Abstract
Poorly healing diabetic wounds are characterized by diminished collagen production and impaired angiogenesis. HoxD3, a homeobox transcription factor that promotes angiogenesis and collagen synthesis, is up-regulated during normal wound repair whereas its expression is diminished in poorly healing wounds of the genetically diabetic (db/db) mouse. To determine whether restoring expression of HoxD3 would accelerate diabetic wound healing, we devised a novel method of gene transfer, which incorporates HoxD3 plasmid DNA into a methylcellulose film that is placed on wounds created on db/db mice. The HoxD3 transgene was expressed in endothelial cells, fibroblasts, and keratinocytes of the wounds for up to 10 days. More importantly, a single application of HoxD3 to db/db mice resulted in a statistically significant acceleration of wound closure compared to control-treated wounds. Furthermore, we also observed that the HoxD3-mediated improvement in diabetic wound repair was accompanied by increases in mRNA expression of the HoxD3 target genes, Col1A1 and β3-integrin leading to enhanced angiogenesis and collagen deposition in the wounds. Although HoxD3-treated wounds also show improved re-epithelialization as compared to control db/db wounds, this effect was not due to direct stimulation of keratinocyte migration by HoxD3. Finally, we show that despite the dramatic increase in collagen synthesis and deposition in HoxD3-treated wounds, these wounds showed normal remodeling and we found no evidence of abnormal wound healing. These results indicate that HoxD3 may provide a means to directly improve collagen deposition, angiogenesis and closure in poorly healing diabetic wounds.
Wound healing is an intricate process involving the communication and interaction between fibroblasts, endothelial cells, keratinocytes, inflammatory cells, and the extracellular matrix. Disruption of these interactions can impair angiogenesis and/or collagen synthesis resulting in wounds that heal slowly and incompletely. 1-3 A major complication of diabetes mellitus is deficient wound repair arising from inadequate collagen deposition and angiogenesis, and attempts to understand the underlying defects to enhance wound healing are the subject of extensive investigation. The leptin receptor-deficient diabetic (db/db) mouse is an established model of deficient wound healing associated with diabetes. 4
Similar to wounds in diabetic humans, diabetic animals display poor wound healing and a reduction in angiogenesis and granulation tissue formation. 4-6 We had previously observed that expression of the homeobox gene, HoxD3, is also compromised in wounds made in db/db animals. 7 HoxD3 is a member of the homeobox (Hox) family of master transcription factors that are expressed during normal embryogenesis, skin development, and during fetal wound healing. 8-10 Hox genes have a conserved 180-bp DNA binding sequence and can modulate expression of a number of genes associated with extracellular matrix remodeling and angiogenesis, two essential aspects of wound repair. 11-15 Our previous studies showed that HoxD3 increases expression of both uPA and the β3-integrin and that HoxD3 promotes endothelial cell migration. 11 More recently, we showed that HoxD3 also stimulates expression of type I collagen (Col1A1). 7 Others have shown that HoxD3 also stimulates expression of ets-1, a transcription factor that regulates expression of many proteolytic enzymes and whose expression is markedly reduced by high glucose levels in diabetic models. 15,16 Together these findings suggest that HoxD3 may play an essential role in normal wound repair.
It is not clear why HoxD3 expression is diminished in wounds of db/db mice. One explanation may be reduced expression of cytokines including platelet-derived growth factor and its receptor platelet-derived growth factor-B, as well as vascular endothelial growth factor, basic fibroblast growth factor (bFGF), and transforming growth factor-β, which are all reduced in diabetic models. 17-21 Our previous studies have shown that expression of HoxD3 is induced by wound-associated cytokines including bFGF and tumor necrosis factor-α. 11 Furthermore, the finding that the ability of bFGF to induce expression of uPA and αvβ3-integrin is blocked in cells lacking HoxD3, suggests that HoxD3 acts as a downstream transcriptional activator of wound repair programs that are normally induced in response to a variety of cytokines. Thus, restoring levels of HoxD3 and its target genes, αvβ3, integrin, uPA, and Col1A1, via gene transfer may represent a novel means to enhance angiogenesis, collagen synthesis, and accelerate wound repair in the compromised diabetic wound environment.
Materials and Methods
Cell Culture, Construction, and Transfection of the HA-HoxD3 Expression Plasmid
For construction of the HA/HoxD3 expression plasmid, the HoxD3 coding sequence cloned into a CMV-driven expression plasmid (pcDNA3) as previously described, 11 was excised using Kpn/NotI and inserted in frame into the pHM6 epitope expression vector under control of the CMV promoter (Boehringer Mannheim, Indianapolis, IN). The sequence of the resulting HA/HoxD3 expression vector was confirmed by ABI sequencing at the Biomolecular Resource Center at UCSF. HMEC-1 cells were cultured as previously described. 12,22 HMEC-1 were transfected with HA/HoxD3 or CMVβgal using Effectene (Qiagen, Valencia, CA) and stable pools of transfected cells were selected using 35 μg/ml of G418.
Preparation of HoxD3 Retroviral Vectors
Human HoxD3 cDNA was isolated by reverse transcriptase-polymerase chain reaction (RT-PCR) using the following primers: forward 5′-agggtcagcaggccctggagc-3′ corresponding to bp 1370 to 1391 and reverse 5′-agagcggggaagggggttcccgaact-3′ corresponding to bp 4598 to 4623 of the human genomic sequence, GenBank accession number D11117. The 1.3-kb PCR product was ligated into the TopoTA cloning vector (Invitrogen, Carlsbad, CA) and the identity of the insert confirmed by sequencing using ABI big dye terminator technology (UCSF Biomolecular Resource Center). The HoxD3 cDNA sequence was excised with HindIII (blunt) and XhoI and ligated into the Hpa/XhoI sites in the pLXSN retroviral vector (Clontech, Palo Alto, CA). Active virus was collected by harvesting the culture media of Phoenix 293 packaging cells, 48 hours after CaPO4 transfection with pLXSNHoxD3 or pLXSN.
Isolation of Primary Mouse Fibroblasts
Fibroblasts were isolated from wild-type C57BL mice by placing explants (1 × 1 mm) on plastic culture dishes that were subsequently cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum after the tissue had dried to the plate. Tissue explants were grown to confluency and passaged as needed.
Collagen Expression in Fibroblasts
For analysis of HoxD3 on collagen expression, wild-type fibroblasts were infected with either control (Plxsn) or HoxD3 retroviral vectors for 24 hours. Another 24 hours later, 1 × 106 control or HoxD3-infected fibroblasts were embedded within three-dimensional collagen gels prepared by incubating 4 ml of a 2.9 mg/ml solution of bovine collagen (Invitrogen), 10× Dulbecco’s modified Eagle’s medium, and 0.34 mol/L NaOH per 100-mm tissue culture dish. Forty-eight hours later RNA was harvested using Trizol Reagent (Life Technologies, Inc., Rockville, MD). Relative HoxD3 levels were confirmed by semiquantitative RT-PCR as previously described. 7 Col1A1 mRNA levels in control and HoxD3-infected cultures were determined using semiquantitative RT-PCR with the following mouse primers: forward primer 5′-GCCAAGAAGACATCCCTGAAG-3′ and the reverse primer 5′-TCATTGCATTGCACGTCATC-3′ were run for 20 cycles at 55°, which yielded a 139-bp product.
