Abstract
A key step in glutamatergic synapse maturation is the replacement of developmentally expressed N-methyl-D-aspartate receptors (NMDARs) with mature forms that differ in subunit composition, electrophysiological properties and propensity to elicit synaptic plasticity. However, the mechanisms underlying the removal and replacement of synaptic NMDARs are poorly understood. Here we demonstrate that NMDARs containing the developmentally regulated NR3A subunit undergo rapid endocytosis from the dendritic plasma membrane in cultured rat hippocampal neurons. This endocytic removal is regulated by PACSIN1/syndapin1, which directly and selectively binds the carboxy-terminal domain of NR3A through its NPF motifs and assembles a complex of proteins including dynamin and clathrin. Endocytosis of NR3A by PACSIN1 is activity dependent, and disruption of PACSIN1 function causes NR3A accumulation at synaptic sites. Our results reveal a new activity-dependent mechanism involved in the regulation of NMDAR expression at synapses during development, and identify a brain-specific endocytic adaptor that confers spatiotemporal and subunit specificity to NMDAR endocytosis.
Stabilization and maturation of synapses is central to neural circuit formation. During development, excitatory synapses in the central nervous system are remodeled in response to neuronal activity, ultimately resulting in the formation of fast and durable connections. One prominent example of such remodeling is the switch from developmental to mature forms of NMDARs during the postnatal period. Glutamatergic synapses acquire NMDARs early in development1. Subsequently, during an activity-dependent critical period, the subunit composition of NMDARs changes, altering their synaptic attachment and kinetic properties2-5. This receptor switch is thought to ‘solidify’ neural circuitry and fine-tune the propensity of glutamatergic synapses to undergo plasticity6. And yet, little is known about the molecular mechanisms for removing or replacing NMDARs at the synapse.
The targeting of NMDARs to and from synapses is influenced by the specific subunits that make up the receptor complex7. Most NMDARs studied to date consist of an obligatory NR1 subunit and combinations of four NR2 (A-D) subunits. More recently, a distinct class of nonconventional NMDAR subunits termed NR3 (A-B) has been identified8. Whereas mature NMDAR subtypes typically comprise NR2A subunits, receptors at developing synapses often contain NR2B or NR3A (refs. 2,9). NR3A shares only limited sequence homology with NR1 and NR2, and heteromeric receptors containing NR3A (NR1/NR2/NR3A) possess unique channel properties, including reduced calcium (Ca2+) permeability and low sensitivity to magnesium (Mg2+) blockade8,10,11. In addition, NR3A can assemble with NR1 (ref. 10) to form functional glycine receptors12. Although intracellular carboxy-terminal domains of NR1 and NR2 subunits have been implicated in synaptic targeting, intracellular trafficking, downstream signal transduction and channel regulation7, the role of NR3 intracellular domains in NMDAR function remains unknown. Notably, NR3A is expressed primarily during a narrow time window of postnatal development over which synaptic circuitry is established9, and mice lacking NR3A show increased dendritic spine density8, suggesting a potential role for this subunit in synapse maturation or elimination.
One possible mechanism for eliminating certain NMDARs and incorporating others is to differentially regulate their trafficking between synapses and intracellular compartments through exocytic and endocytic pathways. Precedents for a role of exocytosis and endocytosis in postsynaptic maturation exist at the neuromuscular junction where activity and innervation control the accumulation or loss of nicotinic acetylcholine receptors (nAChRs)13. Substantial evidence also supports a role for exocytic and endocytic receptor cycling in controlling AMPA-type glutamate receptor abundance at synapses during synaptic plasticity, but NMDARs have conventionally been thought to be stably fixed within the postsynaptic density (PSD). Challenging this view, recent studies have revealed that NMDARs can move rapidly in and out of the neuronal surface14-20. Atleast some forms of NMDAR exocytic and endocytic trafficking require synaptic activity21 or ligand binding17,22 ,23, and may initiate synaptic changes that lead to synapse stabilization or elimination during development16,23.
Despite recent progress, our knowledge of how postsynaptic NMDARs are linked to and regulated by the endocytic machinery is only just emerging. The carboxy terminus of NR2B contains a tyrosine-based endocytic motif that interacts with the clathrin adaptor AP-2 in a manner regulated by PSD-95 and phosphorylation24-26. Ligand binding promotes NMDAR downregulation18,22 and synaptic replacement23, and simultaneously stimulates the association of NMDARs with AP-2 (ref. 17), suggesting the regulated recruitment of the clathrin endocytic machinery.
Of particular interest among the accessory proteins that control clathrin-mediated endocytosis is PACSIN1/syndapin1, a neuron-specific multivalent adaptor that coordinates actin remodeling with cargo recruitment and membrane fission during endocytosis27. PACSIN1/syndapin1 was initially identified as a phosphoprotein upregulated during neuronal development28 and a synaptically localized binding partner of the GTPase dynamin29. PACSIN1 contains two NPF motifs essential for binding to Eps15-homology (EH) domains, which are present in several endocytic accessory proteins, and a carboxy-terminal SH3 domain that binds to proline-rich domains of dynamin 1 and the actin organizing protein N-WASP (ref. 29). Although implicated in synaptic vesicle endocytosis as part of a dynamin-associated protein complex, the function of PACSIN1 in postsynaptic compartments has not been examined.
We investigated the events that regulate the synaptic targeting and removal of NR3A subunits in developing neurons. We found that NMDARs containing NR3A are less enriched in the PSD than other NMDAR subtypes, and undergo rapid endocytosis from the dendritic plasma membrane. NR3A endocytosis required synaptic activity and NMDAR activation and was regulated by a specific interaction of the NR3A carboxy terminus with PACSIN1. We show that PACSIN1 is localized postsynaptically at excitatory synapses and that disrupting PACSIN1 function results in increased synaptic accumulation of NR3A-containing NMDARs. Our results reveal the recruitment of a cargo-selective endocytic accessory protein as a new cellular pathway for downregulating NMDARs containing the enigmatic developmentally regulated NR3A subunit.
RESULTS
NR3A localizes to extra- and perisynaptic compartments
NR1 and NR2 subunits are abundant at excitatory synapses, where they concentrate at PSDs (ref. 1), but the subcellular localization of NR3A-containing NMDARs is less clear. To examine the localization and trafficking of NR3A subunits, we expressed NR3A tagged with green fluorescent protein (GFP-NR3A) in cultures of CA1-CA3 hippocampal neurons. GFP was inserted at the extracellular amino terminus to allow the monitoring of surface receptors. GFP-NR3A coassembles with NR1 and NR2 subunits in HEK293 cells and forms functional receptors properly targeted to the plasma membrane10. When expressed in neurons, GFP-NR3A distributed in a punctate pattern throughout the soma and dendrites, and surface staining with antibody to GFP (anti-GFP) revealed efficient targeting to the plasma membrane (Fig. 1a). A fraction of dendritic GFP-NR3A puncta corresponded to intracellular vesicles, as they did not overlap with surface receptors (Fig. 1a, right). No surface expression was detected in transfected astrocytes, which lack endogenous NMDARs, confirming that functional assembly is required for membrane targeting. Visualization of GFP-NR3A in the GFP channel to detect both surface and intracellular receptors revealed that dendritic GFP-NR3A colocalized with native NMDAR subunits (Fig. 1b).
Figure 1.
NMDARs containing NR3A subunits are inefficiently targeted to synapses and reside in intracellular compartments. (a) Expression of GFP-NR3A in hippocampal neurons in culture. Total and surface-labeled GFP-NR3A are shown. Scale bar, 25 μm. Right: single confocal section of a dendrite expressing GFP-NR3A. The accumulation of GFP-NR3A in intradendritic compartments (green only, arrows) was revealed by the lack of colocalization with surface GFP-NR3A (surface puncta appear yellow). Scale bar, 2 μm. (b) GFP-NR3A colocalized with endogenous NR1 subunits (arrows). Scale bar, 5 μm. Right: correlation plot of GFP-NR3A and NR1 pixel intensities. (c) Distribution of GFP-tagged NMDAR subunits at the dendritic surface of hippocampal neurons (DIV21-23). Surface YFP-NR1-1a and GFP-NR2A were concentrated in spines (arrows), whereas surface GFP-NR3A was more uniformly expressed. Scale bar, 5 μm (insets, 2 μm). (d) Quantification of the abundance of surface GFP-tagged NMDAR subunits in dendritic spines. Ratios of spine:shaft fluorescence are the mean of 30-40 spines in 2-4 cells per group (*P < 0.001, t-test). In this and all subsequent figures, error bars represent s.e.m. (e) NR3A subunits were less enriched in postsynaptic densities (PSDs) than NR1 or NR2. Shown are immunoblots of microsomal (LM) and synaptic fractions from forebrain of 3- to 4-week-old rats. LM, light membrane; SPM, synaptic plasma membrane; TX, Triton X-100; PSDI-III, progressively detergent-insoluble PSD fractions. (f) Quantitative analysis of NMDAR enrichment. Data represent band intensities per mg of protein, normalized to total receptor protein in the homogenate (n = 2-4; *P < 0.05 versus NR1; #P < 0.05 versus NR2A/B).
