Abstract
Infection-induced RBC dysfunction has been shown to play a role in the modulation of host response to injury and infection. The underlying biochemical mechanisms are not known. This study investigated alterations in RBC band-3 phosphorylation status and its relationship to anion exchange activity in vitro as well as under in vivo septic conditions induced by cecal ligation and puncture (CLP) in mice. Pervanadate treatment in vitro increased band-3 tyrosine phosphorylation that was accompanied by decreased RBC deformability and anion exchange activity. Following sepsis, band-3 tyrosine phosphorylation in whole RBC ghosts as well as in cytoskeleton-bound or soluble RBC protein fractions were elevated as compared to controls. Although anion exchange activity was similar in RBCs from septic and control animals, band-3 interaction with eosin-5-maleimide (EMA) which binds to band-3 lysine moieties, was increased in cells from septic animals as compared to controls, indicating that sepsis altered band 3 organization within the RBC membrane. Since glucose-6-phosphate dehydrogenase is a major antioxidant enzyme in RBC, in order to assess the potential role of oxidative stress in band-3 tyrosine phosphorylation, sepsis-induced RBC responses were also compared between WT and (G6PD) mutant animals (20% of normal G6PD activity). Band-3 membrane content and EMA staining were elevated in G6PD mutant mice compared to WT under control non-septic conditions. Following sepsis, G6PD mutant animals showed lessened responses in band-3 tyrosine phosphorylation and EMA staining compared to WT. RBC anion exchange activity was similar between mutant and WT animals under all tested conditions. In summary, these studies indicate that sepsis results in elevated band-3 tyrosine phosphorylation and alters band-3 membrane organization without grossly affecting RBC anion exchange activity. The observations also suggest that factors other than oxidative stress are responsible for the sepsis-induced increase in RBC band-3 tyrosine phosphorylation.
Keywords: Sepsis, RBC deformability, protein phosphorylation, anion exchange, infection, G6PD deficiency, oxidative stress
Introduction
Infection-induced RBC dysfunction has been shown to play an important role in the modulation of host response and may contribute to organ dysfunction in septic patients [1-3]. During inflammation, the alterations in RBC functions include decreased RBC deformability [2,4], increased methemoglobin content [5], cell aggregation [6] and augmented RBC attachment to the endothelium and macrophages [7,8]. These RBC alterations may contribute to microcirculatory dysfunction by causing blood congestion and impair oxygen exchange under septic conditions. However, the biochemical mechanisms responsible for RBC dysfunction following inflammation have not been fully elucidated.
Band-3 is a multifunctional RBC membrane protein that plays several important roles in RBC metabolism and morphology. Band-3 membrane abundance and its organization (dimers; tetramers) within the RBC membrane have been shown to be important for normal anion exchange activity, cytoskeletal structure, cell shape and glycolytic activity [9-12]. In contrast, changes in band-3 structure and its membrane organization had been observed after oxidative stress or during physiological aging of RBCs and was accompanied by cell shrinkage, decreased cell deformability and alterations in RBC metabolic activity [13-15]. Thus, band-3 is a potential molecular target in sepsis, and alterations in band-3 may be associated with RBC dysfunction during septic conditions.
Based mainly on in vitro studies, it is believed that band-3 function is regulated by its phosphorylation status. Increased band-3 tyrosine phosphorylation has been shown to stimulate glycolysis [12,16] and alter the cytoskeletal organization by modulating the interactions between band-3 and cytoskeletal anchoring proteins [17-19]. In vitro studies have also shown that elevated band-3 tyrosine phosphorylation increases its anion transport activity [20]. Hemoglobinopathies have been shown to be accompanied by elevated band-3 tyrosine phosphorylation [21,22] however, it is largely unknown whether band-3 phosphorylation is altered during acute pathophysiological challenges in vivo. Likewise, whereas it is well documented that sepsis or endotoxemia causes RBC dysfunctions, alterations in band-3 phosphorylation or functional status have not been reported under these conditions.
Therefore, based on these observations, we hypothesized that the RBC dysfunction observed during inflammation would be associated with alterations in band-3 phosphorylation status. To test this hypothesis, we employed a polymicrobial septic model in which RBC dysfunctions, including decreased cell deformability, is well documented [4,6,23,24]. Additionally, we tested whether the sepsis-induced increase in band-3 tyrosine phosphorylation is accompanied by alterations in RBC anion transport activity. Furthermore, because oxidative stress has been shown to play an important role in causing RBC dysfunction during sepsis or endotoxemia, we also utilized a glucose 6-phosphate dehydrogenase (G6PD) mutant mouse model in which organ and cell responses display increased sensitivity to oxidative stress [23,25,26].
Materials and Methods
Animals
Male 12-15 weeks old WT and G6PD mutant mice from our breeding colony at Taconic Farm (Germantown, NY) were used in the experiments. Animals were phenotyped by G6PD activity in whole blood using a kit as well as by genotyping as described in detail earlier [23,27]. The studies were performed in accordance with the Guide for the Care and Use of Laboratory Animals [DHEW Publication No. (NIH) 85-23, Revised 1985, Office of Science and Health Reports, DRR/NIH, Bethesda, MD 20205] and were approved by the Institutional Animal Care and Use Committee of the New Jersey Medical School.
Cecal ligation and puncture (CLP)
Polymicrobial sepsis was induced using the CLP model as described earlier [28]. Briefly, animals were anesthetized by a subcutaneous injection of Nembutal (5mg/100g bw). A midline abdominal incision was made. The cecum was exposed, ligated and punctured with a 20-gauge needle in two places. Animals were resuscitated by the subcutaneous injection of isotonic, pyrogen-free saline solution (0.025ml/g bw) postoperatively, and also at 22h post CLP. Blood was collected into heparinized tubes for analyses 24h after the surgical procedures. In this model, animals begin to expire at 24-30h with an overall mortality of 70% by 72-96h. The 24h sampling for the studies prevented introducing survival bias in the studies.
