Abstract
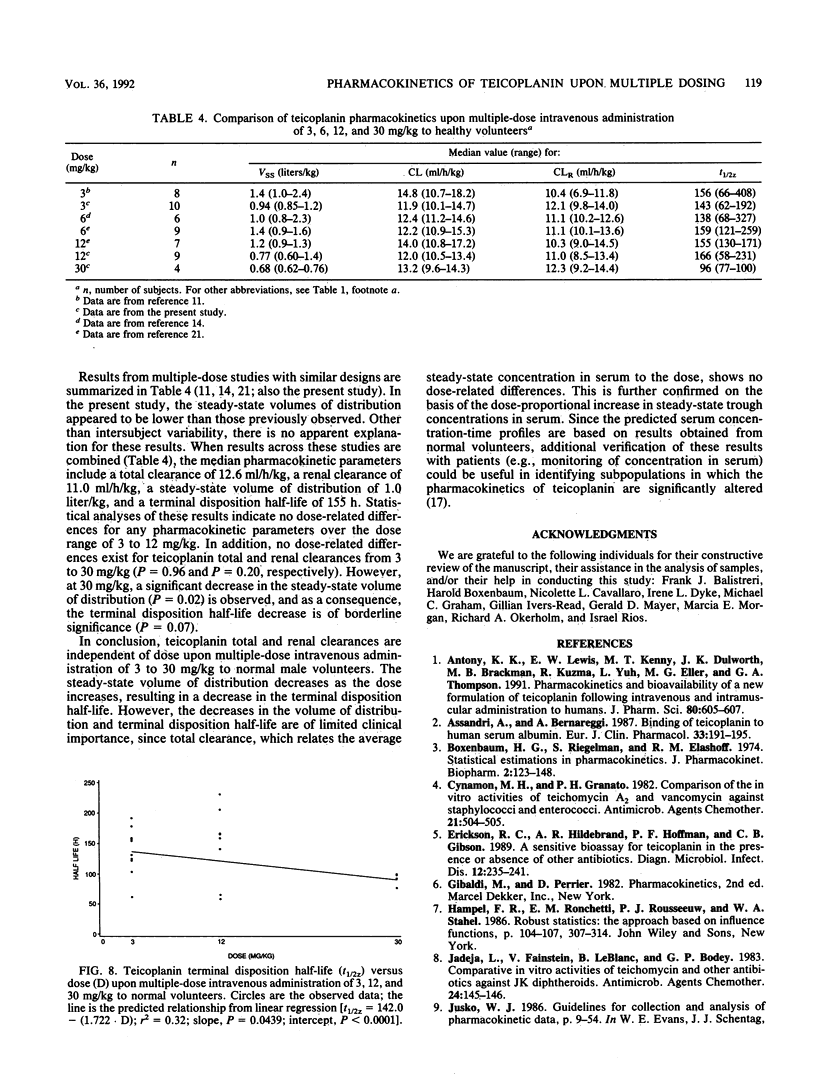
Teicoplanin pharmacokinetics were evaluated after multiple-dose intravenous administration to healthy male volunteers by using a randomized, double-blind, parallel design. Doses of 3, 12, or 30 mg of teicoplanin per kg of body weight were administered every 24 h for 14 days as 60-min constant-rate intravenous infusions. Blood and urine samples were collected over 21 days and analyzed by a microbiological assay. Twenty-three subjects were included in the pharmacokinetic analysis. The median pharmacokinetic parameters upon multiple-dose intravenous administration of 3, 12, and 30 mg/kg included steady-state volumes of distribution of 0.94, 0.77, and 0.68 liter/kg; total clearances of 11.9, 12.0, and 13.2 ml/h/kg; and terminal disposition half-lives of 143, 166, and 96 h, respectively. Renal clearance accounted for approximately 95% of total clearance. No dose-related differences existed for teicoplanin total or renal clearance. The steady-state volume of distribution decreased significantly with increasing doses. As a result of the decrease in the volume of distribution, the terminal disposition half-life at 30 mg/kg was significantly decreased. However, the decreases in the volume of distribution and terminal disposition half-life are of limited clinical importance, since steady-state trough concentrations in serum increase in proportion to dose. Combined results of all multiple-dose studies with similar durations of sample collection indicate no dose-related differences for any pharmacokinetic parameters from 3 to 12 mg/kg. As observed in the present study, no dose-related differences exist for teicoplanin total and renal clearances from 3 to 30 mg/kg. However, at 30 mg/kg, a significant decrease in the steady-state volume of distribution is observed. As a consequence of the reduction in the volume of distribution at 30 mg/kg with no change in clearance, the terminal disposition half-life is decreased.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antony K. K., Lewis E. W., Kenny M. T., Dulworth J. K., Brackman M. B., Kuzma R., Yuh L., Eller M. G., Thompson G. A. Pharmacokinetics and bioavailability of a new formulation of teicoplanin following intravenous and intramuscular administration to humans. J Pharm Sci. 1991 Jun;80(6):605–607. doi: 10.1002/jps.2600800621. [DOI] [PubMed] [Google Scholar]
- Assandri A., Bernareggi A. Binding of teicoplanin to human serum albumin. Eur J Clin Pharmacol. 1987;33(2):191–195. doi: 10.1007/BF00544566. [DOI] [PubMed] [Google Scholar]
- Boxenbaum H. G., Riegelman S., Elashoff R. M. Statistical estimations in pharmacokinetics. J Pharmacokinet Biopharm. 1974 Apr;2(2):123–148. doi: 10.1007/BF01061504. [DOI] [PubMed] [Google Scholar]
- Cynamon M. H., Granato P. A. Comparison of the in vitro activities of teichomycin A2 and vancomycin against staphylococci and enterococci. Antimicrob Agents Chemother. 1982 Mar;21(3):504–505. doi: 10.1128/aac.21.3.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson R. C., Hildebrand A. R., Hoffman P. F., Gibson C. B. A sensitive bioassay for teicoplanin in serum in the presence or absence of other antibiotics. Diagn Microbiol Infect Dis. 1989 May-Jun;12(3):235–241. doi: 10.1016/0732-8893(89)90020-5. [DOI] [PubMed] [Google Scholar]
