Abstract
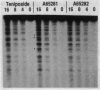
A number of quinolones and related antibacterial compounds were screened for activity against calf thymus topoisomerase II by using the P4 unknotting and DNA breakage assays. Several compounds from different structural classes which inhibited DNA unknotting with 50% inhibitory concentrations ranging from 8 to 25 micrograms/ml were identified. Two experimental isothiazoloquinolones from this group, designated A-65281 and A-65282, were also found to induce considerable DNA breakage mediated by calf thymus topoisomerase II, with 32P-end-labeled pBR322 as the substrate. These compounds were nearly as potent as teniposide, with DNA breakage activity evident at concentrations as low as 4 micrograms/ml. However, some differences in DNA cleavage patterns from those with teniposide were evident. These studies have thus identified a new class of agents which have activity against both bacterial and eukaryotic type II topoisomerases. The implications of these data for the selectivity of topoisomerase-directed compounds and the potential toxicity of such compounds developed as antibacterial agents are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett J. F., Gootz T. D., McGuirk P. R., Farrell C. A., Sokolowski S. A. Use of in vitro topoisomerase II assays for studying quinolone antibacterial agents. Antimicrob Agents Chemother. 1989 Oct;33(10):1697–1703. doi: 10.1128/aac.33.10.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredberg A., Brant M., Jaszyk M. Ciprofloxacin-induced inhibition of topoisomerase II in human lymphoblastoid cells. Antimicrob Agents Chemother. 1991 Mar;35(3):448–450. doi: 10.1128/aac.35.3.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G. L., Yang L., Rowe T. C., Halligan B. D., Tewey K. M., Liu L. F. Nonintercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase II. J Biol Chem. 1984 Nov 10;259(21):13560–13566. [PubMed] [Google Scholar]
- Chu D. T., Fernandes P. B., Claiborne A. K., Shen L., Pernet A. G. Structure-activity relationships in quinolone antibacterials: design, synthesis and biological activities of novel isothiazoloquinolones. Drugs Exp Clin Res. 1988;14(6):379–383. [PubMed] [Google Scholar]
- Cozzarelli N. R. DNA gyrase and the supercoiling of DNA. Science. 1980 Feb 29;207(4434):953–960. doi: 10.1126/science.6243420. [DOI] [PubMed] [Google Scholar]
- Domagala J. M., Hanna L. D., Heifetz C. L., Hutt M. P., Mich T. F., Sanchez J. P., Solomon M. New structure-activity relationships of the quinolone antibacterials using the target enzyme. The development and application of a DNA gyrase assay. J Med Chem. 1986 Mar;29(3):394–404. doi: 10.1021/jm00153a015. [DOI] [PubMed] [Google Scholar]
- Drake F. H., Zimmerman J. P., McCabe F. L., Bartus H. F., Per S. R., Sullivan D. M., Ross W. E., Mattern M. R., Johnson R. K., Crooke S. T. Purification of topoisomerase II from amsacrine-resistant P388 leukemia cells. Evidence for two forms of the enzyme. J Biol Chem. 1987 Dec 5;262(34):16739–16747. [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Gootz T. D., Barrett J. F., Sutcliffe J. A. Inhibitory effects of quinolone antibacterial agents on eucaryotic topoisomerases and related test systems. Antimicrob Agents Chemother. 1990 Jan;34(1):8–12. doi: 10.1128/aac.34.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halligan B. D., Edwards K. A., Liu L. F. Purification and characterization of a type II DNA topoisomerase from bovine calf thymus. J Biol Chem. 1985 Feb 25;260(4):2475–2482. [PubMed] [Google Scholar]
