Abstract
Only a subset of cleavage stage blastomeres in the Xenopus embryo is competent to contribute cells to the retina; ventral vegetal blastomeres do not form retina even when provided with neuralizing factors or transplanted to the most retinogenic position of the embryo. These results suggest that endogenous maternal factors in the vegetal region repress the ability of blastomeres to form retina. Herein we provide three lines of evidence that two vegetal-enriched maternal factors (VegT, Vg1), which are known to promote endo-mesodermal fates, negatively regulate which cells are competent to express anterior neural and retinal fates. First, both molecules can repress the ability of dorsal-animal retinogenic blastomeres to form retina, converting the lineage from neural/retinal to non-neural ectodermal and endo-mesodermal fates. Second, reducing the endogenous levels of either factor in dorsal-animal retinogenic blastomeres expands expression of neural/retinal genes and enlarges the retina. The dorsal-animal repression of neural/retinal fates by VegT and Vg1 is likely mediated by Sox17α and Derriere but not by XNr1. VegT and Vg1 likely exert their effects on neural/retinal fates through at least partially independent pathways because Notch1 can reverse the effects of VegT and Derriere but not those of Vg1 or XNr1. Third, reduction of endogenous VegT and/or Vg1 in ventral vegetal blastomeres can induce a neural fate, but only allows expression of a retinal fate when both BMP and Wnt signaling pathways are concomitantly repressed.
Keywords: VegT, Vg1, Xnr1, derriere, sox3, Notch1, rx1, endodermin, Xbra, retina specification
Introduction
The elucidation of the molecular and cellular mechanisms that control how early embryonic cells are specified to express different fates is central to our understanding of how vertebrate embryos develop into different tissues. The vertebrate retina is a particularly good model for studying fate specification because of its accessibility during developmental stages, a wealth of tissue and stage-specific markers and a number of mutations and diseases that affect its development and function. The developmental program that regulates the specification of retinal fate is comprised of multiple steps that influence the competence of embryonic cells to form retina (reviewed in Williams and Moody, 2004; Zaghloul et al., 2005). Although events during neural plate and optic cup formation are critical for defining the retina-specific stem cells and the many cell phenotypes that are derived from them (Marquardt, 2003; Hatakeyama and Kageyama, 2004), earlier developmental decisions have been shown to significantly impact the ability of embryonic cells to express a retinal fate in Xenopus, in whose embryos specific precursors of the retina can be identified at early cleavage stages.
The potential to form retina is restricted to the dorsal-animal quadrant at early cleavage stages in normal Xenopus embryos (Fig. 1; Huang and Moody, 1993). These cells are a subset of those that give rise to the entire nervous system (Moody, 1987a, b). Other cells in the animal and equatorial tiers that normally contribute to the CNS but not to the retina are competent to form retina if they are moved to the dorsal-animal retinogenic region by experimental manipulations (Huang and Moody, 1993). Further, locally increasing or decreasing the activity of several signaling pathways demonstrated that the early restriction of retinogenic potential to the dorsal-animal quadrant depends upon the level of local BMP signaling experienced by a cell (Moore and Moody, 1999), consistent with the proposal that eye specific genes require a higher level of neural inductive signaling in order to form retinal tissue (Chow et al., 1999; Kenyon et al., 2001). However, there also is a vegetal repression of retinal fate in cleavage blastomeres (Huang and Moody, 1993; Moore and Moody, 1999); vegetal-tier blastomeres in the 32-cell embryo do not form retina when transplanted to the dorsal-animal retinogenic region, even when supplemented with neural, anteriorizing or dorsal mesoderm promoting signaling molecules (Fig. 1; Moore and Moody, 1999). It is particularly interesting that these manipulations do allow the vegetal-tier blastomeres to express neural fates, suggesting that acquiring a retinal fate requires additional information that is repressed in vegetal cells. In this study we set out to define which maternal molecules are responsible for this block of retinal competence.
Figure 1.
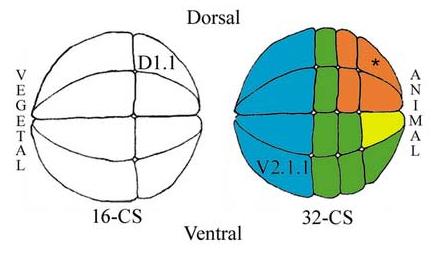
Side views of 16- and 32-cell stage (CS) Xenopus embryos noting the positions of the two blastomeres manipulated in the described experiments (D1.1, V2.1.1). The 32-cell embryo shows four of the eight blastomeres that are highly retinogenic (orange), and the one that is slightly retinogenic (yellow); asterisk indicates the most retinogenic cell. Several other blastomeres are competent to form retina but normally do not (green) and vegetal blastomeres are not competent to make retina (blue).
In Xenopus several maternal molecules are enriched in the vegetal hemisphere and are involved in specifying the germ line, mesoderm and endoderm (reviewed in Sullivan et al., 1999). We investigated two of these (VegT, Vg1) because they promote mesodermal and endodermal fates, and thus might endogenously repress neural and retinal fates in vegetal cells. VegT is a T-box transcription factor that plays an essential role in the formation of mesoderm and endoderm (Zhang and King, 1996; Zhang et al. 1998; Clements et al., 1999; Kofron et al., 1999; Chang and Hemmati-Brivanlou, 2000; Xanthos et al. 2001). It appears to regulate endoderm formation by regulating members of Sox17, GATA, and Mix/Bix/Mixer families (Hudson et al., 1997; Henry and Melton, 1998; Tada et al., 1998; Casey et al., 1999; Clements et al., 1999; Weber et al., 2000; Xanthos et al., 2001) and to promote ventral mesoderm via Nodal signaling (Clements et al., 1999; White et al., 2002; Loose and Patient, 2004; Taverner et al., 2005). Vg1, a member of the TGF-β superfamily distinct from but closely related to the Nodal signaling proteins XNr1, XNr2, XNr4 and Derriere (Kimelman and Griffin, 2000; Xanthos et al., 2001; White et al., 2002), also appears necessary for dorsal mesoderm and endoderm formation (Kessler and Melton, 1995; Henry and Melton, 1998; Kessler, 1999). Thus, both molecules are in the right place at the right time and have an appropriate function to be candidates for the repression of retinal fates in vegetal blastomeres.
To examine whether VegT and/or Vg1 repress retinal fate three types of experiments were performed. First, the factors were over-expressed in dorsal-animal precursors of the CNS and retina to determine whether they repress these fates. Secondly, their endogenous levels were reduced in dorsal-animal blastomeres to determine whether neural and/or retinal fates were expanded. Third, their endogenous levels in ventral vegetal blastomeres were reduced to test whether this is sufficient to permit expression of neural and/or retinal fates at this ectopic position. We show that both VegT and Vg1 modulate the extent of neural/retinal fate in dorsal-animal lineages by converting cells from neural ectoderm to non-neural ectoderm, mesoderm and endoderm fates. These effects appear to be mediated cell autonomously by sox17α, which converts the lineage to endoderm, and non-autonomously via Derriere signaling. The effects of both VegT and Derriere, but not Vg1, are reversed by co-expression of notch1, which is known to activate eye-specific genes (Kumar and Moses, 2001; Onuma et al., 2002), suggesting that those factors act in at least partially independent pathways. In contrast, reduction of VegT and/or Vg1 in ventral vegetal blastomeres can allow these lineages to give rise to small numbers of neural progeny, but this is not sufficient to produce retinal tissue. Similarly, reduction of both BMP and Wnt signaling in the ventral vegetal lineages can give rise to neural but not retinal tissue (Moore and Moody, 1999). However, the combined repression of VegT/Vg1 and BMP/Wnt in ventral vegetal cells leads to formation of retinal cells. Thus, in order for an animal lineage to become a precursor of the retina, it must not express endo-mesoderm promoting factors; for a vegetal lineage the correct, endogenous signaling environment must additionally be provided.
Materials and methods
Embryo collection and blastomere injection of mRNAs
Fertilized Xenopus laevis eggs were obtained by gonadotropin-induced natural matings of adult frogs and prepared for injection as described (Moody, 2000). mRNAs were synthesized in vitro and injected at the indicated concentrations: tALK7 (2.0ng, Reissmann et al., 2001), β-gal (100pg), tBR (250pg, Graff et al., 1994), cerberus-long (cer-L; 200pg; Piccolo et al., 1999); cerberus-short (cer-S; 200pg; Piccolo et al., 1999), derriere (50pg; Sun et al., 1999), cm-derriere (500pg; Sun et al., 1999), gfp (200pg), an activated form of notch1 (XNICD, 50-500pg, Coffman et al., 1993), Xnr1 (25pg, Osada and Wright, 1999), noggin (50pg, Smith and Harland, 1992), sox17α (5-200 pg, Hudson et al., 1997), VegT (5-200pg, Zhang and King, 1996), an activated form of Vg1 (BVg1; 5-50 pg, Joseph and Melton, 1998), dnVg1 (100 pg, Joseph and Melton, 1998) and dnWnt8 (500pg, Hoppler et al., 1996). Test mRNAs mixed with lineage tracer mRNA or lineage tracer mRNA alone were injected into one blastomere of the 16- or 32-cell embryo as described (Moody, 2000; Fig. 1).
Morpholino antisense oligonucleotides
An antisense VegT morpholino oligonucleotide (VegTMO) that is known to deplete maternal VegT (Zhang et al., 1998), the corresponding sense MO as a control (cVegTMO) and a standard control MO (cMO) were obtained commercially (Gene Tools). Blastomeres were injected with 16ng of MO mixed with lineage tracer (β-gal or gfp) mRNA.
Whole mount in situ hybridization
Embryos were fixed, stained for expression of the β-Gal lineage tracer and processed for whole mount in situ hybridization according to standard protocols (Sive et al., 2000) using Digoxigenin-labeled RNA probes for foxD5 (Sullivan et al., 2001), otx2 (Blitz and Cho, 1995), sox3 (Penzel et al., 1997), notch1 (Coffman et al., 1990), rx1 (Mathers et al., 1997), en2 (Hemmati-Brivanlou et al., 1991), Krox20 (Bradley et al., 1993), keratin (Jonas et al., 1989), Xbra (Smith et al., 1991), sox17α (Hudson et al., 1997) and endodermin (edd) (Sasai et al., 1996). In some cases the widths of the expression domains of sox3 on both the injected (β-Gal positive) and uninjected sides were measured with an eyepiece micrometer at 40X. The mean percent difference between sides was compared to similar measurements from lineage tracer injected, control embryos by the Student's t-test.
Cell fate analyses
Embryos were raised in Steinberg's solution, fixed in 4% paraformaldehyde, 3% sucrose in PBS at stage 37/38 (Nieuwkoop and Faber, 1994) and serially sectioned with a cryostat. Serial sections were analyzed for the presence of GFP-labeled cells using epifluorescence microscopy. The distribution of GFP-positive progeny was compared to established fate maps (Moody, 1987a, b; Huang and Moody, 1993) and to control embryos injected with gfp mRNA. The frequencies that embryos contained labeled cells in different tissues in experimental and control groups were compared using the z-test of proportions as described (Moore and Moody, 1999). In some data sets, the size of the D1.1 contribution to the retina was estimated from the area of the retina containing GFP-labeled cells, as previously reported (Moore and Moody, 1999). To quantify effects on the size of the retina, its area was measured from serial tissue sections and volumes calculated as previously described (Moore and Moody, 1999). Measurements were compared between samples by the Student's t-test.
Blastomere transplantation
VegTMO (16ng) mixed with gfp mRNA (200pg) was injected bilaterally into the vegetal pole at the 2-cell stage. When embryos reached the 32- to 64-cell stage, one ventral vegetal blastomere (V2.1.1; Fig. 1) was transplanted to the position of the most retinogenic blastomere (* in Fig. 1) in an unlabeled host embryo as described (Huang and Moody, 1993; Moore and Moody, 1999; Moody, 1999). Embryos were cultured in Steinberg's solution plus gentamycin (1:1000) until stages 26-28 and fixed for cell fate analyses.
