Abstract
NKX3.1 is a homeobox gene which exhibits prostate and testis specific expression. Loss of NKX3.1 expression has been implicated in prostate development and tumorigenesis, but the role of NKX3.1 in testis biology is not known. Here we show that NKX3.1 expression is dramatically down-regulated in testicular cancer of germ cell origin. Immunohistochemical analysis on a tissue microarray containing 510 testicular tissue samples indicate that NKX3.1 is expressed at high levels in normal germ cells and in carcinoma in situ, but is sharply decreased or absent in most seminomas and all embryonal carcinomas. However, NKX3.1 is expressed in a subset of the more differentiated nonseminomas. We provide evidence that these changes in NKX3.1 protein levels are mainly due to transcriptional effects. These results suggest that NKX3.1 is essential for normal testis function and that its loss of expression is highly associated with the invasive phenotype of testicular germ cell tumors.
Testicular germ cell tumor (TGCT) is the most common malignancy among adolescents and young adult men in Western industrialized countries, and the incidence has increased dramatically over the past fifty years. 1,2 TGCTs are subgrouped into two main histological types, seminomas and nonseminomas, both of which develop from premalignant non-invasive carcinoma in situ (CIS, or intratubular malignant germ cells). Seminomas resemble the CIS cells, but are not constrained within the tubules. Whereas seminomas are believed to only rarely differentiate, nonseminomas develop through an undifferentiated but pluripotent embryonal carcinoma stage, which frequently differentiates into cells and tissue types of all three primary germ layers at various stages of differentiation (somatically differentiated teratomas and extra-embryonally differentiated choriocarcinomas and yolk sac tumors). Although several chromosomal regions and a few genes are known to be frequently altered, the molecular mechanisms involved in testicular tumorigenesis remain mostly unknown. 3 One of the genes that has highly restricted expression to the testis, along with the prostate, is the homeobox gene NK3 transcription factor-related locus 1 (NKX3.1). 4-6 The gene product of NKX3.1 is a positive effector of differentiation in prostate cancer, 4,6 and the gene is under both androgen and 17β-estradiol regulation. 4,5,7,8 A large proportion of human prostate carcinomas have lost the NKX3.1 protein expression, and this is most commonly seen in the advanced stages. 6 In mice, deletion of the Nkx3.1 gene results in developmental defects in the prostate, leading to hyperplasia and dysplasia. 9-12 In testis, however, little information is currently available on the possible deregulation of NKX3.1 expression during testicular dysgenesis or tumorigenesis. In this study, we have assessed in situ NKX3.1 expression using a tissue microarray representing normal testis, CIS, and invasive TGCT of all histological subgroups and clinical stages. In a small series of tumors we further evaluated whether the altered protein expression could be explained by transcriptional regulation.
Materials and Methods
Tissue Microarray
We used sections from a tissue microarray block that we previously constructed to contain 510 testicular tissue cores punched from formalin-fixed and paraffin-embedded testicular specimens from 279 individuals. 13 The 279 cases included 278 orchiectomy specimens from patients diagnosed with TGCT of adolescents and young adult males and one testicular autopsy from a patient with no known history of cancer. The patients had TGCT of various clinical stages, including 174 with localized TGCT and 104 with metastatic disease. The numbers of tissue cores acquired from the various histological subtypes were as follows: normal testis, 28; CIS, 21; seminoma, 184; embryonal carcinoma, 102; choriocarcinoma, 16; yolk sac tumor, 69; and teratoma, 90. The use of the testicular tissue samples was approved by the Regional Committee for Medical Research Ethics in Norway.
The Instrumedics (Instrumedics, Hackensack, NJ) tape-transfer method was used to transfer 4-μm sections of the tissue microarray block to glass slides. Hematoxylin and eosin-stained tissue microarray sections were evaluated to check the consistency with the originally assigned histology. Histological classification was performed according to the recommendations of the World Health Organization. 14 Distinction of CIS from normal tissue was assisted by immunohistochemical staining of a parallel section using anti-PLAP antibodies, targeting germ cell and placental alkaline phosphatases, extensively present in CIS but not in normal germ cells. 15
Rabbit Polyclonal Antiserum
NKX3.1 antiserum was raised in rabbits using a recombinant NKX3.1 protein spanning the whole coding region produced in Escherichia coli (Korkmaz CG, Korkmaz KS, Onody T, Seller WR, Loda M, Saacioglu F, submitted manuscript). The antiserum was purified on IgG affinity columns before use.