Animals
Genetically diabetic C57BL/KsJ-db/db mice and their nondiabetic littermates were obtained from Jackson Laboratories (Bar Harbor, ME). Animals were housed in the University of California, San Francisco, animal care facility. The Committee on Animal Research approved all procedures. All mice were between 8 and 12 weeks of age at time of wounding.
Preparation of DNA/Methylcellulose Pellets and Application to Wounds
Twenty-five μg of HoxD3 DNA or CMV βgal plasmid DNA was resuspended in a volume of 25 μl of ddH20 and mixed with an equal volume of 1% methylcellulose prepared in ddH20. Fifty μl of this solution was spotted onto bacterial plates and allowed to dry at room temperature for 2 hours. The dehydrated pellets were removed intact from the plates with forceps. Immediately after wounding, the methylcellulose pellet containing the HoxD3 or βgal plasmid was applied to the wound. The 0.8-cm wounds received one 25-μg pellet whereas the 2.5-cm wounds received four 25-μg pellets.
Wounding and Measurement of Wound Closure
Wild-type and diabetic mice were anesthetized with 0.04 cc of ketamine: xylazine (50 mg/ml: 2.5 mg/ml). The dorsum of the mouse was shaved and an open wound was excised including the panniculus carnosus layer. For wound biopsy purposes, animals received bilateral 0.8-cm wounds. The HA/HoxD3 plasmid was placed on one side and the βgal plasmid on the other. Animals were sacrificed on days 2, 5, 7, 10, 14, and 17 after wounding after which wounds were harvested and processed for RNA, in situ hybridization, or immunohistochemistry. A total of five wild-type mice and five db/db mice were used at each time point. For wound closure measurements, a single 2.5-cm wound was created on each animal and treated with either HA/HoxD3 or βgal plasmid. The open wounds were measured immediately after wounding and then every 7 days until the wound was healed. The NIH Image J analyzer was used to determine the area of the wound tracing. The areas of the wounds were compared using the paired Student’s t-test.
Immunohistochemistry
Five-μm paraffin sections were deparaffinized by heating at 80°C and washing in CitriSolv then blocked with 3% hydrogen peroxide and 1% goat serum. Tissues were treated with a 1:200 dilution of polyclonal anti-von Willebrand factor antibody (DAKO, Carpinteria, CA) followed by a 1:500 dilution of biotin-conjugated anti-rabbit secondary antibody for 1 hour. Tissue sections were then treated with Vectastain ABC reagent. Von Willebrand Factor was then detected with diaminobenzidine (Zymed, South San Francisco, CA) or Nova Red (Vector Laboratories, Burlingame, CA). Immunohistochemistry for HA/HoxD3 staining was performed on 7-μm frozen sections of db/db wound tissue. Tissues were permeabilized with acetone and blocked in 0.3% normal goat serum and 0.3% H202 for 1 hour. Sections were incubated with a 1:150 dilution of a rabbit polyclonal antibody against HA (SC-805; Santa Cruz Biotechnology, Santa Cruz, CA) followed by a biotinylated goat anti-rabbit antibody and Vectastain ABC reagent (Vector Laboratories). Color was developed using the Nova Red substrate kit (Vector Laboratories) and tissues were counterstained using Gils hematoxylin (Fisher, Pittsburgh, PA).
To detect angiogenesis in the wounds, 10-μm frozen sections were incubated with a 1:100 dilution of rat anti-mouse CD31 (PECAM) antibody (Pharmingen, San Diego, CA) followed by a 1:250 dilution of biotin-conjugated goat anti-rat IgG (Jackson Laboratories, Bar Harbor, ME) and color developed using the Vectastain ABC Reagent (Vector Laboratories) and Nova Red and tissues were counterstained using Gills hematoxylin no. 3 (Fisher).
Vascular Labeling in the Skin with Lycopersicon esculentum
Six days after creation of bilateral wounds and placement of either the HoxD3 or βgal cDNA plasmid, animals were prepared for vascular labeling with FITC-conjugated L. esculentum (Vector Laboratories, Burlingame, CA). Animals were anesthetized with inhalation isofluorane. The femoral vein was isolated via a groin incision and cannulated with a 30-gauge needle after which 100 μl of L. esculentum was injected. This was allowed to perfuse for 3 minutes in the animal. After 3 minutes, the chest was entered, the left ventricle was cannulated and the right ventricle was incised. The animal was perfused and fixed with 1× phosphate-buffered saline, 1.0% paraformaldehyde, 0.5% glutaraldehyde, pH 7.4, 0.44-μm filtered at 7 ml/minute for 3 minutes. The wounds were then harvested from the dorsum of the animal and processed for histochemistry.
Trichrome Staining
Tissue samples were harvested and processed by the Masson-Goldner trichrome method for analysis of total collagen as previously described. 7
Histological Score
Wounds (0.8 cm) were harvested 7 days after application of either control (βgal) or HoxD3 expression plasmids. Histological scores were assigned in a blinded manner according to the method of Greenhalgh and colleagues. 5 Briefly, each specimen was given a score of 1 to 12: 1 to 3, none to minimal cell accumulation and granulation tissue or epithelial migration; 4 to 6, thin, immature granulation dominated by inflammatory cells but with few fibroblasts, capillaries, or collagen deposition and minimal epithelial migration; 7 to 9, moderately thick granulation tissue, ranging from being dominated by inflammatory cells to more fibroblasts and collagen deposition; and 10 to 12, thick, vascular granulation tissue dominated by fibroblasts and extensive collagen deposition.
RNA Isolation and Northern Blot Analysis
Mouse wound tissue was excised, snap-frozen in liquid nitrogen, and homogenized with Trizol Reagent (Life Technologies, Rockville, MD) for RNA isolation. For isolation of cellular RNA, the Qiagen RNAeasy kit was used as described. 11 For Northern blot analysis, a total of 5 to 10 μg of total RNA was electrophoresed through 1% agarose gels. Blots were probed with 1 × 106 cpm of labeled cDNA probe against human Col1A1 (American Type Culture Collection, Rockville, MD) or β3 integrin (11) and washed three times under low-stringency conditions of 2% standard saline citrate/0.1% sodium dodecyl sulfate for 30 minutes at 45°C. Membranes were exposed to Kodak BioMax film at −70°C. mRNA levels were quantitated by scanning densitometry of the film and normalized to ethidium bromide staining of total ribosomal RNA using Chem-Imager 4000 software (Alpha Innotech, San Leandro, CA).
Immortalized MK Keratinocytes
MK keratinocytes (obtained from Dr. David Morris, UCSF, San Francisco, CA) were cultured in low-calcium EMEM (BioSource) containing 10% dialyzed fetal calf serum (Hyclone, Logan, UT) and 50 ng of epidermal growth factor (R&D Systems, Minneapolis, MN). After infection with either control or HoxD3 retroviral vectors, stably expressing cells were selected using 100 μg/ml of G418.
Scratch Wound Assay
Scratch wounds (1 mm) were generated in confluent cultures of either control or HoxD3-expressing MK keratinocytes using a sterile 1-ml pipette tip. Cultures were photographed 24 hours later and the number of cells that had migrated into the denuded area was counted.
DNA Synthesis Assay
Cells were labeled in serum-free media containing 10 μmol/L 5-bromo-2′-deoxy-uridine (BrdU) for 8 hours and fixed in a 70% ethanol and stained with the anti-BrdU kit (Boehringer Mannheim) according to the manufacturer’s instructions. Cells were subsequently stained with 4,6-diamidino-2-phenylindole and the number of BrdU-positive nuclei were counted in each of five random fields and expressed as a percentage of total cells present in each field.