- Hoshino K., Sato K., Une T., Osada Y. Inhibitory effects of quinolones on DNA gyrase of Escherichia coli and topoisomerase II of fetal calf thymus. Antimicrob Agents Chemother. 1989 Oct;33(10):1816–1818. doi: 10.1128/aac.33.10.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiang Y. H., Hertzberg R., Hecht S., Liu L. F. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem. 1985 Nov 25;260(27):14873–14878. [PubMed] [Google Scholar]
- Hussy P., Maass G., Tümmler B., Grosse F., Schomburg U. Effect of 4-quinolones and novobiocin on calf thymus DNA polymerase alpha primase complex, topoisomerases I and II, and growth of mammalian lymphoblasts. Antimicrob Agents Chemother. 1986 Jun;29(6):1073–1078. doi: 10.1128/aac.29.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami Y., Takeuchi N., Hanada M., Hasegawa Y., Ishii K., Andoh T., Sato T., Suzuki K., Yamaguchi H., Miyazaki S. Topostin, a novel inhibitor of mammalian DNA topoisomerase I from Flexibacter topostinus sp. nov. II. Purification and some properties of topostin. J Antibiot (Tokyo) 1990 Feb;43(2):158–162. doi: 10.7164/antibiotics.43.158. [DOI] [PubMed] [Google Scholar]
- Kohn K. W. Principles and practice of DNA filter elution. Pharmacol Ther. 1991;49(1-2):55–77. doi: 10.1016/0163-7258(91)90022-e. [DOI] [PubMed] [Google Scholar]
- Liu L. F. DNA topoisomerase poisons as antitumor drugs. Annu Rev Biochem. 1989;58:351–375. doi: 10.1146/annurev.bi.58.070189.002031. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Davis J. L., Calendar R. Novel topologically knotted DNA from bacteriophage P4 capsids: studies with DNA topoisomerases. Nucleic Acids Res. 1981 Aug 25;9(16):3979–3989. doi: 10.1093/nar/9.16.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minocha A., Long B. H. Inhibition of the DNA catenation activity of type II topoisomerase by VP16-213 and VM26. Biochem Biophys Res Commun. 1984 Jul 18;122(1):165–170. doi: 10.1016/0006-291x(84)90454-6. [DOI] [PubMed] [Google Scholar]
- Nelson E. M., Tewey K. M., Liu L. F. Mechanism of antitumor drug action: poisoning of mammalian DNA topoisomerase II on DNA by 4'-(9-acridinylamino)-methanesulfon-m-anisidide. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1361–1365. doi: 10.1073/pnas.81.5.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M. J., Martin B. A., Gootz T. D., McGuirk P. R., Moynihan M., Sutcliffe J. A., Osheroff N. Effects of quinolone derivatives on eukaryotic topoisomerase II. A novel mechanism for enhancement of enzyme-mediated DNA cleavage. J Biol Chem. 1991 Aug 5;266(22):14585–14592. [PubMed] [Google Scholar]
- Ross W. E., Bradley M. O. DNA double-stranded breaks in mammalian cells after exposure to intercalating agents. Biochim Biophys Acta. 1981 Jun 26;654(1):129–134. doi: 10.1016/0005-2787(81)90145-3. [DOI] [PubMed] [Google Scholar]