Results
Increased expression of either VegT or Vg1 in dorsal-animal blastomeres represses retina development
Previous studies demonstrated that ventral vegetal blastomeres are not competent to express a retinal fate, and predicted that this was due to the presence of an endogenous maternal factor (Huang and Moody, 1993; Moore and Moody, 1999). If endogenous VegT were involved, then increasing its expression in a major retina-producing blastomere should suppress retina formation. To test this, a series of doses of VegT mRNA was injected into one dorsal-animal blastomere (D1.1; Fig. 1) that normally produces about 60% of retinal cells (Huang and Moody, 1993). The eyes on the injected side of these embryos were smaller compared to those in gfp mRNA-injected control embryos (Fig. 2A, B), and the frequency of this eye defect positively correlated with the dose of injected VegT mRNA (51% at 5pg to 91% at 200pg). To verify that the retinal tissue was smaller the volumes of the retinas were measured; these were significantly reduced on the VegT-expressing side, whereas in GFP-expressing control embryos no significant difference was observed (Fig. 2B).
Figure 2.
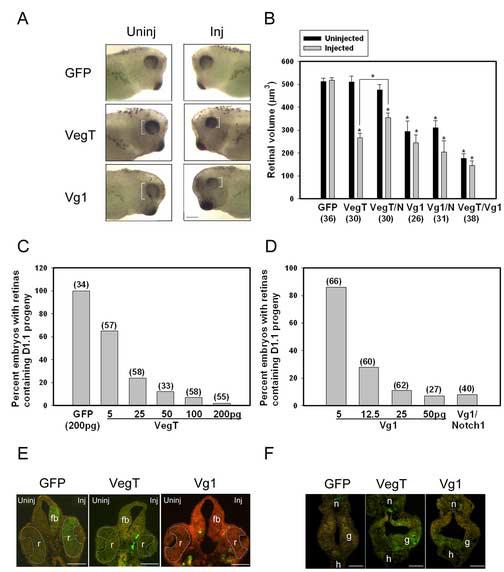
VegT and Vg1 inhibit the retinal fate of the dorsal-animal (D1.1) blastomere.
(A) Expression of VegT or Vg1 in the D1.1 lineage results in a smaller eye (white brackets). Uninj, uninjected side; Inj, injected side. Scale bar = 400μm.
(B) The mean volume of retinas in embryos in which the indicated mRNAs (GFP, VegT, Vg1, notch1 [N]) were expressed. Numbers in parentheses indicate size of sample. * indicates p<0.01 compared to GFP controls. VegT and Vg1 both significantly reduced the size of the retina on the injected side; Vg1 additionally reduced the retina on the uninjected side. Co-injection of notch1 significantly increased the retinal volume of VegT-injected embryos, compared to VegT alone (bracket, p<0.01), although they remained smaller than the GFp controls. Co-injection of notch1 did not increase the retinal size of Vg1-injected embryos compared to Vg1 alone. Co-injection of VegT and Vg1 mRNAs further reduced the size of the retina compared to VegT alone (p<0.01) or Vg1 alone (p<0.05).
(C) Percentage of embryos in which D1.1 progeny populate the retina. The number is dramatically reduced in a dose-dependent manner in embryos injected with VegT mRNA.
(D) Percentage of embryos in which D1.1 progeny populate the retina. The number is dramatically reduced in a dose-dependent manner in embryos injected with Vg1 mRNA; this is not reversed by co-injection of notch1 (500pg).
(E) The retina (r) on the injected (right) side of VegT and Vg1 embryos is smaller, and the D1.1 progeny (green) that normally contribute large numbers to the retina and forebrain (fb) in GFP controls are missing in VegT and Vg1 embryos. Scale bar = 200μm.
(F) The size of the D1.1 clone (green) in the gut (g) is larger in VegT- and Vg1-injected embryos compared to GFP controls. h, heart; n, notochord. Scale bar = 200μm
The reduced size of the retina could result from either death of the VegT-expressing cells or their conversion to a different fate. To distinguish between these possibilities, the GFP-labeled, VegT-expressing cells were fate mapped from serial tissue sections. First, no evidence of dying cells (as described in detail in Moody, 1999) was observed. Second, large numbers of differentiated GFP-labeled cells were found in the embryos, but their presence in the retina was dramatically reduced by VegT (Fig. 2C, E). Whereas 100% of GFP-injected control embryos contained abundant labeled D1.1 descendants in the retina, as expected from normal fate maps (Huang and Moody, 1993), VegT expression significantly reduced this frequency in a dose dependent manner (Fig. 2C). Additionally, the size of the D1.1 clone in the retina was reduced; for embryos injected with 5pg of VegT mRNA, the D1.1 retinal clones were about one-third the size of normal clones and for those injected with 100-200pg of VegT mRNA the D1.1 retinal clones consisted of less than 10 cells. Interestingly, effects on the D1.1 lineage were not confined to the retina. Fate mapping of D1.1 clones injected with 100pg VegT mRNA showed that this lineage, which normally contributes large numbers of cells to ectodermal derivatives and smaller number of cells to mesodermal or endodermal derivatives (Moody, 1987a), also contributed significantly less frequently to other neural structures (forebrain, 4.2%; midbrain, 8.3%; hindbrain and spinal cord, 37.5%; olfactory pit 0%; cranial ganglia, 8.3%) compared to controls (100% for all except cranial ganglia, 93.3%; p<0.05). These clones contributed significantly more frequently to some mesodermal structures (e.g., pronephros, 41.7% vs. 6.2% in controls) (p<0.05). The amount of endoderm populated by the VegT-expressing D1.1 clone appeared larger than in controls (Fig. 2F).
Expression of an activated form of Vg1 (BVg1, which contains a cleavage site necessary for protein activation; Joseph and Melton, 1998) in blastomere D1.1 also resulted in significantly smaller retinas (Fig. 2A, B); the effect often was bilateral as expected for a secreted protein. Vg1 reduced the frequency that the D1.1 lineage contributed to the retina in a dose-dependent manner (Fig. 2D, E). The size of the D1.1 clone in the retina also was reduced; for embryos injected with 12.5pg of BVg1 mRNA, the D1.1 retinal clones were about one-quarter the size of normal clones and for those injected with 100-200pg of BVg1 mRNA the D1.1 retinal clones contained less than 10 cells. Fate-mapping of Vg1-expressing D1.1 clones showed a significantly less frequent contribution to other neural structures (olfactory placode, 0%; forebrain, 10%; midbrain, 0%; hindbrain and spinal cord, 0%; cranial ganglia, 15%) compared to controls (p<0.05), and the amount of endoderm populated by the Vg1-expressing D1.1 clone appeared larger than in controls (Fig. 2F). These results demonstrate that both VegT and Vg1 antagonize retinal fate, but these effects were not specific to the retina. Both factors also caused significantly smaller contributions to other neural tissues and enhanced contributions to the endoderm.
VegT and Vg1 both alter retinal fates by converting lineages to non-neural tissues
The fate mapping results indicate that VegT and Vg1 suppress neural/retinal fates and expand mesoderm and endoderm fates, consistent with several previous studies. To determine whether these effects are the result of alterations in germ layer induction, neural induction, retina-specific induction or neural plate patterning, the expression patterns of several genes during gastrulation and neural plate/neural fold stages were analyzed. VegT expression in the D1.1 lineage repressed two neural ectoderm genes that are expressed during gastrulation (foxD5, otx2), whereas genes expressed in non-neural ectoderm (ker), mesoderm (Xbra) and endoderm (sox17α, edd) were ectopically induced (Fig. 3A; Table 1). At later neural plate stages, the expression domains of two pan-neural plate genes (sox3, notch1) were smaller on the VegT-expressing side (Fig. 3B) in a significant number of embryos (Table 1); the mean width of the neural plate on the VegT-expressing side was reduced by 30% (sox3) and 28% (notch1) compared to β-Gal-expressing controls (p<0.001). Region-specific gene expression patterns at neural plate/neural fold stages also were disrupted. Eye field (rx1), forebrain (otx2), midbrain (en2) and hindbrain (Krox20) genes were significantly repressed on the injected side (Fig. 3B; Table 1), suggesting that the effect is on early establishment of the neural ectoderm rather than regionalization of that tissue. Vg1 expression in the D1.1 lineage caused identical changes in all of the monitored genes except keratin; there was no evidence of ectopic or expanded non-neural ectoderm (Fig. 3A; Table 1). These results indicate that increased expression of either VegT or Vg1 in the major retina-producing lineage (D1.1) reduces the size of the neural ectoderm and promotes formation of the other germ layers during gastrulation; these early changes in fate subsequently affect the expression of later neural genes including one that is necessary for retina formation (rx1).
Figure 3.
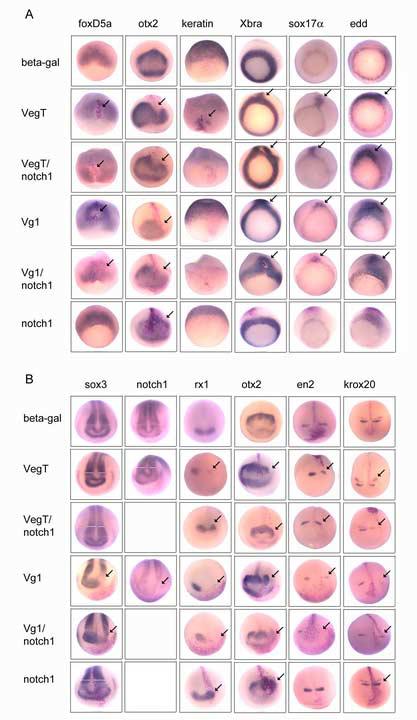
VegT and Vg1 alter cell fates at gastrulation stages and these changes affect later neural patterning.
(A) The expression domains of markers of the germ layers (top labels) during gastrulation after injection of one D1.1 blastomere with mRNAs indicated on the left. Early neural ectoderm is identified by foxD5 and otx2, non-neural ectoderm by keratin, mesoderm by Xbra and endoderm by sox17α and edd. Red cells indicate the D1.1 progeny expressing the injected mRNA, and arrows indicate regions of gene repression or ectopic expression. Frequencies of phenotypes are presented in Table 1.
(B) The expression domains of pan-neural (sox3, notch1), eye field (rx1), forebrain (otx2), midbrain (en2) and hindbrain (krox20) genes during neural plate stages. White bars indicate the width of the expression domains on the injected (right) versus uninjected (left) side of the neural plate. Arrows are as above. Frequencies of phenotypes are presented in Table 1.
Table 1.
