Abstract
Ground glass hepatocyte (GGH) represents a histological hallmark of chronic hepatitis B virus infection and contains surface antigens in the endoplasmic reticulum (ER). Several types of GGHs are recognized at different hepatitis B virus replicative stages. The recent identification of pre-S mutants from GGHs encourages us to investigate whether different GGHs may harbor specific mutants and exhibit differential biological activities. In this study, we applied laser capture microdissection to isolate specific GGHs from a total of 50 samples on eight resected liver specimens. The surface genes in two major types of GGHs were analyzed. Type I GGHs expressed an inclusion-like pattern of hepatitis B surface antigens and harbored mutants with deletions over pre-S1 region, whereas type II GGHs, distributed in clusters and emerged at late replicative phase, contained mutants with deletions over pre-S2 region that defines a cytotoxic T lymphocyte (CTL) immune epitope, and may represent an immune escape mutant. Transfection of pre-S mutants in Huh7 revealed decreased syntheses of middle and small S proteins with accumulation of large surface antigen in ER, which in turn led to the activation of ER stress response with differential activities for different mutants. This study therefore demonstrates that different GGHs may contain specific mutants and exhibit differential biological activities.
Heptatitis B virus (HBV) is a small DNA virus with a partially double-stranded genome of 3.2 kb in size. 1 The majority of HBV patients recover, although 10 to 20% of young children will become chronic carriers and have a high risk to develop cirrhosis and hepatocellular carcinoma. 2 A histopathological hallmark of chronic HBV infection is to recognize the characteristic, glassy or ground glass hepatocytes (GGHs) that represent hepatitis B surface antigen (HBsAg)-containing liver cells. 3-7 Ultrastructurally, GGH is characterized by an abundance of smooth endoplasmic reticulum (ER), among which HBsAg is accumulated. In the past years, many studies have attempted to correlate the expression patterns of HBV antigens with the replicative phases of chronic HBV infection in the liver. 8,9 The GGHs at different replicative stages of chronic HBV infection are different in morphology and distribution in the liver. 8-10 Two major types (types I and II) of GGHs have been recognized. Type I GGHs usually scatter sporadically in liver lobules and occur throughout the replicative phases. Typically, they have slightly eccentric nuclei with accumulation of ground glass substances or an inclusion-like expression of HBsAg in the cytoplasm. 3-10 Distinct from type I GGHs, type II GGHs usually emerge at late nonreplicative stage or in cirrhotic liver and are distributed in large clusters with a marginal expression of HBsAg. 9-11 Although the morphological difference between type I and type II GGHs is readily apparent, their virological and biological significance, however, remains to be elucidated.
Heptatitis B virus encodes three envelope proteins in the pre-S/S open reading frame that are named large, middle, and small (major) surface proteins. In the past years, many investigators strive to elucidate the components of surface antigens in GGHs at different replicative stages. By immunohistochemical studies, GGHs are consistently demonstrated to contain pre-S1 or large surface antigen. 11 The characteristic ground glass appearance of hepatocytes could be in vitro induced by the overproduction of large surface antigen in ER. 12,13 Transgenic mice that strongly overproduce large surface antigen can also form GGHs in the liver. 14 Although GGHs are consistently associated with large surface antigen, the molecular mechanism leading to the accumulation of large surface antigen and the formation of ground glass appearance remain to be clarified. Dienes and colleagues 15 speculate that the accumulation of large surface antigen is associated with HBV integration that may increase the expression of pre-S1 by a highly active cellular promoter. Xu and colleagues 16,17 proposed that the mutation over S promoter is probably one contributing cause of GGHs during chronic HBV infection, because the pre-S region involves the binding sites for transcriptional factors such as NF-1 and SP1. The deletion over these promoter regions will totally or partially remove the binding sites and affect the expression of middle and small surface proteins, 18-20 resulting in intracellular accumulation of pre-S1 or large surface protein. 21,22
The direct confirmation of the existence of pre-S mutants in GGHs in liver tissues, however, is difficult because of the technological limitation to specifically isolate GGHs. In a previous study using manual dissection method, we identified a pre-S2 deletion mutant in cirrhotic nodules that contained large clusters of type II GGHs. 18 This interesting finding encourages us to explore in depth the molecular and biological features of different types of GGHs. In this study, we used a laser capture microdissection (LCM) method to selectively isolate type I and type II GGHs and sampled for molecular analysis of the pre-S gene. Interestingly, we demonstrated for the first time that different types of GGHs consistently harbored specific pre-S mutants in ER, resulting in the activation of ER stress signals such as endoplasmic reticulum resident kinase (PERK), c-jun amino-terminal kinase, glucose-regulated proteins (GRP) 78, and GRP94, 23,24 especially in type I GGHs.
Materials and Methods
Liver Histology, Immunohistochemical Staining, and Monoclonal Antibodies
Liver histology was performed on eight surgically resected specimens from hepatocellular carcinoma patients, in which six were HBsAg-seropositive and two were HBsAg-seronegative. The nontumorous liver tissues were sampled for studies. For immunohistochemical staining, 5-μm tissue sections or transfected cells grown on chamber slides were fixed with ice-cold acetone, and immunostained with primary antibodies. A biotinylated anti-mouse secondary antibody (DAKO Corp., Carpinteria, CA) was then applied. The slides were then incubated with peroxidase-conjugated streptavidin, chromogenized by 3-amino-9-ethylcarbazol, and counterstained with Mayer’s hematoxylin. The monoclonal antibodies of surface antigens used were as follows: anti-pre-S1, MA18/7, a generous gift from Professor Wolfram H. Gerlich, 25 (Institute of Medical Virology, Justus Liebig University Giessen, Giessen, Germany) that binds specifically to gp42 and p39 of large surface proteins; anti-pre-S2 (S26; Chemicon International, Inc., Temecula, CA), a mouse monoclonal antibody that specifically recognizes gp36 and p33 of middle surface protein, and anti-HBs antibody, monoclonal mouse anti-HBsAg against the “a” determinant of small surface antigen (M3506, DAKO) that can identify gp27 and p24 of small surface proteins.