- Ross W. E., Glaubiger D. L., Kohn K. W. Protein-associated DNA breaks in cells treated with adriamycin or ellipticine. Biochim Biophys Acta. 1978 Jun 22;519(1):23–30. doi: 10.1016/0005-2787(78)90059-x. [DOI] [PubMed] [Google Scholar]
- Ross W. E., Glaubiger D., Kohn K. W. Qualitative and quantitative aspects of intercalator-induced DNA strand breaks. Biochim Biophys Acta. 1979 Mar 28;562(1):41–50. doi: 10.1016/0005-2787(79)90124-2. [DOI] [PubMed] [Google Scholar]
- Ross W., Rowe T., Glisson B., Yalowich J., Liu L. Role of topoisomerase II in mediating epipodophyllotoxin-induced DNA cleavage. Cancer Res. 1984 Dec;44(12 Pt 1):5857–5860. [PubMed] [Google Scholar]
- Shen L. L., Kohlbrenner W. E., Weigl D., Baranowski J. Mechanism of quinolone inhibition of DNA gyrase. Appearance of unique norfloxacin binding sites in enzyme-DNA complexes. J Biol Chem. 1989 Feb 15;264(5):2973–2978. [PubMed] [Google Scholar]
- Shen L. L., Pernet A. G. Mechanism of inhibition of DNA gyrase by analogues of nalidixic acid: the target of the drugs is DNA. Proc Natl Acad Sci U S A. 1985 Jan;82(2):307–311. doi: 10.1073/pnas.82.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausfeld U., Richter A. Simultaneous purification of DNA topoisomerase I and II from eukaryotic cells. Prep Biochem. 1989;19(1):37–48. doi: 10.1080/10826068908544895. [DOI] [PubMed] [Google Scholar]
- Sugino A., Peebles C. L., Kreuzer K. N., Cozzarelli N. R. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewey K. M., Chen G. L., Nelson E. M., Liu L. F. Intercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase II. J Biol Chem. 1984 Jul 25;259(14):9182–9187. [PubMed] [Google Scholar]
- Tewey K. M., Rowe T. C., Yang L., Halligan B. D., Liu L. F. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science. 1984 Oct 26;226(4673):466–468. doi: 10.1126/science.6093249. [DOI] [PubMed] [Google Scholar]
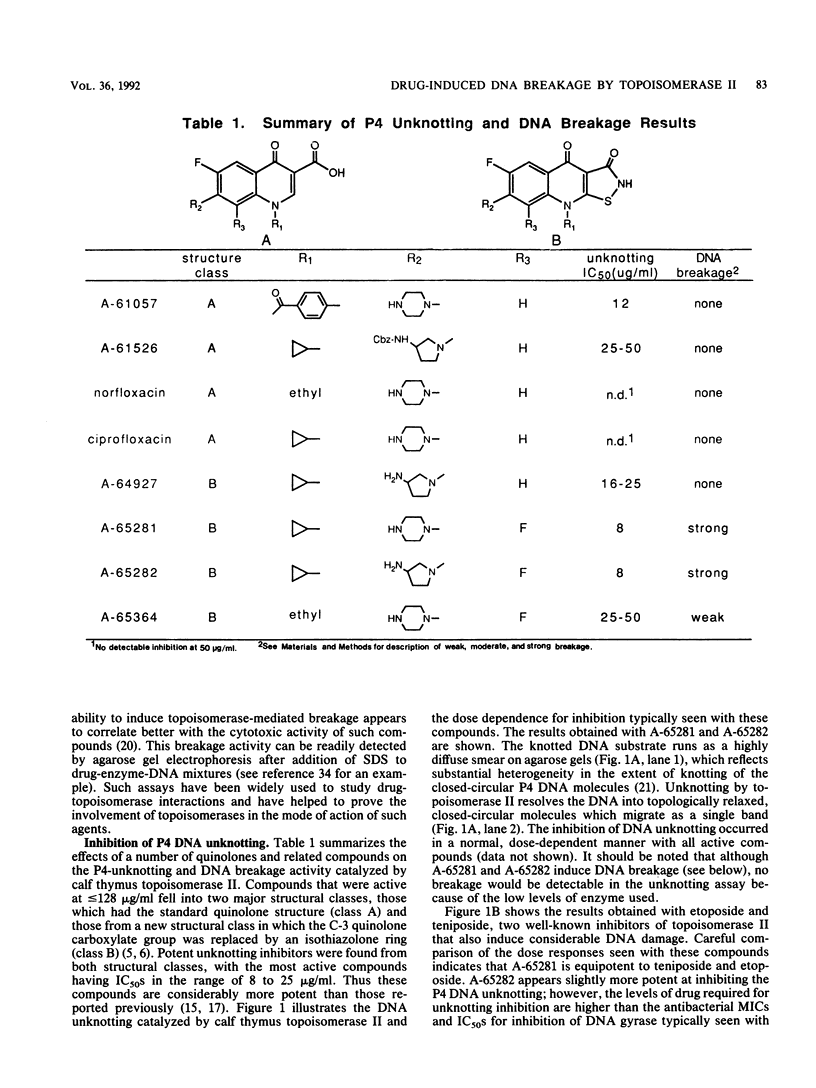
- Walton L., Elwell L. P. In vitro cleavable-complex assay to monitor antimicrobial potency of quinolones. Antimicrob Agents Chemother. 1988 Jul;32(7):1086–1089. doi: 10.1128/aac.32.7.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. Fluoroquinolone antimicrobial agents. Clin Microbiol Rev. 1989 Oct;2(4):378–424. doi: 10.1128/cmr.2.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita Y., Kawada S., Fujii N., Nakano H. Induction of mammalian DNA topoisomerase I and II mediated DNA cleavage by saintopin, a new antitumor agent from fungus. Biochemistry. 1991 Jun 18;30(24):5838–5845. doi: 10.1021/bi00238a005. [DOI] [PubMed] [Google Scholar]
- Zwelling L. A., Michaels S., Erickson L. C., Ungerleider R. S., Nichols M., Kohn K. W. Protein-associated deoxyribonucleic acid strand breaks in L1210 cells treated with the deoxyribonucleic acid intercalating agents 4'-(9-acridinylamino) methanesulfon-m-anisidide and adriamycin. Biochemistry. 1981 Nov 10;20(23):6553–6563. doi: 10.1021/bi00526a006. [DOI] [PubMed] [Google Scholar]
- Zwelling L. A., Michaels S., Kerrigan D., Pommier Y., Kohn K. W. Protein-associated deoxyribonucleic acid strand breaks produced in mouse leukemia L1210 cells by ellipticine and 2-methyl-9-hydroxyellipticinium. Biochem Pharmacol. 1982 Oct 15;31(20):3261–3267. doi: 10.1016/0006-2952(82)90560-3. [DOI] [PubMed] [Google Scholar]