Abstract
The effect of opioid blockade on nociceptive flexion reflex (NFR) activity and subjective pain ratings was examined in 151 healthy young men and women. Using a within-subjects design, NFR threshold was assessed on two days after administration of either placebo or a 50 mg dose of naltrexone. Electrocutaneous pain threshold and tolerance levels were measured after NFR threshold assessment on each day. Results indicated that administration of naltrexone was consistently associated with hypoalgesic responding. Specifically, participants exhibited lower levels of NFR activity and reported lower pain ratings for electrocutaneous stimulation delivered at pain threshold and tolerance levels following administration of naltrexone as compared to placebo. These findings indicate that opiate blockade using the current standard dose may elicit hypoalgesia. A potential moderating effect of dose of opiate blockade medication and level of endogenous opioid activation should be carefully examined in future research.
Keywords: Pain, Nociceptive Flexion Reflex (NFR), Opiate Blockade, Naltrexone
1. Introduction
Since the discovery of endogenous opiates in the 1970's (Pert and Snyder 1973; Hughes et al. 1975; Goldstein 1976; Terenius 1977), the endogenous opiate system has become an important focus in the study of pain modulation. In human studies, opioid antagonists, such as naloxone and naltrexone, are typically administered as an indirect measure of the contribution of endogenous opioid activity to the experience of pain during noxious stimulation. This paradigm is based on the notion that endogenous opiate involvement in pain perception should be evidenced by an increase in pain following pharmacological blockade of opiate receptors.
Support for the opiate-blockade paradigm is clearly demonstrated in the animal literature. Numerous studies show that naloxone and naltrexone increase nociceptive responding across diverse noxious stimuli for a variety of species, including mice (Jacob et al. 1974; Grevert and Goldstein 1977; Jacob and Ramabadran 1977), rats (Jacob et al. 1974; Walker et al. 1977; Woolf 1980), cats (Goldfarb and Hu 1976; Bell and Martin 1977; Willer et al. 1982a), dogs (Jacob and Michaud 1976), and rabbits (Catley et al. 1983).
In contrast to the animal literature, the opiate-blockade paradigm has yielded mixed findings in studies of healthy human participants. Early studies by Buchsbaum and colleagues demonstrated naloxone-related hyperalgesia (Buchsbaum et al. 1977; Davis et al. 1978; Buchsbaum et al. 1983), although these effects were limited to specific individuals (e.g., “pain insensitive” participants) or conditions (e.g., prolonged or intense stimulation). Other studies have reported that naloxone has no effect on forearm ischemic pain (Grevert and Goldstein 1977; 1978; Grevert et al. 1983a; Grevert et al. 1983b; Posner and Burke 1985), cold pressor pain (Grevert and Goldstein 1978; McCubbin and Bruehl 1994), or electrocutaneous pain in response to stimulation applied to the finger (Bromm et al. 1983), forearm (El-Sobky et al. 1976), ear (Stacher et al. 1988), or teeth (Ernst et al. 1986). Finally, a few studies have suggested that, in some cases, opioid antagonists may actually inhibit pain (Volavka et al. 1979; Tassorelli et al. 1995; Janssen and Arntz 1997). More recent studies have also either failed to observe effects of opioid blockade on pain (McCubbin and Bruehl 1994; Mikkelsen et al. 1999; Bruehl et al. 2002; Edwards et al. 2004), or have reported that the effects of opioid blockade varied as a function of subgroup membership (Schobel et al. 1998; Bruehl and Chung 2006; McCubbin et al. 2006). For example, Schoebel et al. (1998) reported that naloxone increased mechanical pain responses in normotensives, but not borderline hypertensives. In contrast, McCubbin and colleagues (2006) reported that naltrexone increased cold pressor pain ratings among young adults with high-normal resting blood pressure, but decreased pain ratings for those with low-normal blood pressure.
To further examine the effects of opiate blockade on nociceptive responding in human participants, the present study tested the effect of naltrexone on nociceptive flexion reflex (NFR) activity and subjective pain ratings in healthy young men and women during exposure to noxious electrocutaneous stimulation. Because prior research suggests that endogenous opioids may be activated only in response to prolonged or intense pain stimulation, both painful and maximally tolerable levels of electrocutaneous stimulation were used to evaluate the effect of opiate blockade on nociceptive responding. It was hypothesized that more intense stimulation would elicit greater pain and nociceptive responding and that endogenous opiate blockade would exacerbate these reactions.
2. Methods
2.1. Participants
Healthy young adults (n = 151) were recruited from college campuses located in Athens, Ohio and Duluth, Minnesota. Participants in the study were predominantly male (55%) and had a mean age of 19.4 (SE = .13) years. All participants received compensation of $20 per hour of testing.
