Abstract
Quiescent full-grown Xenopus oocytes remain arrested at the G2/M border of meiosis I until exposed to progesterone, their natural mitogen. Progesterone triggers rapid, nontranscriptional responses that lead to the translational activation of stored mRNAs, resumption of the meiotic cell cycles, and maturation of the oocyte into a fertilizable egg. It has long been presumed that progesterone activates the oocyte through a novel nontranscriptional signaling receptor. Here, we provide evidence that a conventional transcriptional progesterone receptor cloned from Xenopus oocytes, XPR-1, is required for oocyte activation. Overexpression of XPR-1 through mRNA injection increases sensitivity to progesterone and accelerates progesterone-activated cell cycle reentry. Injection of XPR-1 antisense oligonucleotides blocks the ability of oocytes to respond to progesterone; these oocytes are rescued by subsequent injection of XPR-1 or the human progesterone receptor PR-B. Antisense-treated oocytes can be activated in response to inhibition of protein kinase A, one of the earliest known changes occurring downstream of progesterone stimulation. These results argue that the conventional progesterone receptor also functions as the signaling receptor that is responsible for the rapid nontranscriptional activation of frog oocytes.
Full-grown frog oocytes arrest at the G2/M border of meiosis I until exposed to progesterone, their physiological mitogen (reviewed in refs. 1–4). Progesterone induces oocyte maturation, initiating oocyte activation, resumption of the meiotic cell cycles, and development into the mature, fertilizable egg. In somatic cells, the best-understood effects of progesterone and other steroid hormones are their abilities to regulate transcription: they cross the plasma membrane and bind to a member of the nuclear receptor superfamily, which either resides in the nucleus or moves into the nucleus upon hormone binding, where they regulate the activity of specific target genes (reviewed in refs. 5–8). In frog oocytes, however, progesterone activates the cells through a cytoplasmic signaling pathway that does not depend on transcription (reviewed in refs. 1–4 and 9).
Rapid, nontranscriptional effects of steroids have been documented for many cell types, but, in most cases, little is known about either the physiological significance or the molecular mechanisms underlying those effects (reviewed in refs. 10 and 11). Amphibian and fish oocytes provide some of the most compelling examples known of rapid, nontranscriptional regulation by steroid hormones (reviewed in refs. 3, 4, 12, and 13). In frogs, progesterone is the mitogen that initiates oocyte maturation. In response to pituitary hormones, follicle cells surrounding the oocyte synthesize and secrete progesterone, which induces both oocyte maturation and release of the oocyte from the follicle (14–19). Oocyte activation, as monitored by activation of preexisting cytoplasmic stores of cyclin B/cdc2 (M phase promoting factor, MPF) and reentry into meiosis I, occurs even when transcription is blocked by Actinomycin D or physical enucleation (15, 18–23). Instead, progesterone rapidly activates a poorly understood cytoplasmic signaling pathway that initiates the translational activation of preexisting stored mRNAs whose products then lead to MPF activation (reviewed in refs. 3, 4, and 24).
Within minutes of progesterone addition, adenylyl cyclase activity and cAMP levels drop, both in vivo and in crude plasma membrane preparations (reviewed in ref. 25). Consistent with a role for this drop in cAMP acting through protein kinase A (PKA), injection of the PKA catalytic subunit blocks the ability of progesterone to activate oocytes, whereas injection of the inhibitory subunit induces oocyte maturation in the absence of progesterone (26–28). Progesterone also induces rapid changes in several other enzymes (reviewed in refs. 3 and 4; see also refs. 9, 29, and 30). Through an unknown pathway, this leads to activation of the kinase Eg2 (31). Eg2 then phosphorylates CPEB (32), a protein bound to the cytoplasmic polyadenylation element (CPE) found in the 3′ untranslated region of mos and several other stored mRNAs (reviewed in refs. 33 and 34). Oocytes contain little or no detectable mos protein, whose synthesis is required for oocyte activation (reviewed in refs. 35 and 36). Phosphorylation of CPEB by Eg2 induces the cytoplasmic polyadenylation of mos mRNA, which leads to its translational activation (32). Newly made mos protein, a MAP kinase kinase kinase, leads to activation of preexisting MEK and MAP kinase, and to activation of preexisting MPF, thus catalyzing entry into M phase of meiosis I (reviewed in refs. 4, 24, and 36).
In contrast to the downstream parts of the pathway, the identity of the oocyte progesterone receptor has remained elusive. Early work suggested that progesterone interacts with a receptor at or near the oocyte surface. Injection of progesterone into oocytes usually does not cause oocyte activation (1, 21, 37), whereas exposure to polymer-bound hormone can induce oocyte activation (38, 39). Because of those results, the ability of progesterone to induce oocyte activation in the absence of transcription, and progesterone's ability to induce rapid cytoplasmic signaling events leading to translational activation of mos mRNA, it has long been assumed that the oocyte progesterone receptor must be a novel, surface-associated receptor. Several candidates have been described but none has been shown to meet all of the expected criteria (reviewed in refs. 3 and 40).