NMDARs are uniformly distributed along dendrites early in development and, as neurons mature, they cluster at synapses, often in spines30. We found that surface-targeted GFP-NR3A puncta were evenly distributed over the dendritic plasma membrane, both in dendritic shafts and spine-like protrusions, throughout the culture period (days in vitro (DIV) 10-23; Fig. 1c). This more uniform distribution of NR3A contrasted with the bright discrete clusters of surface-labeled NR1-1a tagged with yellow fluorescent protein (YFP-NR1-1a) or GFP-NR2A present on dendritic spines of mature hippocampal neurons (Fig. 1c). Quantification of spine:shaft fluorescence ratios confirmed a selective enrichment of surface-expressed NR1-1a and NR2A, but not NR3A, in dendritic spines (Fig. 1d).
To determine whether the more uniform distribution of NR3A at the neuronal surface reflects decreased physical association with the PSD, we performed biochemical fractionation on rat brains. As expected, all NMDAR subunits were enriched in synaptic plasma membranes (SPMs) (Fig. 1e,f). However, within SPM subfractions, NR3A was present primarily in the Triton-soluble and PSDI fractions, whereas NR1 and NR2 were more abundant in the highly detergent-insoluble fractions PSDII and PSDIII (Fig. 1e,f), which correspond to the ‘core’ of the PSD. NR3A was also prominent in light membrane fractions that contain intracellular organelles including endoplasmic reticulum, Golgimembranes and endosomes (Fig. 1f), in agreement with our observation that a substantial fraction of transfected GFP-NR3A is located in intracellular compartments.
The presence of NR3A-containing NMDARs in nonsynaptic and intracellular compartments suggests that these receptors may form part of a mobile receptor pool in neurons. To examine if NR3A is present at synapses, we compared the distribution of GFP-NR3A to the postsynaptic scaffold Shank. GFP-NR3A puncta showed only modest colocalization with Shank (Fig. 2a), consistent with the extrasynaptic and vesicular localization of NR3A (Fig. 1), whereas GFP-NR2A was highly concentrated at synapses (Fig. 2b). Quantification of the percent of GFP clusters overlapping Shank demonstrated a significantly (P < 0.001) decreased accumulation of GFP-NR3A at synapses when compared to GFP-NR2A (Fig. 2c). We obtained similar results when the presynaptic marker synapsin I was used to assess synaptic localization.
Figure 2.
NR3A localizes to extrasynaptic and perisynaptic membrane domains. (a-c) Synaptic targeting of GFP-tagged NR3A and NR2A subunits in hippocampal neurons (DIV21). Scale bars, 5 μm (insets, 2 μm). (a)GFP-NR3A showed limited colocalization with Shank. Arrows, GFP-NR3A puncta located away from the PSD; arrowheads, colocalization; asterisks, NR3A puncta adjacent to but not overlapping with the PSD. (b) GFP-NR2A was efficiently targeted to synapses labeled by Shank. Arrowheads, colocalization. (c) Quantification of the postsynaptic localization of NMDAR subunits (n = 8-9 neurons from two independent cultures per group; **P < 0.001, t-test). (d-g) Pre-embedding silver-enhanced immunogold labeling showing the ultrastructural localization of NR3A in layer IV of adult rat somatosensory cortex. Note that NR3A is localized to extrasynaptic membranes (arrows in d and e) or perisynaptic membranes at variable distances from the PSD (asterisks). An example of NR3A labeling associated with the central region of the PSD is shown in g. Scale bar, 1 μm. d, dendrite; s, spine; t, presynaptic terminal. (h) Quantitative analysis of NR3A distribution relative to the PSD. The distance between each particle and the edge of each PSD was measured, and immunoparticles were allocated into 60-nm bins. Data represent percentage of particles located within the indicated distance intervals (n = 95 particles at 30 cortical synapses). Note that the peak of the distribution corresponds to the perijunctional zone, defined arbitrarily as less than 120 nm from the PSD.
We occasionally detected GFP-NR3A puncta adjacent to, but not overlapping with, Shank-labeled PSDs (Fig. 2a), a pattern similar to that observed for clathrin coats at endocytic zones in dendritic spines31. This perisynaptic pattern was not observed for GFP-NR2A clusters, which showed precise overlap with Shank (Fig. 2b). To corroborate our light microscopy data and relate the distribution of GFP-NR3A in mature neurons to endogenous NR3A in vivo, we performed pre-embedding immunogold electron microscopy on the adult rat brain. At asymmetric synapses, NR3A labeling was preferentially observed at perisynaptic (less than 0.1 μm away from the PSD) or extrasynaptic (more than 0.1 μm away from the PSD) domains of the plasma membrane (Fig. 2d-f). A quantitative analysis revealed that NR3A was most concentrated at the lateral margin of the PSD, within 120 nm of the edge of the synaptic junction, but a fraction of membrane-associated particles was present at the PSD (Fig. 2g,h).
To rule out the possibility that the predominant peri- and extra-synaptic localization of NR3A resulted from the low sensitivity of pre-embedding techniques to detecting synaptic antigens, we used postembedding immunogold electron microscopy. As with the pre-embedding methods, NR3A was found at synaptic junctions but was preferentially distributed peri- and extrasynaptically (Fig. 3a-d). Immunoparticles for NR3A were also found associated with intracellular vesicles in spines (Fig. 3e,f). Double immunogold labeling showed that NR3A colocalized with NR2 subunits at both perisynaptic and synaptic locations (Fig. 3g,h). This pattern of NR3A distribution contrasted with that of the NR1 and NR2A/B subunits, which concentrated at postsynaptic sites (Fig. 3i,j). An analysis of tangential distributions revealed that NR3A not only differs from NR1 and NR2 in terms of its predominant peri- and extrasynaptic localization, but also shows an inverse distribution gradient within the PSD domain, where it concentrates at the edge of the postsynaptic specialization (Fig. 3k). Together, these data indicate that, although not excluded from synapses, NR3A is predominantly found in perisynaptic, extra-synaptic and intracellular localizations in spines, suggesting the existence of an active mechanism for the removal of NR3A-containing receptors. Moreover, these findings demonstrate a graded and reciprocal topological organization of NMDAR subtypes within the PSD.
Figure 3.
Ultrastructural localization of NR3A at CA1 hippocampal synapses of adult rat using postembedding immunogold labeling. (a-d) Immunoparticles for NR3A were found along the extrasynaptic plasma membrane (double arrows), at perisynaptic sites (arrowheads) and at synaptic junctions (arrows) on dendritic spines. (e,f) Immunoparticles for NR3A were also found associated with intracellular vesicles in dendritic spines (double arrowheads). (g,h) Immunoparticles for NR3A colocalized with NR2A/B-labeled immunogold particles at synaptic and perisynaptic sites. (i,j) NR1 and NR2A/B labeling was concentrated at the PSD (arrows). (k) Tangential distribution of immunoparticles for NR1 and NR3A along the PSD of CA1 synapses. The radial location of immunoparticles was measured from the midline of the PSD and normalized across the synapse population to control for variable size. The distribution obtained was mirrored across the midline for display. Labeling for NR1 was heavier at the center of the PSD. In contrast, immunoparticles for NR3A were less abundant in the central area and accumulated at the periphery of the PSD. Scale bars, 0.2 μm. s, spine; t, presynaptic terminal.
NR3A undergoes activity-dependent endocytosis
The relative lack of synaptic enrichment, together with the perisynaptic localization of NR3A near sites of endocytosis and in intracellular vesicles in spines, led us to hypothesize that receptors containing NR3A are selectively removed from synapses by endocytosis. To test this, we monitored the internalization of GFP-NR3A in hippocampal neurons (DIV12-14) using antibody uptake assays. The internalized NR3A thus measured represents heteromeric NR3A complexes, as GFP-NR3A by itself is unable to reach the plasma membrane. Under basal conditions, NR3A-containing receptors underwent robust endocytosis from the neuronal plasma membrane (Fig. 4a-c). Internalized NR3A colocalized with the early endosomal marker EEA1, and a large fraction of dendritic EEA1-labeled endosomes contained live-labeled GFP-NR3A receptors (mean ± s.e.m. = 67.0 ± 1.8% of endosomes colabeled with GFP-NR3A after 15 min at 37 °C, 354 endosomes from 11 neurons, Supplementary Fig. 1 online); this indicated transport into early endosomes and verified that the fluorescence signal measured corresponded to internalized receptors.