2.2. Initial Screening
A series of screening questions was used to identify healthy individuals with no history of major medical problems or routine use of medication (other than birth control). Those who met these inclusion criteria, expressed an interest in participating, and completed an informed consent were then scheduled for a brief medical screening to confirm the absence of any medical contraindications to testing. None of the potential participants was excluded based on the medical screening. After successfully completing the medical screening participants were scheduled for two 3-hour laboratory sessions, which were scheduled on average 5 ± 0.7 days apart to allow for clearance of naltrexone. To control for potential menstrual cycle phase effects, women were tested within 2-7 days after the onset of menses. All participants were asked to refrain from caffeine, nicotine, alcohol, and strenuous exercise for at least four hours before their arrival at the laboratory, and from analgesic medication for 24 hours prior to testing.
2.3. Laboratory protocol
The experimental procedure was administered by a female experimenter at each testing site. To start each testing session, participants completed a brief questionnaire to assess compliance with the requested dietary, exercise, and medication restrictions. In addition, women took a One-Step E.P.T.™ pregnancy test to confirm that they were not pregnant. Once participants were cleared to continue, they completed a ten-minute resting baseline of blood pressure and heart rate readings. They then consumed a gel capsule that contained either a 50 mg dose of naltrexone or placebo, with drug order randomly assigned and the experimenter blind to assignment. A 50mg dose of naltrexone was chosen because it is a standardized dose commonly used in clinical settings to treat individuals with narcotic and alcohol addiction. Participants then sat quietly for one hour to allow time for drug absorption. During this interval they completed a number of questionnaires, read quietly, and then had stimulating and recording electrodes attached according to the procedures described below. Following the absorption period, a second ten-minute baseline of blood pressure and heart rate was obtained. Three nociceptive flexion reflex (NFR) assessments were then conducted, followed by electrocutaneous pain threshold and tolerance assessments as described below.
2.4. Electrode attachment
To prepare participants for stimulating and recording electrode application, the skin at the electrode sites was cleaned with alcohol and then abraded with Omni Prep electrode paste. An electrode impedance of less than 10KOhm, verified using a UFI Checktrode (model MKII), was achieved before proceeding. Electromyographic (EMG) activity was recorded from the biceps femoris muscle of the left leg using a DelSys, Bagnoli-2 differential amplifier. The active electrode was placed over the left biceps femoris muscle 10 cm superior to the popliteal fossa, and a reference electrode attached over the lateral epicondyle of the femur. EMG was recorded and processed using a CED Micro1401 analog-to-digital converter and Spike2 software. A Nicolet bar electrode (anode inferior) was attached to the left leg over the retromalleolar pathway of the sural nerve and electrical stimulation was delivered using a Digitimer, DS7A constant-current stimulator.
2.5. Electrocutaneous pain threshold and tolerance
Participants were seated in a Hi-Seat rehabilitation chair (model 2000) with a leg rest adjusted to maintain knee flexion at approximately 60 degrees from horizontal. As part of a separate investigation (France et al. 2005), NFR threshold was assessed three times with each assessment lasting approximately 5 min and followed by a 5 min rest period. Upon completion of the NFR threshold assessments, electrocutaneous pain threshold and tolerance levels were measured. Specifically, sural nerve stimulation trials were delivered as a volley of five 1 ms rectangular pulses with a 3 ms interpulse interval (total duration = 17 ms). Stimulation intensity began at 0 mA and increased in 2 mA steps until a maximum stimulation intensity of 40mA was reached or the participant reached their tolerance threshold. Following each trial, participants rated the perceived stimulation intensity using a verbal rating scale with anchors of 1 (sensory threshold), 25 (uncomfortable), 50 (painful), 75 (very painful), and 100 (maximum tolerable). Pain threshold (in mA) was defined as the first stimulation intensity that received a rating of 50 or greater. Pain tolerance (in mA) was defined as the maximum stimulation intensity that a participant was willing to receive or rated as a 100.
2.6. Data reduction
To allow for a comparison of naltrexone effects on different intensities of electrocutaneous pain, we identified the stimulation intensities that corresponded to pain threshold and pain tolerance on each day. We then compared the observed stimulation intensities across the placebo and naltrexone days, and saved the NFR waveform and pain ratings corresponding to the lower intensity of the two days (e.g., if pain tolerance was 32 mA on one day and 34 mA on the other, then responses to the 32 mA stimulation were saved on each day). This procedure allowed for a direct comparison of responses to the same stimulation intensity on each day. Analyses were conducted separately for pain ratings and nociceptive flexion reflex activity. Consistent with prior studies conducted in our laboratory (Page and France 1997; France and Suchowiecki 2001), nociception flexion reflex activity was defined as EMG activity during the 90 to 150 ms post-stimulation interval (i.e., total area under the curve). This interval corresponds to reflex activation related to stimulation of A-delta nociceptive fibers, while avoiding possible interference from the low-threshold cutaneous flexor reflex, startle reactions, and voluntary movements.