More recent findings prompted us to reexamine the idea that the oocyte relies on a novel progesterone receptor. Two conventional receptors, those for estrogen and progesterone, can also rapidly activate cytoplasmic signaling in somatic cells. For example, in MCF-7 breast cancer cells that express the conventional estrogen receptor (ER), estradiol rapidly induces c-src and MAP kinase activation (41). Cos cells normally lack ER and the conventional progesterone receptor (PR), and they fail to respond to either hormone. After transfection of ER, addition of estradiol results in rapid c-src and MAP kinase activation (42). After transfection with both ER and PR, progesterone addition also activates both kinases in an ER-dependent manner (43). Additional work indicates that rapid activation of the MAP kinase pathway by estrogen may be involved in controlling cell proliferation in certain cells (44). The existence of binding sites for steroids on surface membranes of somatic cells has been reported for many cell types, but, in most cases, the nature of those receptors remains unidentified. Recent work suggests that a small amount of the conventional ER localizes at or near the surface of somatic cells (e.g., refs. 45–48). Finally, it is notable that while progesterone-induced oocyte activation does not depend on transcription, nuclear contents are essential for progression into meiosis II, and transcription during meiosis is required for eggs to develop the capacity for DNA synthesis after fertilization (22, 23). Thus, in oocytes, progesterone induces both signaling and transcriptional changes that culminate in the formation of a mature, fertilizable egg. In light of these findings, we asked whether a conventional PR might also be responsible for activating the cytoplasmic signaling pathway that results in the translational activation of mos mRNA and progression into meiosis I.
Here, we have cloned a Xenopus oocyte cDNA encoding a conventional PR, as judged by its sequence similarity to conventional PRs and by its ability to mediate progesterone-dependent transcription from a well-characterized progesterone-responsive reporter construct. Overexpression of this protein, which we term XPR-1, increases the oocyte's sensitivity to progesterone and accelerates progesterone-stimulated oocyte activation. Depletion of endogenous XPR-1 using antisense oligonucleotides blocks the oocyte's ability to respond to progesterone; subsequent injection of XPR-1 or human PR (hPR) restores progesterone responsiveness. These results argue that XPR-1 is required for oocyte activation.
Materials and Methods
Cloning XPR-1 cDNA.
Poly(A)+ mRNA from collagenase-treated oocytes (polyA pure mRNA isolation kit; Ambion, Austin, TX) was used as template to amplify the ligand binding domain (LBD) of XPR-1 by nested reverse transcription (RT)-PCR (Roche Molecular Biochemicals). The primers were derived from a partial cDNA encoding the LBD obtained by hybridization screening of a Xenopus oocyte cDNA library (gift of D. Melton, Harvard University, Cambridge, MA) by using a radiolabeled hPR-LBD cDNA probe. The outer set of primers are: xpr9s, 5′-CTGGGAGAGAAATCGATACAG-3′, and xpr12as, 5′-GCGATCTTGACTGCAGGAATG-3′; the inner set: xpr10s, 5′-CAGTTGTTCTTCAGTCGCCAC-3′, and xpr11as, 5′-CTGCAGGAATGTGTTGAGACA-3′. The remaining portions of the cDNA were amplified by 5′ and 3′ rapid amplification of cDNA ends (RACE; Roche Molecular Biochemicals and CLONTECH kits). Primers used in the two-step 5′-RACE are: step 1, xpbd3as, 5′-CTGGGCAGAGATCACTTCCGAC-3′, and xpbd2as, 5′-CATGCACAGGAACTCCTCGTGG-3′; step 2, xpr14as, 5′-GAGACGATCCGTAATCCTGACCC-3′, and xpr15as, 5′-AGACCTTTGGCCACCTTCTTCCC-3′. Gene-specific primers used in the 3′-RACE are: xpbd1s, 5′-GGGATGGAGGTCGTATCAACATG-3′, and xpbd2s, 5′-CCACGAGGAGTTCCTGTGCATG-3′. Finally, the whole coding region of XPR-1 was amplified from oocyte double-stranded cDNA by nested PCR using PfuTurbo polymerase (Stratagene) and cloned into PCRII zero blunt vector (Invitrogen). The final set of primers used are: XPR-UTR2, 5′-GTAAGAACCCCCGACACTATGGAGG-3′, and XPR-UTR3, 5′-GATGTTCACTTTTTGTGAAACACAAGTGG-3′. The cloned XPR-1 cDNA was sequenced in both directions by using an automatic DNA sequencer.
Plasmids.
Full-length XPR-1 coding sequence was subcloned into pCS2+ vector for in vitro RNA synthesis using SP6 mMessage mMachine kit (Ambion). The coding region of XPR-1 was amplified by PCR with PfuTurbo polymerase (Strategene) using primers E-1s, 5′-TGAATTCCCGACACTATGGAGGAG-3′ and 733AS-X, 5′-TCTCGAGTCACTTTTTGTGAAACACAAG-3′. The resulting PCR fragment was purified and digested with EcoRI and XhoI and ligated into pCS2+ vector. The C-terminal LBD of XPR-1 was fused with glutathione S-transferase (GST) to produce GST fusion protein (GST-XPRC) for antibody production. The two primers used in the PCR to amplify this C-terminal region of XPR-1 () are: Bam-PBDN, 5′-TGGATCCAACAGTTCCGCTCAGGA-3′, and PBDC-ER, 5′-TGAATTCACTTTTTGTGAAACACAAGTG-3′. The PCR fragment was digested with EcoRI and BamHI and ligated into pGEX-2T vector (Amersham Pharmacia).
GST-XPR Fusion Proteins and Antibodies.
GST-XPRC fusion protein was purified on glutathione-Sepharose 4B (Amersham Pharmacia) and used to generate antibodies in rabbits (Charles River Breeding Laboratories).
Oocytes.