Preparation of RBC suspensions, ghosts and sub-fractions
Fresh heparinized blood was centrifuged at 900 × g for 10 min and the plasma and buffy coat removed. To ensure removal of leukocytes and platelets the packed red cells were washed 3 times with PBS containing 5.5 mM glucose and 0.08% bovine serum albumin. At every washing step, the upper 1/5 volume of the packed red cells was removed. The final RBC pellet was resuspended to a final hematocrit of 10% and used for experiments testing whole RBC responses.
For preparation of RBC ghosts, washed packed red blood cells were lysed with ice cold 5mM sodium phosphate buffer, pH 8.0, containing 1 mM EGTA, 1 mM sodium orthovanadate, and 1 mM phenylmethylsulfonyl fluoride (lysis buffer) and incubated at 4°C for 10 min. RBC ghosts were washed three times by centrifugation (20,000 × g for 20 min at 4°C) to obtain “white” ghosts [29].
For the preparation of “cytoskeleton-bound” and “unbound” protein fractions [29], aliquots of washed ghosts were mixed with an equal volume of 0.5% octaethylene glycol n-dodecyl monoether (C12E8) in the lysis buffer, and left on ice for 10 min. The detergent extracts were layered over a cushion of 35 % sucrose, 0.5% C12E8 in lysis buffer and centrifuged at 20,000 × g for 90 min and 4°C. The resulting pellet contained the detergent insoluble protein fraction (termed “cytoskeleton-bound”) whereas the supernatant contained the soluble protein fraction (termed “unbound”). Aliquots from RBC ghosts, “cytoskeleton-bound” and “unbound” fractions were solubilized in 5% SDS. Protein concentration was determined by using the micro bicinchoninic acid assay, and equal amounts (15μg) of protein were loaded per lane of each gel. Electrophoresis was performed according to the method of Fairbanks [30] using 5.6% polyacrylamide gels containing 1% SDS.
Western Blot analyses
Samples were transferred to Hybond ECL nitrocellulose (Amersham). Nonspecific binding sites were blocked by incubating the nitrocellulose membrane in SuperBlock T-20 (Bio Rad) for 1 h at room temperature prior to the addition of antibodies then incubated with either anti-phosphotyrosine antibody clone 4G10 (Upstate) diluted to a final concentration of 1.5μg/ml, or anti-band 3 antibody (Alpha Diagnostics Inc) diluted to a final concentration of 0.5 μg/ml in SuperBlock T-20 overnight at 4°C. Membranes were washed 3 times with Tris Buffered Saline containing 0.1% Tween 20 and then incubated with the appropriate horseradish peroxidase conjugated secondary antibody diluted in SuperBlock T-20. Finally the membranes were incubated with West Pico chemiluminescence reagent (Pierce) and exposed to X-ray film.
Sulfate uptake and incubations
The anion transport activity was measured as described by Jarolim et al [31]. Washed RBCs were subjected to three additional washes in 84 mM trisodium citrate, 1 mM EGTA, pH 6.5 buffer. Cells were resuspended to a hematocrit of 50%. The cell suspension was mixed 1:1 with 4 mM sodium sulfate, 84 mM sodium citrate, and 1 mM EGTA at pH 6.5 containing 6 μCi of disodium [35] S sulfate (ICN Biomedicals). Samples were incubated at 26°C and aliquots were taken at 5, 10, 15, and 20 minutes of incubation. The band-3 specific anion transport inhibitor 4,4'-diisothiocyano-4,4'-diisothiocyanostilbene-2,2'-disulfonic acid DIDS, (Calbiochem) was administered 10 min prior to initiating the incubation with the radioactive tracer. Maximum inhibitory DIDS concentration was calculated by linear regression analysis of inhibitory curves. For studies using pervanadate, cell suspensions were pre-incubated with pervanadate (0.5, 1 and 2 mM) or vehicle for 10 min at 26°C prior to initiating cell extractions for Western blotting, incubation with the radioactive tracer or testing RBC for cell deformability.
Determination of RBC deformability
RBC deformability was analyzed with a laser-assisted ektacytometer (LORCA) as previously described [24]. Briefly, an aliquot containing approximately 30 million RBCs was suspended in 1 ml of 5% polyvinylpyrrolidone (mol wt 360,000; Sigma, St. Louis, MO) in PBS at a final viscosity of 30 mPa. After gently mixing for 15 min at room temperature, cell deformability was determined at 37°C. Cell deformability was assessed by calculating the elongation index (EI) at shear stresses ranging form 0.3 to 30 Pa as described previously [24].
Flow cytometry
EMA, (eosin-5-maleimide) was added to heparinized whole blood at a final concentration of 0.3 mg/ml and incubated at room temperature for 1 hour in the dark. After cell sedimentation and removal of supernatants, cells were washed twice with 500 ul of PBS containing 0.5 % BSA. Samples are analyzed by a Fluorescence Activated Cell Sorter (FACS-Caliber, Becton-Dickinson, CA, and USA). Cell gating was set to include only RBCs (exclude white blood cells and lymphocytes). After excitation at 488 nm, green fluorescence of gated RBC (15 000 events) were collected and analyzed [32].
Reagents
When applicable, cell culture grade buffers, media and reagents were used. Hank's Balanced Salt Solution, without phenol red, and Dulbecco's Phosphate Buffered Saline were purchased from Life Technologies, Grand Island, NY. Buffers were sterile filtered and de-gassed before use.
Statistical analysis
Statistical calculations were performed using JMP software (SAS Institute Inc., Cary, NC). Results were analyzed using ANOVA followed by t-test for pairwise comparisons or Tukey-Kramer's test for multiple comparisons. Data are expressed as mean ± SE. Statistically significant difference was concluded at p<0.05.