Results
Expression of HA-HoxD3 in Culture and in Vivo
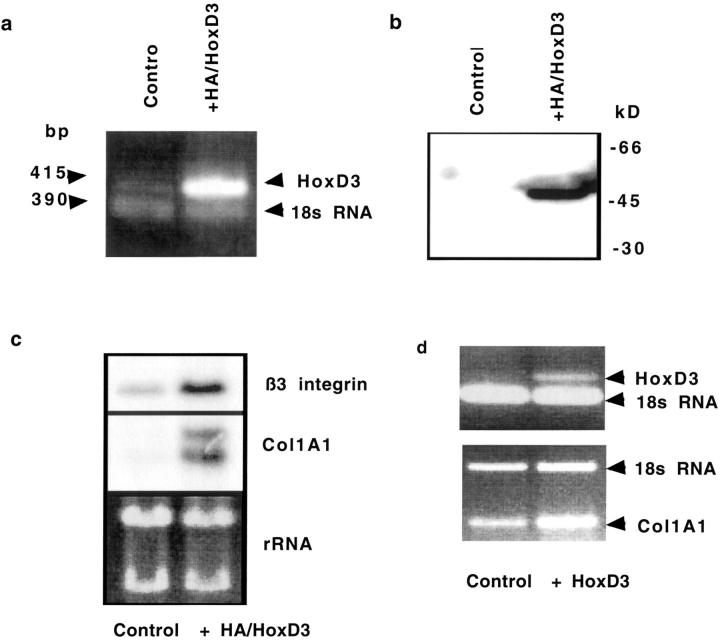
We generated a HA epitope-tagged HoxD3 with the HA tag fused to the N-terminal. We stably transfected HMEC-1 endothelial cells that resulted in an increase in HoxD3 mRNA expression (Figure 1a) ▶ . The resulting fusion protein of ∼45 kd, which corresponds to the predicted MW for HoxD3, could be immunoprecipitated with an antibody against HA (Figure 1b) ▶ . Increased expression of HA/HoxD3 resulted in a corresponding increase in the HoxD3 target genes: collagen (Col1A1) and β3-integrin mRNA expression (Figure 1c) ▶ similar to our previous results using an untagged HoxD3 expression plasmid. 11 In addition, primary mouse fibroblasts transiently infected with a HoxD3-expressing retrovirus also show an increase in Col1A1 expression (Figure 1d) ▶ .
Figure 1.
Expression of HoxD3 in transfected cells. a: RT-PCR showing increased expression of a 415-bp band corresponding to HoxD3 in HMEC-1 transfected with HA/HoxD3 expression plasmids. Total RNA loading is shown by the 390-bp band corresponding to 18s ribosomal RNA, amplified by competitive RT-PCR. b: Immunoprecipitation of HA-HoxD3 in transfected HMEC-1 and subsequent immunoblotting with an anti-HA antibody shows expression of an ∼45-kd protein, corresponding to the size of the HoxD3 protein. c: Top: Northern blot analysis of β3-integrin; middle: Col1A1 mRNA expression levels in control and HA/HoxD3-transfected HMEC-1; bottom: relative loading of total RNA as visualized by ethidium bromide staining of ribosomal RNA (rRNA). d, Top: RT-PCR analysis of HoxD3 mRNA expression in mouse primary fibroblasts, 96 hours after transient infection of primary mouse fibroblasts with either a control or HoxD3-expressing retrovirus. Relative RNA loading is shown by the lower band corresponding to 18s RNA. d, Bottom: RT-PCR analysis of relative levels of Col1A1 mRNA expression in primary fibroblasts 96 hours after infection with either control or HoxD3-expressing retrovirus. Relative RNA loading is shown by semiquantitative RT-PCR for 18s RNA.
To transfect cells in the wound we applied methylcellulose pellets containing HA/HoxD3 cDNA to one wound and the control plasmid DNA (CMVβgal) to the contralateral wound. The uptake and expression of the HA/HoxD3 by cells in the wound was detected using an antibody against the HA epitope. βgal-treated (control) db/db wounds do not show any specific staining for the HA epitope after 7 days (Figure 2, a and c) ▶ . In contrast, the HA/HoxD3-treated wounds showed areas of positive staining beginning at 2 days and continuing until at least 7 days (Figure 2, b and d) ▶ . Furthermore, RT-PCR for HoxD3 expression shows that whereas the overall expression of HoxD3 mRNA is markedly decreased in 7-day db/db wound tissue as compared to 7-day wild-type wounds, treatment of db/db wounds with HoxD3 expression plasmid results in marked increase HoxD3 mRNA levels that approaches that of wild-type wounds (Figure 2e) ▶ . In addition, expression of the HoxD3 transgene did not appear to be restricted to any particular cell type but rather was expressed by a variety of proliferating cells resembling fibroblasts, endothelial cells, and basal keratinocytes, respectively (Figure 2; f to h) ▶ . Despite the extensive expression at 7 days, by 10 days expression of the HA/HoxD3 had markedly declined and was barely detectable in cells of the wounds (not shown).
Figure 2.
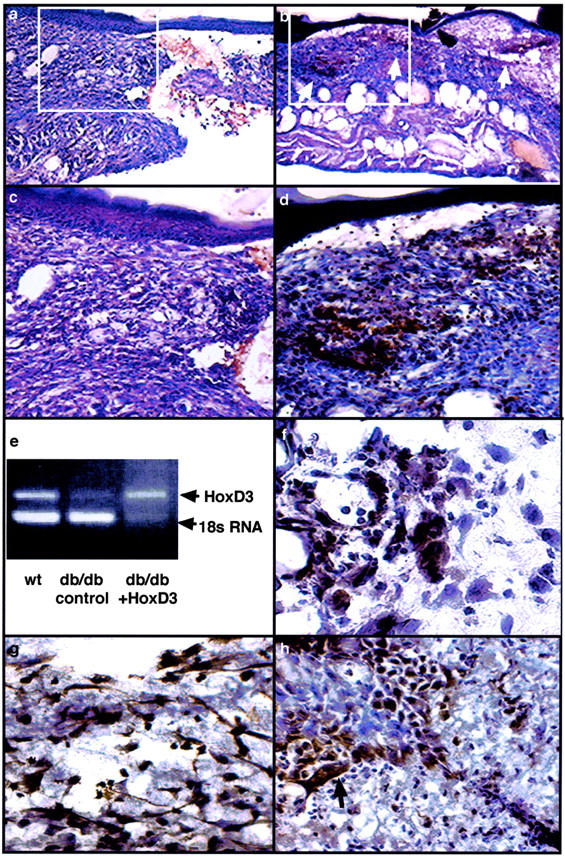
Expression of HA/HoxD3 in wounds. a: Immunoperoxidase staining for HA in frozen sections from control wounds in db/db mice 7 days after treatment with pellets containing βgal cDNA and stained with a polyclonal antibody against HA. Only background staining (red/brown color) derived from extravasated red blood cells is observed in the tissue. b: Immunoperoxidase staining for HA/HoxD3 in frozen sections from wounds in db/db mice 7 days after treatment with pellets containing an HA epitope-tagged HoxD3 DNA expression plasmid. Tissues were stained with a polyclonal antibody against HA. Positive staining (red/brown color) is observed in numerous areas of granulation tissue (arrows) that has formed at the edge of the wound. c: Higher magnification of control plasmid-treated wounds (a) showing a lack of positive staining in the tissue. d: Higher magnification of HA/HoxD3-treated wounds (b) showing positive cellular staining. e: RT-PCR showing relative levels of HoxD3 mRNA in 7-day wound tissue harvested from wild-type, db/db mice treated with control plasmid or db/db wounds treated with HoxD3. f: Immunoperoxidase staining for HA/HoxD3 in granulation tissue of wounds 5 days after application of HA-HoxD3 plasmids. Positive staining is observed in both fibroblasts and endothelial cells. g: Immunoperoxidase staining for HA/HoxD3 in the looser granulation tissue of 7-day wounds shows an increased number of transfected fibroblasts as compared to 2-day wounds. h: Immunoperoxidase staining for HA/HoxD3 in wounds 7 days after application of HA epitope-tagged HoxD3 DNA. Positive staining is observed in basal keratinocytes (arrow) at the edge of the wound.
