Abstract
We have recently reported increased transforming growth factor (TGF)-β1 gene transcription in proximal tubular cells within 12 hours of exposure to 25 mmol/L d-glucose, with a requirement for a second stimulus such as platelet-derived growth factor (PDGF) to increase its translation in short-term experiments. In the current study we investigated the effect on TGF-β1 production of prolonged exposure of proximal tubular cells to high glucose concentrations. Enzyme-linked immunosorbent assay of cell culture supernatant showed significant increase in latent TGF-β1 only after 7 days exposure to high glucose. Radiolabeling of glucose-stimulated cells with 3H amino acids and subsequent immunoprecipitation of TGF-β1 demonstrated de novo synthesis from day 5 of high glucose exposure onwards. Similarly, polysome analysis showed enhanced translation of TGF-β mRNA after 4 or more days of high glucose exposure. TGF-β1 synthesis, following addition of glucose, was inhibited by blockade of the PDGF-α receptor subunit. Glucose did not alter PDGF expression, nor expression of PDGF α-receptors. Activation of the receptor following addition of 25 mm d-glucose could be demonstrated suggesting increased sensitivity to endogenous PDGF. Exposure to glucose activated p38MAP kinase, and inhibition of this activation abrogated both glucose induced TGF-β1 transcriptional activation and TGF-β1 synthesis. Inhibition of p38MAP kinase did not influence the effect of exogenous PDGF when cells were stimulated sequentially by glucose and PDGF. We postulate that glucose induces an early increase in TGF-β1 transcription via activation of p38MAP kinase. In addition, glucose causes a late increase in PDGF-dependent TGF-β1 translation by enhancing cellular sensitivity to PDGF. This provides a potential explanation for the clinical observation that prolonged poor glycemic control may contribute to progression of diabetic nephropathy.
Diabetic nephropathy is now the most common cause of end-stage renal disease and accounts for 30% to 40% of all patients requiring renal replacement therapy. Furthermore, the incidence of diabetic nephropathy continues to increase in part due to the improved survival of type 2 diabetic patients as the cardiovascular mortality in this group declines. 1 Although there is a strong correlation between glomerular structure and renal function across the spectrum of normoalbuminuria-microalbuminuria-overt proteinuria, 2 an increase in fibrillary collagen and interstitial fibrosis occurs with more advanced disease and correlates with deterioration in renal function. 3 Much of our work has focused on the possible mechanisms of the pathogenesis of interstitial fibrosis in diabetic nephropathy, and in particular the role of proximal tubular epithelial cells.
A key molecule that has been implicated in the pathogenesis of diabetic nephropathy is the pro-fibrotic cytokine transforming growth factor-β1 (TGF-β1). Elevated glucose concentration as seen in diabetes is known to stimulate TGF-β1 synthesis within the glomerulus. 4 In contrast we have previously demonstrated that exposure of cultured human renal proximal tubular epithelial cells (HPTC) to elevated d-glucose concentrations increased the expression of a poorly translated TGF-β1 transcript without any associated change in TGF-β1 protein synthesis. 5 More recently we have demonstrated that pretreatment of PTC with elevated glucose concentrations followed by stimulation with growth factors such as platelet-derived growth factor (PDGF) had synergistic effects on TGF-β1 synthesis. 6 PDGF at low doses did not influence TGF-β1 transcription, but led to alteration in TGF-β1 mRNA stability and translation. Without prior glucose-induced increase in the amount of TGF-β1 transcript, PDGF did not stimulate significant TGF-β1 protein synthesis. At a high dose (100 ng/ml) PDGF stimulated TGF-β1 synthesis independent of glucose concentration. This was associated with increased TGF-β1 gene transcription and alteration in TGF-β1 mRNA translational efficiency. This suggests that in diabetic nephropathy, the role of glucose is to lower the threshold at which a stimulus such as PDGF stimulates TGF-β1 protein synthesis. The data also suggest that independent regulation of TGF-β1 transcription and translation by glucose and PDGF account for their synergistic effect on TGF-β1 protein synthesis.
To further examine the role of the hyperglycemia in progressive renal disease, we sought to determine the effect of long-term exposure of renal proximal tubular epithelial cells to elevated glucose concentration, on their synthesis of TGF-β1. The data demonstrate that prolonged culture of PTC under conditions of high glucose stimulated de novo TGF-β1 synthesis. Furthermore we have shown that this involved p38MAP kinase-mediated glucose-dependent increase in TGF-β1 transcription, and stimulation of PDGF α receptor signaling which facilitated TGF-β1 mRNA translation. This data therefore provides some mechanistic insight into how prolonged poor glycemic control may contribute to acceleration in the rate of decline of renal function in diabetic nephropathy.
Materials and Methods
Materials
Kinase inhibitors and antibodies and their sources (in parentheses) were as follows: AG1296, a potent and selective inhibitor of PDGF receptor tyrosine kinase activity (Calbiochem, San Diego, CA) 7 ; SB203580, a highly specific, cell-permeable inhibitor of p38 kinase (Calbiochem) 8,9 ; PD98059, a specific and permeable inhibitor of MAP kinase kinase (MEK) (Calbiochem) 10 ; monoclonal mouse antiphosphotyrosine antibody (Clone PT-66) (Sigma, Poole, UK); polyclonal goat anti-PDGF receptor antibody (AF-307-NA) (R&D Systems, Oxford, UK); polyclonal rabbit anti-TGF-β1 antibody (sc-146) (Santa Cruz Biotechnology, Inc., Wiltshire, UK); polyclonal rabbit anti-phospho-p38 MAP kinase (number 9211) (Cell Signaling Technology, Beverly, MA); mouse monoclonal anti-E-cadherin antibody (Transduction Laboratories, Lexington, KY); polyclonal rabbit anti-p38 MAP kinase (number 9212) (Cell Signaling Technology).
Cell Culture
All experiments were performed using HK-2 cells (American Type Culture Collection number CRL-2190), which are human proximal tubular epithelial cells immortalized by transduction with human papilloma virus 16 E6/E7 genes. 11 Cells were cultured in Dulbecco’s modified Eagle’s medium/Ham’s F12 (Life Technologies, Paisley, UK) supplemented with 10% bovine calf serum (Biological Industries Ltd., Cumbernauld, UK), HEPES, L-glutamine, insulin, transferrin, sodium selenite, and hydrocortisone (Sigma). Fresh growth medium was added to cells every 3 to 4 days until confluent. All experiments were performed using cells at passage 30 or below and, with the exception of the cells used for transfection and reporter gene analysis, cells were growth-arrested in serum- and insulin-free medium for 48 hours before use in experiments. All experiments were performed in serum- and insulin-free conditions.
Quantification of TGF-β1
Total TGF-β1 in the cell culture supernatant was measured by specific enzyme-linked immunosorbent assay (ELISA) (R&D Systems) of cell culture supernatant samples collected from growth-arrested HK-2 cells, stimulated under serum-free conditions. Active TGF-β1 is measured directly and latent TGF-β1 can be measured indirectly following acid activation of samples. This assay has <1% cross-reactivity for TGF-β2 and TGF-β3. TGF-β1 concentration was normalized to number of cells, determined by cell counting with a hemocytometer. Data are expressed as picograms of TGF-β1 per milliliter normalized to the control value.