LCM of GGHs Expressing Different Patterns of HBsAg
Tissue sections were first immunostained by anti-HBs (M3506), dehydrated with ethanol and xylene, and subsequently left air-dried. Different types of GGHs were then selectively isolated and collected by the Arcturus LCM system (Arcturus Engineering Inc., Mountain View, CA). For type I GGHs, a total of 20 hepatocytes were LCM-isolated for analysis. Type II GGHs usually clustered in groups and could be easily isolated.
Sample Preparation, Polymerase Chain Reaction (PCR) Amplification, and Sequencing of the Pre-S and Major S Genes
The LCM-harvested samples were digested in 30 μl of digestion buffer containing 0.04% proteinase K, 10 mmol/L Tris-HCl (pH 8.0), 1 mmol/L ethylenediaminetetraacetic acid, and 1% Tween 20. The reaction was performed at 37°C overnight followed by incubation at 95°C for 8 minutes to inactivate proteinase K. Two sets of primers were designed to amplify the pre-S and major S gene: the pre-S sense primer 5′-GCGGGTCACCATATTCTTGG-3′ (nucleotides 2818 ∼ 2837); the pre-S anti-sense primer 5′-GAGTCTAGACTCTGCGGTAT-3′ (nucleotides 236 ∼ 255); the small S sense primer 5′-CATCTCGTCAATCTCCGCGA-3′ (nucleotides 112 ∼ 131); the small S anti-sense primer 5′-TCCTGTGGCAAAGTTCCCCA-3′ (nucleotides 898 ∼ 927). The PCR products will then cover the whole S gene. The BstEII and XbaI sites used for subcloning are underlined.
In Vitro Expression, Synthesis, and Secretion of the Cloned Pre-S Mutants in Huh7 Cell Line
Construction of the Expression Plasmids of Pre-S Mutants
The pre-S mutants were constructed by using plasmid p(3A)SAg 18,22 as a template to amplify the representative types of deletions. After double digestion by restriction endonucleases BstEII and XbaI, the pre-S PCR fragments were subcloned into p(3A)SAg. For the cassette exchange experiment, the digested products were substituted for the BstEII-XbaI fragment of surface gene carried on p(3A)SAg. The detailed procedures have been described in detail previously. 18,22 The pre-S deletion mutants were amplified by using pairs of primers designated as pre-S sense primer and Δ1 anti-sense primer (5′-AATTGTTGA-CACTGTTGCTCCCACTCCTACTTGGT-3′); pre-S anti-sense primer and Δ1 sense primer (5′-GTAGGAGTGGGA-GCAACAGTGTCAACA-ATTCCTCC-3′); pre-S sense primer and Δ2 anti-sense primer (5′-CTGGAGCCACCAGCAGAATTCCA-CTGTATGGCCTG-3′); pre-S anti-sense primer and Δ2 sense primer (5′-CCATAC-AGTGGAATTCTGCTGGTGGCTCCAGTTCAG-3′). After PCR amplification, the products of both reactions were gel-purified and applied to further amplification by using pre-S sense and anti-sense primers. The resulting PCR products were further cloned into p(3A)SAg.
Western Blot Analyses of Surface Proteins in Huh7 Cells
The cells were first transiently transfected with FuGENE6 (Boehringer Mannheim GmbH, Mannheim, Germany) for 48 hours. Protein lysates were harvested by using freezing and thawing method. Twenty-five μg of total proteins were resolved on polyacrylamide gels and transferred to polyvinylidene difluoride membranes. The membrane was incubated with the primary antibody and then the second antibody conjugated to horseradish peroxidase. The proteins were detected by using ECL chemiluminescence kit (Perkin-Elmer Life Science, Boston, MA). The primary antibodies used for Western blot were as follows: anti-pre-S1, MA18/7; anti-pre-S2, S26; anti-HBs, A10F1, kindly provided by Professor Shern-Chun Lee (Institute of Molecular Medicine, National Taiwan University Medical College, Taiwan).
Extracellular Secretion of Mutant Surface Proteins in Culture Supernatants
For the detection of surface proteins in culture supernatants, the supernatants were collected 48 hours after transfection. Cell debris in the medium was removed by centrifugation. The proteins in the culture supernatant were collected and concentrated by the phenol-ether precipitation for a quantitative recovery from culture supernatants. 26 The Huh7 cells were then transfected with p(3A)SAg and a β-galactosidase control plasmid. The amounts of surface proteins in culture supernatants were quantified by using the enzyme-linked immunosorbent assay (ELISA) kit (Abott, Abott Park, IL). The supernatants were diluted to keep the amount of HBsAg within the linear range of the assay and the result values were then normalized to β-galactosidase activity. All transfections and quantitations were repeated independently at least three times.
Pon-A-Inducible Expression of the Cloned Pre-S Mutants
Construction of an Inducible Gene Expression System
The pre-S/S region (BstEII/EcoRV fragment) of the previous constructs were blunt-end ligated into the EcoRV predigested pEGSH vector, which was an insect hormone analog system (Stratagene, La Jolla, CA) containing ponasterone A (pon-A)-controlling elements. These constructs were then co-transfected with vector pERV3 into Huh7 cells, and stable clones were selected by using G418 and hygromycin B. The expression levels of mutant surface antigens were assayed by reverse transcriptase (RT)-PCR with the defined pre-S primers 20 hours after pon-A induction.
Assay of ER Stress Signals
ER Stress Signals on Inducible Huh7 Cells
Total RNAs were extracted from cells under 8 μmol/L of pon-A induction with REzol C&T (PROtech Technology, Taipei, Taiwan). Cells treated with 2 mg/ml of Brefeldin A (Sigma) as positive control of ER stress induction. Two μg of total RNA were analyzed through a 1% MOPS/formaldehyde gel (1× MOPS, 0.45 mol/L formaldehyde), followed by transfer to nylon membrane. For hybridization, biotin-labeled DNA probes were used for detection. The probe for pre-S1 gene was PCR product-amplified from the p(3A)SAg plasmid. The probes for ER stress signal genes GRP78 and GRP94 were RT-PCR products amplified by specific primers as follows: GRP78 sense primer 5′-TCCTATGTCGCCTTCACTCC-3′, GRP78 anti-sense primer 5′-GTTTTGCAGCTTTTCTCCAATC-3′, the GRP94 sense primer 5′-TCTCTCGTGTGTTTCTTCTTTC-3′, GRP94 anti-sense primer 5′-GTTTGCAGTTTTCTCCAATC-3′. These gene transcripts were detected by using the RNADetector Northern blotting Kit (Kirkeggard & Perry Laboratories, Inc., Gaithersburg MD). The expression levels of genes were expressed and normalized by that of GAPDH mRNA. For detection of PERK expression and JNK phosphorylation, polyclonal goat anti-PERK antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and mouse monoclonal phospho-JNK antibody (Santa Cruz) were used. The actin expression, as an internal control of protein loading, was detected by mouse monoclonal antibody (Santa Cruz).