HoxD3 Expedites Wound Closure in Diabetic Animals
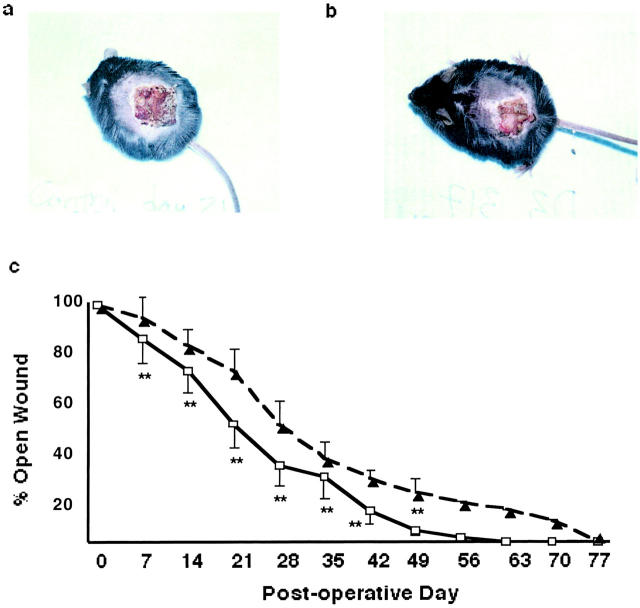
To measure effect of HoxD3 treatment of diabetic wounds on wound closure, 2.5-cm2 wounds were treated with either HoxD3 or control DNA. A representative photograph of the wounds in db/db mice 21 days after wounding showed a smaller wound in the HoxD3-treated mice relative to control (Figure 3, a and b) ▶ .
Figure 3.
Closure of wounds in db/db mice is accelerated by application of HoxD3. a: Representative photograph showing wound appearance of a 2.5-cm wound 21 days after treatment with βgal (control). b: Representative photograph showing reduced size of a 2.5-cm wound 21 days after gene transfer of HoxD3. c: Wound closure in control (▴) versus HoxD3 (□)-treated diabetic wounds. Wound diameter was measured every 7 days after excision of a 2.5-cm wound in db/db mice. Mice treated with HoxD3 showed a significantly (**, P < 0.05) greater degree of closure at days 7, 14, 21, 28, 35, 42, and 49 days as compared to control DNA-treated wounds (HoxD3, n = 10; control DNA, n = 10).
HoxD3 treatment resulted in a statistically significant smaller wound from day 7 until closure; with the greatest difference between 14 to 28 days as compared to control DNA-treated animals (Figure 3c) ▶ . On average, the control diabetic wounds closed by day 77 (±14 days) and the HoxD3-treated wounds closed 2 weeks earlier at day 63 (±5 days), whereas nondiabetic wild-type wounds require only 21 days to show complete closure (S. L. Hansen, unpublished). Interestingly, application of HoxD3 to wild-type wounds that show normal induction of HoxD3 expression after wounding did not result in any significant improvement in wound closure (data not shown).
Increased Angiogenesis in HoxD3-Treated Wounds
Because HoxD3 can promote an angiogenic phenotype in endothelial cells 11 we examined whether the HoxD3-mediated improvement in wound closure in db/db mice was accompanied by an increase in angiogenesis. We administered bilateral 0.8-cm wounds and harvested tissue after 7 days. Wounds treated with HoxD3 were more compact and displayed an increased amount of granulation tissue as compared to the contralateral control DNA-treated wounds at 7 days after wounding (Figure 4; a, b, d, and f) ▶ . The improvement in wound appearance and granulation tissue was confirmed by the significantly increased in histological score of HoxD3 as compared to control-treated wounds (Figure 4c) ▶ . Staining of serial sections of control (Figure 4, d and e) ▶ and HoxD3-treated wounds (Figure 4, f and g) ▶ with the endothelial-specific marker, PECAM (CD31) revealed an increase in microvascular density in HoxD3 as compared to control-treated animals by 7 days. In addition, perfusion with the endothelial lectin, L. esculentum revealed an increase in vascular density in HoxD3 as compared to control-treated wounds (Figure 4; h to k) ▶ .
Figure 4.
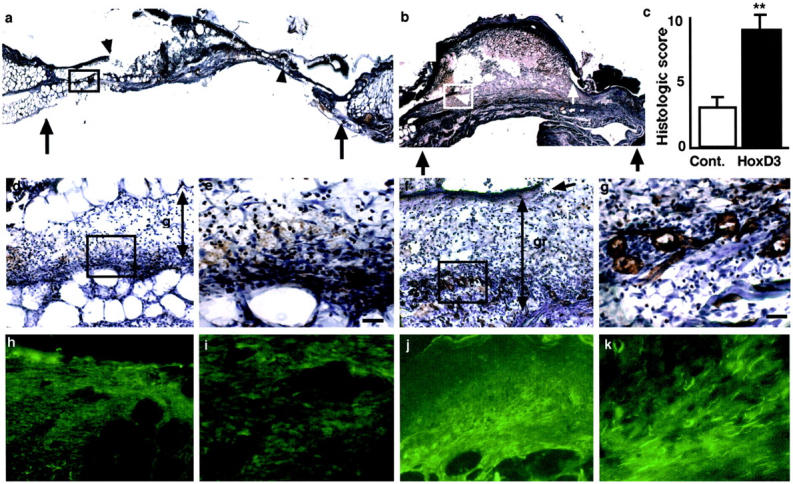
Increased angiogenesis in HoxD3-treated animals. a: Photomicrograph of a frozen section of a 7-day, 0.8-cm wound treated with control DNA. Black arrows show the wound margins and the smaller arrowheads show the leading edge of the keratinocytes. b: Photomicrograph of a frozen section of the corresponding contralateral 7-day, 0.8-cm wound treated with HoxD3 cDNA. Black arrows show the wound margins and the white arrowheads show the leading edge of the keratinocytes. c: Histological score of 7-day, 0.8-cm wounds from control (□)- or HoxD3 (▪)-treated mice (**, P < 0.001; n = 3). d: Serial section of the same 7-day wound treated with control DNA treated corresponding to the boxed region shown in a. The section was stained with an antibody against PECAM (CD31) and only diffuse staining (red/brown) is observed throughout the relatively thin layer of granulation tissue (gr ↕). e: Higher magnification of d showing a lack of well-formed vessels and many erythrocytes within the granulation tissue. f: Serial section of the contralateral 7-day wound treated with HoxD3 corresponds to the boxed region shown in b. The section was stained with an antibody against PECAM and numerous small vessels are observed within the granulation tissue formed under the leading edge of the keratinocytes (arrow). The relative thickness of the granulation tissue is also indicated (gr ↕). g: Higher magnification of f shows positively stained capillaries within the granulation tissue of HoxD3-treated wounds. h: Low-power (×10) magnification of granulation tissue near the edge of 7-day wounds in animal treated with control cDNA after perfusion of L. esculentum. i: Higher magnification of h showing relatively few positive endothelial cells in the granulation tissue. j: Low-power (×10) view of granulation tissue near the edge of the contralateral 7-day wound after treatment with HoxD3 and perfusion with L. esculentum. k: Higher magnification of j showing abundant lectin-positive endothelial cells and vessel within the granulation tissue of HoxD3-treated wounds. Scale bars, 15 μm (e, g).
