Abstract
The antitumor effects of 1,25-dihydroxyvitamin D3 (calcitriol) are being exploited for prevention and treatment of prostate cancer (CaP). These studies examined antitumor effects of calcitriol in primary cell cultures derived from transgenic adenocarcinoma of mouse prostate (TRAMP) mice chronically treated with calcitriol (20μg/kg) or vehicle 3x/week (MWF) from 4 weeks-of-age until palpable tumors developed. This is a report on the response of 2 representative control (vitamin D naïve, naïve) and calcitriol-treated (vitamin D insensitive, VDI) cells to calcitriol. VDI cells were less sensitive to calcitriol based on less cell growth inhibition and less inhibition of DNA synthesis as measured by MTT and BrdU incorporation assays. Similarly, VDI cells were also less sensitive to growth inhibition by the vitamin analog, 19-nor-1α,25-dihydroxyvitamin D2 (paricalcitol). There was no change in apoptosis following treatment of naïve and VDI cells with calcitriol. Vitamin D receptor (VDR) expression was up-regulated by calcitriol in both naïve and VDI cells. Calcitriol induced the vitamin D metabolizing enzyme, 24-hydroxylase (cyp24) mRNA and enzyme activity similarly in naïve and VDI cells as measured by RT-PCR and HPLC respectively. In summary, VDI cells are less responsive to the antiproliferative effects of calcitriol. Understanding vitamin D insensitivity will further clinical development of vitamin D compounds for prevention and treatment of CaP.
Keywords: 1,25-Dihydroxyvitamin D3; Calcitriol; Vitamin D Insensitivity; TRAMP; Prostate cancer
INTRODUCTION
Prostate cancer (CaP) is one of the leading causes of cancer and cancer-related mortality in American men [1]. 1,25-Dihydroxyvitamin D3 (calcitriol) is currently being developed for CaP prevention and treatment. Calcitriol has antitumor activities in CaP [2–6]. The mechanisms of action of calcitriol in CaP include induction of cell cycle arrest [7], apoptosis [8, 9] and differentiation [2, 10]; and inhibition of invasion [11] and metastasis [4]. Clinical trials have been conducted in men with CaP using calcitriol alone or in combination with other antitumor agents [12–14]. Calcitriol is inactivated and degraded by 24-hydroxylase (cyp24) [15]. Thus availability of calcitriol in CaP cells is inversely proportional to cyp24 activity [16].
Evidence of insensitivity to the antiproliferative effects of calcitriol has been described in some malignant cells. Following chronic exposure to calcitriol, MCF-7 human breast cancer cells become resistant to the growth inhibitory effects of calcitriol and other vitamin D analogs [17, 18]. The vitamin D resistant chronic myelogenous leukemia cell line, JMRD3, is less sensitive to growth inhibition by calcitriol compared to parental RWLeu-4 cells [19]. Development of vitamin D insensitivity and the molecular mechanisms of vitamin D insensitivity in CaP is poorly understood. These studies are a report on primary cell cultures established from the transgenic adenocarcinoma of mouse prostate (TRAMP) model that were used to study vitamin D insensitivity in CaP. The TRAMP model was developed using the minimal rat probasin promoter to target expression of SV40 early genes (T, t) specifically to the prostatic epithelium [20]. These mice progressively develop disease in the prostate ranging from prostatic intraepithelial neoplasia (PIN) to poorly differentiated cancer and metastasis. Prostate tumors of calcitriol-treated (vitamin D insensitive, VDI) and vehicle-treated (vitamin D naïve, naïve) mice were digested to generate primary cell cultures. This is a report on the response of a representative pair of naïve and VDI primary cells to calcitriol.
MATERIALS AND METHODS
Chemicals and reagents
Calcitriol and paricalcitol (Abbott Laboratories, Chicago, IL) were dissolved in 100% ethanol and stored at −80ºC. Calcitriol was always handled with indirect light. Test compounds were diluted in DMEM media ((Invitrogen, Frederick, MD) before use.
