Abstract
Objective—To clarify whether endothelium derived nitric oxide contributes to exogenous bradykinin induced dilatation of human epicardial and resistance coronary arteries in vivo. Design—Quantitative coronary angiography and Doppler flow velocity measurements were used to determine the effects of the nitric oxide synthesis inhibitor, NG-monomethyl-L-arginine (L-NMMA), on bradykinin induced dilatation of the epicardial and resistance coronary arteries. Setting—Hiroshima University Hospital. Patients—20 patients (16 men and four women, mean (SD) age 56 (9) years) with angiographically normal smooth epicardial coronary arteries. Interventions—Serial infusions of bradykinin (0.5, 1.5, and 2.5 µg/min) were given into the left coronary ostium before and after L-NMMA infusion (60 µmol/min). Main outcome measures—Epicardial coronary diameter, coronary blood flow, and coronary vascular resistance. Results—Bradykinin-induced epicardial coronary vasodilatation after L-NMMA (dilatation by 2.5 µg/min, 3.8(1.4)% in the proximal and 5.9(1.8)% in the distal segments, mean (SEM)) was less (p < 0.001, respectively) than before L-NMMA (11.7(2.5)% and 15.1(2.0)%, respectively). In contrast, L-NMMA did not affect the bradykinin induced increase in coronary blood flow and decrease in coronary vascular resistance. Conclusions—Endothelium derived nitric oxide contributes to bradykinin induced dilatation of epicardial coronary arteries, but may be less important in coronary resistance vasodilatation. Keywords: bradykinin; nitric oxide; coronary artery; coronary blood flow
Full Text
The Full Text of this article is available as a PDF (144.9 KB).
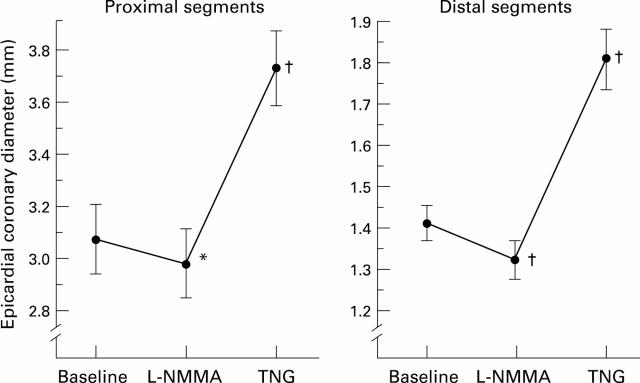
Figure 1 .
Epicardial coronary diameter changes with NG-monomethyl-L-arginine (L-NMMA) and glyceryl trinitrate (TNG) in the proximal and the distal coronary segments. L-NMMA caused significant vasoconstriction in the proximal and distal segments. Glyceryl trinitrate caused significant endothelium independent vasodilatation in the proximal and distal segments. *p < 0.05, †p < 0.001 v baseline.
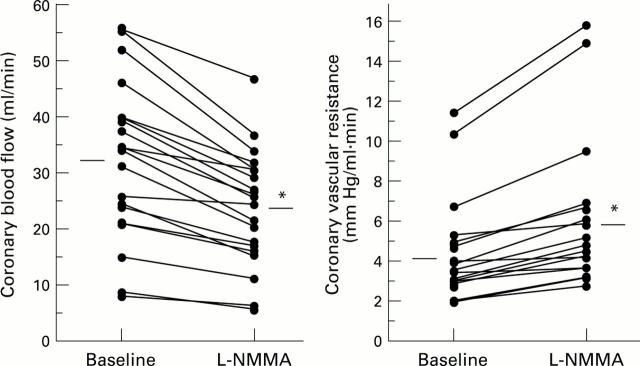
Figure 2 .
Coronary blood flow and coronary vascular resistance changes with NG-monomethyl-L-arginine (L-NMMA). L-NMMA significantly decreased coronary blood flow and increased coronary vascular resistance. *p < 0.001 v baseline.