- Jadeja L., Fainstein V., LeBlanc B., Bodey G. P. Comparative in vitro activities of teichomycin and other antibiotics against JK diphtheroids. Antimicrob Agents Chemother. 1983 Aug;24(2):145–146. doi: 10.1128/aac.24.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Labthavikul P. In vitro activity of teichomycin compared with those of other antibiotics. Antimicrob Agents Chemother. 1983 Sep;24(3):425–428. doi: 10.1128/aac.24.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oie S., Tozer T. N. Effect of altered plasma protein binding on apparent volume of distribution. J Pharm Sci. 1979 Sep;68(9):1203–1205. doi: 10.1002/jps.2600680948. [DOI] [PubMed] [Google Scholar]
- Outman W. R., Nightingale C. H., Sweeney K. R., Quintiliani R. Teicoplanin pharmacokinetics in healthy volunteers after administration of intravenous loading and maintenance doses. Antimicrob Agents Chemother. 1990 Nov;34(11):2114–2117. doi: 10.1128/aac.34.11.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallanza R., Berti M., Goldstein B. P., Mapelli E., Randisi E., Scotti R., Arioli V. Teichomycin: in-vitro and in-vivo evaluation in comparison with other antibiotics. J Antimicrob Chemother. 1983 May;11(5):419–425. doi: 10.1093/jac/11.5.419. [DOI] [PubMed] [Google Scholar]
- Rosenbaum P. R., Rubin D. B. The bias due to incomplete matching. Biometrics. 1985 Mar;41(1):103–116. [PubMed] [Google Scholar]
- Rowland M. Clinical pharmacokinetics of teicoplanin. Clin Pharmacokinet. 1990 Mar;18(3):184–209. doi: 10.2165/00003088-199018030-00002. [DOI] [PubMed] [Google Scholar]
- Rybak M. J., Lerner S. A., Levine D. P., Albrecht L. M., McNeil P. L., Thompson G. A., Kenny M. T., Yuh L. Teicoplanin pharmacokinetics in intravenous drug abusers being treated for bacterial endocarditis. Antimicrob Agents Chemother. 1991 Apr;35(4):696–700. doi: 10.1128/aac.35.4.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson G. A., Shumaker R. C. MLTIDOSE: a multiple-dose simulation program for linear systems characterized by exponential functions. Drug Metab Rev. 1989;21(3):463–469. doi: 10.3109/03602538909030306. [DOI] [PubMed] [Google Scholar]
- Traina G. L., Bonati M. Pharmacokinetics of teicoplanin in man after intravenous administration. J Pharmacokinet Biopharm. 1984 Apr;12(2):119–128. doi: 10.1007/BF01059273. [DOI] [PubMed] [Google Scholar]
- Verbist L., Tjandramaga B., Hendrickx B., Van Hecken A., Van Melle P., Verbesselt R., Verhaegen J., De Schepper P. J. In vitro activity and human pharmacokinetics of teicoplanin. Antimicrob Agents Chemother. 1984 Dec;26(6):881–886. doi: 10.1128/aac.26.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J. G. Linear pharmacokinetic equations allowing direct calculation of many needed pharmacokinetic parameters from the coefficients and exponents of polyexponential equations which have been fitted to the data. J Pharmacokinet Biopharm. 1976 Oct;4(5):443–467. doi: 10.1007/BF01062831. [DOI] [PubMed] [Google Scholar]