Results
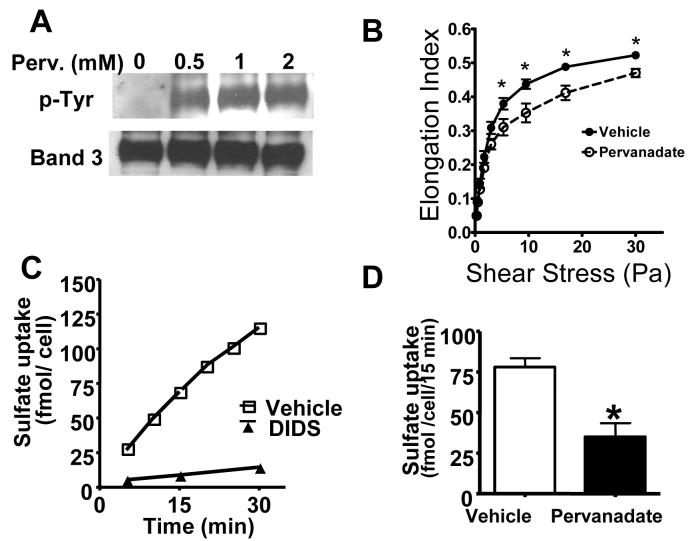
First, in a series of in vitro experiments, we tested the relationship among RBC band-3 tyrosine phosphorylation status, sulfate uptake and cell deformability. RBCs from unchallenged animals were isolated and incubated with pervanadate that increases tyrosine phosphorylation by inhibiting tyrosine phosphatases. After the treatments, cells were processed and analyzed for protein tyrosine phosphorylation, cell deformability and sulfate uptake in parallel. Fig 1A shows that in the absence of pervanadate (vehicle), band-3 tyrosine phosphorylation was below detection limit (but was measurable after longer exposures) as determined by anti-phospho-tyrosine antibody staining in RBC ghosts (Fig 1A lane1, upper row). Pervanadate treatment resulted in a marked and concentration dependent increase in band-3 tyrosine phosphorylation (Fig 1A lanes 2-4 upper row). Fig 1A lower row indicates band-3 content in each lane as determined by reprobing the membranes by anti-band-3 antibody. Testing the effect on RBC elongation in response to prevailing sheer stress indicated decreased cell deformability in the presence of pervanadate as compared to vehicle-treated controls (Fig 1B). Fig C indicates that RBC sulfate uptake was time dependent and DIDS, a known inhibitor of band-3 dependent anion exchange, almost completely inhibited sulfate uptake. Pervanadate treatment inhibited sulfate uptake as compared to vehicle-treated controls (Fig 1D). Whereas pervanadate may influence other cellular functions, these in vitro observations suggested an association between elevated band-3 tyrosine phosphorylation and decreases in RBC deformability and anion transport activity.
Figure 1.
Relationship of band-3 tyrosine phosphorylation, RBC deformability, and sulfate uptake. RBCs were incubated in the absence or presence of pervanadate (0-2.0 mM) and tested for protein tyrosine phosphorylation, cell deformability and sulfate uptake as described in the materials and methods section. Part A: Protein extracts from RBC ghosts were subjected to SDS polyacrylamide electrophoresis and probed with anti phospho-tyrosine antibody (Panel A upper row) or band-3 content using an anti rat band-3 antibody (Panel A lower row). Part B: RBC elongation in response to increasing shear stress in the presence of pervanadate (2.0 mM). Part C: Time dependence of sulfate uptake and the effect of DIDS (10 μM). Part D: The effect of pervanadate (2.0 mM) on RBC sulfate uptake. Mean ± SEM, from 3-6 independent assays, *significant difference compared to vehicle.
Since studies have shown decreased RBC deformability during sepsis, in the next series of experiments, we tested whether the septic condition also increases band-3 tyrosine phosphorylation status and whether this condition was associated with changes in anion transport activity. RBCs were collected from septic (24h) and control (untreated) animals and processed for protein analyses in RBC ghosts (total band-3) as well as in detergent insoluble (cytoskeleton-bound) and detergent soluble (unbound) fractions. Fig 2A shows that band-3 content was similar between septic and control animals in RBC ghosts as well as RBC detergent extracts. In contrast, band-3 tyrosine phosphorylation (normalized to band-3 content in each prep) was markedly increased in all RBC fractions from septic animals compared to controls (Fig 2B). A representative finding of Western blot analyses is shown in Fig 2C. Probing RBC protein extracts for serine phosphorylation from septic and control animals showed no remarkable increase in band-3 tyrosine phosphorylation after sepsis (data not shown).
Figure 2.
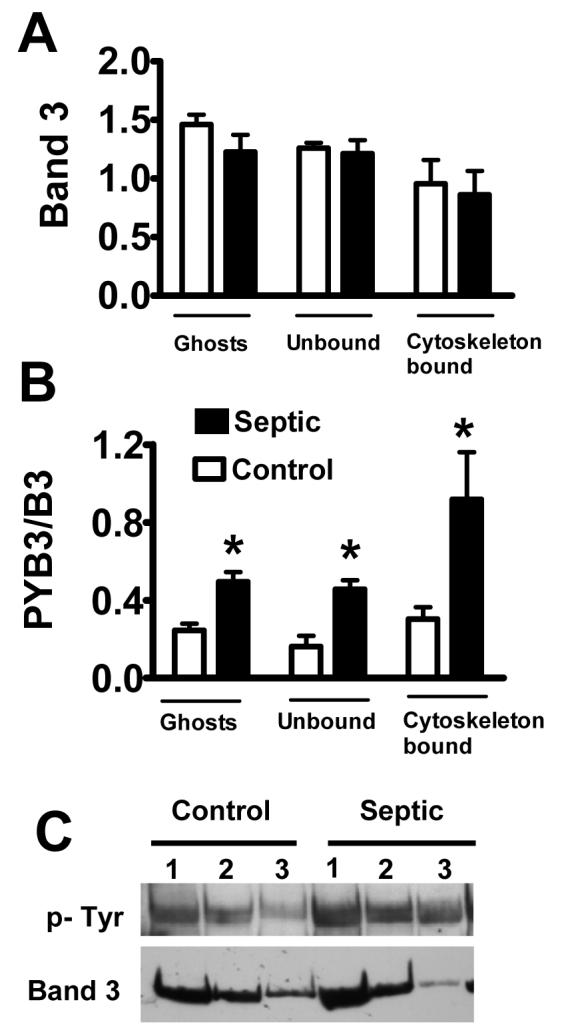
Sepsis increases band-3 tyrosine phosphorylation in RBCs. RBC ghosts obtained from control and septic mice were sub-fractionated and then subjected to SDS PAGE and analyzed for band-3 content using antibodies against band-3 (A) or tyrosine-phosphorylated proteins (B). The intensity of tyrosine phosphorylated band 3 was normalized to total band-3 content obtained in the same lane for each fraction (PYB3/B3). Mean ± SEM from 6-8 animals in each treatment group. * Significant difference compared to control. Part C depicts a typical finding in ghosts (lane 1), unbound (lane 2) and cytoskeleton-bound (lane 3) fractions from control and septic animals as indicated.