ER Stress Signals on Hepatocytes in Liver Tissue
To analyze the ER stress, the expression of GRP78 was performed on the LCM-harvested type I and type II GGHs and HBs-negative hepatocytes. RNA extraction from the LCM-harvested hepatocytes was done according to the manufacturer’s instruction (Arcturus). The expression level of GRP78 was assayed by RT-PCR as described above.
Results
Liver Histology and HBsAg Immunostaining of GGHs
Table 1 ▶ summarizes the status of HBsAg, liver histology, and the presence of GGHs in the eight surgically resected specimens. The nonneoplastic livers showed chronic active hepatitis in two cases, chronic active hepatitis with early cirrhosis in three cases, and inactive cirrhosis in three cases.
Table 1.
Liver Histology and Pre-S Mutants Isolated from Laser Capture Microdissection (LCM)-Harvested Liver Samples in Six HBsAg-Seropositive and Two Seronegative Cases with Hepatocellular Carcinoma
| Case no. | Liver histology | Serum HBsAg | Types of GGHs | No. of samples | No. of clones | Types of pre-S deletion mutants |
|---|---|---|---|---|---|---|
| 1 | CAH | − | ND | 3 | 0 | |
| 2 | Cirrhosis | − | ND | 3 | 0 | |
| 3 | Early cirrhosis | + | N | 3 | 1 | WT |
| I | 3 | 4 | Δ3040∼3111 (x2), Δ3041∼3097, WT | |||
| II | 2 | 3 | Δ3218∼53, Δ4∼57, WT | |||
| 4 | Early cirrhosis | + | N | 5 | 2 | WT |
| I | 1 | 2 | Δ3040∼3111, WT | |||
| II | 2 | 3 | Δ4∼57(x2), WT | |||
| 5 | Cirrhosis | + | N | 4 | 2 | WT |
| I | 3 | 4 | Δ3040∼3111 (x2), Δ2950∼3090, WT | |||
| II | 2 | 3 | Δ2∼55 (x2), WT | |||
| 6 | Cirrhosis | + | N | 5 | 2 | WT |
| I | 2 | 2 | Δ3040∼3111, WT | |||
| II | 3 | 4 | Δ2∼55(x2), Δ3∼56, WT | |||
| 7 | CAH | + | N | 4 | 2 | WT |
| I | 2 | 1 | Δ3040∼3111, WT | |||
| 8 | Early cirrhosis | + | N | 3 | 1 | WT |
The samples were taken from nontumorous parts of the liver specimens. The numbers and types of pre-S clones identified were shown.
CAH, chronic active hepatitis; ND, not detectable; GGH, ground glass hepatocyte; N, negative for GGH, I, type I GGH; II, type II GGH; WT, wild-type pre-S gene.
Morphologically, GGHs were characterized by a homogeneous glassy cytoplasm comparable to the window glass with a granular surface appearance. Type I GGH had an eccentric nucleus and a finely granular, faintly eosinophilic inclusion-like appearance (Figure 1A) ▶ and could be identified in five of six HBsAg-seropositive cases. Type I GGHs usually scattered singly throughout the liver lobules. Type II GGHs were characterized by the clustering distribution of GGHs and the submembranous or marginal localization of glassy substance (Figure 1B) ▶ and were recognized in four of six HBsAg-seropositive cases.
Figure 1.

The histology and expression patterns of surface antigens in two distinct types (type I and type II) of GGHs. Sequential sections for H&E histology and immunostaining studies on the nontumorous part of a HBsAg-positive liver specimen. Histology of type I (A) and type II (B) GGHs. Type I GGHs (arrows) have an inclusion-like glassy appearance and usually scatter singly, whereas type II GGHs usually cluster in groups. Immunostaining with anti-pre-S1 antibody (C and D) revealed an intense staining for both types of GGHs. The immunostaining of anti-pre-S2 antibody revealed a weak staining for type I GGHs (E) but negligible or absent for type II GGHs (F). The immunostaining intensity for S was strong for both types of GGHs (G and H) and could delineate better the different morphology of GGHs. The tissue distributions of both types of GGHs in liver of case 3 (I) and case 4 (J) were shown. Original magnifications: ×400 (A–H); ×100 (I, J).
Immunohistochemically, type I GGHs were immunostained strongly positive for pre-S1 or large S (Figure 1C) ▶ and small or major S (Figure 1G) ▶ . The immunostaining intensity for middle S antigen is relatively weak (Figure 1E) ▶ . Type II GGHs showed a marginal expression pattern of pre-S1 and small surface proteins (Figure 1, D and H) ▶ . Notably, the middle S or pre-S2 protein was either absent or only faintly detectable in type II GGH (Figure 1F) ▶ . The co-existence of two types of GGHs on liver samples of case 3 (Figure 1I) ▶ and case 4 (Figure 1J) ▶ were shown. In this study, type II GGHs consistently occurred in large clusters with enhanced accumulation of surface proteins at the periphery or margin of hepatocytes, much distinct from that of type I GGHs. No GGHs could be detected in one HBsAg-seropositive (case 8) and two seronegative specimens (cases 1 and 2).