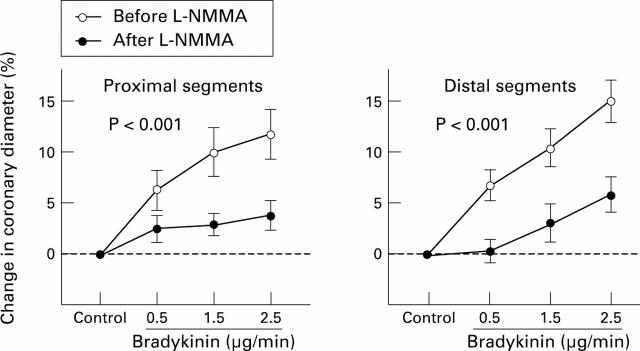
Figure 3 .
Percentage change in epicardial coronary diameter with bradykinin in the proximal and the distal segments before (empty circles) and after (filled circles) NG-monomethyl-L-arginine (L-NMMA) infusion. Although bradykinin induced epicardial coronary vasodilatation in a dose dependent manner, the dilator effects were significantly attenuated after L-NMMA infusion in the proximal and distal segments (p < 0.001 for both).
Figure 4 .
Percentage changes in coronary blood flow and coronary vascular resistance with bradykinin before (empty circles) and after (filled circles) NG-monomethyl-L-arginine (L-NMMA) infusion. Bradykinin caused significant increases in coronary blood flow and decreases in coronary vascular resistance in a dose dependent manner before and after L-NMMA infusion (p < 0.001, respectively). These effects of bradykinin on coronary blood flow and coronary vascular resistance before and after L-NMMA infusion did not differ significantly.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassenge E., Busse R. Endothelial modulation of coronary tone. Prog Cardiovasc Dis. 1988 Mar-Apr;30(5):349–380. doi: 10.1016/0033-0620(88)90003-5. [DOI] [PubMed] [Google Scholar]
- Boulanger C., Hendrickson H., Lorenz R. R., Vanhoutte P. M. Release of different relaxing factors by cultured porcine endothelial cells. Circ Res. 1989 Jun;64(6):1070–1078. doi: 10.1161/01.res.64.6.1070. [DOI] [PubMed] [Google Scholar]
- Cherry P. D., Furchgott R. F., Zawadzki J. V., Jothianandan D. Role of endothelial cells in relaxation of isolated arteries by bradykinin. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2106–2110. doi: 10.1073/pnas.79.6.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucette J. W., Corl P. D., Payne H. M., Flynn A. E., Goto M., Nassi M., Segal J. Validation of a Doppler guide wire for intravascular measurement of coronary artery flow velocity. Circulation. 1992 May;85(5):1899–1911. doi: 10.1161/01.cir.85.5.1899. [DOI] [PubMed] [Google Scholar]
- Egashira K., Katsuda Y., Mohri M., Kuga T., Tagawa T., Kubota T., Hirakawa Y., Takeshita A. Role of endothelium-derived nitric oxide in coronary vasodilatation induced by pacing tachycardia in humans. Circ Res. 1996 Aug;79(2):331–335. doi: 10.1161/01.res.79.2.331. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gilligan D. M., Guetta V., Panza J. A., García C. E., Quyyumi A. A., Cannon R. O., 3rd Selective loss of microvascular endothelial function in human hypercholesterolemia. Circulation. 1994 Jul;90(1):35–41. doi: 10.1161/01.cir.90.1.35. [DOI] [PubMed] [Google Scholar]
- Groves P., Kurz S., Just H., Drexler H. Role of endogenous bradykinin in human coronary vasomotor control. Circulation. 1995 Dec 15;92(12):3424–3430. doi: 10.1161/01.cir.92.12.3424. [DOI] [PubMed] [Google Scholar]
- Hutchinson P. J., Palmer R. M., Moncada S. Comparative pharmacology of EDRF and nitric oxide on vascular strips. Eur J Pharmacol. 1987 Sep 23;141(3):445–451. doi: 10.1016/0014-2999(87)90563-2. [DOI] [PubMed] [Google Scholar]
- Kamiya A., Togawa T. Adaptive regulation of wall shear stress to flow change in the canine carotid artery. Am J Physiol. 1980 Jul;239(1):H14–H21. doi: 10.1152/ajpheart.1980.239.1.H14. [DOI] [PubMed] [Google Scholar]
- Komori K., Lorenz R. R., Vanhoutte P. M. Nitric oxide, ACh, and electrical and mechanical properties of canine arterial smooth muscle. Am J Physiol. 1988 Jul;255(1 Pt 2):H207–H212. doi: 10.1152/ajpheart.1988.255.1.H207. [DOI] [PubMed] [Google Scholar]
