Abstract
Trophinin, tastin, and bystin have been identified as molecules potentially involved in human embryo implantation. Both trophoblasts and endometrial epithelial cells express trophinin, which mediates apical cell adhesion through homophilic trophinin-trophinin binding. We hypothesized that trophinin’s function in embryo implantation is unique to humans and investigated the expression of trophinin, tastin, and bystin in ectopic pregnancy, a condition unique to humans. In tubal pregnancies, high levels of all three were found in both trophoblasts and fallopian tubal epithelia. Trophinin expression in maternal cells was particularly high in the area adjacent to the trophoblasts, whereas trophinin was barely detectable in intact fallopian tubes from women with in utero pregnancies or without pregnancies. When explants of intact fallopian tube were incubated with the human chorionic gonadotrophin (hCG), trophinin expression was enhanced in epithelial cells. Since the trophectoderm of the human blastocyst secretes hCG before and after implantation, these results suggest that hCG from the human embryo induces trophinin expression by maternal cells. As both β-subunit of hCG and trophinin genes have diverged in mammals, the present study suggests a unique role of hCG and trophinin in human embryo implantation, including the pathogenesis of ectopic pregnancy.
Ectopic pregnancy occurs at a rate of 0.25 to 1.4% in all pregnancies in humans, and over 95% of all ectopic pregnancies occur in the fallopian tube. 1,2 Extrauterine implantation does not occur naturally or experimentally in other animals including non-human primates. 3-5 It is not known why extrauterine implantation occurs only in humans.
The human embryo enters the uterine cavity 4 days after ovulation. 6 Blastocyst implantation takes place around day 7 after ovulation. During this period, the endometrial luminal epithelium becomes receptive to blastocyst adhesion in response to rising serum progesterone and peptide hormones from the newly formed corpus luteum, opening an implantation window. 7,8
The blastocyst free from the zona pellucida is sticky in vitro. Human trophoblastic cells avidly bind to fibronectin, laminin, and type IV and V collagen, 9 all of which are present in decidualized endometrial stroma. Therefore, injuries to the epithelium that expose this underlying matrix can enable trophoblast elements to adhere and grow, potentially causing ectopic pregnancy. The receptive human endometrium expresses αV/β3 integrin on the apical surfaces of the luminal epithelium. 8 This integrin type is also found in the normal human fallopian tube epithelium, suggesting the existence of a tubal implantation window. 10 However, given that tubal pregnancy does not occur in animals, it is difficult to explain the mechanism of ectopic pregnancy by the activity of evolutionarily conserved molecules such as integrins.
Previously, we identified and characterized a novel molecular complex, composed of trophinin, tastin and bystin, mediating apical cell adhesion between human trophoblasts and endometrial epithelial cells. 11-17 Trophinin is an integral membrane protein, and part of this polypeptide is exposed on the cell surface. Tastin and bystin are cytoplasmic proteins required for trophinin to exhibit efficient cell adhesion activity. Trophoblastic cells and endometrial epithelial cells expressing these molecules adhere to each other at their respective apical plasma membranes. Human endometrium tightly regulates expression of trophinin, which is expressed only within a restricted region of the apical side of luminal endometrial epithelium at a time coincident with the “implantation window.” 11 In the human placenta at early stages of pregnancy, trophinin, tastin and bystin are strongly expressed in the trophoblast of chorionic villi and in the endometrial glandular epithelium, particularly at the utero-placental interface. 15 Studies of mouse trophinin indicate significant differences from human trophinin in expression patterns and roles in vivo. 16,17 Trophinin is not found in mouse trophoblastic cells during and after implantation. 16,17 Trophinin null mutant mice show no defects in embryo implantation. 17 The in vivo role of human trophinin in embryo implantation remains to be proved.
We hypothesized that trophinin’s function in embryo implantation is unique to humans. The present study was undertaken to evaluate this hypothesis by determining the expression pattern of trophinin in ectopic pregnancy, a condition unique to humans. Here we show that, in tubal pregnancies, trophinin is strongly expressed by both the embryonic trophoblasts and maternal fallopian tube epithelia. The expression pattern of trophinin suggests that a factor derived from the implanting embryo induces trophinin expression by maternal cells. We found that the human chorionic gonadotrophin (hCG) induces trophinin expression by epithelial cells in the fallopian tube, which was determined by quantitative RT-PCR for trophinin transcripts and immunohistochemistry for trophinin protein. Thus the results suggest strongly that trophinin and hCG together play unique roles in human embryo implantation, including the pathogenesis of ectopic pregnancies.