Assessment of de Novo TGF-β1 Synthesis
To determine the kinetics of de novo TGF-β1 synthesis we examined incorporation of radioactive amino acids into newly synthesized protein by metabolic labeling and detection by TGF-β1 immunoprecipitation and autoradiography. Forty μCi of 3H-radiolabeled amino acid mixture (1000 μCi/ml, Amersham Biosciences UK, Little Chalfont, UK) were added to growth-arrested cells in 25-cm2 flasks. Supernatant samples were subsequently collected for TGF-β1 immunoprecipitation. Before immunoprecipitation supernatant samples were pre-cleared with 25 μl of protein A-sepharose beads (Sigma) and 0.25 μg normal rabbit immunoglobulin at 4°C for 1 hour with constant mixing. The supernatant was removed from the beads, and 2 μg polyclonal anti-TGF-β1 antibody (Santa Cruz) was added to each 1 ml of cleared supernatant and incubated with constant mixing at 4°C for 2 hours. Subsequently, 50 μl of protein A-sepharose beads were added, and mixing continued for 12 hours. Samples were centrifuged, the supernatant was removed, and the beads were washed twice with PBS. After the final centrifugation and removal of the second PBS wash, 25 μl of sodium dodecyl sulfate (SDS)/β-mercaptoethanol loading buffer was added, and the samples heated to 95°C for 10 minutes before SDS-polyacrylamide gel electrophoresis (PAGE) in a 10% gel. Gels were fixed by incubating in 10% acetic acid/40% methanol overnight, and then soaking in scintillant (Amplify; Amersham Biosciences) for 30 minutes and dried before visualization of immunoprecipitated TGF-β1 by autoradiography.
Reporter Construct Transfection
The TGF-β1 promoter-luciferase construct pGL3-TGFβ 1 + 11/−1362 was generated as previously described 12 ; the Smad-responsive promoter was a gift from Aristidis Moustakas. 13 For transfection of the reporter constructs, 1.75 × 106 HK-2 cells were seeded per 35-mm dish (this density of cells produced a 90% confluence monolayer the following day). The next day, cells were transfected with 1 μg of plasmid pGL3-TGF-β1 + 11/−1362, or 1 μg of the Smad responsive promoter-luciferase construct, using the mixed lipofection reagent FuGene 6 (Roche) at a ratio of 3 μL Fugene to 1 μg DNA in serum-free and insulin-free medium. Twenty-four hours after transfection, cells were stimulated with 25 mmol/L d-glucose for 12 hours. Following lysis of the cells in reporter lysis buffer (Promega, Madison, WI) luciferase content was quantified by glow-type luminance assay (Bright-Glo; Promega). The data are expressed as relative light units normalized to control (5 mmol/L d-glucose) values.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Alterations in expression of thrombospondin-1, PDGF-A chain, PDGF-B chain and PDGF-α receptor mRNA expression were examined by RT-PCR performed using specific oligonucleotide primers as previously described 5 (Table 1) ▶ . PCR amplification was performed over a range of cycle numbers (26 to 38) to ensure that amplification was in the linear range of the curve. One-tenth of the PCR reaction from both test and control (GAPDH) product were mixed and separated by flat-bed electrophoresis in 3% w/v NuSieve GTG agarose gels (Flowgen Instruments Ltd., Sittingbourne, UK), stained with ethidium bromide (Sigma) and photographed. The negatives were scanned using a densitometer (Model 620 video densitometer; Bio-Rad Laboratories Ltd., Hercules, CA) and the density of the bands compared to those of the housekeeping gene.
Table 1.
Sequence of Oligonucleotide Primers
| Gene | Primer sequence | Product size |
|---|---|---|
| GAPDH | F: 5′-TCCACCACCCTGTTGCTGTAG-3′ | 204 bp |
| R: 5′-CAAGAAGGTGGTGAAGCAGG-3′ | ||
| PDGF-A chain | F: 5′-AATTTCGCCGCCACAGGAGA-3′ | 449 bp |
| R: 5′-ACGGGGGCCAGATCAGGAAG-3′ | ||
| PDGF-B chain | F: 5′-TGCTGGGCGCTCTTCCTG-3′ | 341 bp |
| R: 5′-TTGGCGTTGGTGCGGTCT-3′ | ||
| PDGF-α receptor | F: 5′-GCTCCCACCCTGCGTTCT-3′ | 351 bp |
| R: 5′-TCTGGCCGTGGGTTTTAG-3′ | ||
| Thrombospondin | F: 5′-AAAGCGTCTTCACCAGAG-3′ | 496 bp |
| R: 5′-GCAGATGGTAACTGAGTT-3′ |
F, forward; R, reverse.
Analysis of Efficiency of Translation
Polysome analysis was performed as previously described. 14 Approximately 1.3 × 107 growth-arrested cells per experiment were trypsinized, pelleted, and extracted in 1 ml of ice-cold lysis buffer (10 mmol/L Tris.Cl, pH 8.0, 150 mmol/L NaCl, 1.5 mmol/L MgCl2, 0.5% Nonidet P-40, 500 Uml−1 recombinant RNAsin (Promega). Nuclei were removed by centrifugation at 3000 × g for 2 minutes and the supernatant transferred to a new tube supplemented with 100 μg/ml cycloheximide, 1 mmol/L PMSF, 10 mmol/L DTT, and 0.5 mg/ml heparin, then centrifuged at 13000 × g for 5 minutes to remove mitochondria and membrane debris. The supernatant was layered onto a 10-ml, 15% to 40% linear sucrose gradient containing 10 mmol/L Tris.Cl, pH 7.5, 140 mmol/L NaCl, 1.5 mmol/L MgCl2, 10 mmol/L DTT, 10 mmol/L cycloheximide, and 0.5 mg/ml heparin in a Polyallomer centrifuge tube (Beckman) and centrifuged using an SW41Ti rotor at 36,000 rpm for 2 hours at 4°C. The gradient was fractionated into 22 0.5-ml fractions, each supplemented with 1% SDS, 10 mmol/L EDTA, and 200 μg/ml proteinase K, and incubated at 37°C for 30 minutes to degrade endogenous nucleases. Subsequently, the fractions were mixed with phenol:chloroform:isoamyl alcohol at a ratio of 24:24:1 and the aqueous layer containing the RNA removed. A 5% aliquot of each fraction was analyzed by electrophoresis in a 3% agarose gel to ensure that the RNA was not degraded, and that the tRNA and rRNA species were appropriately distributed through the gradient. RNA was precipitated overnight from the remainder of each fraction with 1 ml 100% ethanol, 50 μl 3 mol/L sodium acetate and 1 μl glycogen (Sigma) and washed once with 70% ethanol before air-drying. Fifty-percent samples of each fraction were run as a single large Northern blot, as above. A single 2.4-kb band was detectable on autoradiography, this was quantified by Densitometry (Chemi Doc; Bio-Rad). Data are expressed as percentage of the total TGF-β1 mRNA for that experiment in each fraction. The actual blot is also shown for comparison.
Western Blot and IP of Phosphorylated PDGF Receptor
Immunoblot analysis of lysate samples was performed by standard methodologies. Briefly, cell extracts were prepared in SDS sample buffer and boiled for 5 minutes at 95°C. Equal numbers of cells were lysed, and the resultant lysates were loaded onto a 10% SDS-PAGE carried out under reducing conditions according to the procedure of Laemmli. 15 After electrophoresis the separated proteins were transferred to a nitrocellulose membrane (Amersham Biosciences). The membrane was blocked with PBS containing 5% nonfat powdered milk for 1 hour and then incubated with the appropriate primary antibody (see materials above) in PBS containing 0.1% Tween 20 (PBS-Tween) for 1 hour at room temperature. The blots were subsequently washed in PBS-Tween and then incubated with an appropriate horseradish peroxidase-conjugated secondary antibody (Sigma) in Tris-buffered saline Tween. Proteins were visualized using enhanced chemiluminescence (Amersham Biosciences) according to the manufacturer’s instructions.