Type I and Type II GGHs Contained Different Pre-S Mutants with Deletions on Pre-S1 and Pre-S2 Regions, Respectively
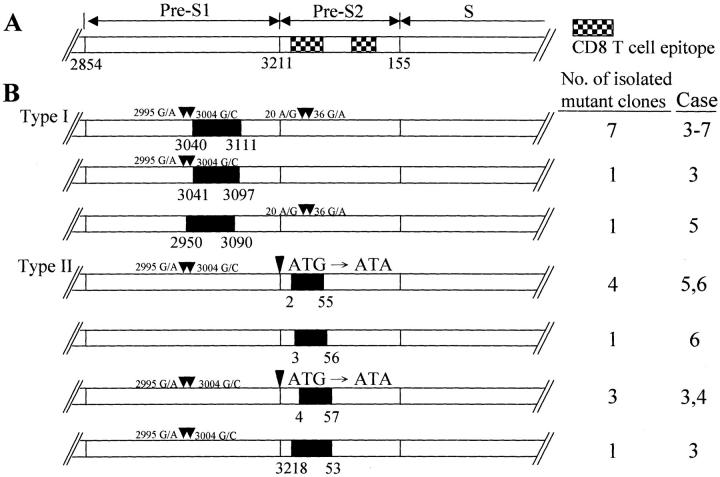
As shown in Table 1 ▶ , a total of 50 samples were LCM-harvested from the eight specimens: 11 from type I GGHs (cases 3 to 7), 9 from type II GGHs (cases 3 to 6), and 24 from hepatocytes without HBsAg in the six HBsAg-seropositive cases. An additional six samples from HBsAg-seronegative cases (cases 1 and 2) were included for controls. The pre-S sequences were amplified and sequenced by the defined primers. In the 11 samples of type I GGHs, 13 distinct bands were identified. Nine of them harbored deletions over pre-S1 regions (3040 to 3111 in seven, 3041 to 3097 in one, 2950 to 3090 in one) (Figure 2) ▶ , while four bands belonged to wild type. In the nine samples obtained from type II GGHs, 13 bands were identified. Nine of them revealed deletions over pre-S2 regions (3218 to 53 in one, 2 to 55 in four, 3 to 56 in one, and 4 to 57 in three). Among the deletion mutants at nucleotides 2 to 55 and nucleotides 4 to 57, an additional point mutation (ATG/ATA) was observed at the start codon of middle surface gene. In the 24 samples harvested from hepatocytes without the expression of HBsAg in the six HBsAg-seropositive specimens (cases 3 to 8), 10 bands could be detected and all 10 bands belonged to wild-type pre-S. It is interesting to note that wild-type pre-S gene co-existed with deletion mutants in eight samples, either in type I or type II GGHs. Notably, multiple point mutations over the pre-S gene were noted in wild-type bands without deletions (Table 1) ▶ . Three mutation hotspots on S region at codon 45 (S→T), codon 113 (T→A), and codon 143 (A→T) were detected in all samples (data not shown). No PCR products could be detected in the six HBsAg-seronegative samples from cases 1 and 2.
Figure 2.
The pre-S mutant clones obtained from laser capture dissection-harvested liver samples. A: Maps of wild-type S gene. The number shown above the map indicates the nucleotide site of the defined genome. The hatched boxes indicate two CD8 T-cell epitopes. B: The pre-S deletion mutants isolated from type I and type II GGHs are shown. Three different deletion clones were isolated from type I GGHs in cases 3 to 7 (nucleotides 3040 to 3111, 3041 to 3097, 2950 to 3090). Most of mutant clones isolated from type I GGHs had pre-S1 deletion, mainly over nucleotides 3040 to 3111. Four deletion mutants in pre-S2 region were isolated from type II GGHs. The deletion regions ranged from nucleotides 2 to 55, 3 to 56, 4 to 57, and 3218 to 53 in pre-S2 region with or without point mutation (arrowhead) (ATG to ATA) at the start codon of middle S gene. Type I deletion mutant (nucleotides 3040 to 3111, Δ1) and type II deletion mutant (nucleotides 4 to 57, Δ2) were further subcloned into p(3A)SAg expression plasmid as described in Materials and Methods. Several mutation hotspots were shown in the map (arrow). The solid boxes indicate the deletion sites.
Synthesis, Expression, and Secretion of the Cloned Pre-S Mutants in Huh7
Construction of the Cloned Mutants
The plasmid p(3A)SAg, which harbors a large S promoter, was used as an expression vector. Two deletions over pre-S1 region (Δ1, nucleotides 3040 to 3111) and pre-S2 region (Δ2, nucleotides 2 to 55) were further cloned and analyzed (Figure 3A) ▶ .
Figure 3.
Synthesis and secretion of surface proteins by wild-type and mutant constructs on Huh7 cells. A: The representative constructs of wild-type (WT), Δ1, and Δ2 deletion. B: Western blot analysis of the intracellular large, middle, and small surface proteins. Protein lysates were harvested in 48 hours after transfection and resolved on SDS-PAGE followed by antibody hybridization. The top panels labeled by gp42/p39 represent glycosylated and unglycosylated large surface proteins detected by anti-pre-S1 antibody, MA18/7. The relative amount of large surface protein for pre-S mutants is ∼40% higher than the wild type. The middle panels labeled by gp36/gp33 represent glycosylated, middle surface proteins detected by anti-pre-S2 antibody. The bottom panels represent small surface proteins (gp27/p24). The relative amounts of each study were shown below. C: Relative extracellular secretion of HBsAg by the cloned wild-type and mutant constructs. The culture medium was collected 48 hours after transfection and the supernatants containing HBsAg were assayed by enzyme-linked immunosorbent assay test. The relative amounts of HBsAg secretion between different expression clones are shown.
Western Blot Analysis of Surface Protein Synthesis by the Cloned Mutants
The synthesis of surface proteins by the cloned mutants was further analyzed by Western blot analysis (Figure 3B) ▶ . Two distinct bands could be identified by anti-pre-S1 antibody, corresponding to the unglycosylated 39-kd and glycosylated 42-kd major surface proteins (p39 and gp42). The intracellular expression of large surface protein was ∼40% higher in levels in both deletion mutants than that of the wild-type clone whereas the molecular sizes were smaller for Δ1 and Δ2 mutants because of the deletion of pre-S regions. An 80% decrease of pre-S2 or middle surface protein was observed for Δ1 as assayed by pre-S2-specific antibody, whereas no middle surface protein could be detected for Δ2-transfected cells because of the presence of a point mutation at the start codon of middle S gene. With A10F1 antibody, the small surface protein could be recognized as two distinct bands as p24 and gp27. A 16% decrease for Δ1 and a 25% decrease for Δ2 of small surface protein expression were observed. The data represented a negative effect of pre-S deletions on the synthesis of small surface protein.