HoxD3 Increases Collagen and β3-Integrin mRNA Expression
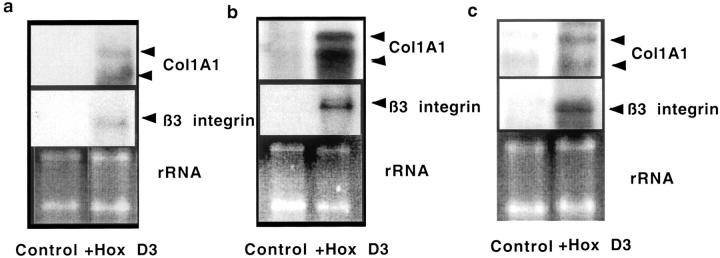
To determine whether the HoxD3-mediated improvement in wound healing and angiogenesis correlated with increased expression of HoxD3 downstream target genes, αvβ3-integrin and Col1A1, in vivo, we performed Northern blot analysis on wound tissue collected on days 3, 7, and 10 after wounding. Whereas β3-integrin and Col1A1 mRNA were barely detectable in wound tissue from the control-treated wound at day 3, the HoxD3-treated wounds showed a marked increase in expression of both Col1A1 and β3-integrin (Figure 5a) ▶ . β3-integrin and Col1A1 mRNA expression levels continued to increase up to 7 days after HoxD3 treatment (Figure 5b) ▶ . It is worth noting that the threefold to fourfold increase in Col1A1 expression induced by HoxD3 over control-treated db/db animals is similar to the threefold to fourfold increase observed in nondiabetic wild-type animals as compared to their diabetic counterparts 7 days after wounding. 7 In other words, HoxD3 treatment of diabetic wounds increased expression of Col1A1 mRNA to the same level seen in wild-type wounds at the same time point. Expression of Col1A1 mRNA remained higher in HoxD3-treated animals at 10 and 14 days (Figure 5c ▶ , and data not shown). Expression of β3-integrin also remained higher in HoxD3 as compared to control-treated animals after 10 days (Figure 5c ▶ , middle).
Figure 5.
Increased collagen and β3-integrin mRNA expression in wound tissue from HoxD3-treated mice. a: Northern blot showing expression of type I collagen mRNA (Col1A1, top) in wounds from a db/db mouse treated with βgal (control) or HoxD3 for 3 days. The blot was stripped and reprobed with a β3-integrin cDNA probe (middle). Corresponding total RNA loading (rRNA) as visualized by ethidium bromide staining is shown at the bottom. b: Northern blot showing expression of type I collagen mRNA (top) at 7 days in wounds treated with either control DNA or HoxD3. The blot was stripped and reprobed with a β3-integrin cDNA probe (middle). The corresponding ribosomal (rRNA) loading controls are shown (bottom). c: Northern blot analysis shows type I collagen mRNA expression (top) in control- or HoxD3-treated wounds from the same animal after 10 days. The blot was stripped and reprobed with a β3-integrin cDNA probe (middle). Bottom: Relative RNA loading as visualized by ethidium bromide staining.
Wound Appearance at 14 Days after Gene Transfer of HoxD3
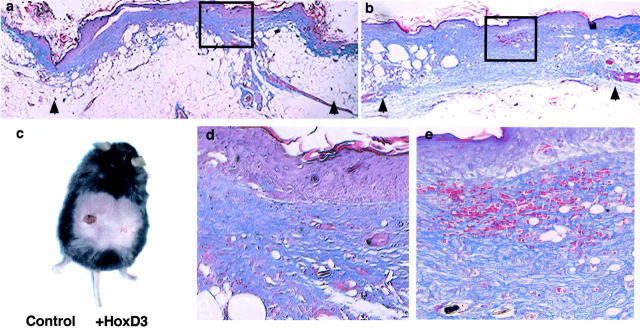
By 14 days after application of HoxD3 or control DNA, differences in the appearance of wounds were readily observed. Trichrome staining was performed on wound biopsies harvested from animals 14 days after application of control cDNA (Figure 6, a and d) ▶ or HoxD3 cDNA to wounds (Figure 6, b and e) ▶ . HoxD3-treated animals showed markedly enhanced collagen deposition as well as increased blood vessel density relative to control DNA-treated animals. A representative photograph of a db/db animal, 14 days after creation of bilateral 0.8-cm wounds is shown (Figure 6c) ▶ . The HoxD3-treated wound on the right side of the mouse revealed complete re-epithelialization by 14 days as compared to control DNA-treated wounds on the left side of the animal.
Figure 6.
Improved wound appearance and increased deposition of collagen after HoxD3 gene transfer. a:. Trichrome staining of wounds 14 days after application of control DNA. Collagen is stained in blue and the arrowhead shows the wound margins. b: Trichrome staining of wounds 14 days after application of HoxD3. Wound margins are indicated by the arrows. c: Photo shows bilateral wounds in two db/db mice 14 days after application of either βgal (control)- or HoxD3-containing pellets on 0.8-cm wounds. Note the HoxD3-treated wounds are closed at this point while the control-treated wound has not completely closed. d: Higher magnification of a. e: Higher magnification of b. Scale bars, 50 μm (d and e).
HoxD3 Does Not Directly Increase Keratinocyte Migration
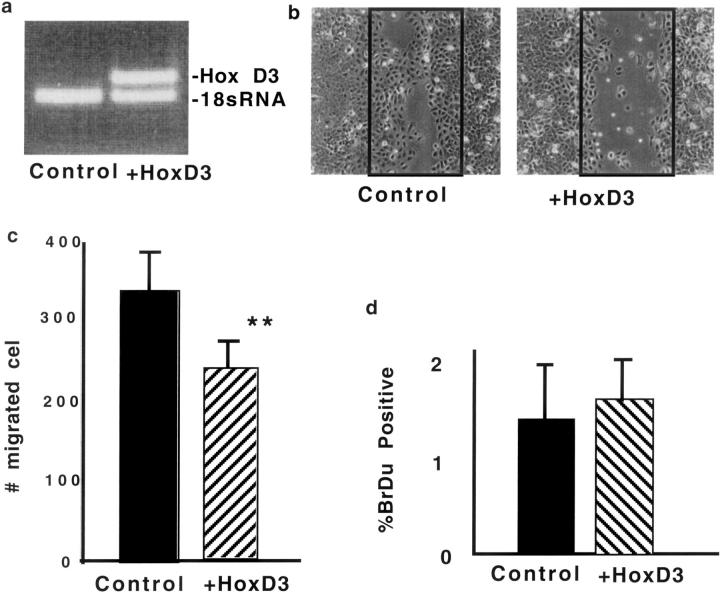
Because we consistently observed an increase in re-epithelialization in HoxD3-treated wounds and observed that HoxD3 expression plasmid could be expressed in keratinocytes (Figure 2h) ▶ , we also determined whether HoxD3 directly enhanced migration of keratinocytes. Immortalized keratinocytes were stably transfected with control or HoxD3-expressing retrovirus (Figure 7a) ▶ . Surprisingly, expression of HoxD3 inhibited migration of keratinocytes as compared to control transfected cells (Figure 7, b and c) ▶ . We also observed a similar trend toward inhibition of migration by HoxD3 in primary keratinocytes infected with a HoxD3 retroviral vector (not shown). To determine whether this was related to an inhibition of proliferation and/or migration, we performed BrdU labeling on stably transfected control or HoxD3-expressing keratinocytes, and observed no difference in DNA synthesis (Figure 7d) ▶ .