Immunohistochemistry on the Tissue Microarray
Tissue microarray sections were stained with the biotin-streptavidin-peroxidase method (Supersensitive Immunodetection System, LP000-UL, BioGenex, San Ramon, CA). Each section was deparaffinized and rehydrated, and high temperature antigen retrieval was achieved by microwaving twice for five minutes at 900W in 10 mmol/L citrate buffer (pH 6.0). The slides were then incubated with 1% hydrogen peroxide (H2O2) for 10 minutes to block the endogenous peroxidase activity before incubation with the polyclonal NKX3.1 antibody (affinity-purified rabbit antiserum against NKX3.1, 1:150) or the monoclonal PLAP antibody (clone 8A9, 1:20; IgG1k, Novocastra Laboratories Ltd., Newcastle, UK) for 30 minutes at room temperature. Afterward, the sections were incubated for 20 minutes with multilink biotinylated anti-immunoglobulins (1:30; BioGenex) and 20 minutes with streptavidin peroxidase (1:30; BioGenex). Finally, the sections were stained for 5 minutes with 0.05% of the peroxidase substrate 3k,3-diaminobenzidine tetrahydrochloride (DAB) freshly prepared in 0.05 mol/L Tris-HCl buffer (pH 7.6) containing 0.01% H2O2, before being counterstained with hematoxylin, dehydrated, and mounted. Negative controls consisted of replacement of the primary polyclonal NKX3.1 antibody with the IgG fraction of a non-immunized rabbit and replacement of the PLAP antibody with mouse myeloma protein of the same subclass and concentration. These controls were negative as expected.
The NKX3.1 immunostaining was nuclear, though weak cytoplasmic staining was also seen in some of the testicular tissues. Tissue cores with staining of more than 5% of the relevant nuclei were considered positive. A tumor was considered positive when one or more of the tissue cores from that specific tumor were positive. Equally, when more than one tissue core of a specific histological component of a tumor was present on the array, that specific component was considered positive if at least one of the samples was scored positive.
Quantitative RT-PCR
For real-time reverse transcriptase-polymerase chain reaction (RT-PCR), total RNA was isolated by use of the Trizol reagent (Invitrogen, Carlsbad, CA) from 12 testicular tissue samples (four of which are present on the tissue microarray), including two from morphologically normal testicular parenchyma adjacent to TGCT, one CIS, seven seminomas, and two embryonal carcinomas. From each RNA sample, first-strand cDNA was synthesized in an oligo(dT)-primed polymerization with SuperScript II reverse transcriptase (Invitrogen). The cDNA was then used as template in quantitative PCR, using LightCycler-FastStart DNA Master SYBR Green I (Roche Diagnostics, Basel, Switzerland), to evaluate the mRNA expression levels of NKX3.1 (primers: 5′-GGCCTGGGAGTCTCTTGACTCCACTAC-3′ and 5′-ATGTGGAGCCCAAACCACAGAAAATG-3′) and the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) control transcript (primers: 5′-GAAGGTGAAGGTCGGAGTC-3′ and 5′-GAAGATGGTGATGGGATTTC-3′). All samples were analyzed simultaneously, and the experiment was repeated twice with consistent results. For each of the amplifications, standard curves were constructed for both NKX3.1 and GAPDH using plasmid templates containing the respective fragments. A calibrated expression value for NKX3.1 was then calculated by dividing its expression value by that of GAPDH. For each sample, the average of the three runs was divided by the average expression from the normal samples to get changes in expression relative to the normal tissues.