The Secretion of Surface Proteins Was Affected by the Pre-S Deletions
The secretion of surface proteins was observed to decrease 48 hours after transfection. We monitored the HBsAg secretion by enzyme-linked immunosorbent assay assay. As shown in Figure 3C ▶ , Δ2 showed a 50% decrease of HBsAg secretion. This phenomenon could result from the decrease of both middle and small surface proteins as shown in Western blot assay. Construct Δ1 also revealed a 22% decrease of HBsAg secretion, probably because of the shortage of the synthesis and secretion of middle surface protein. Such results were consistent in three repeated experiments.
Based on the intracellular synthesis and extracellular secretion of surface antigens by the mutant pre-S genes, it is concluded that the deletions over pre-S regions decreased the synthesis and secretion of small and middle surface proteins, leading to the intracellular retention of large surface protein because the secretion of large surface antigens requires an excess of small surface antigen in normal conditions of HBV assembly and secretion.
The Pre-S1 and Pre-S2 Mutant Transfectants Showed a Similar Pattern to Type I and Type II GGHs, Respectively
Cells transfected with wild-type constructs showed a diffuse cytoplasmic expression of large, middle, and small surface proteins (Figure 4, A to C) ▶ . A relatively strong intensity was observed for Δ1 mutant-transfected cells and the distribution of surface proteins was globular or like an inclusion body in the cytoplasm (Figure 4, D to F) ▶ , simulating the expression pattern of type I GGH in liver tissues (Figure 1, C and G) ▶ . The immunostaining intensity for pre-S2 was relatively weak, as compared to that of anti-pre-S1 and anti-S antibodies in Δ1-transfected cells. The results for Δ2-transfected cells were much different from that of wild-type and Δ1 constructs. The expressions of pre-S1 and small surface antigens showed a blot-like or marginal expression pattern (Figure 4, G to I) ▶ . No pre-S2 expression was observed for Δ2 constructs. The results are consistent with the data in Western blot analysis described above. By immunostaining of the ER marker calregulin, the surface proteins were confirmed to co-localize with calregulin in ER, similar to that observed in liver tissues (data not shown).
Figure 4.

Immunohistochemical staining of pre-S1, pre-S2, and S proteins in wild-type and mutant pre-S-transfected cell lines. Huh7 cells were first seeded on chamber slides and incubated at 37°C overnight. After transient transfection of p(3A)SAg wild-type (WT), Δ1, and Δ2 plasmids, the slides were stained with monoclonal antibodies against pre-S1 (A, D, G), pre-S2 (B, E, H), and small S (C, F, I). Wild-type construct expressed a diffused pattern for large, middle, and small surface proteins (A–C), whereas Δ1 construct expressed a high intensity of large and small surface proteins but a relatively weak expression of middle surface protein, which is similar to the observation on tissue studies (Figure 1E) ▶ . The Δ2 mutant construct expressed a distinct blot-like pattern with enhanced expression at the margin or periphery of the hepatocytes. No middle surface protein was detectable (H). Original magnifications, ×400.
ER Stress Responses Induced by the Pre-S Mutants
The expression of mutant pre-S antigens and ER stress signals in the pon-A-inducible system was analyzed by RT-PCR and Northern blot hybridization. Pon-A induced the surface protein expression in a dosage-dependent manner (data not shown). The treatment with pon-A at 8 μmol/L induced a subpeak level of surface gene expression comparable in intensity to that in liver tissues and it was therefore used throughout this study (Figure 5A) ▶ . Cells treated with 2 μg/ml of Brefeldin A were used as a positive control for ER stress induction. The ER stress signal gene GRP78 was highly induced with Brefeldin A treatment, whereas the induction of GRP94 was relatively mild (Figure 5A) ▶ . As compared to wild type, Δ1 showed an obviously enhanced expression level of GRP78 and GRP94 (sixfold and twofold, respectively). The Δ2 mutant showed a comparable but slightly weak level of GRP78 as compared to Δ1, whereas the induction of GRP94 was much weaker.
Figure 5.
Inducible expression of pre-S mutants and ER stress signals. A: Northern blot analysis of two ER stress genes GRP78 and GRP94 initiated by the wild-type and mutant pre-S genes. Cells were treated with 8 μmol/L of ponA for 20 hours, RNAs were then harvested for Northern analysis. Transcriptional activation of ER stress genes (GRP78 and GRP94) by mutant pre-S genes was observed. Cells treated with 2 μg/ml of Brefeldin A were used as positive control of ER stress induction. Ctrl represents vector control. GAPDH was used as the internal loading control. The level of gene expression was measured by a densitometer and is shown at the right. B: Western blot analysis to demonstrate the expression of PERK and activation of JNK by wild-type and mutant pre-S constructs. Large surface proteins (LHBs) were detected by MA18/7 antibody. PERK expression was analyzed by goat polyclonal anti-PERK antibody. Activation of JNK was determined by the detection of phosphorylated JNK (p-JNK). Actin was used as the internal control of protein loads. The protein expression was quantitated by a densitometer and is shown at the right. A dramatic increase of the JNK activation was demonstrated for both pre-S mutants, particularly for pre-S1. C: GRP78 expression on GGHs. LCM-harvested samples were next used for RT-PCR analysis of GRP78 gene expression. The GRP78 expression levels were normalized with actin expression and compared to GRP78 expression of Huh7 cells.
We next examined the possible activation of PERK and JNK by the pre-S mutants using Western blot assay (Figure 5B) ▶ . Both Δ1 and Δ2 showed enhanced, but not significant, expression of PERK as compared to the control and wild-type constructs. However, the induction of JNK activation by pre-S mutants, especially for pre-S1, is particularly remarkable.