- Kuga T., Egashira K., Mohri M., Tsutsui H., Harasawa Y., Urabe Y., Ando S., Shimokawa H., Takeshita A. Bradykinin-induced vasodilation is impaired at the atherosclerotic site but is preserved at the spastic site of human coronary arteries in vivo. Circulation. 1995 Jul 15;92(2):183–189. doi: 10.1161/01.cir.92.2.183. [DOI] [PubMed] [Google Scholar]
- Lefroy D. C., Crake T., Uren N. G., Davies G. J., Maseri A. Effect of inhibition of nitric oxide synthesis on epicardial coronary artery caliber and coronary blood flow in humans. Circulation. 1993 Jul;88(1):43–54. doi: 10.1161/01.cir.88.1.43. [DOI] [PubMed] [Google Scholar]
- Lenfant C. NHLBI funding policies. Enhancing stability, predictability, and cost control. Circulation. 1994 Jul;90(1):1–1. doi: 10.1161/01.cir.90.1.1. [DOI] [PubMed] [Google Scholar]
- Lenfant C. NHLBI funding policies. Enhancing stability, predictability, and cost control. Circulation. 1994 Jul;90(1):1–1. doi: 10.1161/01.cir.90.1.1. [DOI] [PubMed] [Google Scholar]
- Lüscher T. F., Richard V., Tschudi M., Yang Z. H., Boulanger C. Endothelial control of vascular tone in large and small coronary arteries. J Am Coll Cardiol. 1990 Mar 1;15(3):519–527. doi: 10.1016/0735-1097(90)90619-z. [DOI] [PubMed] [Google Scholar]
- Mombouli J. V., Illiano S., Nagao T., Scott-Burden T., Vanhoutte P. M. Potentiation of endothelium-dependent relaxations to bradykinin by angiotensin I converting enzyme inhibitors in canine coronary artery involves both endothelium-derived relaxing and hyperpolarizing factors. Circ Res. 1992 Jul;71(1):137–144. doi: 10.1161/01.res.71.1.137. [DOI] [PubMed] [Google Scholar]
- Moncada S., Radomski M. W., Palmer R. M. Endothelium-derived relaxing factor. Identification as nitric oxide and role in the control of vascular tone and platelet function. Biochem Pharmacol. 1988 Jul 1;37(13):2495–2501. doi: 10.1016/0006-2952(88)90236-5. [DOI] [PubMed] [Google Scholar]
- Moncada S., Vane J. R. Pharmacology and endogenous roles of prostaglandin endoperoxides, thromboxane A2, and prostacyclin. Pharmacol Rev. 1978 Sep;30(3):293–331. [PubMed] [Google Scholar]
- Nagao T., Vanhoutte P. M. Hyperpolarization as a mechanism for endothelium-dependent relaxations in the porcine coronary artery. J Physiol. 1992 Jan;445:355–367. doi: 10.1113/jphysiol.1992.sp018928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao T., Vanhoutte P. M. Hyperpolarization contributes to endothelium-dependent relaxations to acetylcholine in femoral veins of rats. Am J Physiol. 1991 Oct;261(4 Pt 2):H1034–H1037. doi: 10.1152/ajpheart.1991.261.4.H1034. [DOI] [PubMed] [Google Scholar]
- Nakashima M., Mombouli J. V., Taylor A. A., Vanhoutte P. M. Endothelium-dependent hyperpolarization caused by bradykinin in human coronary arteries. J Clin Invest. 1993 Dec;92(6):2867–2871. doi: 10.1172/JCI116907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofili E., Kern M. J., Tatineni S., Deligonul U., Aguirre F., Serota H., Labovitz A. J. Detection of coronary collateral flow by a Doppler-tipped guide wire during coronary angioplasty. Am Heart J. 1991 Jul;122(1 Pt 1):221–225. doi: 10.1016/0002-8703(91)90780-l. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Panza J. A., García C. E., Kilcoyne C. M., Quyyumi A. A., Cannon R. O., 3rd Impaired endothelium-dependent vasodilation in patients with essential hypertension. Evidence that nitric oxide abnormality is not localized to a single signal transduction pathway. Circulation. 1995 Mar 15;91(6):1732–1738. doi: 10.1161/01.cir.91.6.1732. [DOI] [PubMed] [Google Scholar]