Figure 4.
NR3A-containing NMDARs undergo activity-dependent endocytosis in hippocampal neurons. (a) Hippocampal neurons were transfected at DIV10 with GFP-NR3A, and internalization was measured at DIV14 using fluorescence-based antibody uptake assays. Representative images of total GFP-NR3A (green) and internalized GFP-NR3A (red) are shown. Insets, higher magnification examples of internalized puncta in dendrites. Scale bar, 20 μm (insets, 5 μm). (b) NR3A endocytosis occurred in the soma (left), tips of dendritic filopodia (middle) and spine-like protrusions (right). Arrows, endocytosed NR3A. Scale bar, 5 μm. (c,d) Activity stimulated NR3A endocytosis. Data represent means of NR3A endocytosis normalized to control values at 4 °C (n = 10-20 neurons per condition; *P < 0.001, **P < 0.0001 versus control; #P < 0.001 versus basal). Internalization assays were performed on neurons maintained in normal culture medium at 4 °C or 37 °C (basal), culture medium plus AP5 (100 μM) or TTX (1 μM), or culture medium plus NMDA (20 μM) and glycine (100 μM).
To determine whether ongoing neural activity or receptor activation regulates the endocytosis of NMDARs containing NR3A, we added either D-2-amino-5-phosphonopentanoate (AP5), a selective NMDAR antagonist, or tetrodotoxin (TTX), a sodium (Na+) channel blocker that blocks action potentials. The presence of AP5 or TTX during the internalization period prevented NR3A endocytosis (Fig. 4c,d). In contrast, the selective activation of NMDARs with NMDA (20 μM) plus glycine (100 μM) markedly enhanced NR3A endocytosis (Fig. 4c). These findings indicate that NR3A-containing receptors undergo rapid endocytosis from the dendritic plasma membrane, which requires ongoing neuronal activity and is stimulated by NMDAR activation.
NR3A binds PACSIN1/syndapin1
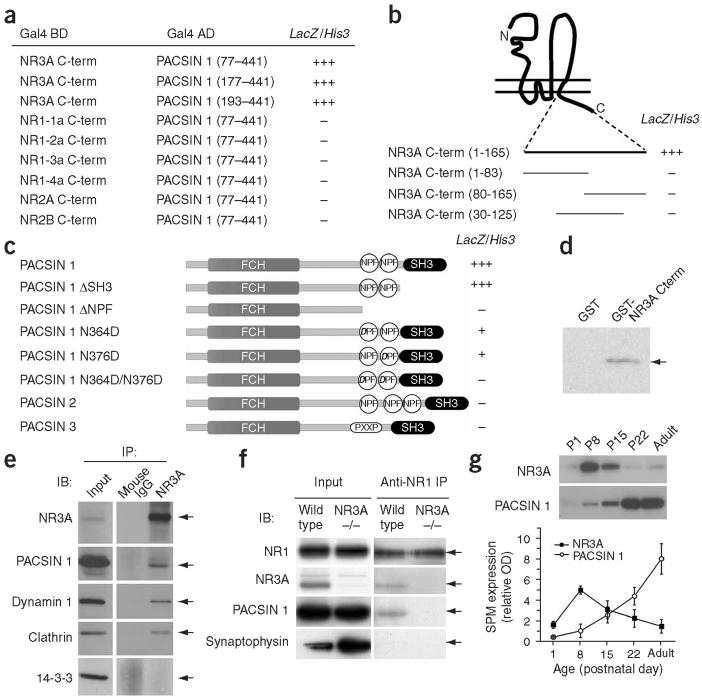
To identify potential molecular mediators of NR3A endocytosis, we performed a yeast two-hybrid screen for binding partners of the intracellular carboxy-terminal domain of NR3A. Three of the clones isolated encoded PACSIN1/syndapin1 (Fig. 5a), a modular protein that contains an N-terminal Fes/CIP4 homology (FCH) domain implicated in actin binding, a coiled-coil region thought to mediate multimerization, two NPF (asparagine-proline-phenylalanine) motifs and a carboxy-terminal SH3 domain that binds dynamin-1 and N-WASP (refs. 28,29).
Figure 5.
NR3A binds the neuron-specific endocytic adaptor PACSIN1/syndapin1. (a)Three independent clones encoding PACSIN1 were isolated by yeast two-hybrid screening of a hippocampal cDNA library using the NR3A C-terminal domain as bait. Interactions between PACSIN1 and C-terminal domains of NR1, NR2 and NR3 subunits were tested in yeast by induction of the reporter genes LacZ (β-galactosidase) and His3.(b) Binding to PACSIN1 required the entire NR3A C terminus. Amino acid numbers refer to positions C-terminal to the last transmembrane segment. (c) Binding to NR3A was mediated by the NPF motifs of PACSIN1 and did not occur with PACSIN2 or PACSIN3. (d) PACSIN1 bound to the C-terminal domain of NR3A in a GST pull-down assay. Extracts of HEK293 cells transfected with Xpress-tagged PACSIN1 were incubated with control GST beads, or beads bound to GST fused to the C-terminal domain of NR3A (GST-NR3A Cterm). Precipitated proteins were detected by immunoblot using antibody to Xpress. (e) PACSIN1 along with dynamin and clathrin coimmunoprecipitated with NR3A from the forebrain of P12-P16 rats. Antibodies used for immunoprecipitation (IP) and immunoblot (IB) are shown. (f) PACSIN1 formed a complex with NR3A-containing NMDARs in the brain. PACSIN1 coimmunoprecipitated with NR1 in wild-type, but not NR3A knockout, mouse brain. (g) NR3A and PACSIN1 showed reciprocal expression patterns during postnatal development. Immunoblot analysis of NR3A and PACSIN1 in synaptic plasma membranes (SPM) isolated from rat forebrain at the indicated postnatal ages. Plotted data represent band intensities from 2-3 independent experiments.
We used yeast two-hybrid assays to examine the selectivity of PACSIN1 binding to NR3A and found that PACSIN1 interacted with the carboxy terminus of NR3A, but not with the carboxy terminus of NR1, NR2A or NR2B (Fig. 5a), consistent with a lack of substantial homology between the NR3A carboxy-terminal domain and corresponding domains in NR1 and NR2. We sought to map the PACSIN1-binding site in NR3A, but none of the smaller fragments tested (Fig. 5b) bound PACSIN1, suggesting a complex interaction dependent on the tertiary structure of the NR3A carboxy-terminal tail. Deletion analyses revealed that the SH3 domain of PACSIN1 was not required for NR3A binding. However, deletion of the two NPF motifs or mutations of the critical asparagine (N) residues to aspartate (D) within one or both PACSIN1 NPF motifs attenuated or abolished the interaction, indicating that PACSIN1 binds NR3A via its NPF motifs (Fig. 5c). Moreover, whereas NR3A interacted with the neuron-specific PACSIN1, no interaction was detected with the ubiquitously expressed PACSIN2 or PACSIN3.
The interaction between PACSIN1 and NR3Awas confirmed using an in vitro binding assay. We fused the carboxy terminus of NR3A to glutathione S-transferase (GST) and used the purified fusion protein (GST-NR3A-Cterm) in pull-down experiments on lysates of HEK293 cells expressing PACSIN1. Consistent with the yeast two-hybrid results, GST-NR3A-Cterm, but not GST alone, pre-cipitated PACSIN1 (Fig. 5d).
NR3A forms a complex with PACSIN1, dynamin and clathrin
We next examined whether NR3A and PAC-SIN1 show coincident spatial distributions in the brain. In situ hybridization showed that PACSIN1 is broadly expressed throughout the brain in young rats (postnatal day (P) 12). At this developmental stage, high levels of both NR3A and PACSIN1 mRNAs were found in the cortex, the CA1 region of the hippocampus, the amygdala and thalamus. Immunohistochemical analysis confirmed the widespread distribution of PACSIN1 protein and its localization to forebrain regions that had high NR3A expression, such as the cortex, amygdala and hippocampal CA1. At the cellular level, NR3A and PACSIN1 were associated with neurons and distributed in a somatodendritic pattern. NR3A staining was denser in the neuropil, whereas PACSIN1 expression was similar in the soma and dendrites (Supplementary Fig. 2 online). Thus, at this level of resolution, PACSIN1 and NR3A show overlapping distribution patterns in vivo.