To compare ER stress signals in type I and type II GGHs in liver tissues, we next analyzed the GRP78 gene expression level in GGHs. Of all the cases we have checked from the LCM-harvested samples, the GRP78 expression was significantly higher in both type I and type II GGHs as compared to HBs-negative hepatocytes (Figure 5C) ▶ . These data indicated that GGHs in liver tissues also revealed increased ER stress.
Discussion
Recent studies have demonstrated the prevalence of pre-S mutants in patients with chronic HBV infection and hepatocellular carcinoma. 18,22,27,28 With the application of LCM, we demonstrated for the first time that different type GGHs harbor specific pre-S deletion mutants. Type I GGHs consistently harbored mutant large surface proteins with deletions over the pre-S1 region (Δ1), whereas type II GGHs contained mutants with deletions over the pre-S2 region (Δ2). These results are consistent, although with minor variations, in 50 samples obtained from eight different specimens. It is unlikely to be nonspecific or artificial. The observation was further supported by the in vitro transfection studies in Huh7, which revealed an expression pattern simulating that of type I and type II GGHs in liver tissues. Because there exists more than two types of GGHs, 10 additional studies are needed to clarify whether all types of GGHs contain specific pre-S or S mutants. In consistence with previous studies, 16 the surface proteins in both types of pre-S mutants are localized in ER as observed by confocal microscopy, although their morphology is remarkably different.
The pre-S deletion mutants have been shown to be competent for HBV replication although some of them may lose the ability of viral secretion and subsequently result in the accumulation of replicative intermediates in the cytoplasm. 22,28,29 Our previous demonstration of pre-S mutants in serum of patients with chronic HBV infection supports the competence of these mutants in HBV replication and secretion. 22 The sequence between amino acids 3 and 77 in the pre-S1 region is involved in virion secretion in the infection step. 30 Because the deletion site of Δ1 (amino acids 63 to 86) partially overlaps with the defined region, type I GGHs will have a mild defect of HBsAg secretion. Besides, the pre-S region involves the binding sites for transcriptional factors. The deletion of these regions will therefore totally or partially remove binding sites of these transcription factors and affect the synthesis of middle and small surface proteins. 19,20 Moreover, the Δ2 mutant combines a point mutation over the pre-S2 start codon (ATG→ATA), which will abrogate the synthesis of middle surface protein. The pre-S deletion mutants will therefore lead to a decreased synthesis of middle and small surface proteins, as demonstrated in this study. Because the secretion of large surface protein can only be accomplished in the presence of excess amounts of small and middle surface proteins, 16,17 the reduced synthesis of middle and small surface proteins in Δ1 and Δ2 will reasonably result in the entrapping of large surface protein in the cytoplasm and result in the formation of GGHs. 31,32
The occurrence of different pre-S mutants or GGHs at different replicative stages may represent the emergence of immune escape mutants during the longtime evolution of chronic HBV infection. Various viral and bacterial pathogens can manipulate ER function to escape immune surveillance. Adenovirus E3-19K protein resides in the ER, where it binds to MHC class I molecules, thereby preventing their transport to the cell surface and inducing an ER overload response. 33 Human herpesvirus gene products can block peptide translocation by TAP on ER membrane, whereas Epstein-Barr virus protein EBNA 1 can block peptide degradation by the proteasome. 34 Therefore, the modulation of ER function may become an important strategy for the immune escape in virus infection. 34,35 The ER retention of pre-S mutants may represent a potential mechanism of immune evasion adopted by HBV proteins. Besides the ER retention mechanism described above, the deletion over the pre-S2 region in type II GGH may lose the HLA-restricted cytotoxic T cell epitope and constitutes one alternative mechanism of immune escape in persons with specific HLA haplotypes. 36-38 It is interesting to note that the GGHs harboring pre-S2 deletion mutants consistently occur at the late replicative phase. By this sense, the emergence of pre-S2 mutant in type II GGHs may escape the immune surveillance and persist to replicate in cirrhotic lesions during chronic HBV infection. 39-41
Besides the interference of immune surveillance, the ER retention of mutant pre-S proteins may induce unfolded protein response and activate ER stress and other cellular signals, including apoptosis, 42 activation of transcription factor nuclear factor-κB, 43 and lipogenic genes. 44 Some ER transmembrane protein kinases (IRE1 and PERK) have been implicated as proximal effectors of the mammalian unfolded protein response, 45,46 protecting cells from stress-induced apoptosis. Recent studies also show that the unfolded proteins in ER could activate JNKs by a mammalian homologue of yeast IRE1 47 . In this study, we demonstrated the induction of ER stress by the retention of mutant pre-S proteins in ER, as revealed by the enhanced expression and activation of GRP78/94, PERK, and JNK in a pon-A inducible expression system. To further confirm the ER stress condition did exist in GGHs, we have checked GRP78 mRNA expression on both types of GGHs in our liver samples. It revealed again that the expression level of GRP78 was increased in GGHs as compared to HBs-negative hepatocytes. Of particular importance is the dramatic activation of the ER stress signal JNK by pre-S mutants, much stronger for pre-S mutants than controls or wild-type surface protein. Whether the activation of JNK by pre-S mutants will lead to apoptosis or other biological effects remains to be clarified.
It is interesting to note, however, that there exist different regulations of ER stress signals by specific pre-S mutants, suggesting that different type GGHs may exhibit differential biological activities. The existence of differential biological activities of these pre-S mutants can be further reflected by the different distribution pattern of GGHs in liver tissues at different replicative stages. Recently, the large surface protein and a C-terminally truncated middle surface protein (MHBst) have been recognized as transactivators that share the same mechanism for transcriptional activation. 48,49 This group of activators can trigger a protein kinase C (PKC)-dependent activation of the c-Raf-1/MAP1-kinase signal cascade, resulting in an activation of transcription factors such as AP-1 and nuclear factor-κB. The functional activity of these activators is dependent on the cytoplasmic orientation of the pre-S2 region, which is also related to their intracellular retention. 50 Whether the pre-S deletion mutants identified in this study may affect the protein folding and induce the same signaling pathway remains to be further characterized.
In conclusion, the virological and biological features of GGHs in chronic HBV infection are unraveled in this study. Different types of GGHs contain specific pre-S mutants that may represent an evolution of the immune escape variants with the replicative stages of chronic HBV infection. The retention of pre-S mutants in ER may disrupt the immune surveillance and in turn initiate ER stress signals, leading to differential biological effects. The relationship between GGHs, particularly for type II GGHs, and HBV-related tumorigenesis remains to be investigated.