Results
Various testicular tissue samples from the tissue microarray are shown in Figures 1 and 2 ▶ with representative immunohistochemical staining of NKX3.1. Whereas all of the normal testicular tissue samples (n = 17) had NKX3.1 immunopositive germ cells, and all of the CIS samples (n = 17) were also positive, only 14% of the seminomas (n = 150; P = 4 × 10−13 [seminoma versus normal, two-sided Fisher’s exact]) and none of the embryonal carcinomas (n = 70; P = 2 × 10−18) or the choriocarcinomas (n = 9; P = 3 × 10−7) expressed NKX3.1. Some of the differentiated nonseminomas expressed NKX3.1, including 15% of the yolk sac tumors (n = 48), and 20% of the teratomas (n = 49), which was significantly different from the complete absence of NKX3.1 in embryonal carcinomas (P = 1 × 10−4).
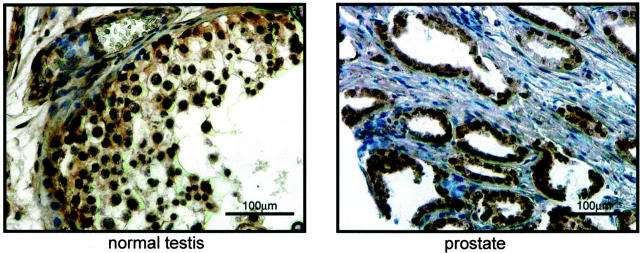
Figure 1.
Normal testicular and prostate tissues are immunopositive for NKX3.1. The antibody specificity is shown demonstrating NKX3.1 positive spermatogenic germ cells within a seminiferous tubule of a non-cancerous testis and immunopositive epithelial cells in a prostatic tissue sample. The prostate tissue was included as a positive control for staining.
The possible correlation of NKX3.1 expression with the metastatic potential of TGCT was evaluated, and NKX3.1 expression tended to be inversely correlated with metastases in patients with seminomas. Whereas 17% of the pure seminomas without metastases at time of diagnosis (n = 89) expressed NKX3.1, there was no NKX3.1 expression among the pure seminomas with metastases at diagnosis (n = 16; P = 0.12). Such a link between NKX3.1 expression and metastases was not observed for the nonseminomas.
The quantitative RT-PCR analyses revealed that all analyzed tumor samples had reduced expression of NKX3.1 mRNA compared with the normal testis tissues (Figure 3) ▶ . For six of ten tumors, NKX3.1 mRNA accumulation was less than five percentage of the average expression in the normal samples, showing that the loss of NKX3.1 in TGCT is at least in part due to loss of NKX3.1 mRNA accumulation. Four of the tumors analyzed by RT-PCR were also present on the tissue microarray. These included two with very low expression (both <0.02 compared to normal testis, and both lacking immunoreactivity to the NKX3.1 antiserum) and two of the seminomas with intermediate expression (0.32 and 0.43, of which one was immunopositive).
Figure 3.
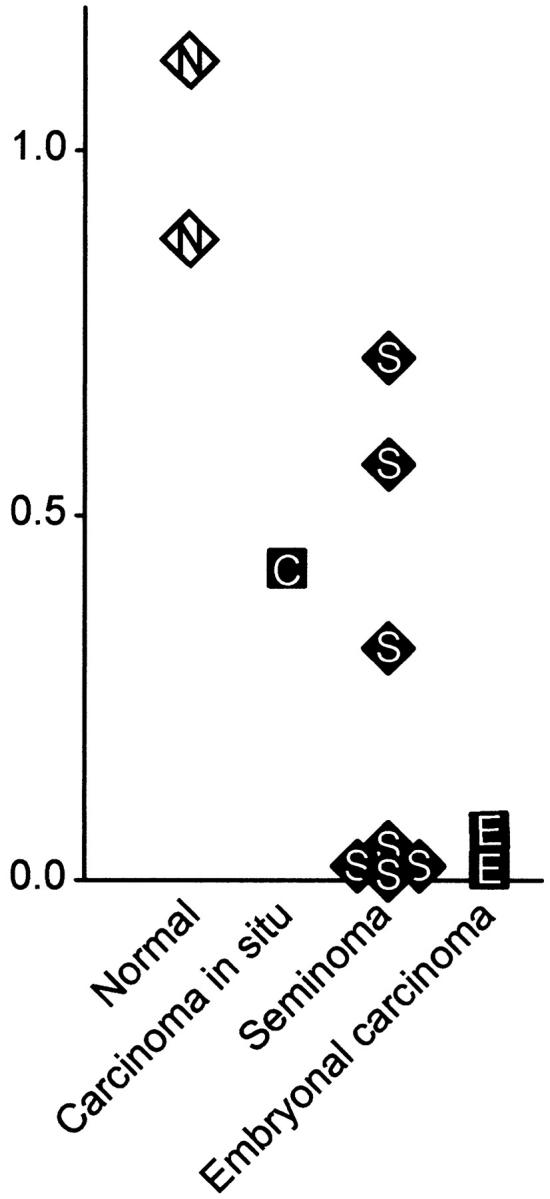
Testicular germ cell tumors have reduced or lost NKX3.1 mRNA expression. Total RNA was isolated from normal testis or testicular germ cell tumors and subjected to quantitative RT-PCR analysis. The mRNA levels are shown relative to the average of the two normal testicular tissues. The experiments were run in triplicate, and the average values are shown.