3. Results
3.1. Electrocutaneous pain ratings
Pain ratings were analyzed using a 2 Sex (female, male) × 2 Drug (naltrexone, placebo) × 2 Stimulation Level (pain threshold, tolerance) repeated measures multivariate analysis of variance (MANOVA). Results of this analysis revealed significant main effects of Drug, Wilks' Lambda = 0.97, F(1, 149) = 4.43, p <.05, and Stimulation Level, Wilks' Lambda = 0.11, F(1, 149) = 1248.26, p <.001. There were no other significant main effects or interactions. As can be seen in Table 1, the main effect of Drug reflected lower pain ratings at both pain threshold and tolerance stimulation levels following administration of naltrexone as compared to placebo. The Stimulation Level effect reflected higher pain ratings during exposure to more intense electrocutaneous stimulation delivered at tolerance versus pain threshold levels. It should be noted that the mean pain threshold and tolerance ratings presented in Table 1 are lower than might be expected given our rating scale (i.e., 50 = painful and 100 = maximum tolerable); however, this result is to be expected given that (a) we compared ratings from the lower of the two intensities of stimulation used across the placebo and naltrexone days, and (b) some participants rated the maximum stimulation intensity (i.e., 40 mA) as less than 100.
Table 1.
Means (Standard Error) for pain ratings and nociceptive flexion reflex (NFR) activity at both electrocutaneous pain threshold and tolerance levels following placebo and naltrexone administration.
| Threshold | Tolerance | |||
|---|---|---|---|---|
| Placebo | Naltrexone | Placebo | Naltrexone | |
| M (SE) | M (SE) | M (SE) | M (SE) | |
| Pain Rating (1-100) | ||||
| Men | 45.0 (1.2) | 41.0 (1.3) | 85.3 (2.2) | 81.5 (2.3) |
| Women | 44.9 (1.3) | 43.3 (1.4) | 86.0 (2.4) | 80.2 (2.6) |
| All | 44.9 (0.9) | 42.1 (0.9) | 85.6 (1.6) | 80.8 (1.7) |
| NFR Activity (μV) | ||||
| Men | 37.7 (4.6) | 33.3 (3.9) | 77.6 (7.5) | 70.4 (7.5) |
| Women | 40.0 (5.1) | 24.3 (4.4) | 64.3 (8.2) | 44.7 (8.3) |
| All | 38.8 (3.4) | 29.3 (2.9) | 71.6 (5.5) | 58.8 (5.6) |
3.2. Nociceptive flexion reflex activity
Similar to the pain analysis, NFR activity was analyzed using a 2 Sex (female, male) × 2 Drug (naltrexone, placebo) × 2 Stimulation Level (pain threshold, tolerance) repeated measures multivariate analysis of variance (MANOVA) of total area under the EMG curve during the 90 to 150 ms post-stimulation interval. Results of this analysis revealed significant effects of Drug, Wilks' Lambda = 0.95, F(1, 149) = 8.33, p <.01, Stimulation Level, Wilks' Lambda = 0.65, F(1, 149) = 78.03, p <.001, and Sex by Stimulation Level, Wilks' Lambda = 0.96, F(1, 149) = 5.49, p <.05. There were no other significant main effects or interactions. As can be seen in Table 1 and Figure 1, the main effect of Drug reflected lower NFR activity in response to both pain threshold and tolerance stimulation levels following administration of naltrexone as compared to placebo. The Stimulation Level effect reflected higher NFR activity in response to the more intense electrocutaneous stimulation delivered at tolerance versus pain threshold, whereas the Sex by Stimulation Level interaction reflected greater NFR activity responses in men versus women in response to tolerance stimulation. This sex difference is consistent with the fact that the tolerance stimulation level for men (M = 32.1; SE = 1.0 mA) was significantly greater than the level for women (M = 25.1; SE = 1.1 mA), t(149) = 4.76, p<.001.
Figure 1.