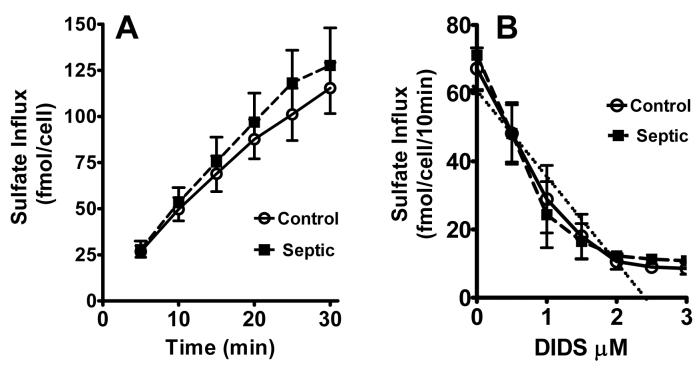
Comparison of sulfate uptake in RBCs from septic and control animals revealed no differences in anion exchange activity (Fig 3A). Furthermore, the DIDS-induced inhibitory curves of sulfate uptake showed similar kinetics between septic and control animals (Fig 3B). Extrapolation of the inhibitory curve resulted in the same maximal inhibitory concentration of DIDS at approximately 2.5 μM under septic and control conditions.
Figure 3.
Anion transport in RBCs from septic mice. Sulfate uptake in RBCs from control and septic mice was determined as described in the materials and methods section. Time course of sulfate uptake in RBCs (Part A) and the effect of prevailing concentration of DIDS (part B) in RBCs from control and septic mice are shown. Extrapolation of the DIDS inhibitory curve indicates an intercept with the X-axis approximately at 2.5 uM in both control and septic animals (dotted line). Mean ± SEM from 3 animals in each group.
Glucose-6-phophate dehydrogenase (G6PD) is a key antioxidant enzyme in RBCs [33]. Because oxidative stress has been implicated in causing RBC dysfunction during sepsis, and the decrease in RBC deformability was more pronounced in septic G6PD deficient than WT animals [23], in the next series of experiments, we compared the sepsis-induced changes in band-3 tyrosine phosphorylation between G6PD mutant and WT animals. Fig 4A indicates that band-3 content was greater in RBC ghosts as well as in RBC detergent fractions of septic G6PD deficient animals as compared to WT. However, the sepsis-induced increase in band-3 tyrosine phosphorylation was less pronounced in G6PD deficient animals as compared to WT especially in the cytoskeleton-bound fraction of band-3 (Fig 4B). Anion exchange activity as assessed by sulfate uptake was similar in RBCs from G6PD deficient and WT animals either under control or septic conditions (data not shown).
Fig 4.
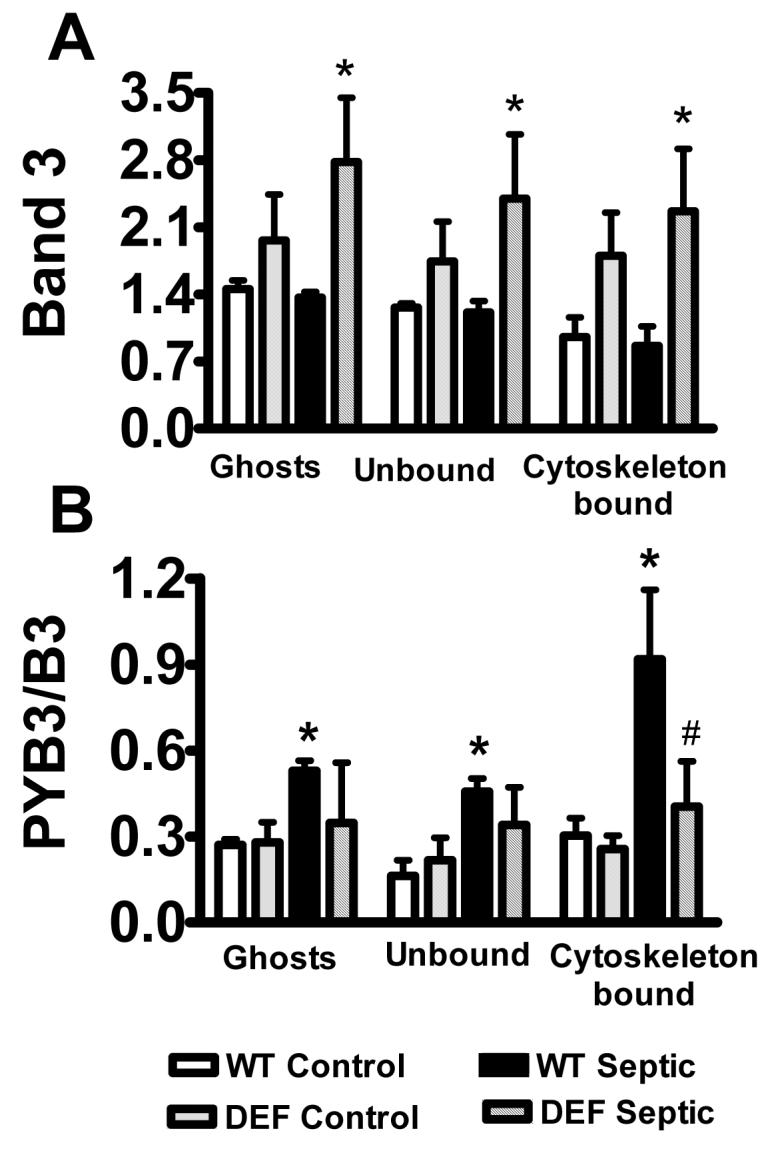
Comparison of band-3 tyrosine phosphorylation between WT and G6PD mutant animals. In separate sets of experiments band-3 tyrosine phosphorylation status was compared between WT and G6PD deficient animals. RBC ghost and cell fractions were analyzed as described for Fig 2. Part A shows results after probing with band-3 antibody; part B indicates tyrosine-phosphorylated band-3. Mean ± SEM from 6 animals in each group. Significant difference, *compared to WT control; #compared to WT septic.