Ovary segments were treated with 1% collagenase (type I; Sigma) in OR2 medium (49) for 4 h at 20–22°C. Oocytes were transferred to a Petri dish and washed with four to six changes of OR2 on a shaker for 1 h to remove remaining follicle cells, as judged by staining with Hoechst 33342 (Roche Molecular Biochemicals). Follicle-free oocytes were incubated in OR2 plus 0.1 mg/ml gentamycin (GIBCO) at 18°C overnight. Oocytes were exposed to water-soluble progesterone (Sigma) at indicated concentrations. Activation was monitored by formation of a white spot in the animal pole, indicative of nuclear envelope breakdown (germinal vesicle breakdown, GVBD), and formation of the meiosis I spindle. In most experiments, confirmation was obtained by fixing and dissecting individual oocytes.
Antisense Injections.
Antisense oligonucleotides targeted to different regions of XPR-1 mRNA were used to deplete XPR-1 message and protein from the oocytes. The 25-mer chimeric phosphorothioate antisense oligos and the positions of their corresponding middle amino acid residues (in parentheses) are: A2, 5′-A*G*G*G*ACACATGTGAGGAGTTA*C*C*C*G-3′ (87); A3, 5′-T*G*C*A*GCTGCCACAAGTCAGGA*C*T*C*C-3′ (381); A4, 5′-A*T*T*C*CAGCGACAGGGTCGGTG*G*C*G*A-3′ (459).
Control oligos were reversals of antisense, e.g., C2 (control for A2), 5′-G*C*C*C*ATTGAGGAGTGTACACA*G*G*G*A-3′. (*, phosphorothioate modification; Integrated DNA Technologies, Coralville, IA). All antisense oligos were HPLC purified and dissolved in 1× injection buffer (88 mM NaCl/15 mM Tris⋅HCl, pH 7.5) at 1 mg/ml. Typically, 25 nl was injected per oocyte and oocytes were cultured in OR2. Rescue experiments were usually performed 4 days after antisense oligo injections, and 25 ng of mRNA (1 mg/ml in water) was injected per oocyte. After 36 h, groups of 20 oocytes were stimulated with 1–1.5 μM progesterone and scored for GVBD.
RT-PCR was used to determine the relative levels of XPR-1 mRNA in control or antisense-injected oocytes. Primers were: XPR-UTR1, 5′- CACTGACCTCGCCTAGAGACCGG-3′; and 14as, 5′-GAGACGATCCGTAATCCTGACCC-3′. A secondary, nested PCR was necessary to visualize the product; the primers used this reaction were: XPR-UTR2, 5′-GTAAGAACCCCCGACACTATGGAGG-3′, and 15as, 5′- AGACCTTTGGCCACCTTCTTCCC-3′.
Luciferase Assay.
Oocytes were injected with 1 ng of XPR-1 mRNA or left uninjected and incubated for 16 h. Then, 3 ng of luciferase reporter construct DNA (PRE2-tata-Luc; a gift from K. Horwitz, University of Colorado, which was derived from PRE2-tata-CAT; ref. 50) was then injected into the nucleus of each oocyte and cells were stimulated with 10 μM progesterone. At the indicated times, groups of 10 injected oocytes were collected and lysed in 50 μl of passive lysis buffer (Promega). Luciferase activity was measured by integrating total light emission over 10 s using a Turner Designs luminometer (TD-20/20). All injections were normalized for injection efficiency by coinjecting 0.3 ng of pRL-TK, a TK-driven Renilla luciferase control plasmid (Promega) per oocyte.
Western Blotting.
Samples were resolved on SDS polyacrylamide gels, transferred to poly(vinylidene difluoride) (PVDF) membranes (semidry blot; Hoefer), and blocked with 5% milk in Tris-buffered saline plus 0.1% Tween 20. The blot was incubated for 1 h with primary antibodies, and blotted with horseradish peroxidase-conjugated secondary antibodies for 1 h. The signal was detected by the enhanced chemiluminescence system (Amersham Pharmacia).
Results
Isolation of cDNA Encoding XPR-1.
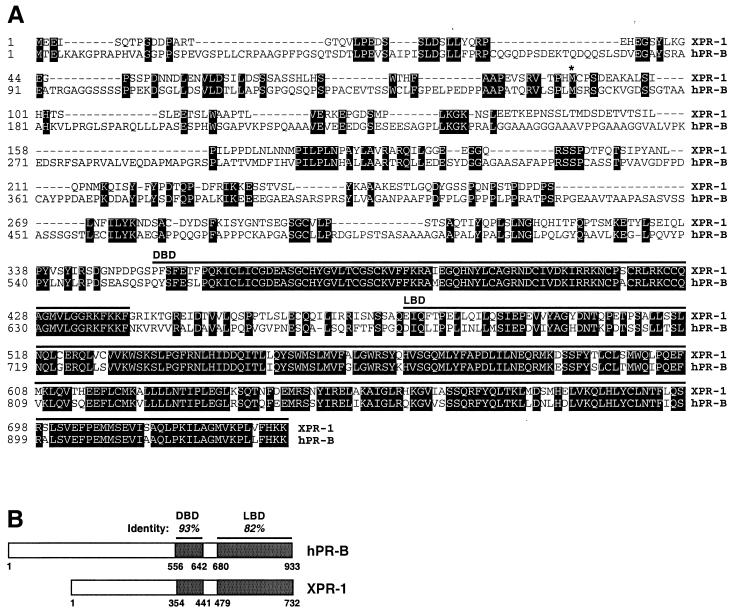
To isolate candidate PRs, we screened for cDNAs encoding proteins containing regions similar to the highly conserved progesterone binding domains of the human and chicken conventional PRs (see Materials and Methods). The longest cDNA clone obtained was 2,611 nt in length, including a 5′ untranslated region (UTR) of 357 nt and a 3′ UTR of 55 nt excluding the poly(A) tail (GenBank accession no. AF279335). The longest ORF, which starts at the first ATG after multiple inframe stop codons, encodes a polypeptide of 732 residues with a predicted molecular weight of 81,969. The C terminus of this protein, which we call XPR-1, shows extremely high sequence similarity with the DNA binding domain (DBD) and LBD of hPR (Fig. 1 A and B). The N-terminal sequences of PRs are considerably more diverse; sequence alignment of XPR-1 with the two human forms suggests that XPR-1 is slightly more similar to hPR-B, which contains additional N-terminal sequences not found on the shorter form, hPR-A (51, 52).