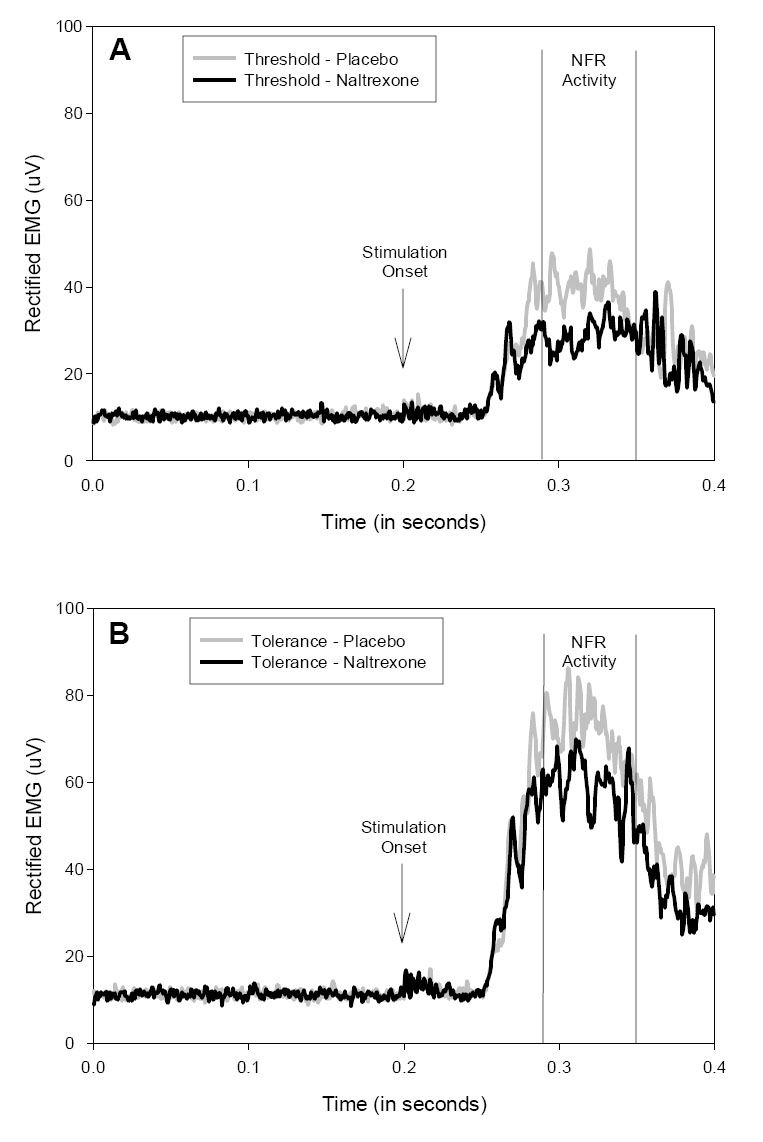
Rectified electromyogram (EMG) recorded during electrocutaneous stimulation at pain threshold (A) and tolerance (B) following placebo and naltrexone administration. Nociception flexion reflex (NFR) activity is defined by the 90 to 150 ms post-stimulation interval.
4. Discussion
In contrast with the notion that blockade of opioid receptors increases pain responding, the results of the present study provide consistent evidence of hypoalgesic responding in healthy young men and women following administration of a 50 mg dose of naltrexone. Given that the central aims of the present study were to evaluate the effect of opiate blockade on pain reports and nociceptive responses at varying intensities of stimulation, it is particularly interesting to note that hypoalgesic responding was evident for every measure.
Prior studies that have examined opiate blockade effects on nociceptive flexion reflex activity have yielded either hyperalgesic responding or no effect of opiate blockade. Evidence of enhanced reflex activity was demonstrated in healthy participants when NFR responses were repeatedly assessed during anticipation of an extremely intense 70 mA electrical shock (Willer et al. 1981). During this period of uncontrolled shock anticipation, an opiate effect on nociceptive responding was demonstrated by a reduction of NFR threshold in the naloxone versus placebo condition. Subsequent investigations that did not incorporate severe shock anticipation reported that opiate blockade had no significant effect on NFR activity (Willer et al. 1982b; Sandrini et al. 1999). In this context, failure to show a hyperalgesic response to naltrexone in the present study may be attributed to the absence of a sufficiently intense or uncontrolled threat that could elicit significant endogenous opiate responsivity. However, this does not explain why we observed hypoalgesia in the absence of such a threat when other studies showed no effect. Although several important methodological distinctions might be relevant, the most important distinction is likely to be the fact that we used a significantly larger sample and this may have increased our power to detect subtle hypoalgesic effects. For example, the study with the next largest sample (Roelofs et al. 2000) reported that naloxone administration was associated with a decrease in both pain reports and NFR activity; however, these changes were non-significant in this between-subjects design with only 15 participants per group. In the present study the observed effect sizes for naltrexone were in the small-to-medium range (i.e., Cohen's d = .35 for pain ratings and .46 for NFR activity). According to power analyses, such effect sizes suggest minimum samples of 120 and 204, respectively, at power = .80 and alpha = .05 levels. In sum, naltrexone may exert a small hypoalgesic effect that requires a relatively large sample size to ensure detection.