Immunoprecipitation was performed by standard methodologies. Briefly, 700 μg of cell protein samples were pre-cleared with 25 μl packed protein A cross-linked 4% beaded agarose (Sigma) at 4°C for 1 hour. The beads were removed by centrifugation (13,000 rpm for 10 minutes) and the supernatant collected. Monoclonal mouse anti-phosphotyrosine antibody at a working dilution of 1 in 200 was added to the cleared supernatant and incubated at 4°C with constant mixing for 1 hour. The immune complex was captured by the addition of 50 μl of packed agarose protein A beads for 8 hours at 4°C. Separation of the beads was achieved by centrifugation (13,000 × g for 25 minutes) and the supernatant removed followed by four washing steps in PBS. Subsequently samples were subject to immunoblot/Western analysis, as described above. Specificity of immunoprecipitation was confirmed by negative control reactions performed with either no primary antibody, or rabbit IgG control.
Results
Long-Term Culture in 25 mmol/L d-Glucose Leads to de Novo Synthesis of Latent TGF-β1
ELISA of cell culture supernatant showed a significant increase in total TGF-β after 7 or more days of exposure to high glucose under serum-free conditions (Figure 1) ▶ (day 7, 481.9 ± 67.9 vs. 703.4 ± 55.2, 5 vs. 25 mmol/L d-glucose, mean ± SEM, n = 4, P < 0.05). Exposure to 5 mmol/L d-glucose together with 20 mmol/L L-glucose, used as the osmotic control, did not lead to increased TGF-β1 generation above that seen following addition of 5 mmol/L d-glucose alone (data not shown). Furthermore there was no difference in the concentration of TGF-β1 in the culture supernatant taken from cells exposed to 5 or 25 mmol/L d-glucose at earlier time points.
Figure 1.
Effect of 25 mmol/L d-glucose on TGF-β1 generation. 25 mmol/L d-glucose was added to confluent monolayers of growth-arrested HK-2 cells under serum-free conditions (▴). In control experiments cells were exposed to 5 mmol/L d-glucose (•). Subsequently TGF-β1 was quantified by ELISA at the time points indicated. Cell counts of the monolayer were performed and the results expressed as TGF-β1 per 105 cells normalized to the control value for each time point. Results represent mean ± SE of six individual experiments. * P < 0.05 vs. 5 mmol/L, ** P < 0.005 vs. 5 mmol/L.
Differences in the quantity of TGF-β1 following addition of either 5 mmol/L or 25 mmol/L d-glucose, as assessed by ELISA, was only detected following acidification of the samples, thus suggesting that TGF-β1 was produced in its latent form. Further confirmation of the latency of TGF-β1 was sought by addition of conditioned medium from HK-2 exposed to either 5 mmol/L d-glucose or 25 mmol/L d-glucose to cells transiently transfected with the Smad-responsive promoter-luciferase construct. No difference in luciferase activity of the promoter construct was seen following addition of either 5 mmol/L or 25 mM d-glucose conditioned medium (Figure 2A) ▶ . Following in vitro activation of latent TGF-β1 performed by repeated cycles of freeze thawing of the samples, a significant increase in Smad-responsive promoter activity was seen when 25 mmol/L d-glucose conditioned medium was added as compared to 5 mmol/L d-glucose conditioned medium (Figure 2A) ▶ .
Figure 2.
Elevated d-glucose stimulates generation of latent TGF-β1. A: Conditioned medium (CM) collected from cells exposed to either 5 mmol/L d-glucose (5CM) or 25 mmol/L d-glucose (25CM) for 7 days was added to HK-2 cells transfected with a Smad-responsive promoter-luciferase construct. In addition, to activate latent TGF-β1, conditioned medium from either 5 mmol/L d-glucose (5CM FT) or 25 mmol/L d-glucose (25CM FT) were subjected to 10 cycles of freeze-thawing before addition to transfected cells. In control experiments, culture medium alone was added to the transfected cells (5 mmol/L). Luciferase activity was quantified 12 hours after the addition of conditioned medium. Results represent means ± SE of four individual experiments. B: To validate TGF-β1 responsiveness of the Smad-responsive promoter-luciferase, a linear increase in luciferase activity was demonstrated following stimulation of Smad-responsive promoter luciferase activity by addition of increasing doses of recombinant TGF-β1 to transfected HK-2 cells. Luciferase activity was quantified 12 hours after the addition of TGF-β1. C: Addition of 25 mmol/L d-glucose had no effect on the expression of thrombospondin-1 mRNA as assessed by RT-PCR. Growth-arrested HK-2 cells were stimulated with 25 mmol/L d-glucose and total mRNA isolated at the time points indicated. In control experiments cells were exposed to 5 mmol/L d-glucose alone. For thrombospondin-1 PCR amplification was performed for 30 cycles. PCR amplification for GAPDH was performed for 25 cycles. Ethidium bromide-stained PCR products were separated on a 3% agarose gel. One representative experiment of three individual experiments performed for both cell types is shown.
Previous studies in mesangial cells, demonstrating generation of active TGF-β1 following exposure to elevated concentration of glucose have demonstrated in addition to stimulation of TGF-β1 generation, that glucose stimulated its activation by transcriptional regulation of thrombospondin (TSP), which is a known activator of TGF-β1. In the current study TSP expression following addition of 25 mmol/L d-glucose was examined by RT-PCR. There was, however, no difference in TSP-mRNA expression following addition of 25 mmol/L d-glucose as compared to cells exposed to 5 mmol/L d-glucose for up to 5 days (Figure 2C) ▶ .
Radiolabeling of glucose-stimulated cells with 3H amino acids and subsequent immunoprecipitation of TGF-β from the cell culture supernatant was used to assess the kinetics of TGF-β protein synthesis. Using this approach increased synthesis of radiolabeled TGF-β1 was demonstrated between days 5 and 7 following addition of 25 mmol/L d-glucose, thus confirming that the increase in TGF-β1 demonstrated by ELISA represented delayed de novo synthesis (Figure 3A) ▶ .
Figure 3.
A: Time course of TGF-β1 synthesis following glucose stimulation. Confluent monolayers of HK-2 cells were growth-arrested before stimulation with 25 mmol/L d-glucose. 3H-labeled amino acids were added to the culture medium at the start of each time point shown. At the end of the time point the medium was removed and the cells were discarded. TGF-β1 protein was extracted from the supernatant by immunoprecipitation as described in Materials and Methods. Following immunoprecipitation, radiolabeled TGF-β1, representative of de novo synthesized and secreted TGF-β1, was detected by autoradiography. B and C: Effect of 25 mmol/L d-glucose on translational efficiency of TGF-β1 mRNA. Confluent monolayers of HK-2 cells were growth-arrested and exposed to 5 mmol/L d-glucose or 25 mmol/L d-glucose for 96 hours before extraction of the cytosol and separation of mRNA on a sucrose gradient as described in Materials and Methods. Fractions 1–16 represent tRNA, free ribosomal subunits, monosomes, untranslated and poorly translated mRNA in the form of messenger ribonucleoprotein particles. Fractions 16–22 represent polysomes of increasing size, containing well translated mRNA. B: Northern blot of the fractionated mRNA, probed for TGF-β1. C: Graphical representation of the polysome distribution of TGF-β1 mRNA, expressed as percentage of the total TGF-β1 mRNA detected on a given blot in each fraction with control-5 mmol/L d-glucose shown as shaded area, and 25 mmol/L d-glucose enclosed by solid line.