Figure 1.
Amino acid sequence and domain structure of XPR-1. (A) Predicated protein sequence of XPR-1 and alignment with hPR-B (GenBank accession no. M15716). Identical residues are shaded. Regions comprising the DBD and LBD are indicated by lines above the sequences. * indicates the start of the A form of hPR; −, alignment gap. (B) Comparison of XPR-1 and hPR-B domain structure. Percent sequence identities in the LBD and DBD are indicated.
XPR-1 mRNA levels in oocytes were sufficiently low to be detectable only by RT-PCR (see below). Similarly, currently available antibodies generated against XPR-1 or human PR failed to reveal any reliably crossreacting bands, suggesting that XPR-1 protein levels may also be very low (data not shown). When XPR-1 was overexpressed by injecting XPR-1 mRNA into oocytes, XPR-1 protein was detected in nuclear, cytoplasmic, and cortical fractions (data not shown).
XPR-1 Is a Progesterone-Responsive Transcriptional Regulator.
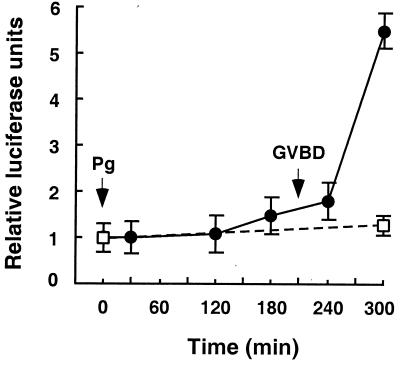
To test whether XPR-1 can function as a progesterone-dependent transcription factor in oocytes, plasmid DNA containing a luciferase reporter gene under the control of two copies of the progesterone response element (PRE; 50) was injected into nuclei of oocytes expressing extra XPR-1 from preinjected mRNA. As shown in Fig. 2, progesterone addition led to a large increase in luciferase activity. In contrast, no significant increase was seen in oocytes not supplied with extra XPR-1. These results indicate that XPR-1 can function as a bona fide progesterone-inducible transcription factor. The absence of detectable progesterone-stimulated luciferase transcription in oocytes lacking exogenously expressed XPR-1 could be because of the low levels of endogenous XPR-1 in these oocytes. It is also possible that most of the endogenous XPR-1 protein is restricted from functioning as a transcription factor for this particular reporter construct.
Figure 2.
XPR-1-regulated transcriptional activation of a luciferase reporter construct. Oocytes were either injected with 1 ng of XPR-1 mRNA (●) or left uninjected (□) and incubated for 16 h. Then, 3 ng of luciferase reporter construct DNA (PRE2-tata-Luc) was injected into the nucleus of each oocyte and cells were stimulated with 10 μM progesterone. At the indicated times, groups of 10 injected oocytes were collected and assayed for luciferase activity as described in Materials and Methods. Similar results were seen in two separate experiments.
Injection of XPR-1 Makes Oocytes More Sensitive to Progesterone and Accelerates Progesterone-Induced Oocyte Activation.
Earlier work has suggested that the Xenopus oocyte progesterone receptor functions at or near the cell surface, predicting the existence of a nontranscriptional receptor. However, as outlined in the introduction, many steroids are now known to induce rapid, nontranscriptional responses in somatic cells, and two conventional steroid receptors, those for estrogen and progesterone, can also rapidly activate cytoplasmic signal transduction on transfection into somatic cells (42, 43). Furthermore, increasing evidence supports the idea that a subset of conventional ER and PR proteins may be located near the cell surface in certain cells (45–47). Thus, it seemed possible that XPR-1 could also function as the oocyte's signaling receptor.
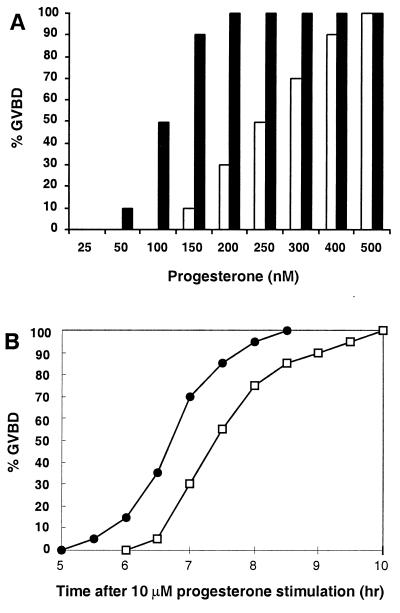
To test this idea, we asked whether introducing extra XPR-1 into oocytes could enhance their response to progesterone. As shown in Fig. 3A, oocytes injected with XPR-1 mRNA were considerably more sensitive to progesterone. For example, when challenged with the subthreshold concentration of 150 nM progesterone, only 10% of control oocytes reached GVBD, whereas 90% of oocytes overexpressing XPR-1 went through GVBD. Furthermore, when exposed to a high dose of progesterone, oocytes containing additional XPR-1 reached GVBD significantly earlier than controls (Fig. 3B). These effects were seen repeatedly with healthy oocytes from unprimed frogs during the peak of mating season, but were not obvious or consistent in gonadotropin-primed oocytes which, in our hands, mature faster than unprimed oocytes. These observations provided an initial indication that XPR-1 might function in the nontranscriptional signaling pathway that results in oocyte activation.