Footnotes
Address reprint requests to Dr. Ih-Jen Su, Division of Clinical Research, NHRI, 12C Ward, National Cheng Kung University Hospital, 138, Sheng-Li Rd., Tainan, Taiwan. E-mail: suihjen@nhri.org.tw.
Supported by grants from the National Science Council and National Health Research Institutes, Taiwan (to I.-J. Su).
References
- 1.Lee WM: Hepatitis B virus infection. N Engl J Med 1997, 337:1733-1745 [DOI] [PubMed] [Google Scholar]
- 2.Beasley RP: Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer 1988, 61:1942-1956 [DOI] [PubMed] [Google Scholar]
- 3.Shikata T: Australia antigen in liver tissue—an immunofluorescent and immunoelectron microscopic study. Jpn J Exp Med 1973, 43:231-245 [PubMed] [Google Scholar]
- 4.Hadziyannis S, Gerber MA, Vissoulis C, Popper H: Cytoplasmic hepatitis B antigen in “ground-glass” hepatocytes of carriers. Arch Pathol 1973, 96:327-330 [PubMed] [Google Scholar]
- 5.Popper H: The ground glass hepatocyte as a diagnostic hint. Hum Pathol 1975, 6:517-520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gudat F, Bianchi L, Sonnabend W, Thiel G, Aenishaenslin W, Stalder GA: Pattern of core and surface expression in liver tissue reflects state of specific immune response in hepatitis B. Lab Invest 1975, 32:1-9 [PubMed] [Google Scholar]
- 7.Bianchi L: Liver biopsy interpretation in hepatitis. Part I: Presentation of critical morphologic features used in diagnosis (glossary). Pathol Res Pract 1983, 178:2-19 [DOI] [PubMed] [Google Scholar]
- 8.Su IJ, Kuo TT, Liaw YF: Hepatocyte hepatitis B surface antigen. Diagnostic evaluation of patients with clinically acute hepatitis B surface antigen-positive hepatitis. Arch Pathol Lab Med 1985, 109:400-402 [PubMed] [Google Scholar]
- 9.Su IJ, Lai MY, Hsu HC, Chen DS, Yang PM, Chuang SM, Sung JL: Diverse virological, histopathological and prognostic implications of seroconversion from hepatitis B e antigen to anti-HBe in chronic hepatitis B virus infection. J Hepatol 1986, 3:182-189 [DOI] [PubMed] [Google Scholar]
- 10.Hsu HC, Lai MY, Su IJ, Chen DS, Chang MH, Yang PM, Wu CY, Hsieh HC: Correlation of hepatocyte HBsAg expression with virus replication and liver pathology. Hepatology 1988, 8:749-754 [DOI] [PubMed] [Google Scholar]
- 11.Thung SN, Gerber MA, Kasambalides EJ, Gilja BK, Keh W, Gerlich WH: Demonstration of pre-S polypeptides of hepatitis B virus in infected livers. Hepatology 1986, 6:1315-1318 [DOI] [PubMed] [Google Scholar]
- 12.Chisari FV, Filippi P, McLachlan A, Milich DR, Riggs M, Lee S, Palmiter RD, Pinkert CA, Brinster RL: Expression of hepatitis B virus large envelope polypeptide inhibits hepatitis B surface antigen secretion in transgenic mice. J Virol 1986, 60:880-887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chisari FV, Klopchin K, Moriyama T, Pasquinelli C, Dunsford HA, Sell S, Pinkert CA, Brinster RL, Palmiter RD: Molecular pathogenesis of hepatocellular carcinoma in hepatitis B virus transgenic mice. Cell 1989, 59:1145-1156 [DOI] [PubMed] [Google Scholar]
- 14.Huang SN, Chisari FV: Strong, sustained hepatocellular proliferation precedes hepatocarcinogenesis in hepatitis B surface antigen transgenic mice. Hepatology 1995, 21:620-626 [PubMed] [Google Scholar]
- 15.Dienes HP, Gerlich WH, Worsdorfer M, Gerken G, Bianchi L, Hess G, Meyer zum Buschenfelde K.H.: Hepatic expression patterns of the large and middle hepatitis B virus surface proteins in viremic and nonviremic chronic hepatitis B. Gastroenterology 1990, 98:1017-1023 [DOI] [PubMed] [Google Scholar]
- 16.Xu Z, Yen TS: Intracellular retention of surface protein by a hepatitis B virus mutant that releases virion particles. J Virol 1996, 70:133-140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Z, Jensen G, Yen TS: Activation of hepatitis B virus S promoter by the viral large surface protein via induction of stress in the endoplasmic reticulum. J Virol 1997, 71:7387-7392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan YF, Lu CC, Chang YC, Chang TT, Lin PW, Lei HY, Su IJ: Identification of a pre-S2 mutant in hepatocytes expressing a novel marginal pattern of surface antigen in advanced diseases of chronic hepatitis B virus infection. J Gastroenterol Hepatol 2000, 15:519-528 [DOI] [PubMed] [Google Scholar]
- 19.Lu CC, Yen TS: Activation of the hepatitis B virus S promoter by transcription factor NF-Y via a CCAAT element. Virology 1996, 225:387-394 [DOI] [PubMed] [Google Scholar]
- 20.Raney AK, Le HB, McLachlan A: Regulation of transcription from the hepatitis B virus major surface antigen promoter by the Sp1 transcription factor. J Virol 1992, 66:6912-6921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melegari M, Scaglioni PP, Wands JR: The small envelope protein is required for secretion of a naturally occurring hepatitis B virus mutant with pre-S1 deleted. J Virol 1997, 71:5449-5454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan YF, Lu CC, Chen WC, Yao WJ, Wang HC, Chang TT, Lei HY, Shiau AL, Su IJ: Prevalence and significance of hepatitis B virus (HBV) pre-S mutants in serum and liver at different replicative stages of chronic HBV infection. Hepatology 2001, 33:277-286 [DOI] [PubMed] [Google Scholar]
- 23.Little E, Ramakrishnan M, Roy B, Gazit G, Lee AS: The glucose-regulated proteins (GRP78 and GRP94): functions, gene regulation, and applications. Crit Rev Eukaryot Gene Expr 1994, 4:1-18 [DOI] [PubMed] [Google Scholar]