Figure 7.
HoxD3 impairs keratinocyte migration. a: RT-PCR showing relative levels of HoxD3 mRNA expression in keratinocytes infected with control or HoxD3-expressing retrovirus. The top band shows the specific 415-bp band corresponding to HoxD3 and the bottom 395-bp band shows the relative levels of 18s RNA in each sample. b: Photomicrographs showing the relative degree of migration by control or HoxD3-expressing keratinocytes 24 hours after application of scratch wounds (original magnification, ×10). The black boxes indicate the initial wound area. c: Quantitative analysis of keratinocyte migration after infection with control (▪) or HoxD3 ( )-expressing retrovirus. **, P < 0.05, n = 4. d: DNA synthesis in control (▪) or HoxD3 (
)-expressing retrovirus. **, P < 0.05, n = 4. d: DNA synthesis in control (▪) or HoxD3 ( )-expressing keratinocytes as measured after incorporation of 10 μmol/L BrdU, n = 5.
)-expressing keratinocytes as measured after incorporation of 10 μmol/L BrdU, n = 5.
HoxD3 Gene Transfer Is Associated with Normal Tissue Remodeling
Finally, because HoxD3 induces such a robust expression and deposition of collagen, we investigated whether these wounds would eventually display evidence of abnormal wound healing, angiogenesis, or altered cellular phenotypes. Histological analysis of 0.8-cm wounds was performed 21 and 42 days after treatment with HoxD3. After 21 days (Figure 8, a and b) ▶ trichrome staining reveals significant amounts of collagen deposited in the HoxD3-treated wounds. However, by 42 days (Figure 8, c and d) ▶ the relative amount of collagen present at the site of repair was reduced and accompanied by a decrease in vascular density in the healed wound area consistent with normal wound repair and remodeling. The HoxD3-treated wounds appear similar to control cDNA-treated wounds after 42 days, which were also healed at this time point (Figure 8, e and f) ▶ .
Figure 8.
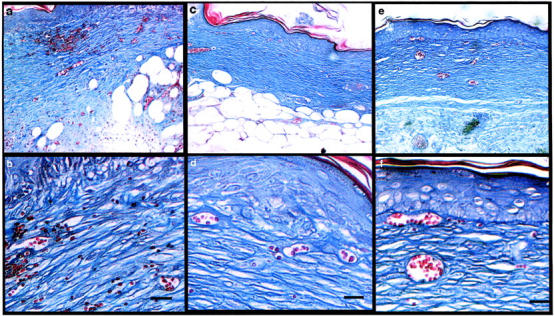
Appearance of wounds 21 and 42 days after gene transfer. a: Histological appearance of healed wounds 21 days after application of HoxD3. Collagen is stained blue. b: Higher magnification of a showing organized collagen fibrils and numerous small capillaries in the area. c: Appearance of healed wound tissue 42 days after application of HoxD3. d: Higher magnification of c showing organized collagen fibrils (blue) and small blood vessels. e: Appearance of healed wound tissue 42 days after application of βgal (control) expression plasmid. f: Higher magnification of e. Scale bars, 35 μm (b, d).
Discussion
In our previous studies, we demonstrated that expression of the homeobox transcription factor, HoxD3, is decreased in wounds of genetically diabetic mice with impaired healing as compared to wild-type mice with normal healing wounds. 7 We now show that restoring expression of HoxD3 in diabetic wounds via gene transfer improves wound healing by enhancing collagen production, angiogenesis and wound closure in db/db mice.
Efficient wound repair is a coordinated process that not only requires an increase in the synthesis and deposition of collagen and angiogenesis, but also migration of fibroblasts and keratinocytes into the wound area. Not surprisingly, impaired wound healing in db/db mice has been linked with reduced expression and deposition of type I collagen (Col1A1) and angiogenesis. 4 Our previous work showed that overexpression of HoxD3 can induce a marked increase in Col1A1 mRNA in cultured endothelial cells 7 and our current studies indicate that HoxD3 also enhances Col1A1 mRNA expression and deposition in vivo. Furthermore, we have shown that HoxD3 also increases expression of the proangiogenic β3-integrin as well as expression of uPA to promote EC migration. 11 Thus not surprisingly, administration of HoxD3 in vivo also leads to an increased β3-integrin expression and angiogenesis in db/db wounds. Our findings are also consistent with recent studies in which mice genetically deficient in PAI-1, an inhibitor of uPA-mediated proteolysis, show a significantly accelerated rate of wound closure. 23
We show that a HA-tagged HoxD3 fusion construct was able to transfect the cells of the open wound and that expression of the epitope-tagged HoxD3 was observed for at least 7 days after wounding. In addition to expression in endothelial cells in the granulation tissue, we also observed expression of the Hox transgene in fibroblasts as well as basal keratinocytes of the diabetic wound. Our results also suggest that HoxD3 is primarily stimulating the repair programs in endothelial cells and fibroblasts and only indirectly enhancing keratinocyte migration. Our previous studies demonstrated that HoxD3 is particularly effective in promoting an angiogenic phenotype in cultured microvascular endothelial cells. 11 We also show that HoxD3 can stimulate collagen synthesis in transfected fibroblasts and thus it is likely that uptake and expression of HoxD3 by wound fibroblasts plays a significant role in collagen synthesis in our model of diabetic wound healing. It is also worth noting that we did not observe any differences in the ability of HoxD3-expressing fibroblasts to contract collagen gels as compared to control-transfected fibroblasts (N. Boudreau, unpublished observations). HoxD3 however does not directly enhance migration of keratinocytes in vitro. Therefore, the increased rate of re-epithelialization observed in HoxD3-treated wounds in vivo may be attributed to the increased deposition of collagen. Previous studies showed that adhesion to collagen could activate nonmotile keratinocytes and plating keratinocytes on type I collagen could directly promote migration. 24,25 Likewise transforming growth factor-β1, a cytokine known to enhance extracellular matrix and collagen production, accelerates healing in diabetic animals, despite the fact that it can inhibit keratinocyte proliferation. 26,27 We have also observed that although HoxD3 is normally expressed by cultured endothelial cells and fibroblasts, it is not expressed by cultured keratinocytes (N. Boudreau, unpublished) and in fact a recent study suggested that increasing expression of the HoxD3 target gene, αvβ3 in keratinocytes may enhance their adhesion to fibrinogen in the clot and possibly retard sloughing of the eschar. 28 Together these findings suggest that limiting HoxD3 expression to fibroblasts or endothelial cells might further improve wound repair.