Materials and Methods
Tissue Collection
Twenty-three tissue blocks of fallopian tubes resected from 23 patients with ectopic tubal pregnancy were retrieved from the archives of Shinshu University Hospital and Keio University Hospital, Japan. In addition, paraffin blocks of intact fallopian tubes removed from 11 patients with benign uterine or ovarian diseases were also retrieved from the same archives. Three of these 11 patients were found to be associated with in utero pregnancy. All archival samples were obtained with informed consent. Age, gestational age, or menstrual cycle of the patients are listed in Tables 1 and 2 ▶ . These specimens were fixed for 48 hours in 20% buffered formalin (pH 7.4), embedded in paraffin, and sectioned serially at 3-μm thickness for immunohistochemistry or at 7-μm thickness for in situ hybridization.
Table 1.
Expression of Trophinin, Tastin, and Bystin Proteins in the Fallopian Tube from Patients Unrelated to Tubal Pregnancy
| Patient no. | Age | Menstrual cycle or gestational age (day) | Trophinin | Tastin | Bystin |
|---|---|---|---|---|---|
| 1 | 24 | proliferative phase | +* | +++ | − |
| 2 | 28 | 98 days pregnancy | − | ++ | +++ |
| 3 | 42 | 49 days pregnancy | + | +++ | +++ |
| 4 | 19 | secretory phase | − | + | − |
| 5 | 19 | secretory phase | − | ++ | ++ |
| 6 | 48 | secretory phase | + | ++ | ++ |
| 7 | 47 | proliferative phase | + | ++ | ++ |
| 8 | 50 | proliferative phase | − | ++ | +++ |
| 9 | 50 | proliferative phase | − | + | ++ |
| 10 | 40 | 44 days pregnancy | − | +++ | + |
| 11 | 37 | proliferative phase | − | + | ++ |
*−, +, ++, and +++ show no, minimal, moderate, and maximal immunohistochemical reactivity, respectively.
In parallel, four tissue specimens of fresh intact fallopian tubes were obtained from the patients who underwent hysterectomy for uterine diseases after informed consent was obtained at Keio University Hospital. All of the patients were cyclic women, and their ages ranged from 26 to 52 years. The fallopian tube specimens from two patients (26- and 43-years-old) were cut into small pieces and cultured at 37°C in a 5% CO2 incubator in Ham’s F-12 medium (Nissui Pharmaceutical Co, Tokyo, Japan) containing 10% fetal calf serum in the presence of 10 IU/ml or 100 IU/ml hCG (from pregnancy urine, Teikoku Hormone Mfg, Tokyo, Japan). As a control, hCG was omitted from the culture medium. On culturing for 6 and 24 hours, the explants were subjected to quantitative analysis of trophinin mRNA. On the other hand, the fallopian tube specimens from two patients (41- and 52-years-old) were cultured for 24 hours in the presence or absence of hCG in the similar manner as described above, and tissues were analyzed for trophinin protein by immunohistochemistry.
Immunohistochemistry
The tissue specimens were fixed for 48 hours in 20% buffered formalin, embedded in paraffin, and sectioned serially at 3-μm thickness. Immunohistochemistry was performed by an indirect immunoperoxidase method using monoclonal antibodies specific for trophinin, bystin, and tastin, as described. 15 Briefly, tissue sections were immersed in absolute methanol containing 0.3% H2O2 for 30 minutes and then washed with PBS. For primary antibodies, monoclonal anti-trophinin (clone 3–11, mouse IgM), anti-bystin (clone 19, mouse IgM), or anti-tastin (clone 38, mouse IgM) antibodies were used, and for the secondary antibody, goat anti-mouse Ig conjugated with horseradish peroxidase (DAKO, Carpinteria, CA) was used. Peroxidase activity was visualized with a diaminobenzidine-hydrogen peroxide solution. Similarly, immunohistochemistry for hCG was performed using polyclonal anti-hCG antibodies (DAKO) and anti-rabbit Ig conjugated with horseradish peroxidase (DAKO) as a secondary antibody. Control experiments were carried out either by omitting the primary antibodies or using an irrelevant isotype-matched primary antibody; ie, HIK1083 (Kanto Kagaku, Tokyo, Japan) specific for GlcNAcα1→4Galβ→R. 18 No specific signal was noted in these control experiments.