- 24.Nicchitta CV: Biochemical, cell biological and immunological issues surrounding the endoplasmic reticulum chaperone GRP94/gp96. Curr Opin Immunol 1998, 10:103-109 [DOI] [PubMed] [Google Scholar]
- 25.Heermann KH, Goldmann U, Schwartz W, Seyffarth T, Baumgarten H, Gerlich WH: Large surface proteins of hepatitis B virus containing the pre-s sequence. J Virol 1984, 52:396-402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sauve DM, Ho DT, Roberge M: Concentration of dilute protein for gel electrophoresis. Anal Biochem 1995, 226:382-383 [DOI] [PubMed] [Google Scholar]
- 27.Gunther S, Fischer L, Pult I, Sterneck M, Will H: Naturally occurring variants of hepatitis B virus. Adv Virus Res 1999, 52:25-137 [DOI] [PubMed] [Google Scholar]
- 28.Melegari M, Bruno S, Wands JR: Properties of hepatitis B virus pre-S1 deletion mutants. Virology 1994, 199:292-300 [DOI] [PubMed] [Google Scholar]
- 29.Bock CT, Tillmann HL, Manns MP, Trautwein C: The pre-S region determines the intracellular localization and appearance of hepatitis B virus. Hepatology 1999, 30:517-525 [DOI] [PubMed] [Google Scholar]
- 30.Le Seyec J, Chouteau P, Cannie I, Guguen-Guillouzo C, Gripon P: Infection process of the hepatitis B virus depends on the presence of a defined sequence in the pre-S1 domain. J Virol 1999, 73:2052-2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Persing DH, Varmus HE, Ganem D: Inhibition of secretion of hepatitis B surface antigen by a related presurface polypeptide. Science 1986, 234:1388-1391 [DOI] [PubMed] [Google Scholar]
- 32.Ou JH, Rutter WJ: Regulation of secretion of the hepatitis B virus major surface antigen by the preS-1 protein. J Virol 1987, 61:782-786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pahl HL, Sester M, Burgert HG, Baeuerle PA: Activation of transcription factor NF-kappaB by the adenovirus E3/19K protein requires its ER retention. J Cell Biol 1996, 132:511-522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ploegh HL: Viral strategies of immune evasion. Science 1998, 280:248-253 [DOI] [PubMed] [Google Scholar]
- 35.Pahl HL: Signal transduction from the endoplasmic reticulum to the cell nucleus. Physiol Rev 1999, 79:683-701 [DOI] [PubMed] [Google Scholar]
- 36.Chisari FV, Ferrari C: Hepatitis B virus immunopathogenesis. Annu Rev Immunol 1995, 13:29-60 [DOI] [PubMed] [Google Scholar]
- 37.Tai PC, Banik D, Lin GI, Pai S, Pai K, Lin MH, Yuoh G, Che S, Hsu SH, Chen TC, Kuo TT, Lee CS, Yang CS, Shih C: Novel and frequent mutations of hepatitis B virus coincide with a major histocompatibility complex class I-restricted T-cell epitope of the surface antigen. J Virol 1997, 71:4852-4856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carman WF, Zanetti AR, Karayiannis P, Waters J, Manzillo G, Tanzi E, Zuckerman AJ, Thomas HC: Vaccine-induced escape mutant of hepatitis B virus. Lancet 1990, 336:325-329 [DOI] [PubMed] [Google Scholar]
- 39.Missale G, Redeker A, Person J, Fowler P, Guilhot S, Schlicht HJ, Ferrari C, Chisari FV: HLA-A31- and HLA-Aw68-restricted cytotoxic T cell responses to a single hepatitis B virus nucleocapsid epitope during acute viral hepatitis. J Exp Med 1993, 177:751-762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rehermann B, Lau D, Hoofnagle JH, Chisari FV: Cytotoxic T lymphocyte responsiveness after resolution of chronic hepatitis B virus infection. J Clin Invest 1996, 97:1655-1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nayersina R, Fowler P, Guilhot S, Missale G, Cerny A, Schlicht HJ, Vitiello A, Chesnut R, Person JL, Redeker AG, Chisari FV: HLA A2 restricted cytotoxic T lymphocyte responses to multiple hepatitis B surface antigen epitopes during hepatitis B virus infection. J Immunol 1993, 150:4659-4671 [PubMed] [Google Scholar]
- 42.Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J: Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 2000, 403:98-103 [DOI] [PubMed] [Google Scholar]
- 43.Pahl HL, Baeuerle PA: A novel signal transduction pathway from the endoplasmic reticulum to the nucleus is mediated by transcription factor NF-kappa B. EMBO J 1995, 14:2580-2588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foo NC, Yen TS: Activation of promoters for cellular lipogenic genes by hepatitis B virus large surface protein. Virology 2000, 269:420-425 [DOI] [PubMed] [Google Scholar]
- 45.Harding HP, Zhang Y, Ron D: Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 1999, 397:271-274 [DOI] [PubMed] [Google Scholar]
- 46.Cox JS, Shamu CE, Walter P: Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell 1993, 73:1197-1206 [DOI] [PubMed] [Google Scholar]
- 47.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D: Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 2000, 287:664-666 [DOI] [PubMed] [Google Scholar]
- 48.Hildt E, Munz B, Saher G, Reifenberg K, Hofschneider PH: The preS2 activator MHBs(t) of hepatitis B virus activates c-raf-1/Erk2 signaling in transgenic mice. EMBO J 2002, 21:525-535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hildt E, Saher G, Bruss V, Hofschneider PH: The hepatitis B virus large surface protein (LHBs) is a transcriptional activator. Virology 1996, 225:235-239 [DOI] [PubMed] [Google Scholar]
- 50.Hildt E, Hofschneider PH: The preS2 activators of the hepatitis B virus: activators of tumour promoter pathways. Recent Results Cancer Res 1998, 154:315-329 [DOI] [PubMed] [Google Scholar]