Because it was difficult to accurately measure changes in wound healing rates between the HoxD3-treated wounds and control in our smaller (0.8 cm) wound model, we used a larger excisional wound (2.5 cm) to evaluate the wound closure rate. On average, the untreated diabetic wound closed at least 2 weeks later than HoxD3-treated wounds. More rapid wound closure in HoxD3-treated diabetic wounds indicated that increased collagen synthesis and angiogenesis associated with HoxD3 transfection had a measurable affect on the more biologically relevant parameter of wound size. This is not surprising as it correlates to the proliferative phase of wound healing. 29
Remarkably, this improvement in wound closure required only a single application of HoxD3. Because we observed such a robust induction of collagen synthesis and deposition with this single treatment, restoring expression of other factors lacking in the diabetic wound environment, rather than repeated treatment with HoxD3 may be required to further improve the rate of wound closure. It is also worth noting, that in wild-type animals in which HoxD3 is abundantly expressed, 7 topical application of HoxD3 does not further improve healing (S. L. Hansen, unpublished). Although we observed expression of the HoxD3 transgene for up to 10 days after application, we observed positive effects on wound healing for longer periods. It is not clear whether low, almost undetectable levels of HoxD3 protein persist for longer periods or whether the initial application of HoxD3 is sufficient to initiate a cascade of events such as increased formation of granulation tissue that secondarily allows wound healing to proceed. For example, although HoxD3 does not directly promote migration in cultured keratinocytes we observe increased re-epithelialization of wounds in vivo suggesting that HoxD3 creates of a favorable microenvironment for keratinocyte migration. Similarly, previous studies have suggested that oxygen is necessary for sustained fibroblast synthesis of collagen 30 and thus the direct improvement in tissue perfusion via HoxD3-mediated angiogenesis may act to prolong collagen synthesis in the wound.
Previous studies using microinjection of naked DNA or direct administration of plasmid DNA after mild abrasion in the skin also showed a rapid but transient expression of the transgenes with peak expression observed anywhere between 3 to 72 hours and low to undetectable levels by 7 days after gene transfer. 31-34 The longer duration of expression of the HoxD3 transgene in our current study is likely attributed to the methylcellulose carrier that retains DNA at the wound site and slows diffusion of the plasmid into the wound and tissue fluid. A recent study also suggested that a major limitation of plasmid DNA gene transfer was the inability of plasmid DNA to effectively enter the nucleus. 34 However, repeated applications of plasmid DNA did not enhance efficiency but in fact had a detrimental effect on healing. By comparison, the methylcellulose carrier method described here yields a robust, widespread expression of the transgene after a single application and allows the use of relatively lower concentrations of plasmid DNA per mm of wound area.
A number of previous studies have shown that application of either recombinant proteins, adenovirus, or plasmid DNAs expressing a variety of cytokines absent or reduced in diabetic wounds can also improve wound repair. 5,20,34-37 For example addition of platelet-derived growth factor, bFGF, keratinocyte growth factor, epidermal growth factor, transforming growth factor-β1, and vascular endothelial growth factor have all been successfully used to accelerate wound repair. It would be of interest to determine whether cDNAs expressing these cytokines were relatively more effective by using the methylcellulose method described here. Also because many of the previous studies have used different size wounds and a variety of methods to deliver the cytokines, it is not possible to directly compare the effectiveness of HoxD3 with these factors. However, because both bFGF and epidermal growth factor induce expression of HoxD3 in cultured endothelial cells, it is possible that many of these cytokines work by reactivating expression of Hox transcription factors, which subsequently activate expression of the wound repair programs in the appropriate cells. It will also be of interest to determine whether HoxD3 expression is also altered in wounds in humans with type I or type II diabetes.
Despite the robust increase in collagen synthesis and deposition by HoxD3, no evidence of abnormal wound healing was observed in mice up to 90 days after gene transfer. Histologically, the HoxD3-treated wounds showed normal remodeling of collagen and wound tissue throughout time, suggesting that high levels of expression of the transgene during the early stages in wound repair was sufficient to induce a prolonged but not permanent change in cellular phenotype. Our inability to detect the fusion protein after 10 days suggests that the plasmid was only transiently expressed and not permanently integrated into the cellular DNA.
Importantly, although sustained high levels of HoxD3 expression can lead to generation of hemangioma-like structures, we did not observe any evidence of hemangioma or hemorrhage in HoxD3-treated animals. 11 Interestingly, sustained high levels of vascular endothelial growth factor expression arising from implantation of genetically engineered myoblasts into ischemic limbs also generates hemangioma-like structures, and emphasizes the need for controlled, transient expression of proangiogenic genes for effective therapeutic intervention. 38 The lack of abnormal wound healing or vascular malformations along with the normal remodeling seen in HoxD3-treated wounds is consistent with a transient expression of the HoxD3 transgene as suggested by the inability to detect the fusion protein or plasmid DNA after 7 to 10 days.
Together these results indicate that a single application of HoxD3 DNA incorporated into an inert methylcellulose patch not only provides a simple means to enhance angiogenesis and diabetic wound repair but is also devoid of complications arising from prolonged or sustained high levels of angiogenic gene expression associated with other gene transfer protocols.
Acknowledgments
We thank Irene Cheung, Michael Brewer, and Meritxell Carrio for technical assistance.
Footnotes
Address reprint requests to Nancy Boudreau, Surgical Research Laboratory, Dept. of Surgery, Box 1302, University of California San Francisco, San Francisco, CA 94143. E-mail: nancyjb@itsa.ucsf.edu.
Supported by the National Institutes of Health (K08GM00674 to D. M. Y., NRSA F32GM2084901 to S. L. H., and P50GM27345 to N. B.).