Animal studies
Experimental uses of laboratory animals were in accordance with IACUC and NIH guidelines. TRAMP animals were in the C57BL/6 X FVB 50:50 strain background. Breeding colonies were maintained at the Roswell Park Cancer Institute animal facilities. Four week-old TRAMP mice were treated chronically with vehicle (control) or 20μg/kg calcitriol interperitoneally (i.p.) 3x/week (MWF) until they developed tumors.
Cell culture
Primary cell cultures were generated by dissociating poorly differentiated prostate tumors of control mice (vitamin D naïve, naive) and calcitriol-treated mice (vitamin D insensitive, VDI). Prostatic tumors were minced and digested with 2.5mg/ml Collagenase Type II (Sigma-Aldrich, St. Louis, MO), 2.5mg/ml Collagenase Type IV (Sigma) and 1mg/ml DNase I (Sigma). Naïve and VDI cells were cultured in DMEM media supplemented with 10% FBS (Hyclone, Logan, UT), 10−8M dihydrotestosterone (Sigma), 5μg/ml insulin (Sigma) and 25U/ml penicillin/streptomycin (Invitrogen) at 37ºC in a humidified atmosphere containing 5% CO2. VDI cells were cultured with media containing 10−8M calcitriol to maintain selective pressure. The response of 2 representative naïve and VDI cells to calcitriol are presented in this report.
Cell proliferation assays
Naïve and VDI cells were seeded in 96 well plates overnight. Cell growth was measured by treating cells with increasing concentrations of calcitriol or paricalcitol for 96h. Ethanol (0.04%) was used as vehicle control. MTT (Sigma) was added to each well and absorbances were measured at 570nm with an ELISA plate reader (Molecular Devices, Sunnyvale, CA). The concentration that inhibited cell growth by 30% (GI30) was determined using Calcusyn software (Biosoft, Ferguson, MO). DNA synthesis was measured using a bromodeoxyuridine (BrdU) ELISA kit (Roche, Indianapolis, IN) according to manufacturer’s instructions. Briefly, sub-confluent cells were treated with calcitriol (100nM) or vehicle (0.004% ethanol) for 96h. Cells were labeled with BrdU and incubated with anti-BrdU antibody. BrdU incorporation was detected using a colorimetric substrate solution and absorbances (370nm) were measured as described above.
Annexin V staining
Apoptosis was measured using the Annexin V-PE Apoptosis Detection Kit (BD PharMingen) according to manufacturer’s instructions. Briefly, sub-confluent cells were treated with vehicle or 100nM calcitriol for 96h. Cells were harvested, stained with annexin V and 7-AAD; and analyzed by flow cytometry. Cells undergoing early apoptosis stain positive for annexin V, while necrotic cells and cells undergoing late apoptosis stain positive for annexin V and 7-AAD.
Western blot analysis
Sub-confluent cells were treated with vehicle or calcitriol (100nM) for 24h. Cells were lysed, proteins were resolved by SDS-PAGE and transferred overnight to a PVDF membrane. Membranes were blocked for at least 1h in 5% milk and incubated overnight with the following primary antibodies: VDR (Santa Cruz, Santa Cruz, CA) and actin (Calbiochem, San Diego, CA). Membranes were incubated with HRP-conjugated secondary antibodies and proteins were detected using a chemiluminescence reagent.
RT-PCR
Sub-confluent cells were treated with vehicle or 100nM calcitriol for 96h. RNA was isolated from cells using TRIzol Reagent (Invitrogen) and RNAqueous-4PCR kit (Ambion, Austin, TX) according to manufacturer’s protocol. cDNA was synthesized using the SuperScript First-Strand Synthesis System (Invitrogen). RT-PCR was performed using primers (5′-3′) for mouse cyp24(forward): TGGGAAGATGATGGTGACCC; cyp24(reverse): ACTGTTCCTTTGGGTAGCGT.