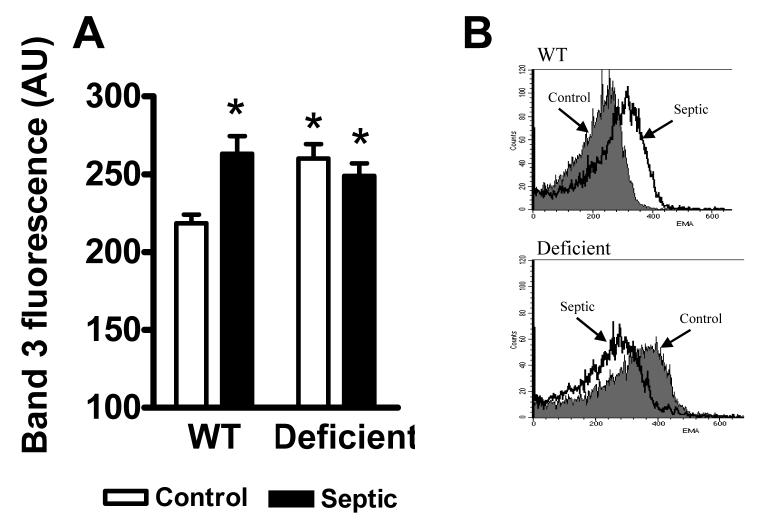
Elevated band-3 tyrosine phosphorylation may affect band-3 organization within the RBC membrane. To test this question on freshly isolated RBCs, we compared the reactivity of band-3 with the fluorescent dye (EMA) that binds to lysine located at one of the extracellular loops of band-3. Cells from WT and G6PD deficient animals under control or septic conditions were collected and analyzed ex vivo using flow cytometry. Fig 5A shows that sepsis resulted in increased RBC EMA fluorescence in WT animals. Comparison of RBCs from G6PD deficient and WT animals under control conditions indicated greater EMA fluorescence in G6PD deficiency compared to WT however, sepsis caused no additional increase in EMA-fluorescence in G6PD deficient RBCs.
Fig 5.
Band-3 EMA reactivity in RBCs. Whole RBC suspensions were prepared from control and septic; WT and G6PD deficient animals and incubated in the presence of EMA followed by flow cytometry analysis as described in the materials and methods section. Part A, summary of results from five animals in each group, mean ± SEM. Part B shows a representative finding. * Significant difference compared to WT control.
Discussion
This study reports that polymicrobial sepsis results in elevated band-3 tyrosine phosphorylation in circulating RBCs. This observation suggests that increased band-3 tyrosine phosphorylation may represent one of the underlying mechanisms contributing to RBC dysfunction during inflammatory conditions. Based on in vitro observations, band-3 tyrosine phosphorylation has been implicated in the regulation of RBC anion exchange activity [34,35]. We also observed an association between increased band-3 tyrosine phosphorylation and decreased anion exchange in vitro; however, we found that band-3 tyrosine phosphorylation was not accompanied by altered RBC sulfate uptake following in vivo sepsis. This indicates that other factors, besides band-3 tyrosine phosphorylation, are required for modulating RBC anion exchange activity in vivo, or repair mechanisms exist under in vivo conditions that are absent following the employed irreversible pharmacological challenge in vitro.
Studies have indicated that hypoxia increases band-3 tyrosine phosphorylation in vitro [36]. Because impaired oxygen exchange in the lung and peripheral tissues is prevalent during sepsis, it is possible that the observed elevation in band-3 tyrosine phosphorylation is associated with decreased RBC oxygenation in this model. It also remains a question whether an elevated band-3 tyrosine phosphorylation is part of the pathology leading to RBC dysfunction during sepsis or alternatively, it is a compensatory event that is part of the cellular defense mechanism under inflammatory conditions. The fact that elevated band-3 tyrosine phosphorylation was shown to stimulate glucose utilization by RBCs supports potential beneficial effects [12]. It remains to be tested whether pharmacological stimulation or inhibition of band-3 tyrosine phosphorylation worsens or alleviates RBC pathology following inflammation.
It has been demonstrated by independent investigations that RBC deformability is decreased during sepsis and endotoxemia [4,6,23,24,37]. The biochemical mechanism responsible for this increased RBC membrane rigidity has not yet been elucidated. However, several studies demonstrated that interactions between band-3 and the cytoskeletal spectrin/actin network play an important function in the maintenance of normal RBC shape and membrane structure [29,38]. Our current finding indicating a similar degree of band-3 increase in RBC ghosts as well as cytoskeletal-bound and unbound fractions suggests that the tyrosine phosphorylation event does not result in grossly increased binding affinity of band-3 to the cytoskeletal network. However, although the employed detergent based RBC fractionation method is commonly used, this procedure might have interrupted weaker interactions between band-3 and the cytoskeletal network. Thus, it remains possible that sepsis-induced tyrosine phosphorylation promotes fine associations between band-3 and the cytoskeletal network. A different organization of band-3 in RBC membranes following sepsis is further supported by the observation that the reactivity of band-3 with EMA was markedly increased after the septic challenge. Although EMA binds to lysine located in the extracellular portion of band-3, the sepsis-induced increase in EMA reactivity may reflect conformational change of band-3 due to altered interactions with the cytoskeletal anchoring proteins as well as changes in band-3 glycosylation status.