Our previous studies have demonstrated that exposure to glucose over a short time-course stimulates TGF-β1 transcription, but that the transcript has an inherently poor translational efficiency. The effect of prolonged culture under conditions of elevated d-glucose concentration on TGF-β1 mRNA translation was therefore examined by polysome analysis to assess the number of ribosomes associated with the mRNA. The results suggest that TGF-β1 mRNA, although poorly translated following short-term exposure to 25 mmol/L d-glucose, is well translated after 4 or more days of high glucose exposure (Figure 3b) ▶ . This is evidenced by a shift in the location of TGF-β1 mRNA, with 42% of the total TGF-β1 mRNA localizing to a region of the gradient corresponding to the heavy polysomes (fractions 16–22), representing well translated mRNA, 16 following 4 days of exposure compared to 14% in the 5 mmol/L d-glucose-treated cells at the same time point.
Identification of PDGF as the Second Messenger Mediating Glucose-Dependent TGF-β1 Synthesis
The delayed kinetics of de novo TGF-β1 synthesis suggests that this is an indirect of glucose requiring a “second messenger”. Previously we have demonstrated a synergistic effect of glucose and PDGF on TGF-β1 synthesis following sequential stimulated in short-term experiments, involving signaling through the PDGF-α receptor subunit. We therefore postulated that prolonged culture under condition of elevated glucose concentration may stimulate a PDGF-dependent facilitation of translation of glucose induced TGF-β1 mRNA.
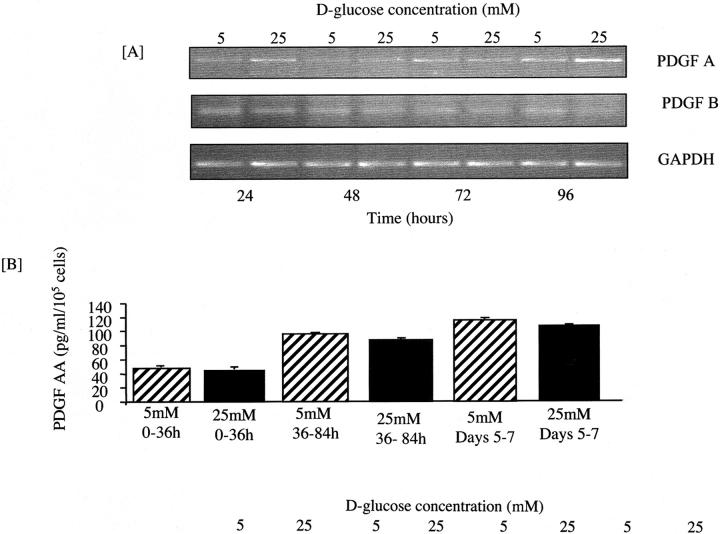
The relationship between PDGF receptor signaling and glucose-stimulated de novo synthesis of TGF-β1, was examined in experiments in which PDGF receptor function was inhibited either chemically (AG1296) or by the use of a specific blocking antibody to the PDGF-α receptor. Addition of either AG1296 or blocking antibody resulted in a dose dependent inhibition of TGF-β1 generation following addition of 25 mmol/L d-glucose for 7 days (Figure 4) ▶ , suggesting that signaling through the PDGF-α receptor was necessary for mediating prolonged effects of glucose. Both PDGF-A and PDGF-B, are known to modulate PDGF-α receptor signaling. There was however no change in the expression of PDGF-A chain mRNA as assessed by RT-PCR, nor protein expression as assessed by specific ELISA (Quantikine Human PDGF AA Sandwich ELISA; R&D Systems) (Figure 5) ▶ . Low-level expression of the PDGF-B chain could be demonstrated by RT-PCR (Figure 5) ▶ , no induction was seen following addition of 25 mmol/L d-glucose, and no PDGF-B chain could be quantified in the culture supernatant collected from cells exposed to either 5 mmol/L or 25 mmol/L d-glucose (Quantikine Human PDGF BB Sandwich ELISA; R&D Systems).
Figure 4.
The role of PDGF in glucose-induced TGF-β1 generation. 25 mmol/L d-glucose was added to confluent monolayers of growth-arrested HK-2 cells under serum-free conditions for 7 days in the presence of increasing doses of a chemical PDGF receptor inhibitor (AG1296) (A). In a separate series of experiments PDGF receptor function was inhibited by the use of a specific blocking antibody to the PDGF-α receptor (B). Subsequently TGF-β1 was quantified by ELISA. Cell counts of the monolayer were performed and the results expressed as TGF-β1 per 105 cells, normalized for the 5 mmol/L d-glucose control value. Results represent mean ± SE of six individual experiments. * P < 0.05 vs. 25 mmol/L alone; ** P < 0.005 vs. 25 mmol/L alone.
Figure 5.
PDGF (A-chain and B-chain) expression following addition of 25 mmol/L d-glucose. Growth-arrested HK-2 cells were stimulated with 25 mmol/L d-glucose under serum-free conditions and total mRNA isolated at the time points indicated (A). In control experiments, cells were exposed to 5 mmol/L d-glucose alone. For PDGF-A chain and PDGF-B chain, PCR amplification was performed for 30 cycles. PCR amplification for GAPDH was performed for 25 cycles. Ethidium bromide-stained PCR products were separated on a 3% agarose gel. One representative experiment of three individual experiments is shown. B: The effect of d-glucose on PDGF-A protein expression was determined by collection of supernatant samples between the time points indicated, following addition of either 25 mmol/L d-glucose or 5 mmol/L d-glucose under serum-free conditions and quantification of PDGF by ELISA. Data represents mean ± SEM of four individual experiments. At each time point there was no significant difference in PDGF concentration between the supernatants collected from cells exposed to either 5 mmol/L or 25 mmol/L d-glucose.
In the absence of up-regulation of PDGF protein expression we postulated that glucose modulated either PDGF-α receptor expression or signaling. Low level expression of PDGF-α receptor subunit was demonstrated by RT-PCR, which did not increase following addition of 25 mmol/L d-glucose (Figure 6A) ▶ . Similarly, although PDGF-α receptor protein expression could be demonstrated by Western analysis, total receptor expression was not altered by exposure to 25 mmol/L d-glucose (Figure 6B) ▶ . The low level of expression was further exemplified by appearance of bands corresponding to the PDGF-α receptor only when total cell protein extracted from 1 × 105 cells was loaded. Lack of increase in receptor expression was also confirmed by FACS analysis (data not shown). In contrast, using phosphotyrosine immunoprecipitation and PDGF-α immunoblot analysis to assess receptor tyrosine phosphorylation, activation of the PDGF-α receptor was increased following exposure of cells to 25 mmol/L d-glucose for 7 days (Figure 6B) ▶ .
Figure 6.