It is important to note that hypoalgesic responding to opiate blockade is not without precedent in the human and laboratory animal literatures. For example, a number of studies have demonstrated that low doses of naloxone and naltrexone may be associated with hypoalgesia (Levine et al. 1979; Woolf 1980; Dickenson et al. 1981; Kayser and Guilbaud 1981; Ueda et al. 1986; Vaccarino et al. 1988; 1989; Kolaric et al. 1999), while still others have demonstrated hypoalgesic responding to more standard doses (Volavka et al. 1979; Tassorelli et al. 1995; Janssen and Arntz 1997; al'Absi et al. 2004). For example, in a design that involved the repeated application of noxious electrocutaneous stimulation to the left ankle (i.e., similar to the pain stimulus applied in the present study), it was observed that a 4 mg dose of naloxone was associated with reduction in pain reports relative to a placebo condition (Janssen and Arntz 1997). Concurrent assessment of plasma endorphin levels revealed no significant changes during the procedure, prompting the authors to conclude that naloxone may lead to hypoalgesia when administered in the absence of significant endogenous opiate activity (Janssen and Arntz 1997). In sum, the likelihood of observed hyperalgesia, hypoalgesia, or no effect may be determined by a number of factors including dose of opiate blockade medication, level of endogenous opiate activation, and the availability of sufficient power to detect subtle differences. Although the exact mechanism underlying hypoalgesic responses to opiate blockade is not known, it has been speculated that low-dose hypoalgesic effects may be attributed to antagonism of presynaptic autoreceptors responsible for down-regulation of opioid release and/or agonistic action at non-mu opioid receptor subtypes (Ueda et al. 1986; Bianchi and Panerai 1993; Kolaric et al. 1999). A collateral disinhibition model, wherein opioid receptor antagonists release a non-opioid hypoalgesic system from tonic opioid inhibition, may also account for hypoalgesic responses. Specifically, the rostral ventromedial medulla contains “on” cells that are excited by noxious stimuli, project to the spinal cord, discharge just prior to withdrawal reflex responses, and are inhibited by opioids (Fields and Basbaum 1999). As a result, administration of opioid antagonists such as naltrexone may release these “on” cells from tonic opioidergic inhibition and in so doing facilitate spinal nociceptive transmission. Such an effect would explain both the observed changes in nociceptive reflex responding and subjective pain reports in the present study.
Although the present study used a standard 50 mg dose of naltrexone to elicit opioid blockade, it must be noted that the absence of a mg/kg dosing protocol may be an important limitation to interpretation of the findings. However, the fact that there were no significant interactions observed between drug and sex suggests that this may not have had a significant impact on the results given that, on average, females would have received relatively higher mg/kg levels of naltrexone than males. An additional limitation of the present study is that, as noted above, participants had previously been exposed to three NFR threshold assessments prior to the assessment of their electrocutaneous pain threshold and tolerance levels. In part, this sequence of events is important in that prior exposure to electrocutaneous stimulation may allow for a more accurate assessment of pain threshold and tolerance levels as participants become more familiar, and less anxious about the stimulation procedure. However, it is also possible that prior exposure to electrical stimulation may influence subsequent responding. Although this notion cannot be tested directly in the present data set, as it would require a comparison of responses with and without prior testing, it should be noted that we previously reported that naltrexone had no significant effect on NFR threshold levels (France et al. 2005). This suggests that, at a minimum, participants in the present study were exposed to similar levels of stimulation to elicit the NFR responses on the naltrexone and placebo days, and hence there was no systematic effect of prior stimulation exposure on the within-subject comparisons performed in the present report. Nonetheless, given that the present design does not allow us to rule out all potential prior exposure effects, future studies should be conducted to determine if similar effects are obtained without prior stimulation.