Cyp24 activity assay
Sub-confluent cells were treated with vehicle or 100nM calcitriol for 24h. Cells were harvested and resuspended in cyp24 buffer (0.19M sucrose, 25mM sodium succinate, 2mM MgCl2, 1mM EDTA Na4, 20mM HEPES; pH 7.4). Protein concentration was determined and samples were incubated for 30min with cyp24 substrate, [26,27-3H]-25-hydroxyvitamin D3 (Perkin Elmer, Boston, MA). Vitamin D metabolites were extracted using tetrahydrofuran (Fisher Scientific, Fair Lawn, NJ) and ethyl acetate (Fisher). Samples were evaporated to dryness and resuspended in HPLC mobile phase (94% hexane and 6% 2-propanol). Radioactive metabolites were separated by HPLC and the amount of radioactivity in each fraction was measured by scintillation counting.
Statistical analysis
ANOVA was used to determine the significance between treatment groups relative to vehicle control using the Statview software (SAS Institute Inc., Cary, NC).
RESULTS
Antiproliferative effects of vitamin D compounds in naïve and VDI cells
Compared to naïve cells, VDI cells were less sensitive to inhibition of cell growth following treatment with calcitriol for 96h (Fig. 1A). Calcitriol (3.6nM) inhibited growth of naïve cells by 30%; however, there was no effect on growth of VDI cells. To determine whether VDI cells are cross-resistant to other vitamin D compounds, cells were treated for 96h with the vitamin D analog, paricalcitol. VDI cells were less responsive to growth inhibition by paricalcitol than naïve cells which were inhibited by 30% with 44.0nM of paricalcitol (Fig. 1B).
Figure 1.
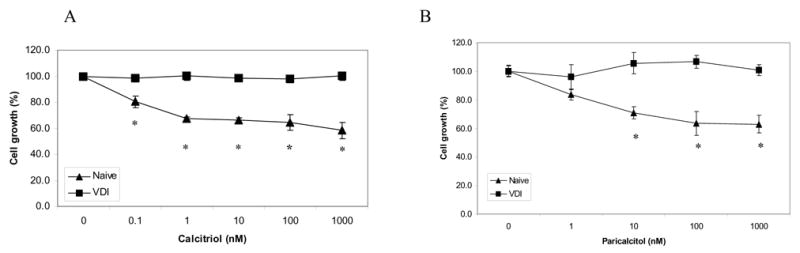
VDI cells are less sensitive to growth inhibition. (A), Naïve and VDI cells were treated with vehicle (control) or different concentrations of calcitriol for 96h. Cell growth was determined by MTT assay and results are reported as mean cell growth (%) of 3 replicate wells ± SD normalized to cell growth at time point zero. (B), Cells were treated with different concentrations of paricalcitol for 96h and cell growth was measured by MTT assay. Results were analyzed as described above. *, P < 0.005 by ANOVA. Results are representative of at least 3 replicate experiments.
Effects of calcitriol on cell proliferation and apoptosis
BrdU incorporation assay was performed to examine the effect of calcitriol (100nM) on DNA synthesis in naïve and VDI cells. Calcitriol inhibited DNA synthesis in naïve cells by ~40%, however, VDI cells were insensitive to the antiproliferative effects of calcitriol (Fig. 2A). To further study the response of naïve and VDI cells to calcitriol, apoptosis was measured by annexin V staining. Calcitriol did not induce apoptosis in either the naïve or VDI cells after 96h of treatment (Fig. 2B).
Figure 2.