To test whether NR3A and PACSIN1 form a complex in vivo, we performed coimmunoprecipitation experiments on rat forebrain lysates. Antibodies to NR3A (anti-NR3A) coprecipitated a complex of proteins including PACSIN1 along with the endocytic proteins dynamin and clathrin (Fig. 5e). In addition, PACSIN1 was present in protein complexes coimmunoprecipitated using antibodies against NR1 subunits (Fig. 5f). We observed no interaction between PACSIN1 and NR1 in mice lacking NR3A (Fig. 5f), suggesting that assembly with NR3A subunits is required for NMDARs to associate with PACSIN1. Whereas immunoprecipitation using NR1 or NR3A antibodies readily coprecipitated PACSIN1, reverse coimmunoprecipitation with PACSIN1 antibodies did not isolate detectable NMDARs, perhaps due to the inaccessibility of PACSIN1 antibodies to PACSIN1 complexed with NR3A or to the presence of a large pool of PACSIN1 not stably associated with NR3A. These data demonstrate the association of NR3A-containing NMDARs with PACSIN1 in the brain and suggest that PACSIN1 recruits NR3A to the clathrin and dynamin endocytic machinery.
A prominent feature of NR3A is its high expression during a critical window of postnatal development in most brain areas with down-regulation at later postnatal stages9. We examined the temporal relation of NR3A and PACSIN1 expression during postnatal development in vivo and during synapse development in vitro. The expression of PACSIN1 was rapidly upregulated in synaptic plasma membranes from rat forebrain over the first postnatal weeks (Fig. 5g). Similarly, PACSIN1 was upregulated between DIV7 and DIV24 in hippocampal and cortical neurons in vitro and, consistent with its proposed role in endocytosis, colocalized with clathrin puncta (Supplementary Fig. 3 online). Upregulation of PACSIN1 coincided with a decline of NR3A abundance in synaptic plasma membranes (Fig. 5g), demonstrating a reciprocal pattern of NR3A and PACSIN1 synaptic expression during postnatal development and consistent with a potential role for PACSIN1 in NR3A downregulation.
PACSIN1 regulates NR3A endocytosis and surface expression
To test whether PACSIN1 regulates NR3A endocytosis, we first analyzed the effect of PACSIN1 on surface expression of NR3A, reasoning that potentiating endocytosis would result in a reduction of surface NR3A whereas defects in endocytosis would drive receptor accumulation at the neuronal surface. Hippocampal neurons were transfected with GFP-NR3A alone or with different PACSIN1 constructs. The experiments were performed in DIV12-14 neurons because at this developmental stage, endogenous PACSIN1 is already present but not yet expressed at maximal levels (Supplementary Fig. 3). Coexpression of PACSIN1 reduced steady-state surface levels of GFP-NR3A (Fig. 6a,b) and this reduction was not associated with changes in total GFP-NR3A expression (data not shown), indicating redistribution of NR3A from the plasma membrane to intracellular compartments. Mutations in the NPF motifs of PACSIN1 or deletion of its SH3 domain abrogated the effect (Fig. 6b). Further, cotransfection of the PACSIN1ΔSH3 mutant, which binds NR3A but cannot link PACSIN1 to the endocytic machinery, significantly (P < 0.01) increased NR3A surface expression, consistent with a dominant-negative effect (Fig. 6b). Expression of wild-type PACSIN1 or the PACSIN1ΔSH3 mutant had no effect on surface levels of the AMPA receptor subunit GluR1 (Fig. 6c) despite robust endocytosis of this receptor in hippocampal neurons, providing further support for the specificity of the NR3A-PACSIN1 association. Because synaptic activity is required for NR3A endocytosis, we asked whether the PACSIN1-induced loss of surface receptors was dependent on ongoing network activity or excitatory synaptic transmission. Indeed, the PACSIN1-induced loss of surface NR3A was abolished upon activity blockade (TTX) or in the presence of glutamate receptor antagonists (Fig. 6d).
Figure 6.
PACSIN1 mediates NR3A endocytosis. (a) PACSIN1 reduced NR3A surface expression. Hippocampal neurons expressing GFP-NR3A with or without PACSIN1 were surface labeled with anti-GFP antibody. Scale bar, 25 μm. (b) Quantification of NR3A surface expression in control neurons or neurons expressing wild-type or mutant PACSIN1. Data represent normalized surface GFP-NR3A fluorescence intensities (n = 3-4 experiments, 30-60 neurons per condition; *P < 0.05, **P < 0.01 versus GFP-NR3A alone). (c) PACSIN1 did not affect the surface expression of GFP-GluR1 (n = 8-10, P < 0.05). (d) The PACSIN1-induced decrease in surface NR3A required neuronal activity and glutamate receptor activation. After transfection, neurons were maintained for 2-3 d in normal culture medium, culture medium plus TTX, or culture medium plus AP5 and kynurenic acid, before surface antibody labeling (n = 2-3 experiments, 15-25 neurons per condition; *P < 0.01 versus NR3A alone; #P < 0.01, ##P < 0.001 versus NR3A + PACSIN1 in untreated neurons). (e) PACSIN1 is required for NR3A endocytosis. Antibody uptake assays were performed on neurons expressing GFP-NR3A alone or together with the dominant-negative PACSIN1-ΔSH3 or a soluble fragment containing the NPF motifs of PACSIN1. Scale bar, 25 μm (insets, 5 μm). (f) Quantification of NR3A endocytosis (n = 10-20; *P < 0.01 versus NR3A alone at 37 °C; #P < 0.01). y(g) Hippocampal neurons were transfected with wild-type or mutant PACSIN1, and tested for overall clathrin-dependent transferrin uptake (n = 8-14 neurons).
To directly examine if disrupting PACSIN1 function affects the internalization of NR3A-containing NMDARs, we used antibody uptake assays. Coexpression of the dominant-negative PACSIN1ΔSH3 significantly (P < 0.01) decreased NR3A endocytosis (Fig. 6e,f). To rule out potential nonspecific effects of expressing a large multifunctional protein, we tested the ability of a smaller PACSIN1 fragment encompassing the NR3Abinding NPF motifs (NPF1/2) to interfere with NR3A endocytosis. Coexpressing the soluble NPF1/2 fragment also caused a significant (P < 0.01) blockade of NR3A internalization (Fig. 6e,f), whereas cotransfection of a construct with the asparagine residues mutated to aspartate (DPF1/2) did not alter internalization (Fig. 6e,f). The effects of PACSIN1 on NR3A surface expression and endocytosis were not due to a general disruption of neuronal endocytic trafficking, as none of the constructs used for interfering with PACSIN1 function affected transferrin uptake under our experimental conditions (Fig. 6g). Taken together, our results demonstrate that PACSIN1 regulates the surface expression of NR3A in neurons in an activity dependent manner and suggest that PACSIN1 acts by coupling NR3A to the endocytic machinery.
Selective regulation of NR1/NR2/NR3A channel expression
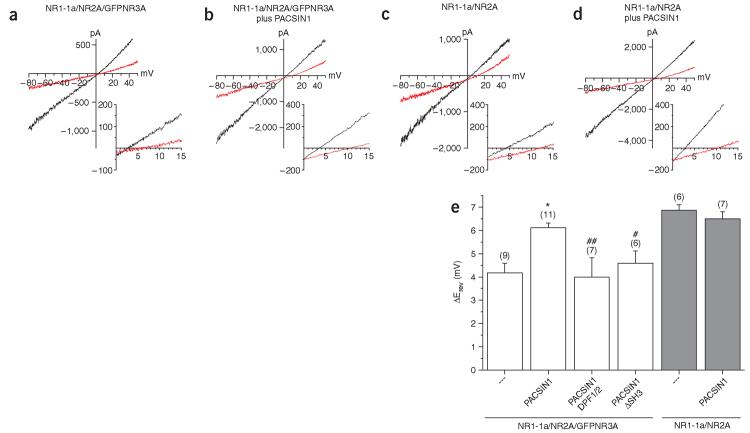
To evaluate whether PACSIN1 regulates the functional surface expression of NR3A-containing NMDARs, GFP-NR3A subunits were coexpressed with NR1-1a and NR2A in HEK293 cells in the absence or presence of PACSIN1. Coexpression of NR1-1a, NR2A and NR3A subunits in heterologous cells yields a mixed population of NMDA-gated channels including NR1/NR2A channels and NR1/NR2A/NR3A channels that both contribute to the whole-cell current8,10. To determine whether PACSIN1 expression can alter the population of surface-expressed NMDARs, we took advantage of the low Ca2+ permeability that distinguishes NR1/NR2/NR3A triheteromeric complexes10.