Changes in expression domains of neural, retinal, ectodermal, mesodermal and endodermal genes after injection of constructs into one dorsal-animal blastomere (D1.1)
|
ISH → Injected ↓ |
foxD5 (n) |
otx2 st 13 (n) |
sox3 (n) |
notch1 (n) |
rx1 (n) |
otx2 st16 (n) |
en2 (n) |
krox20 (n) |
keratin (n) |
Xbra (n) |
sox17α (n) |
edd (n) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VegT | 91.3%↓ (46) |
76.2%↓ (42) |
70.4%↓ (54) |
69.6%↓ (23) |
69.4%↓ (62) |
48.9%↓ (45) |
82.7%↓ (52) |
61.3%↓ (62) |
97.4%↑ (39) |
100%↑ (30) |
27.1%↑ (48) |
86.8%↑ (53) |
| VegT/notch1 | 82.8%↓ (58) |
25.0%↓ (24) |
34.3%↓ (35) |
--- | 24.6%↓ (57) |
28.6%↓ (28) |
66.7%↓ (87) |
70.0%↓ (30) |
12.5%↑ (64) |
92.3%↑ (26) |
29.6%↑ (27) |
90.0%↑ (30) |
| Vg1 | 95.7%↓ (47) |
84.4%↓ (32) |
71.4%↓ (28) |
79.3%↓ (29) |
85.0%↓ (20) |
41.4%↓ (29) |
86.5%↓ (37) |
59.1%↓ (22) |
NC (43) |
97.3%↑ (37) |
38.9%↑ (36) |
96.9%↑ (32) |
| Vg1/notch1 | 96.4%↓ (28) |
85.7%↓ (28) |
75.0%↓ (36) |
--- | 86.1%↓ (36) |
63.3%↓ (30) |
87.0%↓ (23) |
65.0%↓ (40) |
NC (34) |
94.3%↑ (35) |
65.2%↑ (23) |
97.1%↑ (35) |
| notch1 | NC (54) |
42.9%↑ (28) |
56.7%↑ (30) |
--- | 74.1%↑ (27) |
39.3%↑ (28) |
NC (32) |
43.3%↑ (30) |
NC (52) |
NC (33) |
NC (29) |
NC (25) |
| VegT+Vg1 | 96.8%↓ (32) |
91.3%↓ (23) |
87.5%↓ (24) |
90.0%↓ (30) |
91.7%↓ (24) |
83.3%↓ (24) |
90.0%↓ (20) |
87.5%↓ (40) |
NC (28) |
100%↑ (22) |
93.3%↑ (30) |
100%↑ (27) |
| VegT MO | 46.6%↑ (30) |
45.2%↑ (31) |
44.4%↑ (27) |
NC (27) |
NC (23) |
NC (42) |
||||||
| dnVg1 | 54.8%↑ (31) |
42.9%↑ (28) |
50.0%↑ (32) |
62.5%↓ (24) |
NC (36) |
NC (29) |
||||||
| VegTMO/dnVg1 | 82.1%↑ (28) |
77.8%↑ (18) |
85.0%↑ (20) |
|||||||||
| sox17α | NC (30) |
NC (30) |
NC (31) |
NC (32) |
100%↓ (35) |
--- | 83.3%↑ (30) |
|||||
| derriere | 86.1%↓ (72) |
87.5%↓ (24) |
100%↓ (16) |
85.7%↓ (28) |
100%↓ (26) |
47.2%↓ (36) |
76.3%↓ (80) |
90.3%↓ (31) |
100%↑ (16) |
80.0%↑ (45) |
67.4%↑ (46) |
92.5%↑ (40) |
| derriere/notch1 | 80.0%↓ (42) |
20.6%↓ (34) |
27.7%↓ (36) |
--- | 41.4%↓ (29) |
21.9%↓ (32) |
90.5%↓ (21) |
71.1%↓ (45) |
15.0%↑ (40) |
96.6%↑ (30) |
71.0%↑ (31) |
91.7%↑ (36) |
| cm-derriere | 31.3%↑ (32) |
25.0%↑ (32) |
41.0%↑ (39) |
53.1%↓ (32) |
NC (25) |
NC (28) |
||||||
| derriere/VegTMO | 93.8%↓ (16) |
90.0%↓ (20) |
100%↓ (16) |
|||||||||
| VegT/cm-derriere | 6.7%↓ (30) |
NC (32) |
10.7%↓ (28) |
|||||||||
| Xnr1 | 71.4%↓ 23.8%↑ (21) |
35.3%↓ 23.5%↑ (17) |
59.4%↓ 6.3%↑ (32) |
NC (36) |
100%↑ (26) |
96.3%↑ (27) |
100%↑ (27) |
|||||
| Xnr1/notch1 | 63.3%↓ 26.7%↑ (30) |
--- | 73.1%↓ 7.7%↑ (26) |
NC (22) |
96.9%↑ (32) |
100%↑ (32) |
92.5%↑ (40) |
|||||
| tALK7 | 25.6%↑ 23.1%↓ (39) |
7.1%↑ 21.4%↓ (28) |
23.5%↑ 23.5%↓ (34) |
91.7%↓ (24) |
NC (24) |
NC (22) |
notch1 can reverse the repression of neural/retinal fate caused by VegT but not by Vg1
notch1 is expressed throughout the neural plate (Fig. 3B; Coffman et al., 1990), and there is evidence that it acts upstream of retinal-fate specifying genes (Kumar and Moses, 2001; Onuma et al., 2002). To verify that it has a neural/retinal fate promoting effect, we expressed an activated notch1 construct (XNICD, truncated to include only the intracellular signaling domain; Coffman et al., 1993) in the D1.1 lineage. While it had no effect on the expression of foxD5 or en2, it weakly induced other neural plate/neural fold markers and strongly induced rx1 (Fig. 3; Table 1). Therefore, we tested whether the repression of neural and retinal fates in the D1.1 lineage by VegT or Vg1 could be reversed by co-expression of notch1. The frequency of the small eye phenotype caused by 100pg VegT mRNA was reduced by notch1 mRNA in a dose-dependent manner (Fig. 4A), and the proportion of embryos in which VegT-expressing D1.1 progeny populated the retina was increased by co-expression of notch1 in a dose-dependent manner (Fig. 4B). notch1 reduced the frequency and extent of the VegT-induced repression of nearly all neural genes (foxD5 and Krox20 were not rescued), and reduced the ectopic induction of non-neural ectoderm (ker) (Fig. 3; Table 1). The mean width of the neural plate measured by sox3 expression on the side injected with VegT/notch1 mRNAs was only slightly reduced from controls (6.2%), and was significantly larger (p<0.001) than those injected with VegT mRNA alone. The VegT/notch1 retina was significantly larger than the VegT alone retina, although it remained smaller than controls (Fig. 2B). Interestingly, notch1 did not prevent the VegT-induced ectopic expression of Xbra, sox17α or edd (Fig. 3; Table 1), consistent with the observations that notch1 alone did not affect the expression of non-neural ectoderm, mesoderm or endoderm markers (Table 1). These results indicate that most of the effects of VegT on both neural and non-neural ectoderm can be reversed through Notch1 signaling, whereas the VegT effects on mesoderm and endoderm are independent.
Figure 4.
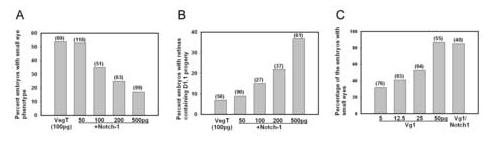
Co-expression of notch1 reverses VegT retinal effects but not those of Vg1.
(A) Co-expression of notch1 reduces the frequency of the VegT small eye phenotype in a dose-dependent manner. Numbers in parentheses indicate size of sample.
(B) The frequency that VegT-expressing D1.1 cells populate the retina increases with increasing doses of notch1.
(C) Vg1 causes a small eye phenotype in a dose-dependent manner, which is not rescued by notch1 (500pg). (See also Figure 2D).
In contrast, the effects of Vg1 were not rescued by notch1. Vg1/notch1-expressing embryos still displayed the small eye phenotype at high frequency (Fig. 4C), their D1.1 progeny rarely populated the retina (Fig. 2D) and retinal volumes were not different from Vg1-injected embryos (Fig. 2B). Neither the repression of neural/retinal genes nor the ectopic expression of mesodermal and endodermal genes was changed compared to Vg1-injected embryos (Fig. 3; Table 1). These results indicate that the ability of Vg1 to repress neural/retinal fates is independent of Notch1 signaling, and therefore likely occurs via a pathway distinct from VegT.
Reduction of either VegT or Vg1 in dorsal-animal blastomeres expands the neural/retinal field
Although VegT mRNA is enriched in the vegetal pole (Lustig et al. 1996; Stennard et al., 1996; Zhang and King, 1996; Horb and Thomsen, 1997), its protein product is proposed to form a concentration gradient from vegetal to animal poles (Kavka and Green, 1997; Green, 2002) and low levels of maternal RNA are reported in animal caps (Stennard et al., 1996). To test whether VegT endogenous to animal blastomeres limits neural/retinal fate, VegT protein was locally reduced by injecting a VegT antisense morpholino (VegTMO) unilaterally into the dorsal-animal region at the 2-cell stage. The retinas on the VegT-depleted side were significantly larger than those on the uninjected side, whereas no differences were observed in control embryos injected with either control MO (cVegTMO, cMO; Fig. 5A, B). In all embryos, large numbers of VegTMO-injected cells contributed to the retina (Fig. 5A). Interestingly, the retinal volume on the uninjected side of VegT-depleted embryos was significantly larger than those in control embryos (Fig. 5B), likely due to the fact that the retina derives cells from both sides of the embryo (Jacobson and Hirose, 1978; Huang and Moody, 1993; see green cells on uninjected side of Fig. 5A). Other regions of the CNS also appeared enlarged in dorsal-animal VegT-depleted embryos (note thicker forebrain in Fig. 5A). This was confirmed by analyzing the expression domains of both pan-neural plate and retinal genes. The domains of sox3, notch1 and rx1 were larger on the VegT-depleted side of embryos (Fig. 5C; Table 1); the mean width of the sox3 (30%) and notch1 (16%) domains were significantly expanded (p<0.05), whereas β-gal mRNA, cMO or cVegTMO did not alter these domains. Because dorsal-animal depletion of VegT did not alter the endogenous domains of mesodermal and endodermal genes (Fig. 5D; Table 1) that could alter the extent of neural induction, the effects on neural/retinal markers appear to be autonomous to the ectoderm.
Figure 5.
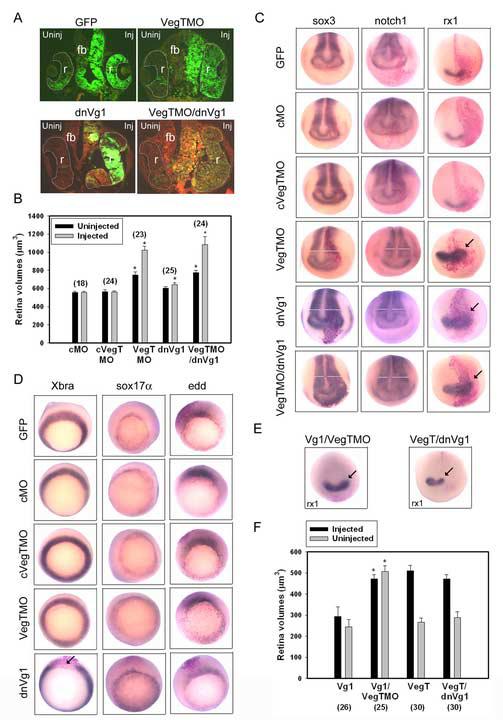
Reduction of VegT and Vg1 in the D1.1 lineage increases the size of the neural ectoderm.
(A) Reduction of VegT by morpholino injection (VegTMO) and of Vg1 by expression of a dominant-negative construct (dnVg1) enlarged the retina (r) and forebrain (fb). Note the abundant D1.1 progeny (green) in both structures (cf. Fig. 2E). Reduction of both factors (VegTMO/dnVg1) resulted in a similar phenotype.
(B) The mean volumes of retinas in embryos in which control morpholinos (cMO, cVegTMO) were injected are not different from GFP controls (Fig. 2B). Those from VegTMO-injected embryos are significantly larger on both sides, and those from dnVg1-injected embryos are significantly larger on the injected side. Those from embryos injected with both constructs (VegTMO/dnVg1) are larger than for dnVg1 alone and similar to those from VegTMO alone. * indicates p<0.01 compared to GFP controls. Numbers in parentheses indicate size of sample.
(C) The expression domains of pan-neural plate (sox3, notch1; white bars indicate measurement of width of domain) and retinal (rx1; arrows) genes are expanded on the side (right) injected with VegTMO or dnVg1. The expansion of neural plate markers is somewhat enhanced in embryos co-injected with VegTMO and dnVg1 (see also Table 1).
(D) The expression domains of mesodermal (Xbra) and endodermal (sox17α, edd) genes after injection into D1.1 of the constructs indicated on the left. The only notable effect is repression of Xbra by dnVg1 (arrow).
(E) The expansion of rx1 expression (arrow) by VegTMO is not altered by co-expression of Vg1, whereas the expansion caused by dnVg1 is reversed by co-expression of VegT.
(F) The small retinal volumes displayed by Vg1-injected embryos were significantly increased by co-injection of VegTMO (* indicates p<0.01). The small retinal volumes displayed by VegTMO-injected embryos were not altered by co-injection of dnVg1 (p>0.05).
To reduce endogenous dorsal-animal Vg1 activity, dnVg1, which reduces both dorsal mesoderm and endoderm development (Joseph and Melton, 1998), was expressed in the D1.1 lineage on one side of the embryo. It caused enlargement of the retina on the injected side (Fig. 5A, B) and in all embryos large numbers of dnVg1-expressing cells populated the retina on the injected side (Fig. 5A). Both pan-neural plate and retinal markers were expanded, whereas in no embryo was ectopic sox17α or edd induced (Fig. 5C, D; Table 1). However, Xbra expression was reduced in a significant number of embryos.