In sum, the present study supports existing evidence of hypoalgesia following opiate blockade, and demonstrates that this effect is consistent across subjective pain ratings and nociceptive flexion reflex responses elicited by intensely aversive electrocutaneous stimulation.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (NHLBI R01 HL64794).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- al'Absi M, Wittmers LE, Ellestad D, Nordehn G, Kim SW, Kirschbaum C, Grant JE. Sex differences in pain and hypothalamic-pituitary-adrenocortical responses to opioid blockade. Psychosomatic Medicine. 2004;66(2):198–206. doi: 10.1097/01.psy.0000116250.81254.5d. [DOI] [PubMed] [Google Scholar]
- Bell JA, Martin WR. The effect of the narcotic antagonists naloxone, naltrexone and nalorphine on spinal cord C-fiber reflexes evoked by electrical stimulation or radiant heat. Eur J Pharmacol. 1977;42(2):147–154. doi: 10.1016/0014-2999(77)90354-5. [DOI] [PubMed] [Google Scholar]
- Bianchi M, Panerai AE. Naloxone-induced analgesia: involvement of kappa-opiate receptors. Pharmacol Biochem Behav. 1993;46(1):145–148. doi: 10.1016/0091-3057(93)90331-m. [DOI] [PubMed] [Google Scholar]
- Bromm B, Meier W, Scharein E. Antagonism between tilidine and naloxone on cerebral potentials and pain ratings in man. Eur J Pharmacol. 1983;87(4):431–439. doi: 10.1016/0014-2999(83)90082-1. [DOI] [PubMed] [Google Scholar]
- Bruehl S, Chung OY. Parental history of chronic pain may be associated with impairments in endogenous opioid analgesic systems. Pain. 2006;124(3):287–294. doi: 10.1016/j.pain.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Bruehl S, Chung OY, Ward P, Johnson B, McCubbin JA. The relationship between resting blood pressure and acute pain sensitivity in healthy normotensives and chronic back pain sufferers: the effects of opioid blockade. Pain. 2002;100(12):191–201. doi: 10.1016/s0304-3959(02)00295-6. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Davis GC, Bunney WE., Jr. Naloxone alters pain perception and somatosensory evoked potentials in normal subjects. Nature. 1977;270(5638):620–622. doi: 10.1038/270620a0. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Davis GC, Naber D, Pickar D. Pain enhances naloxone-induced hyperalgesia in humans as assessed by somatosensory evoked potentials. Psychopharmacology (Berl) 1983;79(23):99–103. doi: 10.1007/BF00427792. [DOI] [PubMed] [Google Scholar]
- Catley DM, Clarke RW, Pascoe JE. Naloxone enhancement of spinal reflexes in the rabbit. J Physiol. 1983;339:61–73. doi: 10.1113/jphysiol.1983.sp014702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GC, Buchsbaum MS, Bunney WE., Jr. Naloxone decreases diurnal variation in pain sensitivity and somatosensory evoked potentials. Life Sci. 1978;23(14):1449–1459. doi: 10.1016/0024-3205(78)90126-1. [DOI] [PubMed] [Google Scholar]
- Dickenson AH, Le Bars D, Besson JM. Endogenous opiates and nociception: a possible functional role in both pain inhibition and detection as revealed by intrathecal naloxone. Neurosci Lett. 1981;24(2):161–164. doi: 10.1016/0304-3940(81)90241-x. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Ness TJ, Fillingim RB. Endogenous opioids, blood pressure, and diffuse noxious inhibitory controls: a preliminary study. Percept Mot Skills. 2004;99(2):679–687. doi: 10.2466/pms.99.2.679-687. [DOI] [PubMed] [Google Scholar]
- El-Sobky A, Dostrovsky JO, Wall PD. Lack of effect of naloxone on pain perception in humans. Nature. 1976;263(5580):783–784. doi: 10.1038/263783a0. [DOI] [PubMed] [Google Scholar]
- Ernst M, Lee MH, Dworkin B, Zaretsky HH. Pain perception decrement produced through repeated stimulation. Pain. 1986;26(2):221–231. doi: 10.1016/0304-3959(86)90077-1. [DOI] [PubMed] [Google Scholar]
- Fields HL, Basbaum AI. Central nervous system mechanisms of pain modulation. In: Wall PD, Melzack R, editors. Book Title|, Vol. Volume|. City|: Publisher|, Year|. p.^pp. Pages|. [Google Scholar]