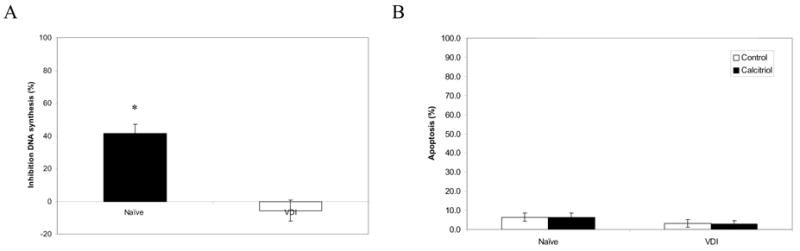
Effects of calcitriol on cell proliferation and apoptosis in naïve and VDI cells. Naïve and VDI cells were treated with vehicle (control) or 100nM calcitriol for 96h. (A), DNA synthesis was measured by BrdU incorporation assay. Results are reported as % inhibition of DNA synthesis relative to vehicle-treated cells. *, P < 0.001 by ANOVA. (B), Apoptosis was measured by Annexin V/7-AAD staining. Results are reported as % apoptosis (Annexin V and Annexin V/7-AAD cell populations). Results are representative of at least 3 replicate experiments.
Effect of calcitriol on vitamin D receptor (VDR) expression, cyp24 mRNA levels and cyp24 enzyme activity
Immunoblot analysis was performed to determine if naïve and VDI cells induce expression of the VDR, the key mediator of the genomic response to vitamin D compounds. Calcitriol increased VDR expression in naïve and VDI cells (Fig 3A). This suggests that decreased sensitivity of VDI cells to calcitriol is not due to lack of VDR expression.
Figure 3.
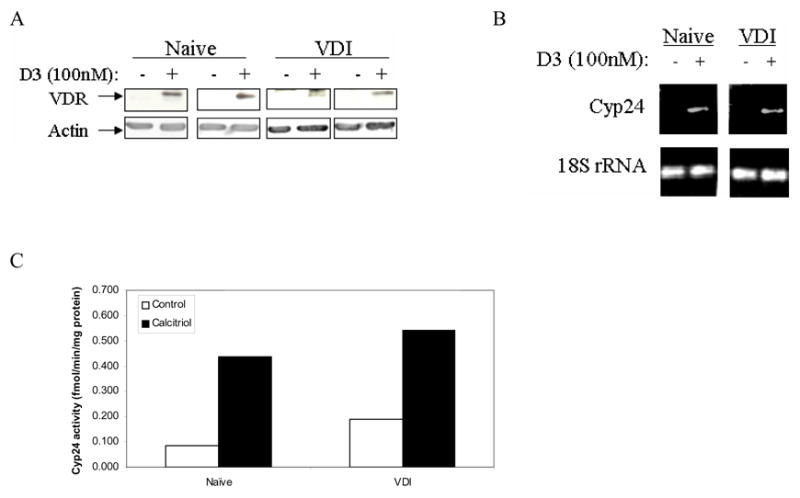
Calcitriol induces VDR and cyp24 expression in naïve and VDI cells. (A), Cells were treated with vehicle or calcitriol (100nM) for 24h. Western blot analysis was performed with anti-VDR antibody (B), Cells were treated with vehicle or 100nM calcitriol for 96h. Cyp24 mRNA levels were detected by RT-PCR. (C), Cells were treated with 100nM calcitriol for 24h. Cyp24 enzyme activity was measured by HPLC. Results are reported as fmol/min/mg protein. Results are representative of 2 replicate experiments.
Increased expression of cyp24, is associated with decreased sensitivity of DU145 human prostate cancer cells to the antitumor effects of calcitriol [16]. Cyp24 expression was examined in naïve and VDI cells to evaluate contribution of altered cyp24 levels in vitamin D insensitivity. Following treatment with calcitriol (100nM) for 96h, cyp24 mRNA levels were increased in both naïve and VDI cells to comparable levels (Fig 3B). Cyp24 enzyme activity was measured in naïve and VDI cells by HPLC. Following treatment with calcitriol (100nM) for 24h, cyp24 activity was increased in naïve and VDI cells by ~3–5 fold (Fig. 3C). There was no difference in induction of cyp24 expression and activity in naïve and VDI cells.