References
- 1.Arbiser JL: Angiogenesis and the skin: a primer. J Am Acad Dermatol 1996, 34:486-497 [DOI] [PubMed] [Google Scholar]
- 2.Gailit J, Clark RA: Wound repair in the context of extracellular matrix. Curr Opin Cell Biol 1994, 6:717-725 [DOI] [PubMed] [Google Scholar]
- 3.Streit M, Velasco P, Riccardi L, Spencer L, Brown LF, Janes L, Lange-Asschendeldt B, Yano K, Hawighorst T, Iruela-Arispe LM, Detmar M: Thrombospondin-1 suppresses wound healing and granulation tissue formation in the skin of transgenic mice. EMBO J 2000, 19:3272-3282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodson WH, Hunt TK: Studies of wound healing in experimental diabetes mellitus. J Surg Res 1997, 22:221-227 [DOI] [PubMed] [Google Scholar]
- 5.Greenhalgh DG, Sprugel KH, Murray MJ, Ross R: PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am J Pathol 1990, 136:1235-1246 [PMC free article] [PubMed] [Google Scholar]
- 6.Werner S, Breeden M, Hubner G, Greenhalgh DG, Longaker MT: Induction of keratinocyte growth factor expression is reduced and delayed during wound healing in the genetically diabetic mouse. J Invest Dermatol 1994, 103:469-473 [DOI] [PubMed] [Google Scholar]
- 7.Uyeno LA, Newman-Keagle JA, Cheung I, Hunt TK, Young DM, Boudreau N: Hox D3 expression in normal and impaired wound healing. J Surg Res 2001, 100:46-56 [DOI] [PubMed] [Google Scholar]
- 8.Stelnicki EJ, Komuves IG, Kwong AO, Holmes D, Klien P, Rozenfeld S, Lawrence HJ, Adzick NS, Harrison M, Largman C: Homeobox genes exhibit spatial and temporal changes in expression during human skin development. J Invest Dermatol 1998, 110:110-115 [DOI] [PubMed] [Google Scholar]
- 9.Stelnicki EJ, Arbeit J, Cass DL, Saner C, Harrison M, Largman C: Modulation of the human homeobox genes Prx-2 and Hox B13 in scarless fetal wounds. J Invest Dermatol 1998, 111:57-63 [DOI] [PubMed] [Google Scholar]
- 10.Reiger E, Bijl JJ, van Oostveen JW, Soyer HP, Oudejans CBM, Jiwa NM, Walboomers JMM, Meijer CJLM: Expression of the homeobox gene Hox C4 in keratinocytes of normal skin and epithelial skin tumors is correlated with differentiation. J Invest Dermatol 1994, 103:341-346 [DOI] [PubMed] [Google Scholar]
- 11.Boudreau N, Andrews C, Srebow A, Ravanpay A, Cheresh DA: Hox D3 induces an angiogenic phenotype in endothelial cells. J Cell Biol 1997, 139:257-264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Myers C, Charboneau A, Boudreau N: Hox B3 promotes capillary morphogenesis and angiogenesis. J Cell Biol 2000, 148:343-352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boudreau N, Bissell MJ: Extracellular matrix signaling: integration of form and function in normal and malignant cells. Curr Opin Cell Biol 1998, 10:640-646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edelman GM, Jones FS: Outside and downstream of the homeobox. J Biol Chem 1993, 268:20683-20686 [PubMed] [Google Scholar]
- 15.Cillo C, Faiella A, Cantile M, Boncinelli E: Homeobox genes and cancer. Exp Cell Res 1999, 248:1-9 [DOI] [PubMed] [Google Scholar]
- 16.Omatu H: Overexpression of human homeobox gene in lung cancer A549 cells results in enhanced motile and invasive properties. Hokkaido Igaku Zasshi 1999, 74:367-376 [PubMed] [Google Scholar]
- 17.Beer HD, Longaker MT, Werner S: Reduced expression of PDGF and PDGF receptors during impaired wound healing. J Invest Dermatol 1996, 109:132-138 [DOI] [PubMed] [Google Scholar]
- 18.Frank S, Hubner G, Breier G, Longaker MT, Greenhalgh DG, Werner S: Regulation of vascular endothelial growth factor expression in cultured keratinocytes. Implication for normal and impaired wound healing. J Biol Chem 1995, 270:12607-12613 [DOI] [PubMed] [Google Scholar]
- 19.Bitar MS, Labbad ZN: Transforming growth factor-B and insulin-like growth factor-I in relation to diabetes-induced impairment of wound healing. J Surg Res 1996, 61:113-119 [DOI] [PubMed] [Google Scholar]
- 20.Tsuboi R, Rifkin DB: Recombinant bFGF stimulates wound healing in healing-impaired db/db mice. J Exp Med 1990, 172:245-251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rivard A, Silver M, Chen D, Kearney M, Magner M, Annex B, Peters K, Isner JM: Rescue of diabetes-related impairment of angiogenesis by intramuscular gene therapy with adeno-VEGF. Am J Pathol 1999, 154:355-363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ades W, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC, Lawley TJ: HMEC-1 establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol 1992, 99:683-690 [DOI] [PubMed] [Google Scholar]
- 23.Chan J, Duszczyszyn DA, Castellino FJ, Ploplis VA: Accelerated skin wound healing in plasminogen activator inhibitor-1-deficient mice. Am J Pathol 2001, 159:1681-1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo M, Toda K, Grinnell F: Activation of human keratinocyte migration on type I collagen and fibronectin. J Cell Sci 1990, 96:197-205 [DOI] [PubMed] [Google Scholar]
- 25.Woodley DT, Bachmann PM, O’Keefe EJ: Laminin inhibits human keratinocyte migration. J Cell Physiol 1988, 136:140-146 [DOI] [PubMed] [Google Scholar]
- 26.Mustoe TA, Pierce GF, Thomason A, Gramates P, Sporn MB, Deuel TF: Accelerated healing of incisional wounds in rats induced by transforming growth factor-beta. Science 1987, 237:1333-1336 [DOI] [PubMed] [Google Scholar]
- 27.Sarret Y, Woodley DT, Grigsby K, Wynn K, O’Keefe EJ: Human keratinocyte locomotion: the effect of selected cytokines. J Invest Dermatol 1992, 98:2-6 [DOI] [PubMed] [Google Scholar]
- 28.Kubo M, Van de Water L, Plantefaber L, Mosesson MW, Simon M, Tonnesen MG, Taichman L, Clark RAF: Fibrinogen and fibrin are anti-adhesive for keratinocytes: a mechanism for fibrin eschar slough during wound repair. J Invest Dermatol 2001, 117:1369-1378 [DOI] [PubMed] [Google Scholar]
- 29.Hunt TK, Hopf H, Hussain Z: Physiology of wound healing. Adv Skin Wound Care 2000, 13:6-11 [PubMed] [Google Scholar]
- 30.Feng JJ, Hussain MZ, Hunt TK: Angiogenesis in wound healing. J Surg Pathol 1998, 3:1-8 [Google Scholar]
- 31.Hennge UR, Chan EF, Foster RA, Walker PS, Vogel JC: Cytokine gene expression in epidermis with biological effects following injection of naked DNA. Nat Genet 1995, 10:161-166 [DOI] [PubMed] [Google Scholar]
- 32.Hennge UR, Pfutzner W, Williams M, Goos M, Vogel JC: Efficient expression of naked plasmid DNA in mucosal epithelium: prospective for the treatment of skin lesions. J Invest Dermatol 1998, 111:605-608 [DOI] [PubMed] [Google Scholar]
- 33.Yu WH, Kashani-Sabet M, Liggitt D, Moore D, Heath TD, Debs RJ: Topical gene delivery to murine skin. J Invest Dermatol 1999, 112:370-375 [DOI] [PubMed] [Google Scholar]
- 34.Byrnes CK, Khan FH, Nass PH, Hatoum C, Duncan MD, Harmon JW: Success and limitations of a naked plasmid transfection protocol for keratinocyte growth factor-1 to enhance cutaneous wound healing. Wound Rep Regen 2001, 9:341-346 [DOI] [PubMed] [Google Scholar]
- 35.Andree C, Swain WF, Page CP, Macklin MD, Slama J, Hatzis D, Eriksson E: In vivo transfer and expression of a human epidermal growth factor gene accelerates wound repair. Proc Natl Acad Sci USA 1994, 91:12188-12192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benn SI, Whitsett JS, Brodaly KN, Nanney LB, Perkins D, He L, Patel M, Morgan JR, Swain WF, Davidson JM: Particle mediated gene transfer with transforming growth factor beta-1 cDNAs enhance wound repair in rat skin. J Clin Invest 1996, 98:2894-2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romano Di Peppe S, Mangoni A, Zambruno G, Spinetti G, Melillo G, Napolitano M, Capogrossi MC: Adenovirus-mediated VEGF(165) gene transfer enhances wound healing by promoting angiogenesis in CD1 diabetic mice. Gene Ther 2002, 9:1271-1277 [DOI] [PubMed] [Google Scholar]
- 38.Springer ML, Chen AS, Kraft PE, Bednarski M, Blau HM: VEGF gene delivery to muscle: potential role for vasculogenesis in adults. Mol Cell 1998, 2:549-558 [DOI] [PubMed] [Google Scholar]