These results indicate that both endogenous VegT and endogenous Vg1 in the dorsal-animal lineage restrict the extent of both neural and retinal progeny. The VegT and Vg1 gain-of-function (GOF) and loss-of-function (LOF) phenotypes described above are very similar, suggesting that they may act through the same pathway. Although there is no evidence to date that VegT acts through Vg1 signaling, it is known that they both can affect the expression of several similar downstream targets (see next section). Several experiments were performed to determine if these two factors work in concert to affect retinal fate. First, VegT and BVg1 mRNAs were co-expressed in the D1.1 lineage. For nearly every marker gene analyzed the frequency of the effect was increased over that observed after expression of only one of the mRNAs (Table 1). The exceptions were: 1) for two markers (foxD5, Xbra) the effects were already near to 100% for the single mRNA injections; and 2) for keratin the phenotype followed the Vg1 effect (no change) rather than the VegT effect (expansion). Co-expression of VegT and Vg1 also significantly reduced the size of both retinas compared to each factor alone (Fig. 2B). Second, both VegT and Vg1 were depleted in the D1.1 lineage (VegTMO/dnVg1); sox3, notch1 and rx1 expression domains were expanded at a significantly higher frequency compared to depletion of only one factor (Fig. 5C; Table 1). However, the size of the retina did not increase compared to VegTMO alone (Fig. 5A, B; p>0.05), perhaps because single depletion already resulted in maximum tissue expansion. Third, we examined whether each construct could rescue the retinal phenotype of the other. VegT LOF in the D1.1 lineage via injection of VegTMO expanded rx1 expression (Fig. 5C; Table 1); this phenotype was not altered by co-expression of Vg1 (Fig. 5E; 43.8%, n=48). Vg1 LOF in the D1.1 lineage via expression of dnVg1 mRNA also expands rx1 expression (Fig. 5C: Table 1); this phenotype was reversed by co-injection of VegT mRNA (Fig. 5E; 71.7%, n=53). Conversely, VegTMO rescued the Vg1 small eye phenotype but dnVg1 did not rescue the VegT small eye phenotype (Fig. 5F). These results indicate that VegT can compensate for loss of Vg1, but Vg1 does not restore VegT LOF. These observations further support the idea generated from the notch1 rescue experiments that VegT and Vg1 likely regulate early neural/retinal fates in dorsal-animal lineages via partially over-lapping but not identical molecular pathways.
Repression of neural/retinal fate results from a combination of Sox17α and Derriere expression
VegT is known to act through two downstream pathways: 1) endoderm formation is promoted via regulation of sox17 (Hudson et al., 1997; Clements et al., 1999; Yasuo and Lemaire, 1999; Clements and Woodland, 2003); and 2) mesoderm formation is promoted via activation of TGFβ signaling involving in particular XNr1-2, XNr4-6 and Derriere (Clements et al., 1999; Sun et al., 1999; Yasuo and Lemaire, 1999; Kimelman and Griffin, 2000; Takahashi et al., 2000; Xanthos et al., 2001; Rex et al., 2002; White et al., 2002; Sinner et al., 2004). There also is evidence that Vg1 can induce XNr1-2, XNr4 and Derriere (Sun et al, 1999; Agius et al., 2000; Hanafusa et al, 2000). To discern which of the common potential downstream genes might mediate the VegT/Vg1 repression of neural/retinal fate some of the individual components were expressed in the D1.1 lineage.
(Hudson et al., 1997; Clements et al., 1999; Yasuo and Lemaire, 1999; Clements and Woodland, 2003); and 2) mesoderm formation is promoted via activation of TGFβ signaling involving in particular XNr1-2, XNr4-6 and Derriere (Clements et al., 1999; Sun et al., 1999; Yasuo and Lemaire, 1999; Kimelman and Griffin, 2000; Takahashi et al., 2000; Xanthos et al., 2001; Rex et al., 2002; White et al., 2002; Sinner et al., 2004). There also is evidence that Vg1 can induce XNr1-2, XNr4 and Derriere (Sun et al, 1999; Agius et al., 2000; Hanafusa et al, 2000). To discern which of the common potential downstream genes might mediate the VegT/Vg1 repression of neural/retinal fate some of the individual components were expressed in the D1.1 lineage.
In sox17α -injected embryos, retinas developed in all cases, although often they developed deep within the embryo rather than at the surface (Fig. 6A). The volumes of these retinas were not different from controls (Fig. 6B; p>0.05), but they rarely contained sox17α-expressing cells (Fig. 6A, C). The sizes of the expression domains of sox3, notch1 and rx1 were not altered (p>0.05, Fig. 6D; Table 1). However, lineage-labeling demonstrated that these tissues were derived from the adjacent, uninjected blastomeres (i.e., not β-Gal-positive). Thus, although sox17α expression in the D1.1 lineage prevents descendants from populating the retina (Fig. 6C), the remaining lineages are able to compensate to produce normal-sized neural plates and retinas. Consistent with previous studies (Hudson et al., 1997), sox17α reduced the expression domain of Xbra and expanded the expression domain of edd at the sites of the injected clones (Fig. 6D; Table 1). Thus, increased expression of sox17α converts the D1.1 lineage from a predominantly neural/retinal fate to a predominantly endodermal fate. This reproduces the expansion of endoderm by VegT and Vg1 but does not account for their alterations of neural and retinal fates. Therefore we investigated the effects of some of the Nodal-related molecules.
Figure 6.
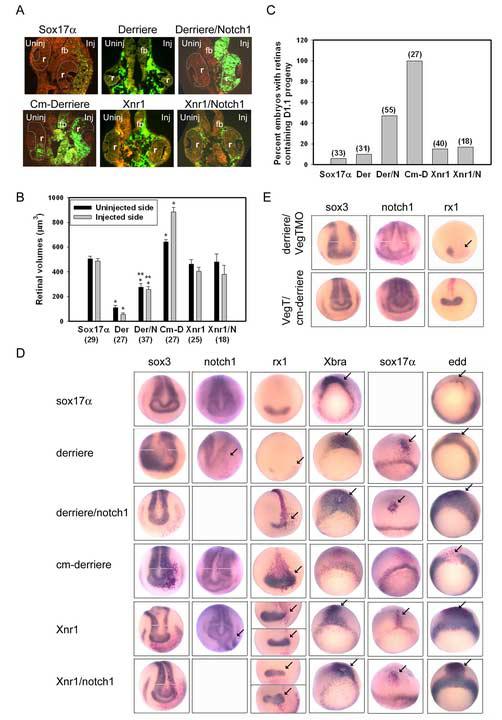
The combination of sox17α and derriere phenocopies the VegT effect on the D1.1 lineage.
(A) The retinas (r) in sox17α and Xnr1 mRNA injected embryos are normal in size, whereas derriere dramatically reduced both retinas. However, D1.1 progeny (green) were not located within the retina in sox17α, derriere or Xnr1 injected embryos. Co-expression of notch1 reversed the derriere phenotype, resulting in large retinas populated by abundant D1.1 progeny (green), but did not alter the Xnr1 phenotype. The expression of a dominant-negative derriere construct (Cm-derriere) resulted in a larger retina populated by large numbers of D1.1 progeny.
(B) The mean volume of retinas in embryos injected with mRNAs for sox17α, derriere (Der), derriere plus Notch1 (Der/N), dominant-negative derriere (Cm-D), Xnr1 and Xnr1 plus notch1 (Xnr1/N). sox17α- and Xnr1-injected embryos do not significantly differ from GFP controls (Fig. 2B), whereas those from derriere embryos are significantly reduced on both sides. Co-expression of Notch1 partially rescues the derriere effect but has no effect on Xnr1 retinas (*, p<0.01 compared to GFP controls; **, p<0.05 compared to derriere). Cm-D significantly increased the size of both retinas (*, p<0.01 compared to GFP controls).
(C) Percentage of embryos in which D1.1 progeny populate the retina is dramatically reduced in embryos injected with sox17α, derriere (Der) or Xnr1 mRNAs. The derriere phenotype is partially rescued by co-expression of notch1 (Der/N), whereas the Xnr1 phenotype is not (Xnr1/N). All Cm-D expressing embryos have D1.1 progeny in the retinas, identical to GFP controls (Fig. 2C)
(D) Domains of pan-neural plate (sox3, notch1), retinal (rx1), mesodermal (Xbra), and endodermal (sox17α, edd) genes after injection of one D1.1 blastomere with constructs listed on the left. The injected side of the embryo is on the right and the uninjected side is on the left. The sites of effects are indicated either by arrows, or in the neural plate by white bars indicating the width of the expression domains. Expression of sox17α does not affect neural/retinal genes, but inhibits Xbra and expands edd, consistent with its role in converting cells to an endodermal fate. Expression of derriere represses neural and retinal genes, and these effects are rescued by notch1. Cm-Derriere expanded neural and retinal markers and repressed Xbra. Xnr1 either represses (sox3, top rx1 panel) or expands (notch1; bottom rx1 panel) neural/retinal genes, and endo-mesoderm markers are dorsally expanded. No Xnr1 phenotype is rescued by notch1.
(E) The expansion of neural (sox3, notch1) and retinal (rx1) expression domains by injection of VegTMO (Fig. 5C) is reversed by co-injection of derriere. Reduction of these markers by VegT (Fig. 3B) is reversed by cm-derriere. (See also Table 1).
In derriere-injected embryos, the volumes of the retinas on both sides of the embryo were dramatically smaller than controls (Fig. 6A, B); these bilateral effects are expected of a secreted protein. Like VegT and Vg1 embryos, the retinas only rarely contained derriere-expressing D1.1 progeny (Fig. 6A, C). derriere also caused a significant reduction in the size of the expression domains for early neural, pan-neural plate (p<0.05 for sox3, notch1) and region-specific neural genes (Fig. 6D; Table 1). Like VegT- but not like Vg1-injected embryos, derriere caused ectopic keratin expression. Like both VegT and Vg1 embryos, derriere caused ectopic or expanded domains of mesodermal and endodermal genes (Fig. 6D; Table 1). Thus, derriere GOF in the D1.1 lineage phenocopies the VegT effect and mostly reproduces the Vg1 effect. In Xnr1-injected embryos, the mean size of the retina was indistinguishable from controls (Fig. 6A, B), even though the D1.1 progeny expressing Xnr1 rarely populated the retina (Fig, 6C). Like VegT and Vg1 embryos, Xnr1 caused ectopic or expanded expression of mesodermal and endodermal genes (Fig. 6D; Table 1), but like Vg1, it had no effect on keratin expression (Table 1). Xnr1 effects on the neural/retinal genes were pleotropic; domains could either reduced or expanded (Fig. 6D; Table 1). This was never observed with VegT or Vg1, suggesting it resulted from the known differential effects of Xnr1 on the mesoderm (Clements et al., 1999; Kofron et al., 1999; Agius et al., 2000). Together these results indicate that derriere is sufficient to mediate the VegT and possibly Vg1 effects on dorsal-animal lineages.
We further tested whether the derriere effects could be attributable to VegT versus Vg1 by determining whether they could be rescued by co-expression of notch1. Both the retinal volume and the proportion of embryos in which derriere-expressing D1.1 progeny populated the retina were significantly increased after notch1 co-injection (Fig. 6B, C; cf. derriere alone). Interestingly, notch1 rescued the retinal phenotypes on both sides of the embryo even though it is not secreted (Fig. 6A, B), suggesting that notch1 may either promote the expression of a secreted factor that antagonizes Derriere or interfere with Derriere upstream of its secretion. These points will require further investigation. Co-expression of notch1 also significantly reduced the repressive effect of derriere on most neural and retinal expression domains (Table 1); like VegT/notch1-injected embryos, there was no rescue of the foxD5 repression. Like VegT/notch1-injected embryos, the ectopic induction of keratin was reversed whereas there was no effect on the derriere-mediated expansion of mesodermal and endodermal genes (Fig. 6D; Table 1). These data indicate that the derriere effects are likely part of the VegT pathway independent of Vg1. In contrast, the effects of Xnr1 on the D1.1 lineage are not rescued by notch1. Retinal volume was unchanged and Xnr1/notch1-expressing cells rarely populated the retina (Fig. 6B, C). notch1 did not alter the pleotropic effects on the sox3 and rx1 expression domains nor prevent the Xnr1-induced expansion of mesodermal and endodermal genes (Fig. 6D; Table 1).