- Pelc L. R., Gross G. J., Warltier D. C. Mechanism of coronary vasodilation produced by bradykinin. Circulation. 1991 Jun;83(6):2048–2056. doi: 10.1161/01.cir.83.6.2048. [DOI] [PubMed] [Google Scholar]
- Quyyumi A. A., Dakak N., Andrews N. P., Gilligan D. M., Panza J. A., Cannon R. O., 3rd Contribution of nitric oxide to metabolic coronary vasodilation in the human heart. Circulation. 1995 Aug 1;92(3):320–326. doi: 10.1161/01.cir.92.3.320. [DOI] [PubMed] [Google Scholar]
- Quyyumi A. A., Dakak N., Andrews N. P., Husain S., Arora S., Gilligan D. M., Panza J. A., Cannon R. O., 3rd Nitric oxide activity in the human coronary circulation. Impact of risk factors for coronary atherosclerosis. J Clin Invest. 1995 Apr;95(4):1747–1755. doi: 10.1172/JCI117852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quyyumi A. A., Mulcahy D., Andrews N. P., Husain S., Panza J. A., Cannon R. O., 3rd Coronary vascular nitric oxide activity in hypertension and hypercholesterolemia. Comparison of acetylcholine and substance P. Circulation. 1997 Jan 7;95(1):104–110. doi: 10.1161/01.cir.95.1.104. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Hodson H. F., Moncada S. A specific inhibitor of nitric oxide formation from L-arginine attenuates endothelium-dependent relaxation. Br J Pharmacol. 1989 Feb;96(2):418–424. doi: 10.1111/j.1476-5381.1989.tb11833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiber J. H., Serruys P. W., Kooijman C. J., Wijns W., Slager C. J., Gerbrands J. J., Schuurbiers J. C., den Boer A., Hugenholtz P. G. Assessment of short-, medium-, and long-term variations in arterial dimensions from computer-assisted quantitation of coronary cineangiograms. Circulation. 1985 Feb;71(2):280–288. doi: 10.1161/01.cir.71.2.280. [DOI] [PubMed] [Google Scholar]
- Shimokawa H., Flavahan N. A., Lorenz R. R., Vanhoutte P. M. Prostacyclin releases endothelium-derived relaxing factor and potentiates its action in coronary arteries of the pig. Br J Pharmacol. 1988 Dec;95(4):1197–1203. doi: 10.1111/j.1476-5381.1988.tb11756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiode N., Morishima N., Nakayama K., Yamagata T., Matsuura H., Kajiyama G. Flow-mediated vasodilation of human epicardial coronary arteries: effect of inhibition of nitric oxide synthesis. J Am Coll Cardiol. 1996 Feb;27(2):304–310. doi: 10.1016/0735-1097(95)00465-3. [DOI] [PubMed] [Google Scholar]
- Sudhir K., Chou T. M., Hutchison S. J., Chatterjee K. Coronary vasodilation induced by angiotensin-converting enzyme inhibition in vivo: differential contribution of nitric oxide and bradykinin in conductance and resistance arteries. Circulation. 1996 May 1;93(9):1734–1739. doi: 10.1161/01.cir.93.9.1734. [DOI] [PubMed] [Google Scholar]
- Tschudi M., Richard V., Bühler F. R., Lüscher T. F. Importance of endothelium-derived nitric oxide in porcine coronary resistance arteries. Am J Physiol. 1991 Jan;260(1 Pt 2):H13–H20. doi: 10.1152/ajpheart.1991.260.1.H13. [DOI] [PubMed] [Google Scholar]
- Zanzinger J., Zheng X., Bassenge E. Endothelium dependent vasomotor responses to endogenous agonists are potentiated following ACE inhibition by a bradykinin dependent mechanism. Cardiovasc Res. 1994 Feb;28(2):209–214. doi: 10.1093/cvr/28.2.209. [DOI] [PubMed] [Google Scholar]