Oxidative stress is an important component of sepsis pathology and has been implicated in cellular and organ dysfunction following inflammation [39]. Likewise, antioxidant therapy prevented infection-induced decrease in RBC deformability in animal models in vivo [40,41]. In the employed model, we demonstrated earlier that sepsis increased plasma glutathione levels and oxidized glutathione content in RBCs, whereas in vivo antioxidant treatment by N-acetyl-cysteine alleviated sepsis-induced decrease in RBC deformability [23]. Therefore, in order to investigate the potential involvement of oxidative stress in causing elevated band-3 tyrosine phosphorylation, we utilized a G6PD mutant mouse model that displays a partial G6PD deficiency (20 % of normal) similar to that observed in the common type-III human G6PD deficiencies [23,33]. We found elevated band-3 abundance in RBCs from G6PD mutant animals compared to WT that is presumably associated with increased RBC turnover in G6PD deficiency consistent with earlier observations in humans [42] and this mouse model [23]. The fact that band-3 reactivity with the fluorescent dye was also elevated in G6PD deficient cells compared to WT under control as well as septic conditions further supports an altered band-3 status in G6PD deficiency. However, somewhat unexpectedly we found a lessened degree of band-3 tyrosine phosphorylation, especially in the cytoskeleton-bound fraction, in G6PD deficient cells compared to WT under septic conditions. These observations clearly indicate that G6PD deficiency modulates sepsis-induced RBC responses, however, the findings suggest complex mechanistic differences between G6PD deficient and WT animals rather than simple augmentation of sepsis-induced changes.
In vitro studies demonstrated that hyperosmotic stress [43-45] promotes band-3 tyrosine phosphorylation in RBCs. The fact that RBC mean corpuscular volume (MCV) was shown to be similar in WT as well as deficient animals either under control or septic conditions [23,24] suggest that RBC volume alterations do not play a major role in the sepsis-induced increase in band-3 tyrosine phosphorylation or account for the observed baseline differences between WT and G6PD deficient animals. It has also been demonstrated that in vitro-stimulated Ca2+ influx augments band-3 tyrosine phosphorylation in RBCs [46-48]. It remains to be elucidated whether subtle changes in RBC Ca2+ metabolism under septic conditions contribute to the increase in band-3 tyrosine phosphorylation or whether differences in Ca2+ homeostasis play a role in modulating band-3 tyrosine phosphorylation in G6PD deficient animals.
In summary, we presented evidence that sepsis is accompanied by increased band-3 tyrosine phosphorylation and potential alterations in band-3 membrane organization without compromising anion exchange activity in RBCs. Partial G6PD deficiency is accompanied by altered band-3 status but it does not augment band-3 tyrosine phosphorylation following sepsis. Under septic conditions, elevated band-3 tyrosine phosphorylation may be associated with decreased RBC deformability or disturbed metabolic status or it may represent a compensatory defensive mechanism supporting RBC functions.
Acknowledgments
This study was supported by grants NIH-NIGMS GM-GM69861 (ZS), VA Seed Grant 0160B2 (MC) and GM69790 (EAD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Langenfeld JE, Machiedo GW, Lyons M, Rush BFJ, Dikdan G, Lysz TW. Correlation between red blood cell deformability and changes in hemodynamic function. Surgery. 1994;116:859–867. [PubMed] [Google Scholar]
- 2.Powell RJ, Machiedo GW, Rush BFJ. Decreased red blood cell deformability and impaired oxygen utilization during human sepsis. Am. Surg. 1993;59:65–68. [PubMed] [Google Scholar]
- 3.Machiedo GW, Powell RJ, Rush BFJ, Swislocki NI, Dikdan G. The incidence of decreased red blood cell deformability in sepsis and the association with oxygen free radical damage and multiple-system organ failure. Arch. Surg. 1989;124:1386–1389. doi: 10.1001/archsurg.1989.01410120032007. [DOI] [PubMed] [Google Scholar]
- 4.Bateman RM, Jagger JE, Sharpe MD, Ellsworth ML, Mehta S, Ellis CG. Erythrocyte deformability is a nitric oxide-mediated factor in decreased capillary density during sepsis. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H2848–H2856. doi: 10.1152/ajpheart.2001.280.6.H2848. [DOI] [PubMed] [Google Scholar]
- 5.Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: Etiology, pharmacology, and clinical management. Ann. Emerg. Med. 1999;34:646–656. doi: 10.1016/s0196-0644(99)70167-8. [DOI] [PubMed] [Google Scholar]
- 6.Baskurt OK, Temiz A, Meiselman HJ. Red blood cell aggregation in experimental sepsis. J. Lab. Clin. Med. 1997;130:183–190. doi: 10.1016/s0022-2143(97)90094-9. [DOI] [PubMed] [Google Scholar]
- 7.Anniss AM, Sparrow RL. Storage duration and white blood cell content of red blood cell (RBC) products increases adhesion of stored RBCs to endothelium under flow conditions. Transfusion. 2006;46:1561–1567. doi: 10.1111/j.1537-2995.2006.00944.x. [DOI] [PubMed] [Google Scholar]
- 8.Raley MJ, Lennartz MR, Loegering DJ. A phagocytic challenge with IgG-coated erythrocytes depresses macrophage respiratory burst and phagocytic function by different mechanisms. J. Leukoc. Biol. 1999;66:803–808. doi: 10.1002/jlb.66.5.803. [DOI] [PubMed] [Google Scholar]