We estimated differences in the relative calcium permeability of NMDARs by monitoring the shift in reversal potential (ΔErev) of recombinant NMDAR currents during steady-state current-voltage (I-V) relations obtained via a voltage ramp protocol in 2 mM and 10 mM extracellular Ca2+ (Fig. 7a-e). The ΔErev of the current measured for NR1-1a/NR2A/GFP-NR3A was 4.17 ± 0.41 mV (Fig. 7a,e). Coexpression of PACSIN1 significantly (P < 0.001) increased ΔErev (6.12 ± 0.21 mV, Fig. 7b,e), reflecting an increase in Ca2+ permeability of the population of surface-expressed NMDARs. The magnitude of ΔErev in the presence of PACSIN1 was nearly as large as that observed when only NR1-1a and NR2A receptors were coexpressed (6.87 ±0.23 mV, Fig. 7c,e), but this PACSIN1-induced increase in ΔErev was not due to a lack of NR3A expression as it was readily observed in the recorded cells by GFP fluorescence. Notably, coexpression of PACSIN1 did not alter the ΔErev of NR1-1a/NR2A diheteromeric receptors (6.50 ± 0.30 mV, Fig. 7d,e), suggesting that PACSIN1 selectively removed NR3A-containing receptors, thus favoring the surface expression of conventional diheteromeric (NR1-1a/NR2A) NMDARs. The removal of NR3A subunits by PACSIN1 was contingent on its ability to bridge NR3A to the endocytic machinery, as both mutation of the NPF motifs and deletion of the SH3 domain prevented the PACSIN1-induced increase in ΔErev (PACSIN1 DPF1/2: 4.00 ± 0.82 mV, n = 6; PACSIN1ΔSH3: 4.59 ± 0.52 mV, n = 5; Fig. 7e). The lack of effect of PACSIN1ΔSH3 in HEK293 cells contrasted dominant-negative effect on NR3A surface expression in neurons (Fig. 6), consistent with the inhibition of ongoing neuron-specific mechanisms not present in HEK293 cells, which lack PACSIN1. Collectively, these data show that PACSIN1 is able to alter the functional composition of NMDARs by selectively facilitating the surface removal of NR3A-containing subtypes.
Figure 7.
PACSIN1 reduces the functional surface expression of NR3A-containing triheteromeric NMDARs. HEK293 cells were transfected with NR1-1a/NR2A and either GFP-NR3A, PACSIN1 or both. The cells were then subjected to whole-cell recordings. (a-d) Representative I-V relationships in 2 mM Ca2+ (black traces) or 10 mM Ca2+ (red traces) for each of the indicated transfection conditions. Insets, expanded views of the region of the I-V curves near Erev. Note the depolarized Erev in 10 mM Ca2+ when PACSIN1 was coexpressed with NR1-1a/NR2A/GFP-NR3A (b) compared to when PACSIN1 was absent (a), indicating the increased Ca2+ permeability of the population of NMDAR channels in the presence of PACSIN1. (e) Summary graph of ΔErev for each of the conditions tested. Numbers in parentheses indicate the number of observations for each condition. *P < 0.001 compared to NR1-1A/NR2A/GFP-NR3A; #P < 0.01 and ##P < 0.05 compared to NR1-1a/NR2A/GFP-NR3A + PACSIN1.
PACSIN1 mediates the synaptic removal of NR3A
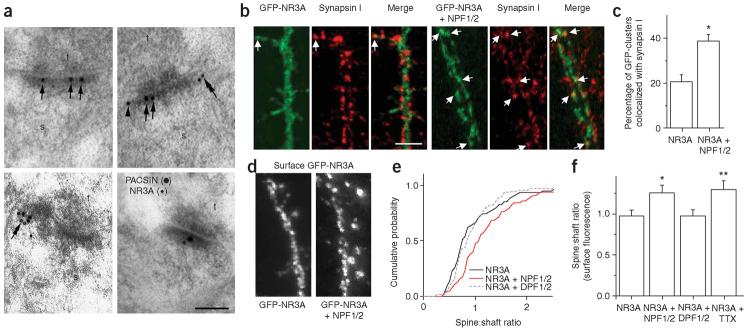
PACSIN1/syndapin1 is specifically expressed in neurons, where it has been localized by light microscopy to presynaptic terminals29. However, the precise ultrastructural localization of PACSIN1 has not been evaluated. To determine the synaptic localization of endogenous PACSIN1, we used postembedding immunogold electron microscopy. In hippocampal CA1, PACSIN1 immunolabeling was observed both presynaptically and postsynaptically at asymmetric synapses. Within dendritic spines, gold particles were found at synaptic and perisynaptic sites, along the extrasynaptic plasma membrane, and associated with intracellular vesicles in lateral domains of the PSD (Fig. 8a). Double-labeling showed NR3A and PACSIN1 immunoparticles colocalizing at the same synaptic specialization (Fig. 8a).
Figure 8.
PACSIN1 mediates synaptic removal of NR3A. (a) Postembedding immunogold labeling showed that PACSIN1 localizes to the PSD (arrows) and perisynaptic sites (arrowheads) at hippocampal CA1 synapses. PACSIN1 immunoparticles were also found associated with intracellular vesicles located in lateral domains of dendritic spines (double arrows). Bottom right, PACSIN1 immunoparticles (20 nm) close to immunoparticles for NR3A (10 nm) in the same synaptic specialization. Scale bar, 0.1 μm. s: spine; t, presynaptic terminal. (b) Hippocampal neurons were transfected on DIV10 with GFP-NR3A alone or GFP-NR3A plus myc-NPF1/2 and fixed and stained for synapsin I on DIV21. Coexpression of the NPF1/2-containing fragment increased the colocalization of NR3A with synapsin I. Scale bar, 5 μm. (c) Quantitative analysis of GFP-NR3A synaptic localization. Data represent percent overlap between GFP-NR3A and synapsin I-immunoreactive puncta (n = 7-8 neurons from two independent cultures; *P < 0.01 versus GFP-NR3A alone). (d) Representative examples of dendritic surface-labeled GFP-NR3A in hippocampal neurons expressing GFP-NR3A alone or GFP-NR3A plus the myc-NPF1/2 fragment. Scale bar, 5 μm. (e) Cumulative probability distributions of the spine:shaft surface GFP-NR3A ratios. (f) Quantification of spine enrichment of surface GFP-NR3A in hippocampal neurons (DIV21). Note that mean spine:shaft ratios were significantly higher in neurons transfected with GFP-NR3A plus myc-NPF1/2. Activity blockade with TTX (1 μM) for 3-4 d caused a similar increase in spine:shaft NR3A surface ratio (n = 64-95 spines from 6-10 cells per group; *P < 0.05, **P < 0.001, t-test).
The ultrastructural localization of PACSIN1 both within the PSD and at lateral membrane zones fits well with recently proposed pathways for postsynaptic endocytosis31-33. In addition, when expressed in hippocampal neurons, recombinant PACSIN1 is targeted to synaptic clusters containing NR3A (Supplementary Fig. 3). If PACSIN1 is involved in endocytic processes that control the synaptic abundance of NR3A-containing NMDARs during development, we reasoned that interfering with PACSIN1-mediated endocytosis should affect the accumulation of NR3A at synapses. To test this, we expressed GFP-NR3A alone or with the dominant inhibitory NPF1/2 fragment of PACSIN1 in hippocampal neurons from early stages of development (DIV10), and measured the colocalization of GFP-NR3A puncta with synapsin I in later stage neurons (DIV21-23). Coexpression of NPF1/2 caused an increase in the synaptic localization of GFP-NR3A that contrasted with its predominantly extrasynaptic and intracellular vesicular distribution in control neurons (Fig. 8b,c). To determine if the accumulation of NR3A at synapses reflected increased surface levels, we compared spine:shaft surface fluorescence ratios in neurons expressing GFP-NR3A alone or with NPF1/2. We found that disruption of PACSIN1 binding by the expression of NPF1/2 increased the enrichment of GFP-NR3A at the spine surface when compared with that at the adjacent dendritic shaft, indicating that PACSIN1 is preferentially involved in the internalization of NR3A-containing NMDARs from the spine plasma membrane (Fig. 8d-f). Coexpression of the corresponding mutated PACSIN1 fragment (DPF1/2) did not alter spine:shaft surface ratios of NR3A (Fig. 8d-f). Activity blockade, which blocks NR3A endocytosis and occludes PACSIN1 changes in the surface expression of NR3A-containing receptors, mimicked the effect of coexpressing the NPF1/2 fragment, causing a similar-magnitude enrichment of NR3A at the spine plasma membrane (Fig. 8f). Together, these findings show that PACSIN1-mediated NR3A endocytosis contributes to the removal of NR3A from glutamatergic synapses.