Expression of PDGF-α receptor subunit did not increase following addition of 25 mmol/L d-glucose (A). Growth-arrested HK-2 cells were stimulated with 25 mmol/L d-glucose under serum-free condition and total mRNA isolated at the time points indicated (A). In control experiments cells were exposed to 5 mmol/L d-glucose alone. For PDGF-α receptor PCR amplification was performed for 45 cycles (at lower cycle number, no expression could be detected). PCR amplification for GAPDH was performed for 25 cycles. Ethidium bromide-stained PCR products were separated on a 3% agarose gel. One representative experiment of three individual experiments is shown. B: Protein expression of the PDGF-α receptor was examined by Western analysis of total cell lysates. Growth-arrested HK-2 cells were exposed to either 5 mmol/L d-glucose or 25 mmol/L d-glucose for 7 days. Cell extracts were prepared in SDS sample buffer and immunoblot analysis was performed under reducing conditions. To demonstrate equal loading of protein, the epithelial cell marker E-cadherin was used as a “housekeeping” control. In parallel experiments activation of the PDGF-α was assessed by phosphotyrosine-immunoprecipitation before immunoblot analysis.
Glucose-Dependent TGF-β1 Generation Requires p38MAP Kinase-Dependent TGF-β1 Gene Transcription
Glucose has recently been shown to activate p38 MAP kinase in vascular smooth muscle cells and mesangial cells in a non-osmotic manner. 17 Activation of p38 MAP kinase may potentially result in increased transcription of TGF-β mRNA. 18
Activation of p38MAP kinase was examined using an antibody to detect the dually phosphorylated (Thr 180 and Tyr 182) peptide sequence representing the catalytic core of the active 38 MAP kinase enzyme. While addition of 25 mmol/L d-glucose had no effect on expression of total p38MAP kinase, there was an increase in activation of p38 MAP kinase manifest as increased phosphorylation apparent 10 minutes following addition of 25 mmol/L d-glucose. There was no change in the expression of either total or phosphorylated p38 MAP kinase following addition of 5 mmol/L d-glucose (Figure 7A) ▶ .
Figure 7.
A: Activation of p38 MAP kinase by 25 mmol/L d-glucose. Growth-arrested HK-2 cells were stimulated with 25 mmol/L d-glucose under serum-free conditions. Cell extracts were prepared in SDS sample buffer and immunoblot analysis was performed under reducing conditions for either phosphorylated p38 MAP kinase or total MAP kinase as indicated. B: p38 MAP kinase mediates glucose-dependent stimulation of TGF-β1 promoter activity. 90% confluent monolayers of HK-2 cells were transfected with the TGF-β1 promoter-luciferase plasmid construct pGL3-TGFβ1 + 11/−1362 before exposure to 5 mmol/L d-glucose (open bar), 25 mmol/L d-glucose (filled bar), 25 mmol/L d-glucose together with 300 ng/ml of the p38MAP kinase inhibitor SB203580 (cross-hatched bar) or 25 mmol/L d-glucose together with 8 μmol/L of the ERK inhibitor PD98059 (stippled bar). After 12 hours total cell protein was extracted and analyzed for luciferase activity. Data are expressed as luciferase activity normalized to the values obtained for control cells. Results represent mean ± SE for three individual experiments; * P < 0.05 vs. 25 mmol/L alone.
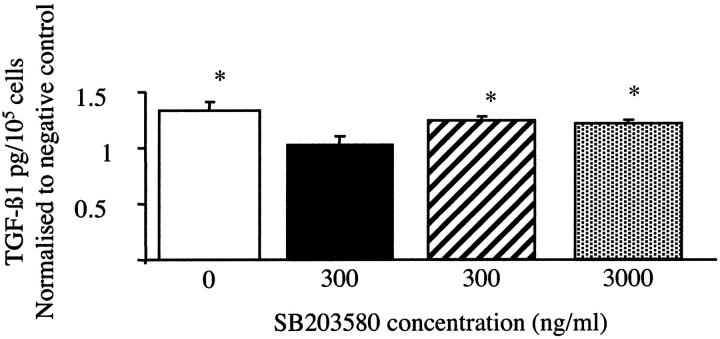
Involvement of p38MAP kinase activation and glucose-induced TGF-β1 gene transcription was assessed by determining the effect of inhibition of p38MAP kinase on glucose-induced activity of the TGF-β1 promoter-luciferase construct pGL3-TGFβ1 + 11/−1362, using a highly specific cell-permeable inhibitor of p38MAP kinase SB203580. Twenty-five mM d-glucose-dependent increase in luciferase was inhibited in a dose-dependent manner by SB203580, confirming that glucose-mediated transcriptional activation of the TGF-β1 gene was p38MAP kinase-dependent (Figure 7B) ▶ . PDGF signaling is known to involve the ERK MAP kinase cascade. To ensure specificity of the p38MAP kinase effect and also to determine whether PDGF signaling was involved in glucose-mediated transcriptional up-regulation of TGF-β1, HK-2 cells transfected with the TGF-β1 promoter-luciferase construct were stimulated with 25 mmol/L d-glucose in the presence of the ERK MAP kinase inhibitor PD98059. In contrast to the p38MAP kinase inhibitor, inhibition of ERK had no effect on glucose-induced TGF-β1 promoter activity (Figure 7B) ▶ . TGF-β1 generation following sequential stimulation by 25 mmol/L d-glucose and PDGF was abrogated by presence of this dose of the inhibitor, confirming its efficacy (data not shown). These results suggest that p38MAP kinase may be involved in either glucose-induced TGF-β1 transcription or modification of PDGF signaling. To differentiate between these possibilities, experiments were performed in which stimulation by 25 mmol/L d-glucose for 48 hours was followed by subsequent addition of PDGF, at doses previously demonstrated to facilitate translation of TGF-β1 mRNA. In these experiments the SB inhibitor was added together with either the glucose during the first 48 hours before its removal, or with PDGF during the second 48 hours. Addition of SB203580 together with 25 mmol/L d-glucose for the initial 48 hours prevented increased TGF-β1 synthesis on subsequent addition of exogenous recombinant PDGF (Figure 8A) ▶ . In contrast, when cells were stimulated with 25 mmol/L d-glucose in the absence of SB203580, increased TGF-β1 protein was detected in the cell culture supernatant, as we have previously described. This was not affected by the addition of SB203580 at the same time as the addition of the PDGF (Figure 8B) ▶ .
Figure 8.
Inhibition of p38MAP kinase prevents glucose-dependent TGF-β1 protein synthesis. Growth-arrested HK-2 cells were stimulated by 25 mmol/L d-glucose for 48 hours, followed by addition of PDGF (25 ng/ml) for another 48 hours. In these experiments the SB inhibitor was added together with either the glucose during the first 48 hours before its removal (solid bar), or with PDGF during the second 48 hours at doses of either 300 ng/ml (cross-hatched bar) or 3000 ng/ml (stippled bar). In the control experiments neither inhibitor was added, and cells were stimulated by 25 mmol/L d-glucose for 48 hours, followed by subsequent addition of 25 ng/ml PDGF (positive control), or 5 mmol/L d-glucose for 48 hours followed by 25 ng/ml PDGF (negative control). At the end of 96 hours, supernatant samples were collected and TGF-β1 quantified by ELISA. Results represent mean ± SE for three individual experiments, and are normalized to the negative control values.
