Abstract
Two new cases of dilated cardiomyopathy (DC) caused by dystrophinopathy are reported. One patient, a 24 year old man, had a family history of X linked DC, while the other, a 52 year old man, had sporadic disease. Each had abnormal dystrophin immunostaining in muscle or cardiac biopsy specimens, but neither had muscle weakness. Serum creatine kinase activity was raised only in the patient with familial disease. Analysis of dystrophin gene mutations showed a deletion of exons 48-49 in the patient with familial DC and of exons 49-51 in the other. Dystrophin transcription in cardiac tissue from the patient with sporadic disease showed abundant expression, predominantly of the muscle isoform. This study, together with previous reports, suggests that some patients with DC have a dystrophinopathy that can be diagnosed using a combination of biochemical and genetic analyses. Keywords: dilated cardiomyopathy; dystrophin; Becker muscular dystrophy
Full Text
The Full Text of this article is available as a PDF (135.0 KB).
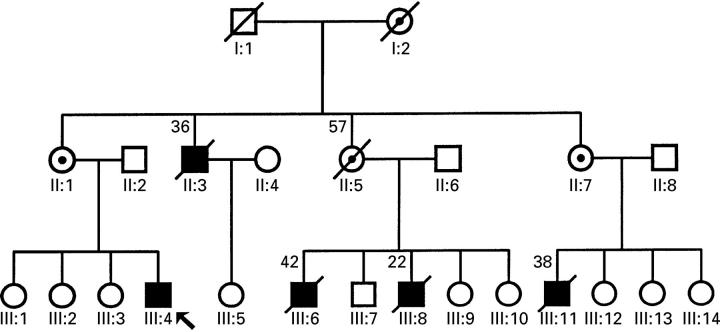
Figure 1 .
Pedigree of family with X linked cardiomyopathy. Open symbols, unaffected individuals; closed circles, affected individuals; barred symbols, deceased (number above is age at death); dotted circles, obligate carriers; arrow, propositus.
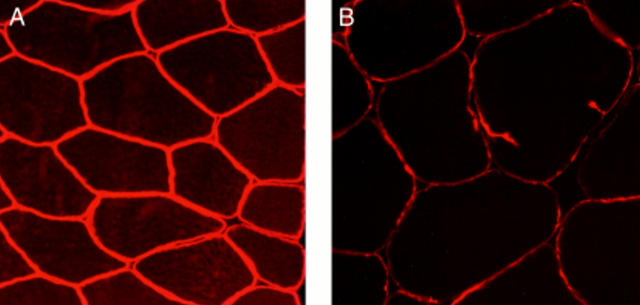
Figure 2 .
Immunohistochemical staining of skeletal muscle with Dys-III N terminus antidystrophin antibodies. (A) Normal muscle: all fibres equally and intensely stained (original magnification ×280). (B) Muscle from patient 1: staining is weaker than control muscle, suggesting a dystrophinopathy (original magnification ×280).
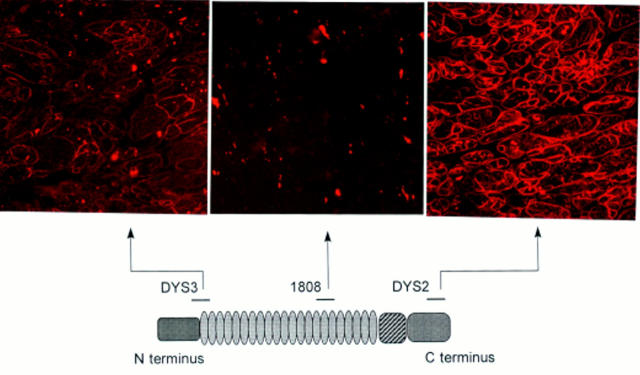
Figure 3 .
Top, immunohistochemical staining of cardiac muscle from patient 2 using antidystrophin antibodies. Bottom, protein dystrophin and epitopes of three of the antibodies used in the study. Top left, N terminus antidystrophin antibodies (Dys3) with a near normal reaction; top middle, no staining was visible with mid-rod domain 1808 antidystrophin antibodies; top right, reduced staining with Dys2 C terminus antidystrophin antibodies (original magnifications ×320).
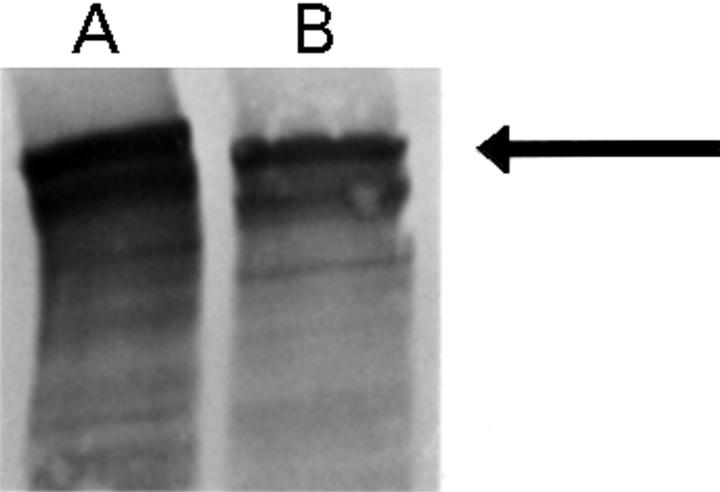
Figure 4 .
Western blot analysis of cardiac biopsy from (A) control and (B) patient 2, probed with mid-rod antidystrophin antibody (P6).22 Note the slight reduction in abundance and molecular weight of dystrophin in the patient (arrow).
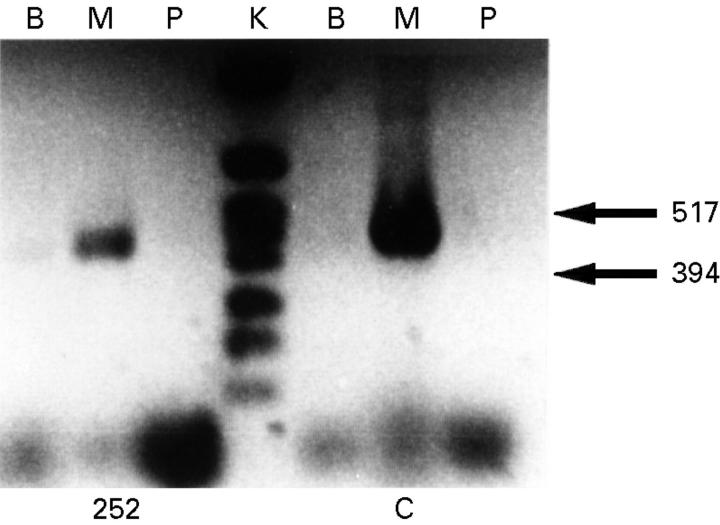
Figure 5 .
Amplification of cDNA from normal cardiac muscle (C) and the cardiac biopsy from patient 1 (252). Dystrophin muscle isoform (M) was amplified as a 494 base pair fragment, the brain isoform (B) as 482 base pairs, and the Purkinje cell isoform (P) as 503 base pairs. The cardiac muscle biopsy from patient 1 showed transcription of trace amount of isoform B and higher expression of isoform M, but no expression of isoform P. The control cardiac tissue showed an identical pattern of isoform transcription with a trace amount of isoform B and high expression of isoform M. Lane K, molecular weight marker.