Discussion
Previous reports have implicated a role for NKX3.1 in human prostate tumorigenesis, possibly as a tumor suppressor protein. 4,6,8,12,16 Although not commented on, it can be found from the data set of a recent study of multiple tumor types that the NKX3.1 protein was absent from a set of TGCTs. 17 We here demonstrate that primary TGCTs display a virtually ubiquitous loss of NKX3.1 expression. Since NKX3.1 expression is still retained in the CIS precursor lesions, this loss is highly associated with the invasive tumor phenotype. Thus, NKX3.1 expression clearly distinguishes the morphologically similar CIS and seminoma cells at the molecular level. The quantitative RT-PCR data suggest that the dramatically lower expression of NKX3.1 protein in testicular cancer compared with normal testis is mostly due to differential transcriptional regulation.
NKX3.1 is located to chromosome band 8p21 that is frequently lost in prostate cancers. 18 Loss of NKX3.1 protein expression has been observed in up to 40% of prostate tumors with correlation to advanced tumor stages. 6 We observed a similar trend toward loss of expression of NKX3.1 in the overall sample set of TGCT. Interestingly, for the seminomas, the NKX3.1 protein was not detected in any of the tissue cores from patients with metastases at time of diagnosis. This suggests that expression of NKX3.1 may be involved in suppressing metastases arising from seminomas.
The loss of NKX3.1 expression in prostate cancer is believed to be due to epigenetic inactivation, 11 a view which is strengthened by the absence of mutations within the exons of NKX3.1. 19,20 In the case of TGCT, we speculate that the loss of NKX3.1 expression is due to epigenetic mechanisms based on the following arguments. Firstly, the chromosome band 8p21 has not been noted as a region with common loss of heterozygosity in this tumor type. 3 Secondly, we found immunopositivity for NKX3.1 in TGCT to be correlated with positive staining of the previously analyzed DNA repair enzyme O6-methylguanine-DNA methyltransferase (MGMT; data not shown). 13 This was evident both across the whole sample series and within specific histological subtypes. Because MGMT was recently reported to be frequently hypermethylated in promoter sequences in TGCT, 21 this correlation suggests an epigenetic silencing mechanism also for NKX3.1. In fact, DNA methylation seems to be a major mechanism for regulating gene expression in TGCT. 22-24 Finally, the expression of NKX3.1 seen in a subset of the most differentiated nonseminomas indicates that the loss of its expression in TGCT is reversible and thus likely caused by epigenetic silencing. The fact that none of the 70 embryonal carcinomas expressed NKX3.1 and the general view that the embryonal carcinomas are precursors for the differentiated nonseminomas support this assumption of reversibility.
The latter observation is consistent with the function of NKX3.1 as a differentiation-specific homeobox protein. However, NKX3.1 may not only reflect the differentiation status of the tissue, but may also be an effector of differentiation itself, as suggested for the prostate gland. 17,25 In light of this, and the fact that Nkx3.1 knockout mice are prone to prostate cancer, 9-11 the loss of NKX3.1 expression can either be a cause or a consequence of the testicular cancer phenotype. More work is needed to differentiate between these two possibilities.