These results were confirmed by LOF experiments. Expression of a dominant-negative Derriere construct (cm-derriere), which has minimal effects on XNr1, XNr2, XNr4 or Vg1 signaling (Sun et al., 1999), in the D1.1 lineage caused enlargement of the retina on both injected and uninjected sides (Fig. 6A, B). In all embryos cm-derriere-expressing D1.1 progeny densely populated the retina (Fig. 6A, C), and pan-neural plate and retinal markers were expanded (Fig. 6D; Table 1). Xbra expression was reduced but endoderm markers were not altered. The neural/retinal results phenocopy both VegT and Vg1 LOF, whereas the effects on Xbra are most similar to those of dnVg1 (Fig. 5D; Table 1). Expression of a dominant-negative XNr1 receptor (tALK7) in the D1.1 lineage did not phenocopy either VegT or Vg1 LOF. As with Xnr1 GOF, the effects on pan-neural plate and retinal markers were pleotropic (Table 1). Xbra expression was significantly repressed, consistent with the proposed function of XNr1 in mesoderm formation (Osada and Wright, 1999; Agius et al, 2000).
Together the GOF, Notch1 rescue and LOF experiments indicate that Derriere is the most likely Nodal-related mediator of either VegT or Vg1 effects on the dorsal-animal lineages. However, reversal of its effects by Notch1 suggests that in this system it likely is predominantly activated by VegT. Our data do not support a strong role for XNr1 in mediating the effects of VegT or Vg1 on D1.1 neural/retinal fates because it did not closely phenotype either gene. However, both VegT and Vg1 can up-regulate the expression of several other Nodal-related genes (Agius et al., 2000; Takahashi et al., 2000; Rex et al., 2002), which could play a role in the phenotypes that our experiments have described. Nonetheless, the above data show that Derriere is sufficient to cause all of the described effects. In addition, sox17α likely contributes to the repression of neural/retinal fates in both VegT and Vg1 pathways by converting cells to endoderm in a cell-autonomous manner. But, sox17α expression alone does not account for the non-autonomous effects on neural/retinal fates; a secreted factor such as Derriere is implicated.
To confirm a role for Derriere as a downstream mediator of the VegT effect on neural/retinal fates, we performed two experiments. Co-injection of VegTMO and derriere mRNA in the D1.1 lineage reversed the VegTMO-induced expansion of sox3, notch1 and rx1 expression domains to significant repression (Fig. 6E; Table 1). Likewise, co-injection of cm-derriere mRNA with VegT mRNA reversed the VegT-induced reduction of these three neural/retinal genes (Fig. 6E; Table 1). These results indicate that Derriere acts downstream of VegT to mediate its effects on neural/retinal fates.
Depletion of endo-mesodermal factors alone in ventral-vegetal blastomeres is not sufficient to induce retinal fates
Previous studies indicated that vegetal blastomeres are not competent to form retina even when transplanted to a retinogenic region of the embryo or when supplemented with neuralizing, anteriorizing or dorsal mesoderm-promoting factors that allow the lineage to express a neural fate (Huang and Moody, 1993; Moore and Moody, 1999). These studies predicted that a vegetal-localized maternal factor is responsible for this inhibition of retinal fate. VegT and Vg1 are both expressed at high levels in the vegetal lineages, and the experiments described above demonstrate that each promote mesodermal and endodermal fates at the expense of neural/retinal fates in dorsal-animal lineages. If these factors were singularly responsible for blocking retinogenic competence of vegetal blastomeres in which their endogenous levels are relatively high, then reducing their activity in the vegetal hemisphere should be sufficient for vegetal lineages to produce retinal cells.
This was tested by reducing VegT and/or Vg1 levels in ventral vegetal blastomeres and assaying for the presence of both neural and retinal descendants in the affected lineages. Confirming previous studies (Xanthos et al., 2001), gut development was dramatically reduced in embryos in which VegT was depleted throughout the vegetal hemisphere by VegTMO injection at the 2-cell stage (not shown). Confining VegT depletion to a single ventral vegetal blastomere (V2.1.1; Fig. 1) likewise resulted in reduced expression domains of Xbra (87%, n=31), sox17 (84%, n=31) and edd (92%, n=36) (Fig. 7A), and the extent of GFP-labeled V2.1.1 progeny in the endoderm appeared reduced in tail bud embryos (Fig. 7B). To test whether the VegTMO-injected V2.1.1 lineage was converted to neural or retinal fates, embryos were assayed for ectopic sox3 and rx1 expression at neural plate stages; no cases of ectopic domains were observed (Fig. 7C; Table 2). Similar data were obtained when V2.1.1 was injected with either dnVg1 mRNA or a combination of VegTMO/dnVg1 mRNA (Fig 7C; Table 2). To determine whether small numbers of V2.1.1 progeny were converted to neural/retinal fates but incorporated into the endogenous tissues (i.e., not ectopically induced), a fate map of GFP-labeled cells in tissue sections from stage 37/38 embryos was constructed. Small numbers of GFP-labeled cells (∼10-20/embryo) were observed infrequently in the caudal spinal cords of VegTMO-, dnVg1- and VegTMO/dnVg1-injected embryos (Table 2). However, none of these manipulations caused the V2.1.1 progeny to contribute to the retina (Table 2). Therefore, while depletion of these two factors can result in small numbers of cells becoming neural, it is not sufficient to promote a retinal fate in the V2.1.1 lineage. To test whether an external neurogenic environment would promote a retinal fate in these cells, V2.1.1 blastomeres that had been injected with VegTMO were transplanted to the most neurogenic/retinogenic region of the 32-cell embryo (* in Fig.1). In no case did the V2.1.1 cells populate the retina (n=14), indicating that the signaling environment within the V2.1.1 lineage needs to be altered cell-autonomously.
(84%, n=31) and edd (92%, n=36) (Fig. 7A), and the extent of GFP-labeled V2.1.1 progeny in the endoderm appeared reduced in tail bud embryos (Fig. 7B). To test whether the VegTMO-injected V2.1.1 lineage was converted to neural or retinal fates, embryos were assayed for ectopic sox3 and rx1 expression at neural plate stages; no cases of ectopic domains were observed (Fig. 7C; Table 2). Similar data were obtained when V2.1.1 was injected with either dnVg1 mRNA or a combination of VegTMO/dnVg1 mRNA (Fig 7C; Table 2). To determine whether small numbers of V2.1.1 progeny were converted to neural/retinal fates but incorporated into the endogenous tissues (i.e., not ectopically induced), a fate map of GFP-labeled cells in tissue sections from stage 37/38 embryos was constructed. Small numbers of GFP-labeled cells (∼10-20/embryo) were observed infrequently in the caudal spinal cords of VegTMO-, dnVg1- and VegTMO/dnVg1-injected embryos (Table 2). However, none of these manipulations caused the V2.1.1 progeny to contribute to the retina (Table 2). Therefore, while depletion of these two factors can result in small numbers of cells becoming neural, it is not sufficient to promote a retinal fate in the V2.1.1 lineage. To test whether an external neurogenic environment would promote a retinal fate in these cells, V2.1.1 blastomeres that had been injected with VegTMO were transplanted to the most neurogenic/retinogenic region of the 32-cell embryo (* in Fig.1). In no case did the V2.1.1 cells populate the retina (n=14), indicating that the signaling environment within the V2.1.1 lineage needs to be altered cell-autonomously.
Figure 7.
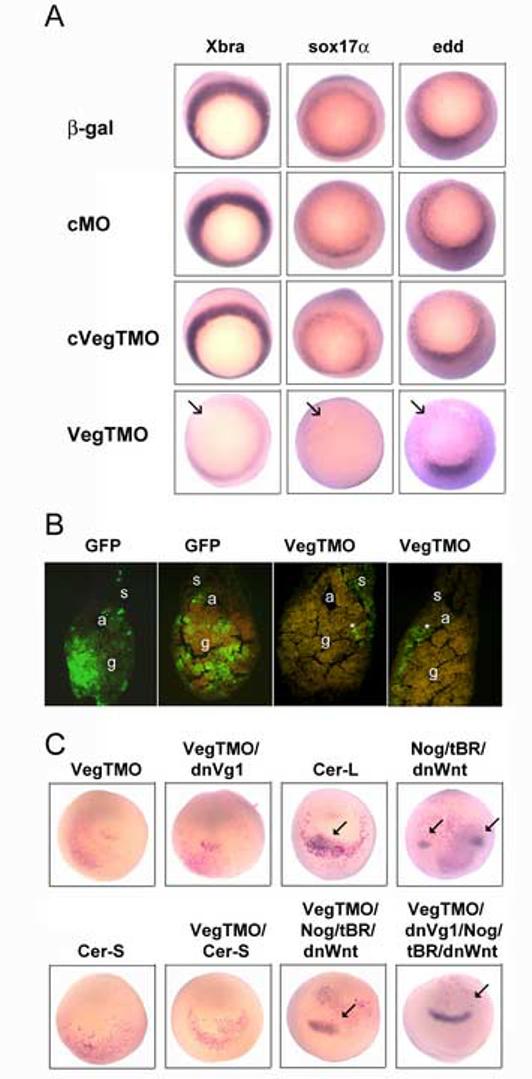
In order to convert the V2.1.1 lineage to a retinal fate endo-mesodermal factors must be blocked in combination with suppression of BMP and Wnt signaling.
(A) Expression domains of mesodermal (Xbra) and endodermal (sox17α, edd) genes are reduced by VegTMO injection into the vegetal pole, whereas controls (β-gal, cMO, cVegTMO) have no effects.
(B) The extent to which the hindgut (g) is populated by V2.1.1 progeny (green) is strikingly reduced in VegTMO embryos (*) compared to controls (GFP). a, archenteron; s, somite.
(C) Ectopic expression of rx1 (blue, arrows) in the vegetal pole was monitored by in situ hybridization after the V2.1.1 blastomere was injected with the indicated constructs. Red cells indicate the V2.1.1 progeny expressing those constructs. Reduction of VegT alone (VegTMO) or in combination with Vg1 (VegTMO/dnVg1) was not sufficient to induce ectopic rx1 expression. The long form of Cerberus (Cer-L), which can inhibit BMP, Wnt and Nodal signaling, was effective whereas the short version of Cerberus (Cer-S), which can inhibit only Nodal signaling, was not. Although reduction of both BMP signaling (by expression of noggin [Nog] and a dominant-negative BMP receptor [tBR]) and Wnt signaling (by expression of a dominant-negative Wnt8 construct [dnWnt]) causes ectopic rx1 expression, the cells expressing rx1 are not derived from the V2.1.1 clone (red cells), demonstrating an indirect consequence of the induction of a secondary head (as reported in Moore and Moody, 1999). However, combining the reduction of VegT or VegT plus Vg1 with the inhibition of BMP and Wnt signaling caused the V2.1.1 clone to ectopically express rx1, equivalent to the Cer-L phenotype.
Table 2.
The ventral vegetal lineage expresses a retinal fate only when vegetal factors, anti-neural factors and posterior-axis promoting factors are inhibited
| Injected mRNAs or morpholinos |
ISH |
Fate Mapping |
||
|---|---|---|---|---|
| ectopic expression (% embryos) |
clone populates (% embryos) |
|||
| sox3 (n) | rx1 (n) | neural (n) | retina (n) | |
| β-gal | 0 (23) | 0 (31) | - | - |
| gfp | - | - | 0 (23) | 0 (23) |
| VegTMO | 0 (32) | 0 (32) | 30 (27) | 0 (27) |
| dnVg1 | 0 (24) | 0 (26) | 32 (25) | 0 (25) |
| VegTMO/dnVg1 | 0 (32) | 0 (34) | 33 (18) | 0 (18) |
| cer-L | 38 (32) | 33 (48) | 80 (30) | 27 (30) |
| nog/tBR/dnWnt | 41 (32) | 32 (38) | 79 (19) | 0 (19) |
| cer-S | - | 0 (37) | - | 0 (31) |
| cm-Xnr2 | - | 0 (46) | - | 0 (42) |
| cer-S/cm-Xnr2 | - | 0 (38) | - | 0 (34) |
| cm-derriere | - | 0 (30) | - | 0 (27) |
| VegTMO/cer-S | - | 0 (26) | - | 0 (34) |
| VegTMO/cm-Xnr2 | - | 0 (38) | - | 0 (28) |
| VegTMO/cm-derriere | - | 0 (34) | - | 0 (22) |
| VegTMO/nog/tBR/dnWnt | 59 (32) | 37 (38) | 50 (29) | 38 (26) |
| VegTMO/tBR | - | 0 (26) | - | 0 (34) |
| VegTMO/dnWnt | - | 0 (31) | - | 0 (27) |
| VegTMO/dnVg1/nog/tBR/dnWnt | 100 (30) | 100 (26) | 100 (6) | 45 (29) |
Legend: Reagents were injected into one V2.1.1 blastomere with appropriate lineage tracer (β-Gal for ISH; GFP for fate mapping). Embryos were fixed at neural plate stages for ISH and stages 37/38 for fate mapping.