DISCUSSION
We have identified a direct interaction between the nonconventional NMDAR subunit NR3A and the endocytic adaptor PACSIN1, and shown that this interaction directs the removal of NR3A-containing NMDARs from synapses. Our findings provide the first instance of selective and regulated trafficking of NMDARs containing the NR3A subunit, demonstrate a new function for the PACSIN/syndapin family of proteins at synapses, and reveal a new mechanism for the endocytosis of a select subset of NMDARs. PACSIN1 is associated with synapses, localizes to dendritic protrusions and is upregulated during neuronal development, coincident with periods of synaptic maturation. We propose that PACSIN1-mediated endocytosis provides a means to control the abundance and subunit composition of NMDARs at synapses during development. Specifically, data presented here support a model whereby the presence of the NR3A subunit in immature NMDAR complexes in young neurons keeps receptors in a mobile pool, and the upregulation of PACSIN1 during development accelerates the removal of NR3A-containing receptors from synapses, allowing the insertion of mature NMDAR subtypes that are more firmly associated with the PSD. At mature synapses, the presence of channels with brief open times and high sensitivity to Mg2+ blockade (that is, NR2A-containing, NR3A-lacking) would ensure more tightly input-specific coding with a requirement for coincident activity (Supplementary Fig. 4 online).
NMDAR mobility and receptor endocytosis
Until recently, NMDARs were generally considered to be immobile and tightly anchored to the postsynaptic membrane. Although this seems to be the case in mature neurons, NMDARs move rapidly into and out of synapses in developing neurons15,18,34. It is likely that synaptic removal of NMDARs involves a combination of lateral diffusion15,34 and endocytosis16-18. Although the mechanisms have been unclear, this process is almost certainly determined by a balance between interactions with scaffolding proteins that anchor receptors at the PSD, and interactions with adaptors that target NMDARs to the endocytic machinery for membrane removal25. Our findings suggest that, whereas NR2 subunits carry molecular determinants for synaptic attachment7,35,36, NR3A subunits impart mobility and direct the synaptic removal of NMDARs via endocytosis by linking the receptor to the endocytic scaffold.
First, our biochemical data show that NMDARs containing NR3A are less tightly associated with PSDs than conventional NMDARs composed of only NR1/NR2. Second, the tangential distribution gradient of NR3A within the PSD is consistent with a higher rate of migration away from synapses, and differs from NR1 and NR2 subunits that display an exactly opposite gradient—that is, the probability of finding NR3A increases steadily towards the postsynaptic edge as compared to NR1, which concentrates at the center of the PSD (Fig. 3k and ref. 37). Notably, the synaptic density of NR3A-containing NMDARs peaks at the lateral edge of the PSD, indicating the presence of a rate-limiting step at this level and consistent with models of the PSD acting as a ‘diffusion trap’. Third, outside the PSD proper, NR3A is often found at perisynaptic sites and intracellular vesicles in spines and undergoes rapid internalization, suggesting the uncoupling of NR3A-containing receptors from the PSD followed by lateral movement toward zones of clathrin assembly and endocytosis localized in the perisynaptic domain31-33. Alternatively, and rather than reflecting receptor mobility, our findings could indicate the targeting of two different populations of NR3A-containing channels, NR1/NR3A and NR1/NR2/NR3A (refs. 10,12), to peri- and extrasynaptic spaces and synaptic spaces, respectively. Our observation that NR3A colocalizes with NR2 at perisynaptic sites, however, suggests that such subsynaptic location is not an exclusive domain of NR1/NR3A complexes. In addition, the gradient of NR3A-containing receptors at membrane domains lateral to the PSD, along with their robust endocytosis, is reminiscent of events that occur at the neuromuscular junction during development or after denervation, upon which nAChRs migrate into perijunctional regions before being internalized13,38.
PACSIN1 as an accessory factor for NR3A endocytosis
Although most endocytic accessory proteins are expressed ubiquitously and are believed to contribute in a general manner to clathrin-mediated endocytosis, a few, including PACSIN1, are enriched at synapses, suggesting a more specialized function29,39. Using a combination of interaction and receptor trafficking assays, we have shown that PACSIN1 has a specific role in NMDAR endocytosis and selectively controls the surface and synaptic expression of NMDARs containing NR3A.
PACSIN1 differs from other endocytic adaptors because of its ability to connect to both the endocytic machinery and the actin cytoskeleton. Recent models of clathrin-mediated endocytosis in neurons suggest that receptors are not internalized directly at the PSD, but rather need to diffuse or be escorted to specialized endocytic domains adjacent to but spatially segregated from PSDs before internalization31-33. Because of its ability to couple endocytic machinery and the actin cytoskeleton, PACSIN1 may act as a bridge between synaptically localized NR3A and the endocytic scaffold, perhaps by propelling or directing cargo to perisynaptically located endocytic zones via actin reorganization. Such a function is supported by the location of PACSIN1 along the endocytic pathway (Fig. 8) and, indeed, our domain mapping analysis showed that binding to NR3A is mediated by the NPF motifs in PACSIN1, leaving the SH3 domain available to bind to endocytic partners and actin regulators. According to this model, PACSIN1 binding would facilitate the lateral movement of NR3A-containing receptors away from the PSD and allow receptor capture by endocytic clathrin-coated pits. Alternatively, PACSIN1 could accelerate the fission of budded vesicles containing the activated receptor by recruiting dynamin and/or the actin cytoskeleton through N-WASP. This is in line with studies implicating PACSIN1 in late stages in the formation of clathrin-coated pits27,40,41. In the latter scheme, NR3A could either diffuse constitutively into the perisynaptic membrane, or be actively shuttled through sequential interactions of PACSIN1 with the actin cytoskeleton (for removal to the endocytic zone) and dynamin (to trigger internalization). In both schemes, PACSIN1 would prevent NR3A from diffusing back into the synapse, potentially allowing for replacement by other NMDAR subtypes (Supplementary Fig. 4).
Notably, the domain arrangement of the PACSINs is closely related to that of nervous wreck (Nwk), a cytoskeletal adaptor that regulates synaptic bouton growth in Drosophila42. Nwk localizes to the periactive zone of Drosophila neuromuscular junctions and interacts with the Drosophila ortholog of WASP. By analogy to Nwk, PACSIN1 may coordinate NMDAR activation and endocytosis with actin remodeling events. Indeed, manipulations of both NR3A and PACSIN1 expression impact actin dynamics: NR3A-null mutants show increased spine density8, and the overexpression of PACSIN1 induces filopodia extension in non-neuronal cells43. Further studies will be necessary to address whether PACSIN1 causes a similar phenotype in neurons, and whether this is mediated by NMDAR signaling.
Endocytosis and synapse development
What are the functional consequences of removing NR3A from synapses? Unlike any of the other known NMDAR subunits, inclusion of NR3A into the NMDAR complex causes the formation of a nonconventional NMDAR channel with low Ca2+ permeability and reduced sensitivity to Mg2+ blockade8,10,11. We now document that NR3A does not undergo the characteristic pattern of synaptic stabilization shown by other NMDARs (ref. 30), which has been largely attributed to a replacement of NR2B by NR2A in the receptor complex and is thought to mark the end of critical periods of experience-dependent plasticity14. But although the NR2B/NR2A switch seems necessary for the acquisition of certain receptive field properties14,44, several lines of evidence indicate that this model is incomplete and suggest the participation of additional mechanisms. First, both NR2Aand NR2B subunits contain relevant information for synaptic targeting7,35,36. Second, NR2A upregulation and NR2B downregulation do not match the end of critical periods45. Third, NR2A knockout mice have critical periods of normal duration in somatosensory and visual cortices44,46. Notably, the onset of PACSIN1 expression and concomitant downregulation of synaptic NR3A (Fig. 5g) correlate with the closure of critical periods, and the synaptic removal of NR3A could be predicted to increase NMDAR stability at PSDs. Moreover, it has been suggested that increases in the voltage dependence of NMDAR responses underlie the developmental decline in plasticity47,48, observations that could be explained, in part, by alterations in the NR3A content of synaptic NMDARs.