Figure 3.
Effects of overexpressing XPR-1 in oocytes. (A) Sensitivity to progesterone. Oocytes were injected with H2O (□) or 1 ng of XPR-1 mRNA (■), incubated overnight, and then stimulated with progesterone at indicated concentrations. Cells were scored for GVBD after 16 h. Ten oocytes from the same unprimed frog were tested at each concentration of hormone, because the EC50 varied significantly from frog to frog. The relative increase in sensitivity to progesterone was similar in three different experiments. (B) Kinetics of GVBD. Groups of 40 oocytes from unprimed females were injected with H2O (□) or 1 ng of XPR-1 mRNA (●), incubated overnight, and then stimulated with 10 μM progesterone. GVBD was scored at indicated times. Similar results were seen in four separate experiments.
Antisense Ablation of XPR-1 Blocks Progesterone-Induced Oocyte Activation.
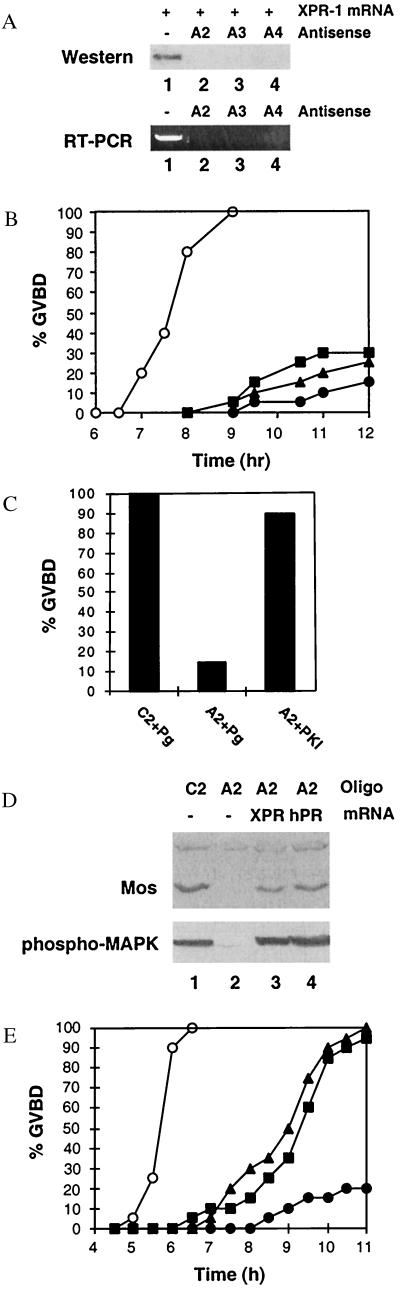
To ask whether XPR-1 is required for oocyte activation, we used antisense depletion experiments. Antisense oligonucleotides complementary to three different regions of XPR-1 mRNA each blocked synthesis of XPR-1 from coinjected mRNA (Fig. 4A Upper). Injection of 20–30 ng of each antisense oligo almost completely depleted the XPR-1 mRNA, as judged by RT-PCR (Fig. 4A Lower). When antisense-injected oocytes were treated with progesterone, 70–85% of the cells failed to undergo GVBD (Fig. 4B). The small number of oocytes that did go through GVBD were much delayed. Injection of control oligonucleotides had no effect on GVBD (not shown). Subsequent injection of the PKA inhibitor PKI into antisense-treated oocytes induced GVBD in the absence of progesterone (Fig. 4C), just as it does in untreated oocyte (26), indicating that the failure of antisense-injected oocytes to undergo GVBD was not because of general toxicity of the antisense constructs. Most importantly, the ability of antisense-treated oocytes to respond to progesterone was restored by subsequent injection of XPR-1 mRNA (Fig. 4 D and E). We analyzed the antisense-treated oocytes in more detail using A2 as an example. After injection with antisense oligo A2, progesterone failed to induce synthesis of mos protein, activation of MAP kinase (Fig. 4D, lane 2), and GVBD (Fig. 4E, ●). When XPR-1 expression was restored to antisense-treated cells and progesterone was added subsequently, these oocytes were again able to synthesize mos protein, to activate MAP kinase (Fig. 4D, lane 3), and go through GVBD (Fig. 4E, ▴). The ability of XPR-1 to rescue antisense-treated oocytes was not affected by incubation in actinomycin D, indicating that XPR-1's effect was not through transcriptional activation of any positive regulators of GVBD (data not shown). Injection of human PR-B mRNA also rescued the ability of antisense-treated oocytes to synthesize mos, to activate MAP kinase (Fig. 4D, lane 4), and to reach GVBD (Fig. 4E, ▪), demonstrating that this well-characterized transcriptional receptor can also connect to the oocyte signaling pathway. These results establish that XPR-1 or a closely related protein, which is structurally and functionally similar to hPR-B and other conventional transcriptional progesterone receptors, is required for progesterone to activate the cytoplasmic signaling pathway that initiates oocyte maturation.
Figure 4.