Previous work demonstrated that in order for the V2.1.1 lineage to express a retinal fate, three signaling pathways (Nodal, BMP and Wnt) need to be repressed (Moore and Moody, 1999). Although vegetal blockade of both Wnt and BMP signaling can cause a secondary head containing eyes to form (Glinka et al., 1997; Piccolo et al., 1999), lineage analysis demonstrated that the vegetal clone contributed sparsely to neural but not at all to retinal tissue (Moore and Moody, 1999). Only the combined repression of Wnt, BMP and Nodal signaling via expression of the long form of Cerberus (Cer-L) caused the injected V2.1.1 lineage to produce retinal progeny (Moore and Moody, 1999). We confirmed these results by showing that Cer-L induced ectopic sox3 and rx1 expression, and that the labeled V2.1.1 clone was part of the ectopic rx1 domain (Fig. 7C; Table 2). In contrast, repression of both BMP and Wnt signaling (via co-expression of nog/tBR/dnWnt8 mRNAs) also induced ectopic sox3 and rx1 expression (Table 2), but the V2.1.1 lineage did not contribute to the ectopic rx1 domain (Fig. 7C). Fate mapping at later stages revealed that GFP-labeled V2.1.1 progeny contributed to the spinal cord in both Cer-L- and nog/tBR/dnWnt8-injected embryos, but they contributed to the retina only in Cer-L embryos (Table 2). Presumably the difference in effects between Cer-L and nog/tBR/dnWnt8 mRNA injections is the additional ability of Cer-L to inhibit Nodals (Glinka et al., 1997; Piccolo et al., 1999). Therefore we tested whether blocking Nodal signaling either alone (using the short form of Cerberus [Cer-S] or various dominant-negative constructs [cm-Xnr2, cm-derriere]) or in various combinations with VegTMO induced a retinal fate. None of the combinations was sufficient to induce ectopic rx1 expression or cause the V2.1.1 lineage to contribute to the retina (Fig. 7C; Table 2).
In contrast, inhibition of VegT in combination with blocking both BMP and Wnt signaling (via injection of VegTMO plus nog/tBR/dnWnt8 mRNAs) induced a retinal fate as frequently as did Cer-L; ectopic domains of rx1 expression were induced within the βgal-expressing V2.1.1 clone and V2.1.1 descendants populated the differentiating retina (Fig. 7C; Table 2). Combining VegTMO with blockade of only one signaling pathway did not cause this effect (Table 2). Interestingly, additional inhibition of Vg1 in this combination significantly increased the frequency of both ectopic sox3 and rx1 expression and contribution to both the spinal cord and the retina by V2.1.1 descendants (Fig. 7C; Table 2). These experiments confirm that while blocking both BMP and Wnt pathways in vegetal cells can transfate the descendants of these cells to produce neural cells, it is not sufficient to transfate them to retina. Likewise, inhibiting endogenous endo-mesodermal factors (VegT/Vg1) can transfate the vegetal lineage to neural but not retinal. A retinal fate requires both sets of factors to be concomitantly reduced.
Discussion
An important question in development is how and when do pluripotent embryonic cells acquire the differential competence to contribute to specific tissues. Is this a stochastic process, or do early factors bias or restrict the selection of embryonic precursors? This question is readily approachable for retinal fate in Xenopus because each retina descends from a subset of nine animal blastomeres at the 32-cell stage, and each of these blastomeres produces characteristic proportions of the retina (Huang and Moody, 1993). Previous work demonstrates that the earliest steps in retina fate specification occur well before the overt morphogenesis of the eye cup (reviewed in Williams and Moody, 2004; Zaghloul et al., 2005). A first step selects a subset of cells to be competent to contribute to the retina (Fig. 1). Transplantation experiments showed that many blastomeres, not just the nine that normally produce the retina, are competent if moved to the appropriate retinogenic environment, i.e., the dorsal-animal quadrant. Thus, the potential to express a retinal fate may simply require the appropriate signaling environment for anterior neural tissues. However, vegetal blastomeres transplanted to this position never contribute progeny to retina (Huang and Moody, 1993). This developmental restriction is not overcome by providing components of signaling pathways involved in neural and anterior fate specification, even under conditions in which ectopic neural and retinal tissues are induced (Moore and Moody, 1999). These results predict that vegetal blastomeres contain one or more maternal molecules that specifically repress the competence of a cell to form the retina. Two maternal factors, VegT and Vg1, are candidates for the repression of retinal competence because both are vegetally-enriched, and both play essential roles in the formation of mesoderm and endoderm (Fig. 8). In this study we demonstrate that both factors negatively regulate the competence of cleavage blastomeres to produce neural and retinal lineages.
Figure 8.
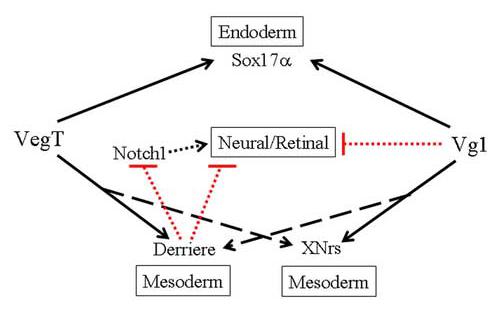
VegT and Vg1 both repress the ability of a cell to express a neural/retinal fate via overlapping but not identical pathways. Both dominantly promote (black arrows) endodermal and mesodermal fates via up-regulation of Sox17α, Derriere and XNrs (broken arrows indicate that the strength of the effect may not be equally strong for VegT and Vg1). Derriere acts downstream of VegT. Notch1, which promotes neural/retinal fates (black arrow), is proposed as an intermediary between VegT/Derriere repression of neural/retinal fate because: a) both VegT and Derriere repress the Notch1 expression domain; and b) Notch1 can rescue the repression of neural/retinal fates by both VegT and Derriere. But, Notch may not be the only pathway by which VegT/Derriere repress neural/retinal fates. Notch1 does not rescue Vg1 or XNr1 repression of neural/retinal fates. Dashed lines indicate that the effects may not be direct.
In dorsal-animal lineages, VegT and Vg1 affect the allocation of cells into the neural ectoderm
Several reports concur that VegT induces: 1) endoderm in a cell autonomous manner via Bix/Mix/Sox17 transcription factors; and 2) mesoderm via the activation of TGFβ signals (Tada et al.1998; Casey et al., 1999; Clements et al., 1999; Yasuo and Lemaire, 1999; Sun et al., 1999; Agius et al., 2000; White et al., 2002; Clements and Woodland, 2003; Loose and Patient, 2004; Taverner et al., 2005). Vg1 is thought to induce endoderm via induction of sox17α, mix1 and eomes (Clements et al., 1999) and mesoderm via mix and Xnrs (Mead et al., 1998; Loose and Patient, 2004). We demonstrate that increased expression of either VegT or Vg1 in a retinogenic lineage represses the formation of the retina, an easily identifiable part of the anterior neural plate whose precursor blastomeres have been quantitatively mapped in the cleavage embryo (Fig. 1). However, by analyzing a large number of marker genes expressed at different times in early neural development, as well as fate maps of embryos containing differentiated tissues, we conclude that this repression is not specific to the retina, but affects the allocation of cells during gastrulation to the various germ layer domains. Markers of the earliest induced neural ectoderm are repressed and for VegT, but not Vg1, non-neural ectoderm is expanded. In addition, markers of early mesoderm and endoderm are expanded or ectopically expressed, consistent with previous reports (Kessler and Melton, 1995; Zhang and King, 1996; Clements et al., 1999; Kessler, 1999). Thus, these two factors both appear to affect retinal fate indirectly by repressing specification of cells to a neural ectodermal fate. Although this might be accomplished by conversion of cells directly to endo-mesodermal fates via expression of the known downstream target genes, there also may be involvement of two maternal sox genes (sox3 in the animal hemisphere and sox7 in the vegetal hemisphere) that are proposed to competitively regulate the Xnr genes to define the germ layer domains (Zhang et al., 2004; 2005). Since VegT and Vg1 also regulate Xnr gene expression, it will be important to determine if their downstream effects intersect with those of the maternal sox genes to allocate the extent of the neural ectodermal domain.
The VegT and Vg1 effects are likely mediated primarily by Sox17α and Derriere
As noted above, both VegT and Vg1 can affect cell fate decisions by promoting mesoderm, via Nodal-related factors including XNr1-2, XNr4-6 and Derriere, and endoderm, via factors including Sox17α. We tested some of these factors for potential involvement in the VegT/Vg1 effects on the dorsal-animal lineages. Part of the VegT/Vg1 GOF phenotypes can be attributed to their ability to induce sox17α, and edd is a direct target of both Sox17α and VegT (Engleka et al., 20001; Clements et al., 2003; Ahmed et al., 2004; Taverner et al., 2005). We demonstrate that exogenous expression of sox17α in the dorsal-animal lineage mimics part of the VegT/Vg1 phenotype in a cell-autonomous manner; it converts the lineage from neural/retinal to endodermal, consistent with its initial description (Hudson et al., 1997). This aspect of the VegT/Vg1 phenotype also may involve other endodermal target genes; for example, expression of bix1 in dorsal-animal cells represses head development and diverts cells from the notochord (Tada et al., 1998). However, similar analyses are not yet available for bix4, edd, mix1, eomes or sox7, the latter of which also regulates edd (Zhang et al., 2005).
Although Sox17α converted the dorsal-animal lineage from neural/retinal to endodermal, these embryos contained normal sized retinas and normal expression domains of neural and retinal genes. These results indicate that signaling factors that affect cells derived from uninjected neighboring blastomeres must be involved in the VegT/Vg1 phenotypes. This interpretation is consistent with the observation that neighboring blastomeres signal each other to reconstitute a normal sized retina after the major retinogenic blastomere is ablated (Huang and Moody, 1993), and with the fact that VegT and Vg1 induce several TGFβ/Nodal signaling proteins (Clements et al., 1999; Kofron et al., 1999; Sun et al., 1999; Yasua and Lemaire, 1999; Agius, 2000; Xanthos et al., 2001; Eimon and Harland, 2001; Onuma et al., 2002; White et al., 2002; Loose and Patient, 2004). We tested two of these, Derriere and XNr1, for their ability to phenocopy the VegT/Vg1phenotypes. The non-autonomous effects on the dorsal-animal lineage appear to be mediated primarily by Derriere because both its ectopic expression and inhibition mimicked the neural/retinal phenotypes of both VegT and Vg1. In contrast, results from similar experiments with XNr1 were very pleiotropic, indicating that this Nodal-related factor is not significantly involved. Further, Derriere GOF and LOF constructs reverse the effects of VegT LOF and GOF constructs, respectively, indicating it acts downstream of VegT. Interestingly, derriere has been shown to be a direct target of VegT (White et al., 2002; Taverner et al. 2005), although its transcription is also regulated by other Tbox proteins and other TGFβ signaling factors (White et al., 2002).