An important finding in the current study is that synaptic activity is required for PACSIN1-dependent endocytosis and synaptic removal of NR3A-containing NMDARs. Through its regulation by synaptic activity, NR3A removal could locally drive the stabilization of inputs that remained active beyond the initial presynaptic contact. Further, the recruitment of PACSIN1 provides the capability to couple changes in NMDAR properties to actin polymerization, thereby translating activity into both functional and structural remodeling. Indeed, maturation of brain circuitry is characterized by the progressive stabilization of dendritic spines, a structural feature altered in NR3A-null mutants8. By recruiting the endocytic machinery, NR3A could behave as an endocytic adaptor for the NMDAR itself, and its regulated synaptic removal might offer an attractive mechanism for directing synapse elimination.
METHODS
cDNA constructs
Generation of GFP-NR3A was described previously10. For expression in mammalian cells, Xpress-tagged PACSIN 1 and myc-tagged PACSIN 1 were subcloned into pRK5. PACSIN1ΔSH3 mutant was generated by introducing a stop codon just before the SH3 domain coding region, and NPF mutants were obtained by site-directed mutagenesis. Soluble fragments of PACSIN1 containing the NPF motifs (aa 340-391) were subcloned into pmyc-CMV (Clontech).
Immunofluorescent analysis
Cultured rat hippocampal neurons were transfected at DIV10 with GFP-tagged NR3A alone or together with myc-tagged PACSIN1 constructs. For synaptic localization, neurons were stained at DIV 21-23 using anti-Shank or synapsin I antibodies. Surface expression and internalization assays were carried out at DIV12-14 after labeling surface-tagged receptors in live neurons with anti-GFP antibody. For transferrin uptake assays, neurons were incubated with Alexa 568-conjugated transferrin, washed with serum-free medium, and incubated with holotransferrin to exchange the surface bound transferrin and selectively monitor the endocytosed fraction. Further details of staining and trafficking assays, along with data analysis methods, are available in Supplementary Methods online.
Biochemical fractionation
Homogenates were prepared from forebrains of 3-4 week old rats, and synaptic fractionation was performed essentially as described49.
Yeast two-hybrid screen and biochemical binding assays
The cDNA encoding the NR3A carboxy terminus was fused in frame with the GAL4 DNA-binding domain of pAS2-1 (Clontech), and used to screen a rat hippocampal cDNA library fused to the GAL4 DNA-activating domain of pACT2 (Clontech). For GST pull down assays, the carboxy terminus of NR3A was subcloned into pGEX-2T (Pharmacia) and expressed in E.coli. Extracts of HEK293 transfected with Xpress-tagged PACSIN1 were incubated with control GST beads, or beads bound to GST fused to the carboxyl terminus of NR3A, washed with buffer containing 150 mM NaCl and 0.1% TX-100, and eluted with SDS. Bound proteins were detected by Western blotting using anti-Xpress antibody. Coimmunoprecipitation experiments were performed as described10.
Histological procedures
In situ hybridization was performed with 33P-labeled RNA probes (rat NR3A-probe: nucleotides 3330-3821, U29873, rat PACSIN1-probe: nt 745-1600, AF104402). For immunohistochemistry, mouse anti-NR3A and rabbit anti-PACSIN1 antibodies were used as primary antibodies.
Electron microscopy
Rats were perfused with 4% paraformaldehyde and 0.05-0.6% glutaraldehyde in phosphate buffer. For pre-embedding immunogold labeling, brain sections (50-70 μm) were cut on a Vibratome and processed for immunocytochemical detection of NR3A using silver-enhanced immunogold techniques. For postembedding immunogold labeling, ultrathin sections (70-90 nm) from three Lowicryl-embedded blocks slices were cut on an Ultramicrotome and processed for immunocytochemical detection of NR3A and/or PACSIN1.
Electrophysiology
HEK293 cells were transfected using calcium phosphate and recorded as described50.
Supplementary Material
ACKNOWLEDGMENTS
We thank J.F. Wesseling for intellectual support; K. Hawk, A. Zandueta, I. López-García, C. Zhang and H. Zhang for technical help; J.R. Naranjo and M. Palczewska for support with yeast assays; and G. Augustine, T. Blanpied, J. Hernandez, B. Philpot, A. Horton and R. Mooney for critical readings of this manuscript. This work was supported by grants from the National Alliance for Research on Schizophrenia and Depression, the Marie Curie International Reintegration Grant (IRG) Program and the Unión Temporal de Empresas project (UTE) at the Centro de Investigación Médica Aplicada (CIMA; I.P.O.), Deutsche Forschungsgemeinschaft (PL233/1-1, M.P.) and the Center for Molecular Medicine (CMMC) of the University of Cologne (TP78, M.P.). Research in D.C.L.’s laboratory is supported by grants from the US National Institutes of Health (NS32742). S.J.T. is supported by grants from the US National Institutes of Health (NS46661); R.L. by grants from Junta de Comunidades de Castilla-La Mancha (SAN-04-008-00, PAI05-040); M.D.E. by grants from the US National Institutes of Health (NS39402, MH64748), the American Heart Association, the Raymond and Beverley Sackler Foundation and the Ruth K. Broad Foundation; and S.F.H. by the US National Institutes of Health (R01 NS28709) and the McKnight and Adler Foundations. M.D.E is an Investigator of the Howard Hughes Medical Institute.
Footnotes
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
References
- 1.Petralia RS, et al. Selective acquisition of AMPA receptors over postnatal development suggests a molecular basis for silent synapses. Nat. Neurosci. 1999;2:31–36. doi: 10.1038/4532. [DOI] [PubMed] [Google Scholar]
- 2.Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- 3.Carmignoto G, Vicini S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science. 1992;258:1007–1011. doi: 10.1126/science.1279803. [DOI] [PubMed] [Google Scholar]
- 4.Crair MC, Malenka RC. A critical period for long-term potentiation at thalamocortical synapses. Nature. 1995;375:325–328. doi: 10.1038/375325a0. [DOI] [PubMed] [Google Scholar]
- 5.Hestrin S. Developmental regulation of NMDA receptor-mediated synaptic currents at a central synapse. Nature. 1992;357:686–689. doi: 10.1038/357686a0. [DOI] [PubMed] [Google Scholar]
- 6.Perez-Otaño I, Ehlers MD. Learning from NMDA receptor trafficking: clues to the development and maturation of glutamatergic synapses. Neurosignals. 2004;13:175–189. doi: 10.1159/000077524. [DOI] [PubMed] [Google Scholar]
- 7.Wenthold RJ, Prybylowski K, Standley S, Sans N, Petralia RS. Trafficking of NMDA receptors. Annu. Rev. Pharmacol. Toxicol. 2003;43:335–358. doi: 10.1146/annurev.pharmtox.43.100901.135803. [DOI] [PubMed] [Google Scholar]
- 8.Das S, et al. Increased NMDA current and spine density in mice lacking the NMDA receptor subunit NR3A. Nature. 1998;393:377–381. doi: 10.1038/30748. [DOI] [PubMed] [Google Scholar]
- 9.Wong HK, et al. Temporal and regional expression of NMDA receptor subunit NR3A in the mammalian brain. J. Comp. Neurol. 2002;450:303–317. doi: 10.1002/cne.10314. [DOI] [PubMed] [Google Scholar]
- 10.Perez-Otaño I, et al. Assembly with the NR1 subunit is required for surface expression of NR3A-containing NMDA receptors. J. Neurosci. 2001;21:1228–1237. doi: 10.1523/JNEUROSCI.21-04-01228.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sasaki YF, et al. Characterization and comparison of the NR3A subunit of the NMDA receptor in recombinant systems and primary cortical neurons. J. Neurophysiol. 2002;87:2052–2063. doi: 10.1152/jn.00531.2001. [DOI] [PubMed] [Google Scholar]
- 12.Chatterton JE, et al. Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature. 2002;415:793–798. doi: 10.1038/nature715. [DOI] [PubMed] [Google Scholar]
- 13.Akaaboune M, Culican SM, Turney SG, Lichtman JW. Rapid and reversible effects of activity on acetylcholine receptor density at the neuromuscular junction in vivo. Science. 1999;286:503–507. doi: 10.1126/science.286.5439.503. [DOI] [PubMed] [Google Scholar]