DISCUSSION
Vitamin D resistance has been observed in some cancers and may limit the efficacy of vitamin D therapies in the clinic. Progression to advanced CaP is associated with insensitivity to vitamin D. There is a need to understand the molecular mechanisms that underlie insensitivity to vitamin D therapies in order to improve the clinical efficacy of vitamin D compounds. Previous studies have indicated that MCF-7 breast cancer cells and RWLeu-4 leukemia cells become insensitive to the antitumor effects of calcitriol following chronic treatment with calcitriol [17, 19]. These studies demonstrate for the first time that chronic treatment of TRAMP mice with calcitriol results in development of vitamin D insensitive (VDI) CaP. VDI cells that were generated from these tumors were less sensitive to cell growth inhibition by vitamin D compounds (calcitriol and paricalcitol). Based on results obtained thus far, decreased sensitivity of VDI cells does not appear to be due to aberrant expression of the vitamin D inactivating enzyme, cyp24. Some of the molecular pathways deregulated in vitamin D resistant variants of MCF-7 breast cancer cells (MCF-7D3RES) and RWLeu-4 leukemia cells (JMRD3) cells include loss of cell cycle regulation [18, 19] and resistance to induction of apoptosis in MCF-7D3RES cells [17]. Similar to VDI cells, MCF-7D3RES and JMRD3 cells express functional VDRs [17, 19], that are uncoupled from downstream antiproliferative pathways. Further studies are underway to dissect the role of metabolism by cyp24, deregulation of downstream molecular pathways (cell cycle and apoptosis) and VDR function/signaling in development of vitamin D insensitive/resistant CaP.
An in vitro model system that will facilitate future studies to elucidate the molecular mechanisms of insensitivity has been successfully created using the TRAMP model. Studying and understanding the difference between vitamin sensitive and insensitive CaP cells is critical for rational design of future clinical trials with vitamin D compounds for prostate cancer prevention and treatment.
ABBREVIATIONS
- CaP
Prostate Cancer
- TRAMP
Transgenic Adenocarcinoma of Mouse Prostate
- MWF
Monday/Wednesday/Friday
- VDI
Vitamin D Insensitive
- HPLC
High Performance Liquid Chromatography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cancer.org ACS website, American Cancer Society.
- 2.Skowronski R, Peehl D, Feldman D. Vitamin D and prostate cancer: 1,25-dihydroxyvitamin D3 receptors and actions in human prostate cancer cell lines. Endocrinology. 1993;132:1952. doi: 10.1210/endo.132.5.7682937. [DOI] [PubMed] [Google Scholar]
- 3.Peehl DM, Skowronski RJ, Leung GK, Wong ST, Stamey TA, Feldman D. Antiproliferative effects of 1,25-dihydroxyvitamin D3 on primary cultures of human prostatic cells. Cancer Res. 1994;54(3):805–810. [PubMed] [Google Scholar]
- 4.Getzenberg R, Light B, Lapco P, Konety B, Nangia A, Acierno J, Dhir R, Shurin Z, Day R, Trump D, Johnson C. Vitamin D inhibition of prostate adenocarcinoma growth and metastasis in the Dunning rat prostate model system. Urology. 1997;50(6):999–1006. doi: 10.1016/S0090-4295(97)00408-1. [DOI] [PubMed] [Google Scholar]
- 5.Oades GM, Dredge K, Kirby RS, Colston KW. Vitamin D receptor-dependent antitumour effects of 1,25-dihydroxyvitamin D3 and two synthetic analogues in three in vivo models of prostate cancer. BJU Int. 2002;90(6):607–616. doi: 10.1046/j.1464-410x.2002.02964.x. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed S, Johnson CS, Rueger RM, Trump DL. Calcitriol (1,25-dihydroxycholecalciferol) potentiates activity of mitoxantrone/dexamethasone in an androgen independent prostate cancer model. J Urol. 2002;168(2):756–761. [PubMed] [Google Scholar]