The above description implies that manipulations of Derriere levels alone account for the VegT/Vg1 effects on dorsal-animal blastomere fate, but several caveats should be noted. First, derriere caused a greater repression of sox3 and rx1 than either VegT or Vg1, and a greater expansion of sox17α. Second, Vg1, VegT and another maternal transcript factor whose function it regulates, Sox7, each induce several XNr factors (Clements et al., 1999; Kofron et al., 1999; Yasuo and Lemaire, 1999; Takahashi et al., 2000; Xanthos et al., 2001; Rex et al., 2002; Clements and Woodland, 2003; Zhang et al., 2005), and the other XNrs and Derriere cooperatively regulate each other (Eimon and Harland, 2002). There also is evidence that Derriere can dimerize with other XNr proteins, so that its effects may depend on the availability of these other related factors (Agius et al., 2000; Eimon and Harland, 2002). Thus, although our experiments support the notion that Derriere, and not XNr1, is sufficient to account for the VegT/Vg1 phenotypes in the dorsal-animal lineage, the effects downstream of Derriere and its interactions with the other XNr family members need to be further investigated.
The VegT and Vg1 pathways affecting neural/retinal fate are not identical
Although there are several similarities between the VegT and Vg1 phenotypes, as expected from the many common downstream factors that they regulate, there are some distinct differences. First, the effects of both VegT and Derriere can be reversed by Notch1, whereas neither Vg1 nor XNr1 effects are altered. There are several potential mechanisms by which Notch1 may reverse VegT/Derrier effects. First, Notch1 may specifically promote retinal fate (Fig. 8); in fly and Xenopus, Notch1 signaling promotes eye gene expression (Kumar and Moses, 2001; Onuma et al, 2002). Second, Notch1 may interfere with the induction of endodermal genes and thus be permissive to alternate fates; in zebrafish, Notch1 activation down-regulates endodermal genes in the marginal zone (Kikuchi et al., 2004). Third, Notch1 is well know in numerous systems to prevent differentiation and hold cells in an immature state (Chiba, 2006); it may delay mesodermal induction and thus allow cells to remain competent to respond to later expressed neural inducers. Interestingly, a recent study identified three Notch1 regulated genes as direct targets of VegT but did not identify Notch1 as a down-regulated gene (Taverner et al., 2005), suggesting that the potential interaction between the VegT and Notch1 pathways is indirect, perhaps via Derriere (Fig. 8). Other differences between VegT and Vg1 are that their increased expression has different effects on keratin, and their decreased expression has different effects on Xbra. Finally, Vg1 can not rescue the VegT LOF retinal phenotype, whereas VegT reverses the Vg1 LOF retinal phenotype. These data support a model of two endo-mesodermal pathways that regulate blastomere competence to produce retina that likely interact and are partially redundant (Fig. 8). Redundancy has also been noted in several studies reporting that VegT and Vg1 synergize to regulate endo-mesodermal fates (Clements et al., 1999; Yasuo and Lemaire, 1999; Agius et al., 2000).
For vegetal lineages to produce retinal progeny multiple endogenous molecules must be inhibited
In their simplest interpretation, the results presented herein demonstrate that if cleavage blastomeres express high levels of endo-mesodermal factors, then they express these tissues dominantly, which by default prevents the expression of neural/retinal fates. If this is the extent of the regulation of neural/retinal fate competence, then to produce retinal precursors from pluripotent embryonic cells, one need only deplete the cells of these factors. This scenario appears to hold for dorsal-animal blastomeres. However, experiments in vegetal cells show that the requirements for inducing neural and retinal fates are not equivalent and that simple depletion of endo-mesodermal factors is not sufficient to impose a retinal fate. Although VegT/Vg1 reduction allowed neural cells to develop within the vegetal lineage, in order for retinal cells to develop both BMP and Wnt pathways also needed to be reduced. Although this might be explained by the fact that anterior neural structures require prolonged BMP blockade (Hartley et al., 2001), transplantation experiments demonstrate that it is not sufficient to move a VegT depleted cell to the native neurogenic/retinogenic region of the embryo. In our hands the BMP and Wnt pathways needed to be inhibited within the vegetal lineage itself, suggesting that some component of the BMP/Wnt signaling that represses retinal fate is autonomous to the vegetal cell. Thus, the requirements for expressing a retinal fate are differentially allocated: dorsal-animal blastomeres can be converted to neural and retinal fates simply by reducing endomesodermal factors, whereas reducing these factors in vegetal blastomeres only minimally converts cells to a neural fate and does not produce a retinal fate unless combined with endogenous repression of both BMP and Wnt signaling.
Acknowledgements
We thank Himani Datta Majumdar for histological assistance, and our many colleagues for sharing gene constructs. This work was supported by NIH grants R01 EY10096 and R01 NS23158.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agius E, Oelgeschlager M, Wessely O, Kemp C, De Robertis EM. Endodermal Nodal-related signals and mesoderm induction in Xenopus. Development. 2000;127:1173–1183. doi: 10.1242/dev.127.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed N, Howard L, Woodland HR. Early endodermal expression of the Xenopus Endodermin gene is driven by regulatory sequences containing essential Sox protein-binding elements. Differentiation. 2004;72:171–184. doi: 10.1111/j.1432-0436.2004.07204005.x. [DOI] [PubMed] [Google Scholar]
- Blitz IL, Cho KW. Anterior neurectoderm is progressively induced during gastrulation: the role of the Xenopus homeobox gene orthodenticle. Development. 1995;121:993–1004. doi: 10.1242/dev.121.4.993. [DOI] [PubMed] [Google Scholar]
- Bradley LC, Snape A, Bhatt S, Wilkinson DG. The structure and expression of the Xenopus Krox-20 gene: conserved and divergent patterns of expression in rhombomeres and neural crest. Mech Dev. 1993;40:73–84. doi: 10.1016/0925-4773(93)90089-g. [DOI] [PubMed] [Google Scholar]
- Casey ES, Tada M, Fairclough L, Wylie CC, Heasman J, Smith JC. Bix4 is activated directly by VegT and mediates endoderm formation in Xenopus development. Development. 1999;126:4193–4200. doi: 10.1242/dev.126.19.4193. [DOI] [PubMed] [Google Scholar]
- Chang C, Hemmati-Brivanlou A. A post-mid-blastula transition requirement for TGFbeta signaling in early endodermal specification. Mech Dev. 2000;90:227–235. doi: 10.1016/s0925-4773(99)00257-9. [DOI] [PubMed] [Google Scholar]
- Chiba S. Notch signaling in stem cell systems. Stem Cells. 2006;24:2437–2447. doi: 10.1634/stemcells.2005-0661. [DOI] [PubMed] [Google Scholar]
- Chow RL, Altmann CR, Lang RA, Hemmati-Brivanlou A. Pax6 induces ectopic eyes in a vertebrate. Development. 1999;126:4213–4222. doi: 10.1242/dev.126.19.4213. [DOI] [PubMed] [Google Scholar]
- Clements D, Woodland HR. VegT induces endoderm by a self-limiting mechanism and by changing the competence of cells to respond to TGF-beta signals. Dev Biol. 2003;258:454–463. doi: 10.1016/s0012-1606(03)00124-6. [DOI] [PubMed] [Google Scholar]
- Clements D, Cameleyre I, Woodland HR. Redundant early and overlapping larval roles of Xsox17 subgroup genes in Xenopus endoderm development. Mech. Dev. 2003;120:337–348. doi: 10.1016/s0925-4773(02)00450-1. [DOI] [PubMed] [Google Scholar]
- Clements D, Friday RV, Woodland HR. Mode of action of VegT in mesoderm and endoderm formation. Development. 1999;126:4903–4911. doi: 10.1242/dev.126.21.4903. [DOI] [PubMed] [Google Scholar]
- Coffman C, Harris W, Kintner C. Xotch, the Xenopus homolog of Drosophila notch. Science. 1990;249:1438–1441. doi: 10.1126/science.2402639. [DOI] [PubMed] [Google Scholar]
- Coffman CR, Skoglund P, Harris W, Kintner C. Expression of an extracellular deletion of Xotch diverts cell fate in Xenopus embryos. Cell. 1993;73:659–671. doi: 10.1016/0092-8674(93)90247-n. [DOI] [PubMed] [Google Scholar]
- Eimon PM, Harland RM. Effects of heterodimerization and proteolytic processing on Derriere and Nodal activity: implications for mesoderm induction in Xenopus. Development. 2002;129:3089–3103. doi: 10.1242/dev.129.13.3089. [DOI] [PubMed] [Google Scholar]
- Engleka MJ, Craig EJ, Kessler DS. VegT activation of Sox17 at the midblastula transition alters the response to nodal signals in the vegetal endoderm domain. Dev. Biol. 2001;237:159–172. doi: 10.1006/dbio.2001.0366. [DOI] [PubMed] [Google Scholar]
- Glinka A, Wu W, Onichtchouk D, Blumenstock C, Niehrs C. Head induction by simultaneous repression of Bmp and Wnt signalling in Xenopus. Nature. 1997;389:517–519. doi: 10.1038/39092. [DOI] [PubMed] [Google Scholar]
- Graff JM, Thies RS, Song JJ, Celeste AJ, Melton DA. Studies with a Xenopus BMP receptor suggest that ventral mesoderm-inducing signals override dorsal signals in vivo. Cell. 1994;79:168–179. doi: 10.1016/0092-8674(94)90409-x. [DOI] [PubMed] [Google Scholar]
- Green J. Morphogen gradients, positional information, and Xenopus: interplay of theory and experiment. Dev. Dyn. 2002;225:392–408. doi: 10.1002/dvdy.10170. [DOI] [PubMed] [Google Scholar]
- Hanafusa H, Masuyama N, Kusakabe M, Shibuya H, Nishida E. The TGF-beta family member derriere is involved in regulation of the establishment of left-right asymmetry. EMBO Rep. 2000;1:32–39. doi: 10.1093/embo-reports/kvd008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley KO, Hardcastle Z, Friday RV, Amaya E, Papalopulu N. Transgenic Xenopus embryos reveal that anterior neural development requires continued suppression of BMP signaling after gastrulation. Dev. Biol. 2001;238:168–184. doi: 10.1006/dbio.2001.0398. [DOI] [PubMed] [Google Scholar]
- Hatakeyama J, Kageyama R. Retinal cell fate determination and bHLH factors. Semin. Cell Dev. Biol. 2004;15:83–89. doi: 10.1016/j.semcdb.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, de la Torre JR, Holt C, Harland RM. Cephalic expression and molecular characterization of Xenopus En-2. Development. 1991;111:715–724. doi: 10.1242/dev.111.3.715. [DOI] [PubMed] [Google Scholar]
- Henry GL, Melton DA. Mixer, a homeobox gene required for endoderm development. Science. 1998;281:91–96. doi: 10.1126/science.281.5373.91. [DOI] [PubMed] [Google Scholar]
- Hoppler S, Brown JD, Moon RT. Expression of a dominant-negative Wnt blocks induction of MyoD in Xenopus embryos. Genes Dev. 1996;10:2805–2817. doi: 10.1101/gad.10.21.2805. [DOI] [PubMed] [Google Scholar]
- Horb ME, Thomsen GH. A vegetally localized T-box transcription factor in Xenopus eggs specifies mesoderm and endoderm and is essential for embryonic mesoderm formation. Development. 1997;124:1689–1698. doi: 10.1242/dev.124.9.1689. [DOI] [PubMed] [Google Scholar]
- Huang S, Moody SA. The retinal fate of Xenopus cleavage stage progenitors is dependent upon blastomere position and competence: studies of normal and regulated clones. J. Neurosci. 1993;13:3193–3210. doi: 10.1523/JNEUROSCI.13-08-03193.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson C, Clements D, Friday RV, Stott D, Woodland HR. Xsox17alpha and -beta mediate endoderm formation in Xenopus. Cell. 1997;91:397–405. doi: 10.1016/s0092-8674(00)80423-7. [DOI] [PubMed] [Google Scholar]