- France CR, al'absi M, Ring C, France JL, Brose J, Spaeth D, Harju A, Nordehn G, Wittmers LE. Assessment of opiate modulation of pain and nociceptive responding in young adults with a parental history of hypertension. Biol Psychol. 2005;70(3):168–174. doi: 10.1016/j.biopsycho.2005.01.012. [DOI] [PubMed] [Google Scholar]
- France CR, Suchowiecki S. Assessing supraspinal modulation of pain perception in individuals at risk for hypertension. Psychophysiology. 2001;38(1):107–113. [PubMed] [Google Scholar]
- Goldfarb J, Hu JW. Enhancement of reflexes by naloxone in spinal cats. Neuropharmacology. 1976;15(12):785–792. doi: 10.1016/0028-3908(76)90009-5. [DOI] [PubMed] [Google Scholar]
- Goldstein A. Opioid peptides endorphins in pituitary and brain. Science. 1976;193(4258):1081–1086. doi: 10.1126/science.959823. [DOI] [PubMed] [Google Scholar]
- Grevert P, Albert LH, Goldstein A. Partial antagonism of placebo analgesia by naloxone. Pain. 1983a;16(2):129–143. doi: 10.1016/0304-3959(83)90203-8. [DOI] [PubMed] [Google Scholar]
- Grevert P, Albert LH, Inturrisi CE, Goldstein A. Effects of eight-hour naloxone infusions on human subjects. Biol Psychiatry. 1983b;18(12):1375–1392. [PubMed] [Google Scholar]
- Grevert P, Goldstein A. Effects of naloxone on experimentally induced ischemic pain and on mood in human subjects. Proc Natl Acad Sci U S A. 1977;74(3):1291–1294. doi: 10.1073/pnas.74.3.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grevert P, Goldstein A. Endorphins: naloxone fails to alter experimental pain or mood in humans. Science. 1978;199(4333):1093–1095. doi: 10.1126/science.343250. [DOI] [PubMed] [Google Scholar]
- Hughes J, Smith T, Morgan B, Fothergill L. Purification and properties of enkephalin - the possible endogenous ligand for the morphine receptor. Life Sci. 1975;16(12):1753–1758. doi: 10.1016/0024-3205(75)90268-4. [DOI] [PubMed] [Google Scholar]
- Jacob JJ, Michaud GM. [Reversal by naloxone of the effects of morphine on the unanesthetized dog] Arch Int Pharmacodyn Ther. 1976;222(2):322–340. [PubMed] [Google Scholar]
- Jacob JJ, Ramabadran K. Opioid antagonists, endogenous ligands and nociception. Eur J Pharmacol. 1977;46(4):393–394. doi: 10.1016/0014-2999(77)90235-7. [DOI] [PubMed] [Google Scholar]
- Jacob JJ, Tremblay EC, Colombel MC. [Enhancement of nociceptive reactions by naloxone in mice and rats (author's transl)] Psychopharmacologia. 1974;37(3):217–223. doi: 10.1007/BF00421535. [DOI] [PubMed] [Google Scholar]
- Janssen SA, Arntz A. No evidence for opioid-mediated analgesia induced by phobic fear. Behavior Research and Therapy. 1997;35(9):823–830. doi: 10.1016/s0005-7967(97)00046-6. [DOI] [PubMed] [Google Scholar]
- Kayser V, Guilbaud G. Dose-dependent analgesic and hyperalgesic effects of systemic naloxone in arthritic rats. Brain Res. 1981;226(12):344–348. doi: 10.1016/0006-8993(81)91110-0. [DOI] [PubMed] [Google Scholar]
- Kolaric S, Makulska-Nowak HE, Gumulka SW, Mizerska K. Paradoxical effects of intracerebroventricular low-dose opioid antagonists in SHR with chronic pain. Life Sci. 1999;65(4):395–402. doi: 10.1016/s0024-3205(99)00260-x. [DOI] [PubMed] [Google Scholar]
- Levine JD, Gordon NC, Fields HL. Naloxone dose dependently produces analgesia and hyperalgesia in postoperative pain. Nature. 1979;278(5706):740–741. doi: 10.1038/278740a0. [DOI] [PubMed] [Google Scholar]
- McCubbin JA, Bruehl S. Do endogenous opioids mediate the relationship between blood pressure and pain sensitivity in normotensives? Pain. 1994;57(1):63–67. doi: 10.1016/0304-3959(94)90108-2. [DOI] [PubMed] [Google Scholar]
- McCubbin JA, Helfer SG, Switzer FS, 3rd, Galloway C, Griffith WV. Opioid analgesia in persons at risk for hypertension. Psychosom Med. 2006;68(1):116–120. doi: 10.1097/01.psy.0000195742.24850.79. [DOI] [PubMed] [Google Scholar]
- Mikkelsen S, Ilkjaer S, Brennum J, Borgbjerg FM, Dahl JB. The effect of naloxone on ketamine-induced effects on hyperalgesia and ketamine-induced side effects in humans. Anesthesiology. 1999;90(6):1539–1545. doi: 10.1097/00000542-199906000-00007. [DOI] [PubMed] [Google Scholar]
- Page GD, France CR. Objective evidence of decreased pain perception in normotensives at risk for hypertension. Pain. 1997;73(2):173–180. doi: 10.1016/S0304-3959(97)00111-5. [DOI] [PubMed] [Google Scholar]