- 9.Zhang D, Kiyatkin A, Bolin JT, Low PS. Crystallographic structure and functional interpretation of the cytoplasmic domain of erythrocyte membrane band 3. Blood. 2000;96:2925–2933. [PubMed] [Google Scholar]
- 10.Tanner MJ. Band 3 anion exchanger and its involvement in erythrocyte and kidney disorders. Curr. Opin. Hematol. 2002;9:133–139. doi: 10.1097/00062752-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Campanella ME, Chu H, Low PS. Assembly and regulation of a glycolytic enzyme complex on the human erythrocyte membrane. Proc. Natl. Acad. Sci. U. S. A. 2005;102:2402–2407. doi: 10.1073/pnas.0409741102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrison ML, Rathinavelu P, Arese P, Geahlen RL, Low PS. Role of band 3 tyrosine phosphorylation in the regulation of erythrocyte glycolysis. J. Biol. Chem. 1991;266:4106–4111. [PubMed] [Google Scholar]
- 13.Arese P, Turrini F, Schwarzer E. Band 3/complement-mediated recognition and removal of normally senescent and pathological human erythrocytes. Cell. Physiol. Biochem. 2005;16:133–146. doi: 10.1159/000089839. [DOI] [PubMed] [Google Scholar]
- 14.Ciana A, Minetti G, Balduini C. Phosphotyrosine phosphatases acting on band 3 in human erythrocytes of different age: PTP1B processing during cell ageing. Bioelectrochemistry. 2004;62:169–173. doi: 10.1016/j.bioelechem.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Pietraforte D, Matarrese P, Straface E, Gambardella L, Metere A, Scorza G, Leto TL, Malorni W, Minetti M. Two different pathways are involved in peroxynitrite-induced senescence and apoptosis of human erythrocytes. Free Radic. Biol. Med. 2007;42:202–214. doi: 10.1016/j.freeradbiomed.2006.10.035. [DOI] [PubMed] [Google Scholar]
- 16.Weber RE, Voelter W, Fago A, Echner H, Campanella E, Low PS. Modulation of red cell glycolysis: interactions between vertebrate hemoglobins and cytoplasmic domains of band 3 red cell membrane proteins. Am. J. Physiol Regul. Integr. Comp. Physiol. 2004;287:R454–R464. doi: 10.1152/ajpregu.00060.2004. [DOI] [PubMed] [Google Scholar]
- 17.Peters LL, Shivdasani RA, Liu SC, Hanspal M, John KM, Gonzalez JM, Brugnara C, Gwynn B, Mohandas N, Alper SL, Orkin SH, Lux SE. Anion exchanger 1 (band 3) is required to prevent erythrocyte membrane surface loss but not to form the membrane skeleton. Cell. 1996;86:917–927. doi: 10.1016/s0092-8674(00)80167-1. [DOI] [PubMed] [Google Scholar]
- 18.Southgate CD, Chishti AH, Mitchell B, Yi SJ, Palek J. Targeted disruption of the murine erythroid band 3 gene results in spherocytosis and severe haemolytic anaemia despite a normal membrane skeleton. Nat. Genet. 1996;14:227–230. doi: 10.1038/ng1096-227. [DOI] [PubMed] [Google Scholar]
- 19.Low PS, Zhang D, Bolin JT. Localization of mutations leading to altered cell shape and anion transport in the crystal structure of the cytoplasmic domain of band 3. Blood Cells Mol. Dis. 2001;27:81–84. doi: 10.1006/bcmd.2000.0352. [DOI] [PubMed] [Google Scholar]
- 20.Jennings ML, Passow H. Anion transport across the erythrocyte membrane, in situ proteolysis of band 3 protein, and cross-linking of proteolytic fragments by 4,4'-diisothiocyano dihydrostilbene-2,2'-disulfonate. Biochim. Biophys. Acta. 1979;554:498–519. doi: 10.1016/0005-2736(79)90387-0. [DOI] [PubMed] [Google Scholar]
- 21.Hosey MM, Tao M. Altered erythrocyte membrane phosphorylation in sickle cell disease. Nature. 1976;263:424–425. doi: 10.1038/263424a0. [DOI] [PubMed] [Google Scholar]
- 22.Terra HT, Saad MJ, Carvalho CR, Vicentin DL, Costa FF, Saad ST. Increased tyrosine phosphorylation of band 3 in hemoglobinopathies. Am. J. Hematol. 1998;58:224–230. doi: 10.1002/(sici)1096-8652(199807)58:3<224::aid-ajh11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 23.Spolarics Z, Condon MR, Siddiqi M, Machiedo GW, Deitch EA. Red blood cell dysfunction in septic glucose-6-phosphate dehydrogenase-deficient mice. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H2118–H2126. doi: 10.1152/ajpheart.01085.2003. [DOI] [PubMed] [Google Scholar]
- 24.Condon MR, Kim JE, Deitch EA, Machiedo GW, Spolarics Z. Appearance of an erythrocyte population with decreased deformability and hemoglobin content following sepsis. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H2177–H2184. doi: 10.1152/ajpheart.01069.2002. [DOI] [PubMed] [Google Scholar]
- 25.Nicol CJ, Zielenski J, Tsui LC, Wells PG. An embryoprotective role for glucose-6-phosphate dehydrogenase in developmental oxidative stress and chemical teratogenesis. Faseb J. 2000;14:111–127. doi: 10.1096/fasebj.14.1.111. [DOI] [PubMed] [Google Scholar]
- 26.Jain M, Cui L, Brenner DA, Wang B, Handy DE, Leopold JA, Loscalzo J, Apstein CS, Liao RL. Increased myocardial dysfunction after ischemiareperfusion in mice lacking glucose-6-phosphate dehydrogenase. Circulation. 2004;109:898–903. doi: 10.1161/01.CIR.0000112605.43318.CA. [DOI] [PubMed] [Google Scholar]
- 27.Wilmanski J, Villanueva E, Deitch EA, Spolarics Z. G6PD deficiency and the inflammatory response to endotoxin and polymicrobial sepsis. Crit. Care Med. 2007 doi: 10.1097/01.CCM.0000254337.50361.2E. [DOI] [PubMed] [Google Scholar]