- Jacobson M, Hirose G. Origin of the retina from both sides of the embryonic brain: a contribution to the problem of crossing at the optic chiasma. Science. 1978;202:637–639. doi: 10.1126/science.705349. [DOI] [PubMed] [Google Scholar]
- Jonas E, Snape A, Sargent T. Transcriptional regulation of a Xenopus embryonic epidermal keratin gene. Development. 1989;106:399–405. doi: 10.1242/dev.106.2.399. [DOI] [PubMed] [Google Scholar]
- Joseph EM, Melton DA. Mutant Vg1 ligands disrupt endoderm and mesoderm formation in Xenopus embryos. Development. 1998;125:2677–2685. doi: 10.1242/dev.125.14.2677. [DOI] [PubMed] [Google Scholar]
- Kavka AI, Green JB. Tales of tails: Brachyury and the T-box genes. Biochim Biophys Acta. 1997;1333:F73–84. doi: 10.1016/s0304-419x(97)00016-4. [DOI] [PubMed] [Google Scholar]
- Kenyon KL, Zaghloul NA, Moody SA. Transcription factors of the anterior neural plate alter cell movements of epidermal progenitors to specify a retinal fate. Dev. Biol. 2001;240:77–91. doi: 10.1006/dbio.2001.0464. [DOI] [PubMed] [Google Scholar]
- Kessler DS, Melton DA. Induction of dorsal mesoderm by soluble, mature Vg1 protein. Development. 1995;121:2155–2164. doi: 10.1242/dev.121.7.2155. [DOI] [PubMed] [Google Scholar]
- Kessler DS. Maternal signaling pathways and the regulation of cell fate. In: Moody SA, editor. Cell Lineage and Fate Determination. Academic Press; San Diego: 1999. pp. 323–340. [Google Scholar]
- Kimelman D, Griffin KJ. Vertebrate mesendoderm induction and patterning. Curr Opin. Genet. Dev. 2000;10:350–356. doi: 10.1016/s0959-437x(00)00095-2. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y, Verkade H, Reiter JF, Kim CH, Chitnis AB, Kuroiwa A, Stainier DY. Notch signaling can regulate endoderm formation in zebrafish. Dev. Dyn. 2004;229:756–762. doi: 10.1002/dvdy.10483. [DOI] [PubMed] [Google Scholar]
- Kofron M, Demel T, Xanthos J, Lohr J, Sun B, Sive H, Osada S, Wright C, Wylie C, Heasman J. Mesoderm induction in Xenopus is a zygotic event regulated by maternal VegT via TGFbeta growth factors. Development. 1999;126:5759–5770. doi: 10.1242/dev.126.24.5759. [DOI] [PubMed] [Google Scholar]
- Kumar JP, Moses K. EGF receptor and Notch signaling act upstream of Eyeless/Pax6 to control eye specification. Cell. 2001;104:687–697. doi: 10.1016/s0092-8674(01)00265-3. [DOI] [PubMed] [Google Scholar]
- Loose M, Patient R. A genetic regulatory network for Xenopus mesendoderm formation. Dev. Biol. 2004;271:467–478. doi: 10.1016/j.ydbio.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Lustig KD, Kroll KL, Sun EE, Kirschner MW. Expression cloning of a Xenopus T-related gene (Xombi) involved in mesodermal patterning and blastopore lip formation. Development. 1996;122:4001–4012. doi: 10.1242/dev.122.12.4001. [DOI] [PubMed] [Google Scholar]
- Marquardt T. Transcriptional control of neuronal diversification in the retina. Prog Retin Eye Res. 2003;22:567–577. doi: 10.1016/s1350-9462(03)00036-3. [DOI] [PubMed] [Google Scholar]
- Mathers PH, Grinberg A, Mahon KA, Jamrich M. The Rx homeobox gene is essential for vertebrate eye development. Nature. 1997;387:603–607. doi: 10.1038/42475. [DOI] [PubMed] [Google Scholar]
- Mead PE, Zhou Y, Lustig KD, Huber TL, Kirschner MW, Zon LI. Cloning of Mix-related homeodomain proteins using fast retrieval of gel shift activities, (FROGS), a technique for the isolation of DNA-binding proteins. Proc. Natl. Acad. Sci. U.S.A. 1998;95:11251–11256. doi: 10.1073/pnas.95.19.11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody SA. Fates of the blastomeres of the 16-cell stage Xenopus embryo. Dev. Biol. 1987a;119:560–578. doi: 10.1016/0012-1606(87)90059-5. [DOI] [PubMed] [Google Scholar]
- Moody SA. Fates of the blastomeres of the 32-cell stage Xenopus embryo. Dev. Biol. 1987b;122:300–319. doi: 10.1016/0012-1606(87)90296-x. [DOI] [PubMed] [Google Scholar]
- Moody SA. Testing the cell fate commitment of single blastomeres in Xenopus laevis. In: Richter J, editor. Advances in Molecular Biology: a comparative methods approach to the study of oocytes and embryos. Oxford University Press; London/New York: 1999. pp. 355–381. [Google Scholar]
- Moody SA. Cell lineage analysis in Xenopus embryos. In: Tuan R, Lo C, editors. Methods in Molecular Biology: Dev. Biol. Protocols. Humana Press Inc.; Totowa, NJ.: 2000. pp. 331–347. 2000. [DOI] [PubMed] [Google Scholar]
- Moore KB, Moody SA. Animal-vegetal asymmetries influence the earliest steps in retina fate commitment in Xenopus. Dev. Biol. 1999;212:25–41. doi: 10.1006/dbio.1999.9338. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus (Daudin) Garland; New York: 1994. [Google Scholar]
- Onuma Y, Takahashi S, Asashima M, Kurata S, Gehring WJ. Conservation of Pax6 function and upstream activation by Notch signaling in eye development of frogs and flies. Proc. Natl. Acad. Sci. U.S.A. 2002;99:2020–2025. doi: 10.1073/pnas.022626999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada SI, Wright CV. Xenopus nodal-related signaling is essential for mesendodermal patterning during early embryogenesis. Development. 1999;126:3229–3240. doi: 10.1242/dev.126.14.3229. [DOI] [PubMed] [Google Scholar]
- Penzel R, Oschwald R, Chen Y, Tacke L, Grunz H. Characterization and early embryonic expression of a neural specific transcription factor xSOX3 in Xenopus laevis. Int. J. Dev. Biol. 1997;41:667–677. [PubMed] [Google Scholar]
- Piccolo S, Agius E, Leyns L, Bhattacharyya S, Grunz H, Bouwmeester T, De Robertis EM. The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature. 1999;397:707–710. doi: 10.1038/17820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissmann E, Jornvall H, Blokzijl A, Andersson O, Chang C, Minchiotti G, Persico MG, Ibanez CF, Brivanlou AH. The orphan receptor ALK7 and the Activin receptor ALK4 mediate signaling by Nodal proteins during vertebrate development. Genes Dev. 2001;15:2010–2022. doi: 10.1101/gad.201801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex M, Hilton E, Old R. Multiple interactions between maternally-activated signalling pathways control Xenopus nodal-related genes. Int. J. Dev. Biol. 2002;46:217–226. [PubMed] [Google Scholar]
- Sasai Y, Lu B, Piccolo S, De Robertis EM. Endoderm induction by the organizer-secreted factors chordin and noggin in Xenopus animal caps. EMBO J. 1996;15:4547–4555. [PMC free article] [PubMed] [Google Scholar]
- Sinner D, Rankin S, Lee M, Zorn AM. Sox17 and beta-catenin cooperate to regulate the transcription of endodermal genes. Development. 2004;131:3069–3080. doi: 10.1242/dev.01176. [DOI] [PubMed] [Google Scholar]
- Sive HL, Grainger RM, Harland RM. Early Development of Xenopus laevis: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2000. [Google Scholar]
- Smith JC, Price BM, Green JB, Weigel D, Herrmann BG. Expression of a Xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell. 1991;67:79–87. doi: 10.1016/0092-8674(91)90573-h. [DOI] [PubMed] [Google Scholar]
- Smith WC, Harland RM. Expression cloning of noggin, a new dorsalizing factor localized in the Spemann Organizer in Xenopus embryos. Cell. 1992;70:829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- Stennard F, Carnac G, Gurdon JB. The Xenopus T-box gene, Antipodean, encodes a vegetally localised maternal mRNA and can trigger mesoderm formation. Development. 1996;122:4179–4188. doi: 10.1242/dev.122.12.4179. [DOI] [PubMed] [Google Scholar]
- Sullivan SA, Moore KB, Moody SA. Early events in frog blastomere fate determination. In: Moody SA, editor. Cell Lineage and Fate Determination. Academic Press; San Diego: 1999. pp. 297–321. 1999. [Google Scholar]
- Sullivan SA, Akers L, Moody SA. foxD5a, a Xenopus winged helix gene, maintains an immature neural ectoderm via transcriptional repression that is dependent on the C-terminal domain. Dev. Biol. 2001;232:439–457. doi: 10.1006/dbio.2001.0191. [DOI] [PubMed] [Google Scholar]
- Sun BI, Bush SM, Collins-Racie LA, LaVallie ER, DiBlasio-Smith EA, Wolfman NM, McCoy JM, Sive HL. derriere: a TGF-beta family member required for posterior development in Xenopus. Development. 1999;126:1467–1482. doi: 10.1242/dev.126.7.1467. [DOI] [PubMed] [Google Scholar]
- Tada M, Casey ES, Fairclough L, Smith JC. Bix1, a direct target of Xenopus T-box genes, causes formation of ventral mesoderm and endoderm. Development. 1998;125:3997–4006. doi: 10.1242/dev.125.20.3997. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Yokota C, Takano K, Tanegashima K, Onuma Y, Goto J-I, Asashimas M. Two novel nodal-related genes initiate early inductive events in Xenopus Nieuwkoop center. Development. 2000;127:5319–5329. doi: 10.1242/dev.127.24.5319. [DOI] [PubMed] [Google Scholar]
- Taverner NV, Kofron M, Shin Y, Kabitschke C, Gilchrist MJ, Wylie C, Cho KW, Heasman J, Smith JC. Microarray-based identification of VegT targets in Xenopus. Mech. Dev. 2005;122:333–354. doi: 10.1016/j.mod.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Weber H, Symes CE, Walmsley ME, Rodaway AR, Patient RK. A role for GATA5 in Xenopus endoderm specification. Development. 2000;127:4345–4360. doi: 10.1242/dev.127.20.4345. [DOI] [PubMed] [Google Scholar]
- White RJ, Sun BI, Sive HL, Smith JC. Direct and indirect regulation of derriere, a Xenopus mesoderm-inducing factor, by VegT. Development. 2002;129:4867–4876. doi: 10.1242/dev.129.20.4867. [DOI] [PubMed] [Google Scholar]
- Williams RW, Moody SA. Developmental and genetic control of cell number in retina. In: Chalupa LM, Werner JS, editors. The Visual Neurosciences. MIT Press; MA: 2004. pp. 63–76. [Google Scholar]
- Xanthos JB, Kofron M, Wylie C, Heasman J. Maternal VegT is the initiator of a molecular network specifying endoderm in Xenopus laevis. Development. 2001;28:167–180. doi: 10.1242/dev.128.2.167. [DOI] [PubMed] [Google Scholar]
- Yasuo H, Lemaire P. A two-step model for the fate determination of presumptive endodermal blastomeres in Xenopus embryos. Curr. Biol. 1999;9:869–879. doi: 10.1016/s0960-9822(99)80391-1. [DOI] [PubMed] [Google Scholar]
- Zaghloul NA, Yan B, Moody SA. Step-wise specification of retinal stem cells during normal embryogenesis. Biol. Cell. 2005;97:321–337. doi: 10.1042/BC20040521. [DOI] [PubMed] [Google Scholar]
- Zhang C, Basta T, Fawcett SR, Klymkowsky MW. SOX7 is an immediate-early target of VegT and regulates Nodal-related gene expression in Xenopus. Dev. Biol. 2005;278:526–541. doi: 10.1016/j.ydbio.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Zhang C, Basta T, Hernandez-Lagunas L, Simpson P, Stemple DL, Artinger KB, Klymkowsky MW. Repression of nodal expression by maternal B1-type SOXs regulates germ layer formation in Xenopus and zebrafish. Dev. Biol. 2004;273:23–37. doi: 10.1016/j.ydbio.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Zhang J, King ML. Xenopus VegT RNA is localized to the vegetal cortex during oogenesis and encodes a novel T-box transcription factor involved in mesodermal patterning. Development. 1996;122:4119–4129. doi: 10.1242/dev.122.12.4119. [DOI] [PubMed] [Google Scholar]
- Zhang J, Houston DW, King ML, Payne C, Wylie C, Heasman J. The role of maternal VegT in establishing the primary germ layers in Xenopus embryos. Cell. 1998;94:515–524. doi: 10.1016/s0092-8674(00)81592-5. [DOI] [PubMed] [Google Scholar]