A recent study of mutant mice that are deficient in both Nkx3.1 and Pten demonstrated that loss of function of these two genes cooperate in prostate cancer development and progression. 11 Mice with defects in the Pten gene spontaneously develop germ cell tumors, 26,27 and interestingly, a human PTEN germ line mutation has been described in an adolescent man with synchronous testicular and extragonadal germ cell tumor. 28 We therefore speculate that there may be a link between NKX3.1 and PTEN disruption also in testicular tumorigenesis.
In conclusion, we have provided evidence that the expression of the NKX3.1 homeobox gene is lost from most human TGCTs. This adds to the molecular understanding of the disease, and raises the possibility that NKX3.1 expression could be used as a marker for invasive testicular tumorigenesis.
Figure 2.
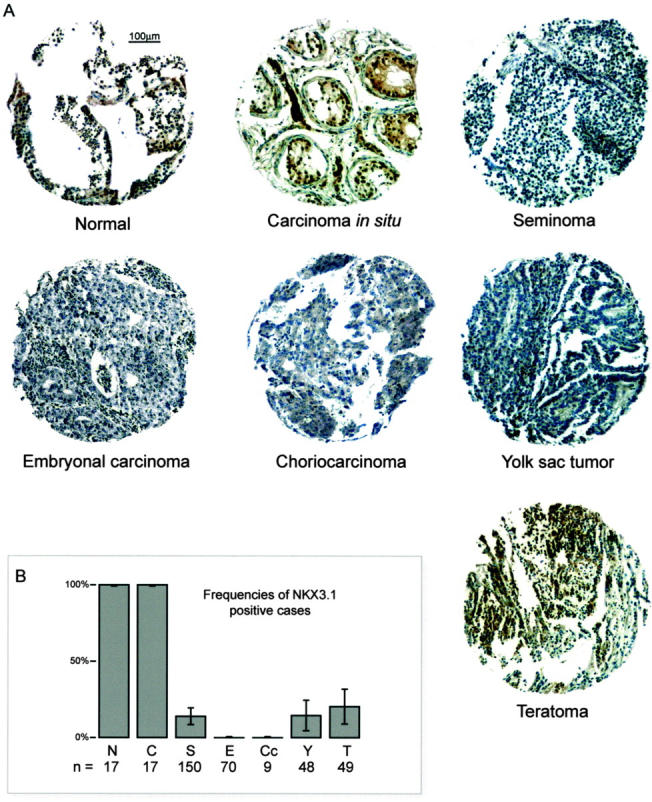
NKX3.1 expression analysis on a testicular germ cell tumor tissue microarray. A: One tissue core with representative staining is shown for each of the histological subtypes. Positive cases include the normal germ cells, carcinoma in situ, and the teratoma. The remaining cases were scored negative. B: The histogram bars represent the frequencies of NKX3.1 immunopositive cases for the subgroups of normal germ cells (N), carcinoma in situ (C), and the various histological subtypes of testicular germ cell tumor (S, seminoma; E, embryonal carcinoma; Cc, choriocarcinoma; Y, yolk sac tumor; T, teratoma). Error bars define 95% confidence intervals.
Footnotes
Address reprint requests to Ragnhild A. Lothe, Department of Genetics, Institute for Cancer Research, The Norwegian Radium Hospital, N-0310 Oslo, Norway. E-mail: rlothe@radium.uio.no; or Fahri Saatcioglu, Department of Biology, University of Oslo, Postboks 1050 Blindern, N-0316, Oslo, Norway. E-mail: fahris@bio.uio.no.
Supported by grants from the Norwegian Cancer Society (to R. I. S., R. A. L., and F. S.) and the Research Council of Norway (to R. I. S., R. A. L.,and F. S.).