Discussion
Diabetic nephropathy is now the commonest cause of end-stage renal disease, and with the ever-increasing number of patients with type II diabetes, the numbers are set to rise further. The etiology of diabetic nephropathy is multifactorial, but the degree of glycemic control is likely to play a major role. There is now clear evidence of a lower incidence of nephropathy associated with improved glycemic control, and a slower transition of incipient-to-overt renal disease. 19,20 It is also clear that there is a close correlation between glycemic control and decline in renal function. 21,22 Although many studies have focused on the association between pathological changes in the glomerulus and renal outcome, it is now clear that progression of renal dysfunction correlates best with the degree of renal interstitial fibrosis. 23 These observations led us to examine how elevated glucose concentrations may alter proximal tubular cell function, which may in turn contribute to these pathological changes.
One of the key cytokines implicated in the pathogenesis of progressive renal fibrosis of diverse etiologies including diabetic nephropathy is TGF-β1. Work in the streptozotocin model of diabetic nephropathy suggests that antagonism of TGF-β1 may provide a potential therapeutic target to ameliorate renal injury. 24 In clinical practice to date, blockade of the renin-angiotensin system either with angiotensin enzyme inhibitors, 25 or angiotensin II receptor antagonists, 26 have proved to delay progression of diabetic nephropathy. Significantly it has also been demonstrated that the beneficial effect of these agents can be predicted by the reduction of serum TGF-β1 concentration. 27 Much of our work has focused on the mechanism by which glucose modulates TGF-β1 generation, and how TGF-β1 may modulate proximal tubular cell function.
In short term in vitro experiments, we have demonstrated that 25 mmol/L d-glucose stimulated TGF-β1 gene transcription; however, the glucose-induced transcript was poorly translated. Subsequent application of a second stimulus, such as PDGF, facilitated translation of the glucose induced transcript. At high doses PDGF was also able to induce synthesis of TGF-β, independent of ambient glucose concentration; however, prior application of 25 mmol/L d-glucose lowered the concentration at which PDGF stimulated TGF-β1 generation. These observations are in keeping with the clinical observation that not all diabetics develop nephropathy, suggesting that the role of glucose may be to prime the kidney to an enhanced pro-fibrotic response in the context of a second stimulus.
In contrast to the short term in vitro experiments described above, the results in the current manuscript suggest that prolonged persisted exposure to elevated d-glucose concentration may be sufficient to generate TGF-β1. Interestingly however TGF-β1 is produced predominantly in its latent form, as it could only be detected by ELISA following acidification of the supernatant samples and similarly conditioned medium from glucose-stimulated cells was only able to stimulate a Smad-responsive promoter-luciferase construct following repeated cycles of freeze-thawing. Both transient acidification and freeze-thawing of samples are well established in vitro mechanisms of activation of latent TGF-β1. 28 In vivo activation has been associated with proteolytic processing of the latent complex, 29,30 and also conformation change of the latent complex, which may be mediated by integrin binding. 31 Glomerular mesangial cells differ from human proximal tubular cells in that addition of 25 mmol/L d-glucose alone is associated with generation of TGF-β1. Again in contrast with proximal tubular cells, the TGF-β1 is generated in a biologically active form. 4 Binding of the latent complex of TGF-β1 to the extracellular matrix glycoprotein thrombospondin-1 has also been shown to regulate TGF-β activation and is thought to have an important role in its activation in vivo. 32 Recent studies have also demonstrated that high-glucose stimulated up-regulation of thrombospondin-1 in mesangial cells is responsible for converting high levels of latent TGF-β1 to bioactive growth factor. 33 In the current study, in contrast to those performed in mesangial cells, we were unable to demonstrate induction of thrombospondin-1.
It is clear that exposure to elevated glucose concentration alone triggers structural changes within the glomerulus. In contrast initiation of pathological changes in the interstitium is more complex in that all diabetics do not exhibit fibrotic changes and thus elevated glucose concentration alone is unlikely to stimulate progressive fibrosis. The differential regulation of thrombospondin-1 in mesangial cells to that shown in this manuscript, with the resulting difference in generation of active as compared to latent TGF-β in the two cell types, may in part explain these clinical observations. This would suggest that factors in addition to elevated glucose concentration are required to generate bioactive TGF-β1 in the cortico-interstitium of the diabetic patient.
Previously, we demonstrated that regulation of TGF-β1 generation by proximal tubular cells may occur at numerous levels including transcription, translation, and secretion of pre-formed protein. 6,34-36 The results in the current manuscript demonstrating increased incorporation of radiolabeled amino acids into TGF-β1, would suggest that prolonged exposure of 25 mmol/L d-glucose leads to de novo protein synthesis rather than release of pre-formed TGF-β1. The kinetics of TGF-β1 generation, however, together with our previous observations that exposure to elevated d-glucose in short-term experiments led to induction of a poorly translated TGF-β1 mRNA transcript, suggest that this was related to an indirect effect of glucose.
In addition to TGF-β1, PDGF has also been implicated in the pathogenesis of glomerulosclerosis, fibrosis, and wound repair, 37-39 as well as the microvascular complications associated with diabetes mellitus. 40,41 Autocrine and paracrine interactions between TGF-β1 and PDGF have been demonstrated in numerous cell types. In this respect TGF-β1 is known to regulate PDGF receptor expression in mesangial cells and fibroblasts, 42-44 as well as its secretion in fibroblasts. 45 With relevance to diabetic nephropathy, high glucose concentration induces the over-expression of TGF-β1 through the activation of a PDGF loop in human mesangial cells. 46 Furthermore we have previously demonstrated synergistic effects between glucose and PDGF that facilitate TGF-β1 synthesis. 5,6,47 More specifically we have shown that the effect of PDGF was mediated via the PDGF-α receptor subunit. 5 From the data presented in the current manuscript it appears that prolonged exposure to 25 mmol/L d-glucose modifies PDGF signaling, which subsequently facilitates the production of TGF-β1. No changes in production of PDGF isoforms (either A or B chains) could be demonstrated and furthermore there were no changes in the expression of the PDGF-α receptors as assessed by immunoblot analysis. That the effects of glucose were mediated through this receptor was, however, supported by blocking experiments using either a chemical inhibitor, or a specific blocking antibody. Furthermore although there was no change in total receptor expression, increased activation, demonstrated by increased phosphorylation, was seen following stimulation with 25 mmol/L d-glucose. This would suggest that glucose sensitized the receptor, rather than altered its expression. In our previous work, we demonstrated transient increase in de novo TGF-β1 protein synthesis following stimulation with a high dose of PDGF. In contrast, pretreatment with 25 mmol/L d-glucose not only lowered the PDGF dose required to stimulate TGF-β1 synthesis, but also led to sustained de novo TGF-β1 synthesis. 6 This is, therefore, consistent with the hypothesis of increased PDGF-α receptor sensitization by glucose.