In Situ Hybridization
To detect trophinin, tastin, and bystin transcripts, in situ hybridization was carried out using RNA probes specific for these molecules, as described previously. 15,19 Briefly, digoxigenin-labeled anti-sense and sense probes were prepared by in vitro transcription from pGEM-3Zf(+) plasmids (Promega, Madison, WI) containing specific DNA fragments of trophinin (nucleotides −3 to +149; the first nucleotide of the initiation codon is defined as +1), tastin (−26 to +124), and bystin (+627 to +796). The deparaffinized tissue sections from tubal pregnancy and intact fallopian tubes were immersed in 0.2 mol/L HCl for 20 minutes, digested with 100 μg/ml proteinase K at 37°C for 20 minutes, and post-fixed with 4% paraformaldehyde. The slides were rinsed with 2 mg/ml glycine, acetylated for 10 minutes in 0.25% acetic anhydride in 0.1 mol/L triethanolamine (pH 8.0), defatted with chloroform, and air-dried. After prehybridization with 50% deionized formamide/2X SSC for 1 hour at 45°C, the slides were hybridized with 0.5 mg/ml of anti-sense or sense probe in 50% deionized formamide, 2.5 mmol/L EDTA (pH 8.0), 300 mmol/L NaCl, 1X Denhardt’s solution, 10% dextran sulfate, and 1 mg/ml brewer’s yeast tRNA at 45°C for 48 hours. After hybridization, the slides were washed in 50% formamide/2X SSC for 1 hour at 45°C and digested with 10 mg/ml RNase A at 37°C for 30 minutes. After sequential washing with 2X SSC/50% formamide at 45°C for 1 hour and 1X SSC/50% formamide at 45°C for 1 hour, the sections were subjected to immunohistochemistry for detection of hybridized probes using an alkaline phosphatase-conjugated anti-digoxigenin antibody (Roche Molecular Biochemicals, Alameda, CA). Digoxigenin was detected by alkaline phosphatase by incubating with 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium in the presence of 0.2 mmol/L levamisole and 10% polyvinylalcohol for 30 minutes at room temperature. No specific signals were detected in the control experiments using sense probes.
Quantitative Analysis of Trophinin mRNA
The intact fallopian tubes obtained from two patients (26- and 43-years-old) on hysterectomy were cultured in the presence or absence of hCG for 6 hours and for 24 hours as described above. Total RNA was isolated from the tissue explants using Isogen (Nippon Gene, Tokyo, Japan). After digestion with DNase I, reverse transcription of the total RNA was performed in a final volume of 20 μl containing 4 μl of 5X first strand buffer, 2 μl of 2 mmol/L dNTPs, 0.2 μl of 0.1 mol/L DTT, 1 μl of RNase inhibitor, 0.5 μl of M-MLV reverse transcriptase, and 1 μl of oligo dT primer at 42°C for 60 minutes.
Quantitative analysis of trophinin mRNA expressed in the fallopian tubes was carried out using an ABI PRISM 7700 Sequence Detection System (PE Applied Biosystems, Foster City, CA). Specific primer pair and probe were designed with the aid of the Primer Express program (PE Applied Biosystems). Forward and reverse primers for human trophinin were 5′-AGGGAAGAGTTAGGCGATGATTC-3′ (nucleotides +22 to +44), and 5′-TTGGGCTCTGGCCTCAATT-3′ (nucleotides +72 to+ 90), respectively. The TaqMan probe, 5′-CAAATGAAAATCTGCTCCAGGCCTG-3′ (nucleotides +46 to +70), which carries 5′-FAM (6-carboxyfluorescein) reporter label and 3′-TAMURA (6-carboxy-N, N, N′, N′-tetramethylrhodamine) quencher group was synthesized by PE Applied Biosystems. A relative standard curve representing tenfold dilutions of a trophinin cDNA (pcDNAI-trophinin 11 ) ranging from 1010 to 101 copies/ml was used for linear regression analysis for trophinin mRNA. A standard curve ranging from 1010 to 101 copies/ml for a housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), was prepared in the same tube that trophinin cDNA was prepared, and this was used for normalizing the samples. 20
Multiplex PCR was carried out in 50 μl of reaction mixture containing 3 μl of cDNA sample, 1X Universal PCR Master Mix (PE Applied Biosystems), 800 nmol/L of the primer pair for trophinin, 800 nmol/L of the primer pair for GAPDH, 200 nmol/L of the TaqMan probe for trophinin, and 200 nmol/L of the TaqMan probe for GAPDH that carries 5′-VIC reporter label and 3′-TAMURA quencher group (PE Applied Biosystems). These reaction tubes were placed in the ABI PRISM 7700 Sequence Analyzer, and preheated at 95°C for 10 minutes, followed by 50 cycles of denaturation at 95°C for 15 seconds and annealing/extension at 60°C for 1 minute. The quantity of trophinin mRNA was determined using the standard curve of trophinin cDNA on normalizing the sample size by GAPDH. The quantitity of trophinin mRNA was expressed as the relative amount of GAPDH mRNA, thus the ratio of trophinin mRNA to GAPDH multiplied by 100. The assays were performed in duplicate, and the mean values of the two experiments were indicated.