- 14.Philpot BD, Sekhar AK, Shouval HZ, Bear MF. Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron. 2001;29:157–169. doi: 10.1016/s0896-6273(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 15.Tovar KR, Westbrook GL. Mobile NMDA receptors at hippocampal synapses. Neuron. 2002;34:255–264. doi: 10.1016/s0896-6273(02)00658-x. [DOI] [PubMed] [Google Scholar]
- 16.Snyder EM, et al. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat. Neurosci. 2001;4:1079–1085. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- 17.Nong Y, et al. Glycine binding primes NMDA receptor internalization. Nature. 2003;422:302–307. doi: 10.1038/nature01497. [DOI] [PubMed] [Google Scholar]
- 18.Li B, et al. Differential regulation of synaptic and extra-synaptic NMDA receptors. Nat. Neurosci. 2002;5:833–834. doi: 10.1038/nn912. [DOI] [PubMed] [Google Scholar]
- 19.Scott DB, Michailidis I, Mu Y, Logothetis D, Ehlers MD. Endocytosis and degradative sorting of NMDA receptors by conserved membrane-proximal signals. J. Neurosci. 2004;24:7096–7109. doi: 10.1523/JNEUROSCI.0780-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Washbourne P, Liu XB, Jones EG, McAllister AK. Cycling of NMDA receptors during trafficking in neurons before synapse formation. J. Neurosci. 2004;24:8253–8264. doi: 10.1523/JNEUROSCI.2555-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mu Y, Otsuka T, Horton AC, Scott DB, Ehlers MD. Activity-dependent mRNA splicing controls ER export and synaptic delivery of NMDA receptors. Neuron. 2003;40:581–594. doi: 10.1016/s0896-6273(03)00676-7. [DOI] [PubMed] [Google Scholar]
- 22.Vissel B, Krupp JJ, Heinemann SF, Westbrook GL. A use-dependent tyrosine dephosphorylation of NMDA receptors is independent of ion flux. Nat. Neurosci. 2001;4:587–596. doi: 10.1038/88404. [DOI] [PubMed] [Google Scholar]
- 23.Barria A, Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron. 2002;35:345–353. doi: 10.1016/s0896-6273(02)00776-6. [DOI] [PubMed] [Google Scholar]
- 24.Lavezzari G, McCallum J, Dewey CM, Roche KW. Subunit-specific regulation of NMDA receptor endocytosis. J. Neurosci. 2004;24:6383–6391. doi: 10.1523/JNEUROSCI.1890-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prybylowski K, et al. The synaptic localization of NR2B-containing NMDA receptors is controlled by interactions with PDZ proteins and AP-2. Neuron. 2005;47:845–857. doi: 10.1016/j.neuron.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roche KW, et al. Molecular determinants of NMDA receptor internalization. Nat. Neurosci. 2001;4:794–802. doi: 10.1038/90498. [DOI] [PubMed] [Google Scholar]
- 27.Kessels MM, Qualmann B. Syndapins integrate N-WASP in receptor-mediated endocytosis. EMBO J. 2002;21:6083–6094. doi: 10.1093/emboj/cdf604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plomann M, et al. PACSIN, a brain protein that is upregulated upon differentiation into neuronal cells. Eur. J. Biochem. 1998;256:201–211. doi: 10.1046/j.1432-1327.1998.2560201.x. [DOI] [PubMed] [Google Scholar]
- 29.Qualmann B, Roos J, DiGregorio PJ, Kelly RB. Syndapin I, a synaptic dynamin-binding protein that associates with the neural Wiskott-Aldrich syndrome protein. Mol. Biol. Cell. 1999;10:501–513. doi: 10.1091/mbc.10.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao A, Kim E, Sheng M, Craig AM. Heterogeneity in the molecular composition of excitatory postsynaptic sites during development of hippocampal neurons in culture. J. Neurosci. 1998;18:1217–1229. doi: 10.1523/JNEUROSCI.18-04-01217.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blanpied TA, Scott DB, Ehlers MD. Dynamics and regulation of clathrin coats at specialized endocytic zones of dendrites and spines. Neuron. 2002;36:435–449. doi: 10.1016/s0896-6273(02)00979-0. [DOI] [PubMed] [Google Scholar]
- 32.Racz B, Blanpied TA, Ehlers MD, Weinberg RJ. Lateral organization of endocytic machinery in dendritic spines. Nat. Neurosci. 2004;7:917–918. doi: 10.1038/nn1303. [DOI] [PubMed] [Google Scholar]
- 33.Petralia RS, Wang YX, Wenthold RJ. Internalization at glutamatergic synapses during development. Eur. J. Neurosci. 2003;18:3207–3217. doi: 10.1111/j.1460-9568.2003.03074.x. [DOI] [PubMed] [Google Scholar]
- 34.Groc L, et al. Differential activity-dependent regulation of the lateral mobilities of AMPA and NMDA receptors. Nat. Neurosci. 2004;7:695–696. doi: 10.1038/nn1270. [DOI] [PubMed] [Google Scholar]
- 35.Mori H, et al. Role of the carboxy-terminal region of the GluR epsilon2 subunit in synaptic localization of the NMDA receptor channel. Neuron. 1998;21:571–580. doi: 10.1016/s0896-6273(00)80567-x. [DOI] [PubMed] [Google Scholar]
- 36.Steigerwald F, et al. C-Terminal truncation of NR2A subunits impairs synaptic but not extrasynaptic localization of NMDA receptors. J. Neurosci. 2000;20:4573–4581. doi: 10.1523/JNEUROSCI.20-12-04573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Racca C, Stephenson FA, Streit P, Roberts JD, Somogyi P. NMDA receptor content of synapses in stratum radiatum of the hippocampal CA1 area. J. Neurosci. 2000;20:2512–2522. doi: 10.1523/JNEUROSCI.20-07-02512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levitt-Gilmour TA, Salpeter MM. Gradient of extrajunctional acetylcholine receptors early after denervation of mammalian muscle. J. Neurosci. 1986;6:1606–1612. doi: 10.1523/JNEUROSCI.06-06-01606.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cottrell JR, Borok E, Horvath TL, Nedivi E. CPG2: a brain- and synapse-specific protein that regulates the endocytosis of glutamate receptors. Neuron. 2004;44:677–690. doi: 10.1016/j.neuron.2004.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simpson F, et al. SH3-domain-containing proteins function at distinct steps in clathrin-coated vesicle formation. Nat. Cell Biol. 1999;1:119–124. doi: 10.1038/10091. [DOI] [PubMed] [Google Scholar]
- 41.Da Costa SR, et al. Impairing actin filament or syndapin functions promotes accumulation of clathrin-coated vesicles at the apical plasma membrane of acinar epithelial cells. Mol. Biol. Cell. 2003;14:4397–4413. doi: 10.1091/mbc.E03-05-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coyle IP, et al. Nervous wreck, an SH3 adaptor protein that interacts with Wsp, regulates synaptic growth in Drosophila. Neuron. 2004;41:521–534. doi: 10.1016/s0896-6273(04)00016-9. [DOI] [PubMed] [Google Scholar]
- 43.Qualmann B, Kelly RB. Syndapin isoforms participate in receptor-mediated endocytosis and actin organization. J. Cell Biol. 2000;148:1047–1062. doi: 10.1083/jcb.148.5.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fagiolini M, et al. Separable features of visual cortical plasticity revealed by N-methyl-D-aspartate receptor 2A signaling. Proc. Natl. Acad Sci. USA. 100:2854–2859. doi: 10.1073/pnas.0536089100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roberts EB, Ramoa AS. Enhanced NR2A subunit expression and decreased NMDA receptor decay time at the onset of ocular dominance plasticity in the ferret. J. Neurophysiol. 1999;81:2587–2591. doi: 10.1152/jn.1999.81.5.2587. [DOI] [PubMed] [Google Scholar]
- 46.Lu HC, Gonzalez E, Crair MC. Barrel cortex critical period plasticity is independent of changes in NMDA receptor subunit composition. Neuron. 2001;32:619–634. doi: 10.1016/s0896-6273(01)00501-3. [DOI] [PubMed] [Google Scholar]
- 47.Kato N, Yoshimura H. Reduced Mg2+ block of N-methyl-D-aspartate receptor-mediated synaptic potentials in developing visual cortex. Proc. Natl. Acad. Sci. USA. 1993;90:7114–7118. doi: 10.1073/pnas.90.15.7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burgard EC, Hablitz JJ. Developmental changes in the voltage-dependence of neocortical NMDA responses. Brain Res. Dev. Brain Res. 1994;80:275–278. doi: 10.1016/0165-3806(94)90113-9. [DOI] [PubMed] [Google Scholar]
- 49.Cho KO, Hunt CA, Kennedy MB. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron. 1992;9:929–942. doi: 10.1016/0896-6273(92)90245-9. [DOI] [PubMed] [Google Scholar]
- 50.Westphal RS, et al. Regulation of NMDA receptors by an associated phosphatase-kinase signaling complex. Science. 1999;285:93–96. doi: 10.1126/science.285.5424.93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.