- 28.Ebong S, Call D, Nemzek J, Bolgos G, Newcomb D, Remick D. Immunopathologic alterations in murine models of sepsis of increasing severity. Infect. Immun. 1999;67:6603–6610. doi: 10.1128/iai.67.12.6603-6610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu SC, Palek J, Yi SJ, Nichols PE, Derick LH, Chiou SS, Amato D, Corbett JD, Cho MR, Golan DE. Molecular basis of altered red blood cell membrane properties in Southeast Asian ovalocytosis: role of the mutant band 3 protein in band 3 oligomerization and retention by the membrane skeleton. Blood. 1995;86:349–358. [PubMed] [Google Scholar]
- 30.Fairbanks G, Steck TL, Wallach DF. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971;10:2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- 31.Jarolim P, Rubin HL, Liu SC, Cho MR, Brabec V, Derick LH, Yi SJ, Saad ST, Alper S, Brugnara C. Duplication of 10 nucleotides in the erythroid band 3 (AE1) gene in a kindred with hereditary spherocytosis and band 3 protein deficiency (band 3PRAGUE) J. Clin. Invest. 1994;93:121–130. doi: 10.1172/JCI116935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King MJ, Smythe JS, Mushens R. Eosin-5-maleimide binding to band 3 and Rh-related proteins forms the basis of a screening test for hereditary spherocytosis. Br. J. Haematol. 2004;124:106–113. doi: 10.1046/j.1365-2141.2003.04730.x. [DOI] [PubMed] [Google Scholar]
- 33.Luzzatto L, Mehta A. Glucose 6phosphate dehydrogenase deficiency. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. NY McGrawHill; New York: 1995. pp. 3367–3398. [Google Scholar]
- 34.Baggio B, Bordin L, Clari G, Gambaro G, Moret V. Functional correlation between the Ser/Thr-phosphorylation of band-3 and band-3-mediated transmembrane anion transport in human erythrocytes. Biochim. Biophys. Acta. 1993;1148:157–160. doi: 10.1016/0005-2736(93)90173-w. [DOI] [PubMed] [Google Scholar]
- 35.Teti D, Crupi M, Busa M, Valenti A, Loddo S, Mondello M, Romano L. Chemical and pathological oxidative influences on band 3 protein anion-exchanger. Cell. Physiol. Biochem. 2005;16:77–86. doi: 10.1159/000087734. [DOI] [PubMed] [Google Scholar]
- 36.Barbul A, Zipser Y, Nachles A, Korenstein R. Deoxygenation and elevation of intracellular magnesium induce tyrosine phosphorylation of band 3 in human erythrocytes. FEBS Lett. 1999;455:87–91. doi: 10.1016/s0014-5793(99)00822-4. [DOI] [PubMed] [Google Scholar]
- 37.Korbut R, Gryglewski RJ. The effect of prostacyclin and nitric oxide on deformability of red blood cells in septic shock in rats. J. Physiol. Pharmacol. 1996;47:591–599. [PubMed] [Google Scholar]
- 38.Hoefner DM, Blank ME, Davis BM, Diedrich DF. Band 3 antagonists, p-azidobenzylphlorizin and DIDS, mediate erythrocyte shape and flexibility changes as characterized by digital image morphometry and microfiltration. J. Membr. Biol. 1994;141:91–100. doi: 10.1007/BF00232877. [DOI] [PubMed] [Google Scholar]
- 39.Crimi E, Sica V, Slutsky AS, Zhang H, Williams-Ignarro S, Ignarro LJ, Napoli C. Role of oxidative stress in experimental sepsis and multisystem organ dysfunction. Free Radic. Res. 2006;40:665–672. doi: 10.1080/10715760600669612. [DOI] [PubMed] [Google Scholar]
- 40.Powell RJ, Machiedo GW, Rush BFJ, Dikdan G. Effect of alpha-tocopherol on red cell deformability and survival in sepsis. Curr. Surg. 1989;46:380–382. [PubMed] [Google Scholar]
- 41.Powell RJ, Machiedo GW, Rush BFJ, Dikdan G. Oxygen free radicals: effect on red cell deformability in sepsis. Crit. Care Med. 1991;19:732–735. [PubMed] [Google Scholar]
- 42.Spolarics Z, Siddiqi M, Siegel JH, Garcia ZC, Stein DS, Ong H, Livingston DH, Denny T, Deitch EA. Increased incidence of sepsis and altered monocyte functions in severely injured type A- glucose-6-phosphate dehydrogenase-deficient African American trauma patients. Crit. Care Med. 2001;29:728–736. doi: 10.1097/00003246-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Bordin L, Zen F, Ion-Popa F, Barbetta M, Baggio B, Clari G. Band 3 tyr-phosphorylation in normal and glucose-6-phospate dehydrogenase-deficient human erythrocytes. Mol. Membr. Biol. 2005;22:411–420. doi: 10.1080/09687860500233679. [DOI] [PubMed] [Google Scholar]
- 44.Haussinger D, Lang F, Gerok W. Regulation of cell function by the cellular hydration state. Am. J. Physiol. 1994;267:E343–E355. doi: 10.1152/ajpendo.1994.267.3.E343. [DOI] [PubMed] [Google Scholar]
- 45.Minetti G, Seppi C, Ciana A, Balduini C, Low PS, Brovelli A. Characterization of the hypertonically induced tyrosine phosphorylation of erythrocyte band 3. Biochem. J. 1998;335(Pt 2):305–311. doi: 10.1042/bj3350305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zipser Y, Piade A, Barbul A, Korenstein R, Kosower NS. Ca2+ promotes erythrocyte band 3 tyrosine phosphorylation via dissociation of phosphotyrosine phosphatase from band 3. Biochem. J. 2002;368:137–144. doi: 10.1042/BJ20020359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor D, Baker R, Hochstein P. The effect of calcium ionophore A23187 on the ATP level of human erythrocytes. Biochem. Biophys. Res. Commun. 1976;76:205–211. doi: 10.1016/0006-291x(77)90712-4. [DOI] [PubMed] [Google Scholar]
- 48.Minetti G, Piccinini G, Balduini C, Seppi C, Brovelli A. Tyrosine phosphorylation of band 3 protein in Ca2+/A23187-treated human erythrocytes. Biochem. J. 1996;320(Pt 2):445–450. doi: 10.1042/bj3200445. [DOI] [PMC free article] [PubMed] [Google Scholar]