Recent studies have demonstrated that glucose may activate the p38MAP kinase pathway. At moderate concentrations, this was via a PKC-dependent pathway, while at higher levels, p38MAP kinase could also be activated via a PKC-independent, osmolarity-dependent pathway. 17 In the current manuscript we have demonstrated glucose-induced activation of p38MAP kinase, and furthermore the data suggests that transcriptional regulation of TGF-β1 may be abrogated by inhibition of this kinase pathway. It is not clear, however, from our experiments if activation is mediated by glucose-specific pathways or via osmolarity. Lack of effect of osmolarity on TGF-β1 generation in this study, and also our previous observations that osmolar controls do not prime for TGF-β1 synthesis following addition of PDGF, 5 would argue against “osmotic” activation of the p38. The demonstration of p38-dependent transcriptional activation of TGF-β1 is compatible with data generated using mesangial cells, in which mechanical stretch-induced TGF-β1 production was also p38MAP kinase-dependent. The data in this manuscript also demonstrate that inhibition of p38MAP kinase-mediated transcriptional activation of TGF-β1 inhibited de novo TGF-β1 synthesis following prolonged exposure to 25 mmol/L d-glucose. In addition, inhibition of p38MAP kinase also abrogated TGF-β1 production following addition of 25 mmol/L d-glucose and subsequent stimulation with exogenous PDGF. From these observations we postulate that in our long-term high-glucose culture model, glucose initially stimulates TGF-β1 transcription. Glucose also sensitized the PDGF receptor, leading to enhanced response to endogenous PDGF, without stimulation of PDGF synthesis. This enhanced sensitivity to PDGF subsequently facilitated PDGF-mediated translation of the glucose-induced TGF-β1 mRNA transcript, which is poorly translated without the synergistic effect of PDGF. In conclusion the data provide some insight into the contribution of prolonged periods of hyperglycemia to progression of renal disease in diabetes mellitus by modification of de novo synthesis of the pro-fibrotic cytokine TGF-β1.
Footnotes
Address reprint requests to Dr. A. O. Phillips, Institute of Nephrology, University of Wales College of Medicine, Heath Park, Cardiff CF14 4XN. E-mail: PhillipsAO@cf.ac.uk.
D. F. is the recipient of a National Kidney Research Fund Training Fellowship; A. O. P. is supported by a Glaxo Wellcome Advanced Fellowship.
References
- 1.Ritz E, Stefanski A: Diabetic nephropathy in type II diabetes Am J Kidney Dis 1996, 27(2):167-194 [DOI] [PubMed] [Google Scholar]
- 2.Caramori ML, Kim Y, Huang C, Fish AJ, Rich SS, Miller ME, Russell G, Mauer M: Cellular basis of diabetic nephropathy: 1. study design and renal structural-functional relationships in patients with long-standing type 1 diabetes Diabetes 2002, 51:506-513 [DOI] [PubMed] [Google Scholar]
- 3.Katz A, Caramori ML, Sisson-Ross S, Groppoli T, Basgen JM, Mauer M: An increase in the cell component of the cortical interstitium antedates interstitial fibrosis in type 1 diabetic patients. Kidney Int 2002, 61:2058-2066 [DOI] [PubMed] [Google Scholar]
- 4.Ziyadeh FN, Sharma K, Ericksen M, Wolf G: Stimulation of collagen gene expression and protein synthesis in murine mesangial cells by high glucose is mediated by autocrine activation of transforming growth factor-β. J Clin Invest 1994, 93:536-542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips AO, Steadman R, Topley N, Williams JD: Elevated D-glucose concentrations modulate TGF-β1 synthesis by human cultured renal proximal tubular cells: the permissive role of platelet-derived growth factor. Am J Pathol 1995, 147:362-374 [PMC free article] [PubMed] [Google Scholar]
- 6.Fraser DJ, Wakefield L, Phillips AO: Independent regulation of transforming growth factor-β1 transcription and translation by glucose and platelet-derived growth factor. Am J Pathol 2002, 161:1039-1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovalenko M, Gazit A, Bohmer A, Rorsman C, Ronnstrand L, Heldin CH, Waltenberger J, Bohmer FD, Levitzki A: Selective platelet-derived growth factor receptor kinase blockers reverse sis-transformation. Cancer Res 1994, 54:6106-6114 [PubMed] [Google Scholar]
- 8.Kramer RM, Roberts EF, Um SL, Borsch-Haubold AG, Watson SP, Fisher MJ, Jakubowski JA: p38 mitogen-activated protein kinase phosphorylates cytosolic phospholipase A2 (cPLA2) in thrombin-stimulated platelets: evidence that proline-directed phosphorylation is not required for mobilization of arachidonic acid by cPLA2. J Biol Chem 1996, 271:27723-27729 [DOI] [PubMed] [Google Scholar]
- 9.Saklatvala J, Rawlinson L, Waller RJ, Sarsfield S, Lee JC, Morton LF, Barnes MJ, Farndale RW: Role for p38 mitogen-activated protein kinase in platelet aggregation caused by collagen or a thromboxane analogue J Biol Chem 1996, 271(12):6586-6589 [DOI] [PubMed] [Google Scholar]
- 10.Pang L, Sawada T, Decker SJ, Saltiel AR: Inhibition of MAP kinase kinase blocks the differentiation of PC-12 cells induced by nerve growth factor. J Biol Chem 1995, 270:13585-13588 [DOI] [PubMed] [Google Scholar]
- 11.Ryan MJ, Johnson G, Kirk J, Fuerstenberg SM, Zager RA, Torok-Storb B: HK-2: an immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int 1994, 45:48-57 [DOI] [PubMed] [Google Scholar]
- 12.Kim SJ, Jeang KT, Glick AB, Sporn MB, Roberts AB: Promoter sequences of the human transforming growth factor-β1 gene responsive to transforming growth factor-β1 auto-induction. J Biol Chem 1989, 264:7041-7045 [PubMed] [Google Scholar]
- 13.Piek E, Moustakas A, Kurisaki A, Heldin CH, ten Dijke P: TGF-β type I receptor/ALK-5 and Smad proteins mediate epithelial to mesenchymal transdifferentiation in NMuMG breast epithelial cells. J Cell Sci 1999, 112:4557-4568 [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Sanz JA, Mikulits W, Livingstone A, Lefkovits I, Mullner EW: Translational control: a general mechanism for gene regulation during T cell activation. FASEB 1998, 12:299-306 [DOI] [PubMed] [Google Scholar]
- 15.Laemmli UK: Cleavage of structural proteins during the assembly the head of bacteriophage T4. Nature 1970, 227:680-685 [DOI] [PubMed] [Google Scholar]
- 16.Allison RSH, Mumy ML, Wakefield LM: Translational control elements in the major human transforming growth factor β1 mRNA. Growth Factors 1998, 16:89-100 [DOI] [PubMed] [Google Scholar]
- 17.Igarashi M, Wakasaki H, Takashara N, Ishii H, Jiang Z, Yamauchi T, Kuboki T, Meier K, Rhodes C, King GL: Glucose or diabetes activates p38 mitogen-activated protein kinase by different pathways. J Clin Invest 1999, 103:185-195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gruden G, Zonca S, Hayward A, Thomas S, Maestrini S, Gnudi L, Viberti G: Mechanical stretch-induced fibronectin and transforming growth factor-β1 production in human mesangial cells is p38MAP kinase-dependent. Diabetes 2000, 49:655-661 [DOI] [PubMed] [Google Scholar]
- 19.: The Diabetes Control and Complications Research Group: Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int 1995, 47:1703-1720 [DOI] [PubMed] [Google Scholar]