Effect of antisense ablation of XPR-1. (A) Injection of XPR-1 antisense oligonucleotides blocks synthesis of injected XPR-1 mRNA and ablates endogenous XPR-1 mRNA. (Upper) Oocytes were injected with 1 ng of XPR-1 mRNA in 1× injection buffer (25 nl) or XPR-1 mRNA plus individual antisense oligos A2, A3, or A4 at 25 ng per oocyte at 1 ng/nl. After 16 h of incubation, the expression levels of XPR-1 protein from two oocytes per condition were determined on immunoblots using antibody against hPR-B (C-19; Santa Cruz Biotechnology). Lane 1, buffer-injected; lane 2, injected with A2; lane 3, injected with A3; lane 4, injected with A4. (Lower) Endogenous XPR-1 mRNA levels were determined by nested RT-PCR, as described in Materials and Methods. Lanes are as in Upper. (B) Effect of antisense oligonucleotides on GVBD. Oocytes were injected with 25 nl of buffer (○) or 1 ng/nl individual antisense oligonucleotides (●, A2; ▴, A3; ■, A4). After 8 days of incubation, 20 healthy-looking oocytes from each group were stimulated with 1.5 μM progesterone and scored for GVBD at the indicated times. Similar results were obtained in five separate experiments. The effects of antisense oligos were obvious by day 4. Control oligos did not delay GVBD (not shown). (C) Effects of the PKA inhibitor PKI on XPR-1-depleted oocytes. Six days after injection of antisense oligonucleotide A2 or its control oligonucleotide C2, oocytes were either stimulated with 1.5 μM progesterone or injected with 10 nl of PKI (9 units/μl) in the absence of progesterone and GVBD was scored after 16-h incubation. (D) mos synthesis and MAP kinase activation. Oocytes were injected with 25 ng of control (C2) or antisense (A2) oligonucleotides and incubated for 4 days. Groups of A2-injected oocytes were then injected again with 20 ng of XPR-1 or hPR-B mRNA as indicated and incubated for another 2 days. Finally, oocytes were treated with 1.5 μM progesterone for 16 h. At 16 h, three oocytes from each group were lysed and immunoblotted with antibodies against mos or phospho-MAP kinase. (E) Rescue of XPR-1-depleted oocytes by injection of XPR-1 or hPR-B mRNA. Oocytes were injected with 25 ng of control (C2) or antisense (A2) oligonucleotides, incubated for 4 days, and then injected with either XPR-1 or hPR-B mRNA as indicated. After 36 h, oocytes were stimulated with 1.5 μM progesterone and GVBD was scored at the indicated times. ○, 25 ng of C2; ●, 25 ng of A2; ■, 25 ng of A2 plus 25 ng of XPR-1 mRNA; ▴, 25 ng of A2 plus 25 ng of hPR-B mRNA.
Discussion
This work establishes three points. First, XPR-1 is identified as a transcription factor: its sequences in the hormone-binding and DNA-binding domains are almost identical to those of well-characterized PRs that function as transcription factors, and its activity in vivo functionally identifies XPR-1 as a progesterone-responsive transcription factor. Second, overexpression of XPR-1 makes oocytes more sensitive to progesterone and accelerates progesterone-induced oocyte activation. Third, antisense ablation of endogenous XPR-1 mRNA blocks the ability of progesterone to induce synthesis of mos, activation of MAP kinase, and entry into meiosis. Taken together, these results indicate that XPR-1 is required for progesterone-dependent oocyte activation, a process that can proceed even when transcription is blocked. These results are consistent with the idea that XPR-1 also functions as the receptor that activates the cytoplasmic signaling pathway that leads to translational activation of mos mRNA, mos-dependent activation of MAP kinase and MPF, and resumption of the meiotic cell cycle.
Numerous well-documented examples of rapid, nontranscriptional effects of steroid hormones have been described in somatic cells and, in some systems, steroids can induce these effects in membrane preparations (reviewed in refs. 10 and 11). In most cases, however, it is not known whether the steroid acts through a novel surface receptor or through the conventional receptor, some of which resides at or near the membrane. Given early experiments showing that polymer-bound progesterone can activate frog oocytes, whereas injected progesterone usually does not (except by leakage; see ref. 1), it has long been presumed that the receptor responsible for rapid signaling will be found at the cell surface. Currently available antibodies are not sensitive enough for us to determine the location of XPR-1 in oocytes by using Western blots or immunofluorescence. When oocytes containing overexpressed XPR-1 are fractionated into nuclear, cytoplasmic, and crude membrane preparations, XPR-1 is found in all three fractions; immunoprecipitation of metabolically labeled proteins gave similar results (J.T., unpublished observations). Although yeast two-hybrid screens using XPR-1 have identified some potentially significant binding partners (S.K., unpublished observations), we are unable to say at this time how progesterone-activated XPR-1 is coupled to any of the known elements of the downstream signaling pathway.
The ability of steroid hormones to induce both cytoplasmic signaling and transcriptional changes, and the demonstrated ability of certain conventional receptors to function in both pathways suggests that, at least in some cases, a single conventional receptor may coordinate cytoplasmic and nuclear responses. In somatic cells, the nuclear pathways have been investigated intensively, whereas the cytoplasmic pathways are not well understood at the molecular level. In oocytes, by contrast, the cytoplasmic pathway has received considerable attention, whereas the nuclear response to progesterone is only beginning to be appreciated in these cells. It is hoped that further work will yield useful insights into the molecular mechanisms by which XPR-1 functions in oocyte activation.