- Pert CB, Snyder SH. Opiate receptor: demonstration in nervous tissue. Science. 1973;179(77):1011–1014. doi: 10.1126/science.179.4077.1011. [DOI] [PubMed] [Google Scholar]
- Posner J, Burke CA. The effects of naloxone on opiate and placebo analgesia in healthy volunteers. Psychopharmacology (Berl) 1985;87(4):468–472. doi: 10.1007/BF00432515. [DOI] [PubMed] [Google Scholar]
- Roelofs J, ter Riet G, Peters ML, Kessels AG, Reulen JP, Menheere PP. Expectations of analgesia do not affect spinal nociceptive R-III reflex activity: an experimental study into the mechanism of placebo-induced analgesia. Pain. 2000;89(1):75–80. doi: 10.1016/S0304-3959(00)00347-X. [DOI] [PubMed] [Google Scholar]
- Sandrini G, Milanov I, Willer JC, Alfonsi E, Moglia A, Nappi G. Different effect of high doses of naloxone on spinal reflexes in normal subjects and chronic paraplegic patients. Neuroscience Letters. 1999;261(12):5–8. doi: 10.1016/s0304-3940(98)01000-3. [DOI] [PubMed] [Google Scholar]
- Schobel HP, Handwerker HO, Schmieder RE, Heusser K, Dominiak P, Luft FC. Effects of naloxone on hemodynamic and sympathetic nerve responses to pain in normotensive vs. borderline hypertensive men. J Auton Nerv Syst. 1998;69(1):49–55. doi: 10.1016/s0165-1838(98)00005-8. [DOI] [PubMed] [Google Scholar]
- Stacher G, Abatzi TA, Schulte F, Schneider C, Stacher-Janotta G, Gaupmann G, Mittelbach G, Steinringer H. Naloxone does not alter the perception of pain induced by electrical and thermal stimulation of the skin in healthy humans. Pain. 1988;34(3):271–276. doi: 10.1016/0304-3959(88)90122-4. [DOI] [PubMed] [Google Scholar]
- Tassorelli C, Micieli G, Osipova V, Rossi F, Nappi G. Pupillary and cardiovascular responses to the cold-pressor test. J Auton Nerv Syst. 1995;55(12):45–49. doi: 10.1016/0165-1838(95)00026-t. [DOI] [PubMed] [Google Scholar]
- Terenius L. Opioid peptides and opiates differ in receptor selectivity. Psychoneuroendocrinology. 1977;2(1):53–58. doi: 10.1016/0306-4530(77)90031-2. [DOI] [PubMed] [Google Scholar]
- Ueda H, Fukushima N, Kitao T, Ge M, Takagi H. Low doses of naloxone produce analgesia in the mouse brain by blocking presynaptic autoinhibition of enkephalin release. Neurosci Lett. 1986;65(3):247–252. doi: 10.1016/0304-3940(86)90269-7. [DOI] [PubMed] [Google Scholar]
- Vaccarino AL, Tasker RA, Melzack R. Systemic administration of naloxone produces analgesia in BALB/c mice in the formalin pain test. Neurosci Lett. 1988;84(1):103–107. doi: 10.1016/0304-3940(88)90345-x. [DOI] [PubMed] [Google Scholar]
- Vaccarino AL, Tasker RA, Melzack R. Analgesia produced by normal doses of opioid antagonists alone and in combination with morphine. Pain. 1989;36(1):103–109. doi: 10.1016/0304-3959(89)90117-6. [DOI] [PubMed] [Google Scholar]
- Volavka J, Mallya A, Bauman J, Pevnick J, Cho D, Reker D, James B, Dornbush R. Hormonal and other effects of naltrexone in normal men. Adv Exp Med Biol. 1979;116:291–305. doi: 10.1007/978-1-4684-3503-0_17. [DOI] [PubMed] [Google Scholar]
- Walker JM, Berntson GG, Sandman CA, Coy DH, Schally AV, Kastin AJ. An analog of enkephalin having prolonged opiate-like effects in vivo. Science. 1977;196(4285):85–87. doi: 10.1126/science.190683. [DOI] [PubMed] [Google Scholar]
- Willer JC, Dehen H, Cambier J. Stress-induced analgesia in humans: endogenous opioids and naloxone-reversible depression of pain reflexes. Science. 1981;212(4495):689–691. doi: 10.1126/science.6261330. [DOI] [PubMed] [Google Scholar]
- Willer JC, Dehen H, Cambier J. [Stress-induced analgesia (author's transl)] Nouv Presse Med. 1982a;11(18):1389–1391. [PubMed] [Google Scholar]
- Willer JC, Roby A, Gerard A, Maulet C. Electrophysiological evidence for a possible serotonergic involvement in some endogenous opiate activity in humans. Eur J Pharmacol. 1982b;78(1):117–120. doi: 10.1016/0014-2999(82)90379-x. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. Analgesia and hyperalgesia produced in the rat by intrathecal naloxone. Brain Res. 1980;189(2):593–597. doi: 10.1016/0006-8993(80)90375-3. [DOI] [PubMed] [Google Scholar]