References
- 1.Devesa SS, Blot WJ, Stone BJ, Miller BA, Tarone RE, Fraumeni JFJ: Recent cancer trends in the United States. J Natl Cancer Inst 1995, 87:175-182 [DOI] [PubMed] [Google Scholar]
- 2.Bergström R, Adami HO, Mohner M, Zatonski W, Storm H, Ekbom A, Tretli S, Teppo L, Akre O, Hakulinen T: Increase in testicular cancer incidence in six European countries: a birth cohort phenomenon. J Natl Cancer Inst 1996, 88:727-733 [DOI] [PubMed] [Google Scholar]
- 3.Skotheim RI, Lothe RA: The testicular germ cell tumour genome. APMIS 2003, 111:136-151 [DOI] [PubMed] [Google Scholar]
- 4.He WW, Sciavolino PJ, Wing J, Augustus M, Hudson P, Meissner PS, Curtis RT, Shell BK, Bostwick DG, Tindall DJ, Gelmann EP, Abate-Shen C, Carter KC: A novel human prostate-specific, androgen-regulated homeobox gene (NKX3.1) that maps to 8p21, a region frequently deleted in prostate cancer. Genomics 1997, 43:69-77 [DOI] [PubMed] [Google Scholar]
- 5.Prescott JL, Blok L, Tindall DJ: Isolation and androgen regulation of the human homeobox cDNA, NKX3.1. Prostate 1998, 35:71-80 [DOI] [PubMed] [Google Scholar]
- 6.Bowen C, Bubendorf L, Voeller HJ, Slack R, Willi N, Sauter G, Gasser TC, Koivisto P, Lack EE, Kononen J, Kallioniemi OP, Gelmann EP: Loss of NKX3.1 expression in human prostate cancers correlates with tumor progression. Cancer Res 2000, 60:6111-6115 [PubMed] [Google Scholar]
- 7.Bieberich CJ, Fujita K, He WW, Jay G: Prostate-specific and androgen-dependent expression of a novel homeobox gene. J Biol Chem 1996, 271:31779-31782 [DOI] [PubMed] [Google Scholar]
- 8.Korkmaz KS, Korkmaz CG, Ragnhildstveit E, Kizildag S, Pretlow TG, Saatcioglu F: Full-length cDNA sequence and genomic organization of human NKX3A: alternative forms and regulation by both androgens and estrogens. Gene 2000, 260:25-36 [DOI] [PubMed] [Google Scholar]
- 9.Abdulkadir SA, Magee JA, Peters TJ, Kaleem Z, Naughton CK, Humphrey PA, Milbrandt J: Conditional loss of Nkx3.1 in adult mice induces prostatic intraepithelial neoplasia. Mol Cell Biol 2002, 22:1495-1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim MJ, Bhatia-Gaur R, Banach-Petrosky WA, Desai N, Wang Y, Hayward SW, Cunha GR, Cardiff RD, Shen MM, Abate-Shen C: Nkx3.1 mutant mice recapitulate early stages of prostate carcinogenesis. Cancer Res 2002, 62:2999-3004 [PubMed] [Google Scholar]
- 11.Kim MJ, Cardiff RD, Desai N, Banach-Petrosky WA, Parsons R, Shen MM, Abate-Shen C: Cooperativity of Nkx3.1 and Pten loss of function in a mouse model of prostate carcinogenesis. Proc Natl Acad Sci USA 2002, 99:2884-2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magee JA, Abdulkadir SA, Milbrandt J: Haploinsufficiency at the NKX3.1 locus: a paradigm for stochastic, dosage-sensitive gene regulation during tumor initiation Cancer Cell 2003, 3:273-283 [DOI] [PubMed] [Google Scholar]
- 13.Skotheim RI, Abeler VM, Nesland JM, Fosså SD, Holm R, Wagner U, Florenes VA, Aass N, Kallioniemi O-P, Lothe RA: Candidate genes for testicular cancer evaluated by in situ protein expression analyses on tissue microarrays. Neoplasia 2003, in press [DOI] [PMC free article] [PubMed]
- 14.Mostofi FK, Sesterhenn IA: World Health Organization International Histological Classification of Tumours: Histological Typing of Testis Tumours. 1998. Springer-Verlag Berlin