Acknowledgments
We are especially grateful to Thorkell Andresson for his efforts in the early days of this project. We thank Cydney Brooks for help in cloning a C-terminal XPR-1 sequence; Katherine Horwitz for constructs; and Jim Maller, Joan Brugge, and Laurinda Jaffe for very helpful discussions. J.T. is a Hoffmann–La Roche Fellow of the Life Sciences Research Foundation. This work was supported by National Institutes of Health Grant HD37636 (to J.V.R.).
Abbreviations
- MPF
M phase promoting factor
- ER
estrogen receptor
- PR
progesterone receptor
- hPR
human PR
- GVBD
germinal vesicle breakdown
- PKA
protein kinase A
- LBD
ligand binding domain
- DBD
DNA binding domain
- RT-PCR
reverse transcription–PCR
- GST
glutathione S-transferase
Note Added in Proof.
We have recently identified a second XPR sequence, XPR-2, whose open reading frame (predicted to be greater than 703 amino acids) is related to but distinct from that of XPR-1.
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF279335).
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.250492197.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.250492197
References
- 1.Masui Y, Shibuya E K. In: Molecular Regulation of Nuclear Events in Mitosis and Meiosis. Schlegel R A, Halleck M S, Rao P N, editors. New York: Academic; 1987. pp. 1–42. [Google Scholar]
- 2.Cork R J, Robinson K R. Zygote. 1994;2:289–299. doi: 10.1017/s0967199400002112. [DOI] [PubMed] [Google Scholar]
- 3.Maller J L. Biol Cell. 1998;90:453–460. [PubMed] [Google Scholar]
- 4.Ferrell J E. BioEssays. 1999;21:833–842. doi: 10.1002/(SICI)1521-1878(199910)21:10<833::AID-BIES5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 5.Xu L, Glass C K, Rosenfeld M G. Curr Opin Genet Dev. 1999;9:140–147. doi: 10.1016/S0959-437X(99)80021-5. [DOI] [PubMed] [Google Scholar]
- 6.McKenna N J, Xu J, Nawaz A, Tsai M J, O'Malley B W. J Steroid Biochem Mol Biol. 1999;69:3–12. doi: 10.1016/s0960-0760(98)00144-7. [DOI] [PubMed] [Google Scholar]
- 7.Weatherman R V, Fletternick R J, Scanlon T S. Annu Rev Biochem. 1999;68:559–581. doi: 10.1146/annurev.biochem.68.1.559. [DOI] [PubMed] [Google Scholar]
- 8.McEwan I J. Biochem Soc Trans. 2000;28:369–373. [PubMed] [Google Scholar]
- 9.Morrill G A, Kostellow A B. Steroids. 1999;64:157–167. doi: 10.1016/s0039-128x(98)00093-2. [DOI] [PubMed] [Google Scholar]
- 10.Wehling M. Annu Rev Physiol. 1997;59:365–393. doi: 10.1146/annurev.physiol.59.1.365. [DOI] [PubMed] [Google Scholar]
- 11.Revelli A, Massobrio M, Tesarik J. Endocr Rev. 1998;19:3–17. doi: 10.1210/edrv.19.1.0322. [DOI] [PubMed] [Google Scholar]
- 12.Nagahama Y. Steroids. 1997;62:190–196. doi: 10.1016/s0039-128x(96)00180-8. [DOI] [PubMed] [Google Scholar]
- 13.Yamashita M, Mita K, Yoshida N, Kondo T. Prog Cell Cycle Res. 2000;4:115–129. doi: 10.1007/978-1-4615-4253-7_11. [DOI] [PubMed] [Google Scholar]
- 14.Masui Y. J Exp Zool. 1967;166:365–375. doi: 10.1002/jez.1401660309. [DOI] [PubMed] [Google Scholar]
- 15.Smith L D, Ecker R E. Dev Biol. 1969;19:281–309. doi: 10.1016/0012-1606(69)90065-7. [DOI] [PubMed] [Google Scholar]
- 16.Fortune J E, Concannon P W, Hansel W. Biol Reprod. 1975;13:561–567. doi: 10.1095/biolreprod13.5.561. [DOI] [PubMed] [Google Scholar]
- 17.Smith L D, Ecker R E, Subtelney S. Dev Biol. 1968;17:627–643. doi: 10.1016/0012-1606(68)90010-9. [DOI] [PubMed] [Google Scholar]
- 18.Merriam R W. J Exp Zool. 1972;180:421–426. [Google Scholar]
- 19.Wasserman W J, Masui Y. Biol Reprod. 1974;11:133–144. doi: 10.1095/biolreprod11.2.133. [DOI] [PubMed] [Google Scholar]
- 20.Schuetz A W. J Exp Zool. 1967;166:347–354. doi: 10.1002/jez.1401660307. [DOI] [PubMed] [Google Scholar]
- 21.Masui Y, Markert C L. J Exp Zool. 1971;177:129–145. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- 22.Iwashita J, Hayano Y, Sagata N. Proc Natl Acad Sci USA. 1998;95:4392–4397. doi: 10.1073/pnas.95.8.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akamatsu E, Tanaka T, Kato J. J Biol Chem. 1998;273:16494–16500. doi: 10.1074/jbc.273.26.16494. [DOI] [PubMed] [Google Scholar]
- 24.Palmer A, Nebreda A R. Prog Cell Cycle Res. 2000;4:131–143. doi: 10.1007/978-1-4615-4253-7_12. [DOI] [PubMed] [Google Scholar]