- 7.Zhuang SH, Burnstein KL. Antiproliferative effect of 1alpha,25-dihydroxyvitamin D3 in human prostate cancer cell line LNCaP involves reduction of cyclin-dependent kinase 2 activity and persistent G1 accumulation. Endocrinology. 1998;139(3):1197–1207. doi: 10.1210/endo.139.3.5770. [DOI] [PubMed] [Google Scholar]
- 8.Modzelewski R, Hershberger P, Johnson C, Trump D. Apoptotic effects of paclitaxel and calcitriol in rat dunning MLL and human PC-3 prostate tumor cells in vitro. Proc Amer Assoc Cancer Res. 1999;40:580a. [Google Scholar]
- 9.Guzey M, Kitada S, Reed JC. Apoptosis Induction by 1α,25-Dihydroxyvitamin D3 in Prostate Cancer. Molecular Cancer Therapeutics. 2002;1:667–677. [PubMed] [Google Scholar]
- 10.Miller GJ, Stapleton GE, Ferrara JA, Lucia MS, Pfister S, Hedlund TE, Upadhya P. The human prostatic carcinoma cell line LNCaP expresses biologically active, specific receptors for 1 alpha,25-dihydroxyvitamin D3. Cancer Res. 1992;52(3):515–520. [PubMed] [Google Scholar]
- 11.Schwartz GG, Wang MH, Zang M, Singh RK, Siegal GP. 1 alpha,25-Dihydroxyvitamin D (calcitriol) inhibits the invasiveness of human prostate cancer cells. Cancer Epidemiol Biomarkers Prev. 1997;6(9):727–732. [PubMed] [Google Scholar]
- 12.Trump DL, Serafine S, Brufsky A, Potter D, Johnson CS. High dose calcitriol (1,25(OH)2 vitamin D3) + dexamethasone in androgen independent prostate cancer (AIPC) Proc Amer Soc Clin Oncol. 2000;19:337a. [Google Scholar]
- 13.Beer TM, Eilers KM, Garzotto M, Egorin MJ, Lowe BA, Henner WD. Weekly high-dose calcitriol and docetaxel in metastatic androgen-independent prostate cancer. J Clin Oncol. 2003;21(1):123–128. doi: 10.1200/jco.2003.05.117. [DOI] [PubMed] [Google Scholar]
- 14.Johnson CS, Hershberger PA, Bernardi RJ, McGuire TF, Trump DL. Vitamin D receptor: a potential target for intervention. Urology. 2002;60(3 Suppl 1):123–130. doi: 10.1016/s0090-4295(02)01591-1. discussion 130-121. [DOI] [PubMed] [Google Scholar]
- 15.Horst RL, Reinhardt TA, Reddy GS. Vitamin D metabolism. In: Feldman D, Pike JW, Glorieux FH, editors. Vitamin D. Elsevier; Oxford: 2005. pp. 15–36. [Google Scholar]
- 16.Miller G, Stapleton G, Hedlund T, Moffat K. Vitamin D receptor expression, 24-hydroxylase activity, and inhibition of growth by 1alpha,25-dihydroxyvitamin D3 in seven human prostatic carcinoma cell lines. Clin Cancer Res. 1995;1(9):997–1003. [PubMed] [Google Scholar]
- 17.Narvaez CJ, Vanweelden K, Byrne I, Welsh J. Characterization of a vitamin D3-resistant MCF-7 cell line. Endocrinology. 1996;137(2):400–409. doi: 10.1210/endo.137.2.8593782. [DOI] [PubMed] [Google Scholar]
- 18.Narvaez CJ, Welsh J. Differential effects of 1,25-dihydroxyvitamin D3 and tetradecanoylphorbol acetate on cell cycle and apoptosis of MCF-7 cells and a vitamin D3-resistant variant. Endocrinology. 1997;138(11):4690–4698. doi: 10.1210/endo.138.11.5545. [DOI] [PubMed] [Google Scholar]
- 19.Lasky SR, Posner MR, Iwata K, Santos-Moore A, Yen A, Samuel V, Clark J, Maizel AL. Characterization of a vitamin D3-resistant human chronic myelogenous leukemia cell line. Blood. 1994;84(12):4283–4294. [PubMed] [Google Scholar]
- 20.Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci. 1995;92:3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]