- 15.Rørth M, Rajpert-De Meyts E, Andersson L, Dieckmann KP, Fosså SD, Grigor KM, Hendry WF, Herr HW, Looijenga LH, Oosterhuis JW, Skakkebæk NE: Carcinoma in situ in the testis. Scand J Urol Nephrol Suppl 2000, 205:166-186 [DOI] [PubMed] [Google Scholar]
- 16.Bhatia-Gaur R, Donjacour AA, Sciavolino PJ, Kim M, Desai N, Young P, Norton CR, Gridley T, Cardiff RD, Cunha GR, Abate-Shen C, Shen MM: Roles for Nkx3.1 in prostate development and cancer. Genes Dev 1999, 13:966-977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gelmann EP, Bowen C, Bubendorf L: Expression of NKX3.1 in normal and malignant tissues. The Prostate 2003, 55:111-117 [DOI] [PubMed] [Google Scholar]
- 18.Bova GS, Carter BS, Bussemakers MJ, Emi M, Fujiwara Y, Kyprianou N, Jacobs SC, Robinson JC, Epstein JI, Walsh PC: Homozygous deletion and frequent allelic loss of chromosome 8p22 loci in human prostate cancer. Cancer Res 1993, 53:3869-3873 [PubMed] [Google Scholar]
- 19.Voeller HJ, Augustus M, Madike V, Bova GS, Carter KC, Gelmann EP: Coding region of NKX3.1, a prostate-specific homeobox gene on 8p21, is not mutated in human prostate cancers. Cancer Res 1997, 57:4455-4459 [PubMed] [Google Scholar]
- 20.Ornstein DK, Cinquanta M, Weiler S, Duray PH, Emmert-Buck MR, Vocke CD, Linehan WM, Ferretti JA: Expression studies and mutational analysis of the androgen regulated homeobox gene NKX3.1 in benign and malignant prostate epithelium. J Urol 2001, 165:1329-1334 [PubMed] [Google Scholar]
- 21.Smith-Sørensen B, Lind GE, Skotheim RI, Fosså SD, Fodstad Ø, Stenwig A-E, Jakobsen KS, Lothe RA: Frequent promoter hypermethylation of the O6-methylguanine-DNA methyltransferase (MGMT) gene in testicular cancer. Oncogene 2002, 21:8878-8884 [DOI] [PubMed] [Google Scholar]
- 22.Koul S, Houldsworth J, Mansukhani MM, Donadio A, McKiernan JM, Reuter VE, Bosl GJ, Chaganti RS, Murty VV: Characteristic promoter hypermethylation signatures in male germ cell tumors. Mol Cancer 2002, 1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smiraglia DJ, Szymanska J, Kraggerud SM, Lothe RA, Peltomäki P, Plass C: Distinct epigenetic phenotypes in seminomatous and nonseminomatous testicular germ cell tumors. Oncogene 2002, 21:3909-3916 [DOI] [PubMed] [Google Scholar]
- 24.Honorio S, Agathanggelou A, Wernert N, Rothe M, Maher ER, Latif F: Frequent epigenetic inactivation of the RASSF1A tumour suppressor gene in testicular tumours and distinct methylation profiles of seminoma and nonseminoma testicular germ cell tumours. Oncogene 2003, 22:461-466 [DOI] [PubMed] [Google Scholar]
- 25.Schneider A, Brand T, Zweigerdt R, Arnold H: Targeted disruption of the Nkx3.1 gene in mice results in morphogenetic defects of minor salivary glands: parallels to glandular duct morphogenesis in prostate. Mech Dev 2000, 95:163-174 [DOI] [PubMed] [Google Scholar]
- 26.Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP: Pten is essential for embryonic development and tumour suppression. Nat Genet 1998, 19:348-355 [DOI] [PubMed] [Google Scholar]
- 27.Kimura T, Suzuki A, Fujita Y, Yomogida K, Lomeli H, Asada N, Ikeuchi M, Nagy A, Mak TW, Nakano T: Conditional loss of PTEN leads to testicular teratoma and enhances embryonic germ cell production. Development 2003, 130:1691-1700 [DOI] [PubMed] [Google Scholar]
- 28.Psiachou-Leonard E: PTEN mutation in an adolescent with synchronous - testicular and retroperitoneal - germ cell tumor and the Bannayan-Riley-Ruvalcaba syndrome. Oncogenomics 1–5-2002, poster abstract C-69