- 25.Maller J L. Cell Differ. 1985;16:211–221. doi: 10.1016/0045-6039(85)90570-6. [DOI] [PubMed] [Google Scholar]
- 26.Maller J L, Krebs E G. J Biol Chem. 1977;252:1712–1718. [PubMed] [Google Scholar]
- 27.Finidori-Lepicard J, Schorderet-Slatkine S, Hanoune J, Baulieu E-E. Nature (London) 1981;292:255–257. doi: 10.1038/292255a0. [DOI] [PubMed] [Google Scholar]
- 28.Sadler S, Maller J. J Biol Chem. 1981;256:6368–6373. [PubMed] [Google Scholar]
- 29.Bandyopadhyay A, Bandyopadhyay J, Choi H H, Choi H S, Kwon H B. Gen Comp Endocrinol. 1998;109:293–301. doi: 10.1006/gcen.1997.7038. [DOI] [PubMed] [Google Scholar]
- 30.Morrison T, Waggoner L, Whitworth-Langley L, Stith B J. Endocrinology. 2000;141:2145–2152. doi: 10.1210/endo.141.6.7510. [DOI] [PubMed] [Google Scholar]
- 31.Andresson T, Ruderman J V. EMBO J. 1998;17:5627–5637. doi: 10.1093/emboj/17.19.5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mendez R, Hake L E, Andresson T, Littlepage L E, Ruderman J V, Richter J D. Nature (London) 2000;404:302–307. doi: 10.1038/35005126. [DOI] [PubMed] [Google Scholar]
- 33.Wickens M, Goodwin E B, Kimble J, Strickland S, Hentze M W. In: Translational Control of Gene Expression, Cold Spring Harbor Monographs. Sonenberg N, Hershey J W B, Mathews M, editors. Vol. 39. Plainview, NY: Cold Spring Harbor Lab. Press; 2000. pp. 295–370. [Google Scholar]
- 34.Richter J D. In: Translational Control of Gene Expression, Cold Spring Harbor Monographs. Sonenberg N, Hershey J W B, Mathews M, editors. Vol. 39. Plainview, NY: Cold Spring Harbor Lab. Press; 2000. pp. 785–806. [Google Scholar]
- 35.Sagata N. BioEssays. 1997;19:13–21. doi: 10.1002/bies.950190105. [DOI] [PubMed] [Google Scholar]
- 36.Murakami M S, Vande Woude G F. Methods Enzymol. 1997;283:584–600. doi: 10.1016/s0076-6879(97)83046-7. [DOI] [PubMed] [Google Scholar]
- 37.Smith L D, Ecker R E. Dev Biol. 1971;25:233–247. doi: 10.1016/0012-1606(71)90029-7. [DOI] [PubMed] [Google Scholar]
- 38.Ishikawa K, Hanaoka Y, Kondo Y, Imai K. Mol Cell Endocrinol. 1977;9:91–100. doi: 10.1016/0303-7207(77)90049-1. [DOI] [PubMed] [Google Scholar]
- 39.Godeau J F, Schorderet-Slatkine S, Hubert P, Baulieu E-E. Proc Natl Acad Sci USA. 1978;75:2353–2357. doi: 10.1073/pnas.75.5.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morrill G, MA, G-Y, Kostellow A. Biochem Biophys Acta. 1997;232:213–217. doi: 10.1006/bbrc.1997.6190. [DOI] [PubMed] [Google Scholar]
- 41.Migliaccio A, Pagano M, Auricchio F. Oncogene. 1993;8:2183–2191. [PubMed] [Google Scholar]
- 42.Migliaccio A, Domenico M D, Castoria G, deFalco A, Bontempo P, Nola E, Auricchio F. EMBO J. 1996;15:1292–1300. [PMC free article] [PubMed] [Google Scholar]
- 43.Migliaccio A, Piccolo D, Castoria G, DiDomenico M, Bioancio A, Lombardi M, Gong W, Beato M, Auricchio F. EMBO J. 1998;17:2008–2018. doi: 10.1093/emboj/17.7.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Castoria G, Barone M V, Domenico M D, Bilancio A, Ametrano D, Migliaccio A, Auricchio F. EMBO J. 1999;18:2500–2510. doi: 10.1093/emboj/18.9.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Razandi M, Pedram A, Greene G L, Levin E R. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- 46.Russell K S, Haynes M P, Sinha D, Clerisme E, Bender J R. Proc Natl Acad Sci USA. 2000;97:5930–5935. doi: 10.1073/pnas.97.11.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clarke C, Norfleet A, Clarke M, Watson C, Cunningham K, Thomas M. Neuroendocrinology. 2000;71:34–42. doi: 10.1159/000054518. [DOI] [PubMed] [Google Scholar]
- 48.Chen A, Yuhanna I S, Galcheva Z, Karas R H, Mendelsohn M E, Shaul P W. J Clin Invest. 1999;103:401–406. doi: 10.1172/JCI5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wallace R A, Jared D W, Dumont J N, Sega M W. J Exp Zool. 1973;184:321–333. doi: 10.1002/jez.1401840305. [DOI] [PubMed] [Google Scholar]
- 50.Takimoto G S, Hovland A R, Tasset D M, Melville M Y, Tung L, Horwitz K B. J Biol Chem. 1996;271:13308–13316. doi: 10.1074/jbc.271.23.13308. [DOI] [PubMed] [Google Scholar]
- 51.Horwitz K B, Alexander P S. Endocrinology. 1983;113:2195–2201. doi: 10.1210/endo-113-6-2195. [DOI] [PubMed] [Google Scholar]
- 52.Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P. EMBO J. 1990;9:1606–1614. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]