronic heart failure. Lancet. 1990 Jan 13;335(8681):63–66. doi: 10.1016/0140-6736(90)90536-e. [DOI] [PubMed] [Google Scholar]
- Coats A. J., Adamopoulos S., Radaelli A., McCance A., Meyer T. E., Bernardi L., Solda P. L., Davey P., Ormerod O., Forfar C. Controlled trial of physical training in chronic heart failure. Exercise performance, hemodynamics, ventilation, and autonomic function. Circulation. 1992 Jun;85(6):2119–2131. doi: 10.1161/01.cir.85.6.2119. [DOI] [PubMed] [Google Scholar]
- Costill D. L., Daniels J., Evans W., Fink W., Krahenbuhl G., Saltin B. Skeletal muscle enzymes and fiber composition in male and female track athletes. J Appl Physiol. 1976 Feb;40(2):149–154. doi: 10.1152/jappl.1976.40.2.149. [DOI] [PubMed] [Google Scholar]
- Donovan C. M., Brooks G. A. Endurance training affects lactate clearance, not lactate production. Am J Physiol. 1983 Jan;244(1):E83–E92. doi: 10.1152/ajpendo.1983.244.1.E83. [DOI] [PubMed] [Google Scholar]
- Donovan C. M., Pagliassotti M. J. Enhanced efficiency of lactate removal after endurance training. J Appl Physiol (1985) 1990 Mar;68(3):1053–1058. doi: 10.1152/jappl.1990.68.3.1053. [DOI] [PubMed] [Google Scholar]
- Edström L., Nyström B. Histochemical types and sizes of fibres in normal human muscles. A biopsy study. Acta Neurol Scand. 1969;45(3):257–269. doi: 10.1111/j.1600-0404.1969.tb01238.x. [DOI] [PubMed] [Google Scholar]
- Favier R. J., Constable S. H., Chen M., Holloszy J. O. Endurance exercise training reduces lactate production. J Appl Physiol (1985) 1986 Sep;61(3):885–889. doi: 10.1152/jappl.1986.61.3.885. [DOI] [PubMed] [Google Scholar]
- Glasheen J. W., McMahon T. A. Arms are different from legs: mechanics and energetics of human hand-running. J Appl Physiol (1985) 1995 Apr;78(4):1280–1287. doi: 10.1152/jappl.1995.78.4.1280. [DOI] [PubMed] [Google Scholar]
- Gollnick P. D., Sjödin B., Karlsson J., Jansson E., Saltin B. Human soleus muscle: a comparison of fiber composition and enzyme activities with other leg muscles. Pflugers Arch. 1974 Apr 22;348(3):247–255. doi: 10.1007/BF00587415. [DOI] [PubMed] [Google Scholar]
- Hills M., Armitage P. The two-period cross-over clinical trial. Br J Clin Pharmacol. 1979 Jul;8(1):7–20. doi: 10.1111/j.1365-2125.1979.tb05903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloszy J. O., Coyle E. F. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol Respir Environ Exerc Physiol. 1984 Apr;56(4):831–838. doi: 10.1152/jappl.1984.56.4.831. [DOI] [PubMed] [Google Scholar]
- Lenfant C. NHLBI funding policies. Enhancing stability, predictability, and cost control. Circulation. 1994 Jul;90(1):1–1. doi: 10.1161/01.cir.90.1.1. [DOI] [PubMed] [Google Scholar]
- Magnusson G., Gordon A., Kaijser L., Sylvén C., Isberg B., Karpakka J., Saltin B. High intensity knee extensor training, in patients with chronic heart failure. Major skeletal muscle improvement. Eur Heart J. 1996 Jul;17(7):1048–1055. doi: 10.1093/oxfordjournals.eurheartj.a015001. [DOI] [PubMed] [Google Scholar]
- Mancini D. M., Ferraro N., Tuchler M., Chance B., Wilson J. R. Detection of abnormal calf muscle metabolism in patients with heart failure using phosphorus-31 nuclear magnetic resonance. Am J Cardiol. 1988 Dec 1;62(17):1234–1240. doi: 10.1016/0002-9149(88)90266-4. [DOI] [PubMed] [Google Scholar]
- Massie B. M., Kramer B., Haughom F. Acute and long-term effects of vasodilator therapy on resting and exercise hemodynamics and exercise tolerance. Circulation. 1981 Dec;64(6):1218–1226. doi: 10.1161/01.cir.64.6.1218. [DOI] [PubMed] [Google Scholar]
- Massie B., Conway M., Yonge R., Frostick S., Ledingham J., Sleight P., Radda G., Rajagopalan B. Skeletal muscle metabolism in patients with congestive heart failure: relation to clinical severity and blood flow. Circulation. 1987 Nov;76(5):1009–1019. doi: 10.1161/01.cir.76.5.1009. [DOI] [PubMed] [Google Scholar]
- Minotti J. R., Johnson E. C., Hudson T. L., Zuroske G., Murata G., Fukushima E., Cagle T. G., Chick T. W., Massie B. M., Icenogle M. V. Skeletal muscle response to exercise training in congestive heart failure. J Clin Invest. 1990 Sep;86(3):751–758. doi: 10.1172/JCI114771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida M., Nishijima H., Yonezawa K., Sato I., Anzai T., Okita K., Yasuda H. Phosphorus-31 magnetic resonance spectroscopy of forearm flexor muscles in student rowers using an exercise protocol adjusted for differences in cross-sectional muscle area. Eur J Appl Physiol Occup Physiol. 1992;64(6):528–533. doi: 10.1007/BF00843763. [DOI] [PubMed] [Google Scholar]
- Pagliassotti M. J., Donovan C. M. Role of cell type in net lactate removal by skeletal muscle. Am J Physiol. 1990 Apr;258(4 Pt 1):E635–E642. doi: 10.1152/ajpendo.1990.258.4.E635. [DOI] [PubMed] [Google Scholar]
- Ploutz L. L., Tesch P. A., Biro R. L., Dudley G. A. Effect of resistance training on muscle use during exercise. J Appl Physiol (1985) 1994 Apr;76(4):1675–1681. doi: 10.1152/jappl.1994.76.4.1675. [DOI] [PubMed] [Google Scholar]
- Sharp R. L., Costill D. L., Fink W. J., King D. S. Effects of eight weeks of bicycle ergometer sprint training on human muscle buffer capacity. Int J Sports Med. 1986 Feb;7(1):13–17. doi: 10.1055/s-2008-1025727. [DOI] [PubMed] [Google Scholar]
- Stratton J. R., Dunn J. F., Adamopoulos S., Kemp G. J., Coats A. J., Rajagopalan B. Training partially reverses skeletal muscle metabolic abnormalities during exercise in heart failure. J Appl Physiol (1985) 1994 Apr;76(4):1575–1582. doi: 10.1152/jappl.1994.76.4.1575. [DOI] [PubMed] [Google Scholar]
- Sullivan M. J., Higginbotham M. B., Cobb F. R. Exercise training in patients with severe left ventricular dysfunction. Hemodynamic and metabolic effects. Circulation. 1988 Sep;78(3):506–515. doi: 10.1161/01.cir.78.3.506. [DOI] [PubMed] [Google Scholar]
- Wilson J. R., Fink L., Maris J., Ferraro N., Power-Vanwart J., Eleff S., Chance B. Evaluation of energy metabolism in skeletal muscle of patients with heart failure with gated phosphorus-31 nuclear magnetic resonance. Circulation. 1985 Jan;71(1):57–62. doi: 10.1161/01.cir.71.1.57. [DOI] [PubMed] [Google Scholar]