- 20.: UK Prospective Diabetes Study Group: Intensive blood glucose control with sulphonylurease or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes Lancet 1998, 352:837-853 [PubMed] [Google Scholar]
- 21.Mulec H, Blohme G, Grande B, Bjorck S: The effect of metabolic control on rate of decline in renal function in insulin-dependent diabetes mellitus with overt diabetic nephropathy. Nephrol Dial Transplant 1998, 13:651-655 [DOI] [PubMed] [Google Scholar]
- 22.Alaveras AE, Thomas SM, Sagriotis A, Viberti GC: Promoters of progression of diabetic nephropathy: the relative roles of blood glucose and blood pressure control. Nephrol Dial Transplant 1997, 12(Suppl 2):71-74 [PubMed] [Google Scholar]
- 23.Bohle A, Wehrmann M, Bogenschutz O, Batz C, Muller GA: The Pathogenesis of chronic renal failure in diabetic nephropathy. Pathol Res Pract 1991, 187:251-259 [DOI] [PubMed] [Google Scholar]
- 24.Sharma K, Jin Y, Guo J, Ziyadeh FN: Neutralization of TGF-β by anti-TGF-β antibody attenuates kidney hypertrophy and the enhanced extracellular matrix gene expression in STZ-induced diabetic mice. Diabetes 1996, 45:522-530 [DOI] [PubMed] [Google Scholar]
- 25.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD: The effect of angiotensin-converting enzyme inhibition on diabetic nephropathy. N Engl J Med 1993, 329:1456-1462 [DOI] [PubMed] [Google Scholar]
- 26.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I: Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001, 345:851-860 [DOI] [PubMed] [Google Scholar]
- 27.Sharma K, Eltayeb BO, McGowan TA, Dunn SR, Alzahabi B, Rohde R, Ziyadeh FN, Lewis EJ: Captopril-induced reduction of serum levels of transforming growth factor β1 correlates with long-term renoprotecion in insulin dependent diabetic patients. Am J Kidney Dis 1999, 34:818-823 [DOI] [PubMed] [Google Scholar]
- 28.Brown PD, Wakefield L, Levinson AD, Sporn MB: Physiochemical activation of recombinant latent transforming growth factor β’s 1, 2 and 3. Growth Factors 1990, 3:35-43 [DOI] [PubMed] [Google Scholar]
- 29.Sato Y, Okada M, Seguchi T, Kuwano M, Sato S, Furuya A, Hanai N, Tamaoki T: The mechanism for the activation of latent TGF-β during co-culture of endothelial cells and smooth muscle cells: cell-type specific targeting of latent TGF-β to smooth muscle cells. J Cell Biol 1993, 123:1249-1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu Q, Stamenkovic I: Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-β and promotes tumor invasion and angiogenesis. Genes Dev 2000, 14:163-176 [PMC free article] [PubMed] [Google Scholar]
- 31.Munger JS, Huang X, Kawaskatsu H, Griffiths MJD, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D: The integrin αvβ6 binds and activates latent TGF-β1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 1999, 96:319-328 [DOI] [PubMed] [Google Scholar]
- 32.Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SMF, Lawler J, Hynes RO, Bolvin GP, Bouck N: Thrombospondin-1 is a major activator of TGF-β1 in vivo. Cell 1998, 93:1159-1170 [DOI] [PubMed] [Google Scholar]
- 33.Yevdokimova N, Wahab N, Mason R: Thrombospondin-1 is the key activator of TGF-β1 in human mesangial cells exposed to high glucose. J Am Soc Nephrol 2001, 12:703-712 [DOI] [PubMed] [Google Scholar]
- 34.Morrisey K, Evans RA, Wakefield L, Phillips AO: Translational regulation of renal proximal tubular epithelial cell transforming growth factor β1 generation by insulin. Am J Pathol 2001, 159:1905-1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phillips AO, Topley N, Steadman R, Morrisey K, Williams J: Induction of TGF-β1 synthesis in d-glucose primed human proximal tubular cells: differential stimulation by the macrophage derived pro-inflammatory cytokines IL-1β and TNFα. Kidney Int 1996, 50:1546-1554 [DOI] [PubMed] [Google Scholar]
- 36.Phillips AO, Topley N, Morrisey K, Williams JD, Steadman R: Basic fibroblast growth factor stimulates the release of pre-formed TGF-β1 from human proximal tubular cells in the absence of de-novo gene transcription or mRNA translation. Lab Invest 1997, 76:591-600 [PubMed] [Google Scholar]
- 37.Floege J, Eng E, Young BA, Alpers CE, Barrett TB, Bowen-Pope DF, Johnson RJ: Infusion of platelet-derived growth factor or basic fibroblast growth factor induces selective glomerular mesangial cell proliferation and matrix accumulation in rats. J Clin Invest 1993, 92:2952-2962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pierce GF, Mustoe TA, Lingelbach J, Masakowski VR, Griffin GL, Senior RM, Deuel TF: Platelet-derived growth factor and transforming growth factor-β enhance tissue repair activities by unique mechanisms. J Cell Biol 1989, 109:429-440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pierce GF, Vande Berg J, Rudolph R, Tarpley J, Mustoe TA: Platelet-derived growth factor-BB and transforming growth factor β1 selectively modulate glycosaminoglycans, collagen and myofibroblasts in excisional wounds. Am J Pathol 1991, 138:629-646 [PMC free article] [PubMed] [Google Scholar]
- 40.Mizutani M, Okuda Y, Yamaoka T, Tsukahara K, Isaka M, Bannai C, Yamashita K: High glucose and hyperosmolarity increase platelet-derived growth factor mRNA levels in cultured human vascular endothelial cells. Biochem Biophys Res Com 1992, 187:664-669 [DOI] [PubMed] [Google Scholar]
- 41.Guillausseau PJ, Dupuy E, Bryckaert MC, Timsit J, Chanson P, Tobelem G, Caen JP, Lubetzki J: Platelet-derived growth factor in type 1 diabetes mellitus. Eur J Clin Invest 1989, 19:172-175 [DOI] [PubMed] [Google Scholar]
- 42.Battegay EJ, Raines EW, Seifert RA, Bowen-Pope DF, Ross R: TGF-β Induces bimodal proliferation of connective tissue cells via complex control of an autocrine PDGF loop. Cell 1990, 63:515-524 [DOI] [PubMed] [Google Scholar]
- 43.Haberstroh U, Zahner G, Disser M, Thaiss F, Wolf G, Stahl RAK: TGF-β stimulates rat mesangial cell proliferation in culture: role of PDGF β-receptor expression. Am J Physiol 1993, 264:F199-F205 [DOI] [PubMed] [Google Scholar]
- 44.Ishikawa O, LeRot EC, Trojanowska M: Mitogenic effect of transforming growth factor β1 on human fibroblasts involves the induction of platelet-derived growth factor α receptors. J Cell Physiol 1990, 145:181-186 [DOI] [PubMed] [Google Scholar]
- 45.Soma Y, Grotendorst GR: TGF-β stimulates primary human skin fibroblast DNA synthesis via an autocrine production of PDGF-related peptides. J Cell Physiol 1989, 140:246-253 [DOI] [PubMed] [Google Scholar]
- 46.Paolo S, Gesualdo L, Ranieri E, Grandaliano G, Schena FP: High glucose concentration induces the over-expression of transforming growth factor β through the activation of a platelet-derived growth factor loop in human mesangial cells. Am J Pathol 1996, 149:2095-2106 [PMC free article] [PubMed] [Google Scholar]
- 47.Phillips AO, Morrisey R, Steadman R, Williams JD: Polarity of stimulation and secretion of TGF-β1 by proximal tubular cells. Am J Pathol 1997, 150:1101-1111 [PMC free article] [PubMed] [Google Scholar]