Results
Expression of Trophinin, Tastin, and Bystin by Trophoblasts of Chorionic Villi during Tubal Pregnancy
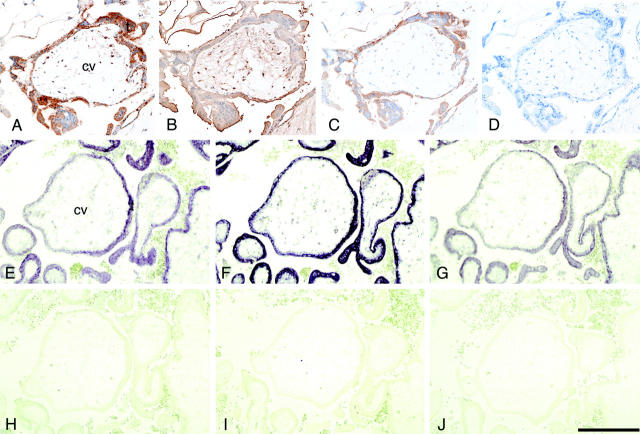
Immunohistochemistry of the fallopian tube from ectopic pregnancy patients using anti-hCG antibodies showed expression of hCG, a trophoblast marker, in the chorionic villous trophoblasts but not in the tubal epithelia (data not shown). Immunohistochemistry showed strong expression of trophinin, tastin, and bystin proteins in trophoblasts of the chorionic villi (Figure 1, A to C) ▶ . These results were confirmed by in situ hybridization, demonstrating that transcripts of these molecules are strongly expressed in trophoblastic cells (Figure 1, E to G) ▶ . The expression patterns of these proteins and transcripts are similar to those observed previously in the human placenta in normal pregnancy. 15
Figure 1.
Expression of trophinin, tastin, and bystin proteins and their transcripts in the chorionic villi from tubal pregnancy. Immunohistochemistry for trophinin (A), tastin (B), bystin (C), second antibody alone (D), and in situ hybridization for trophinin (E, antisense; H, sense), tastin (F, antisense; I, sense), and bystin (G, antisense; J, sense) using digoxigenin-labeled RNA probes. All photographs presented are in the same magnification, and the bar in (J) indicates 200 μm. (cv, chorionic villi; t, trophoblasts).
Overexpression of Trophinin Protein in Maternal Epithelial Cells in Tubal Pregnancy
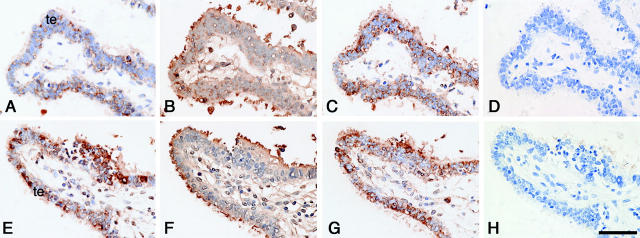
Immunohistochemistry of intact fallopian tubes from non-pregnant and in utero pregnant women showed barely detectable levels of trophinin protein in tubal epithelial cells (Figure 2A ▶ , Table 1 ▶ ). On the other hand, various amounts of tastin and bystin proteins were detected in the cilia and the cytoplasm of the intact fallopian tube epithelia, respectively, regardless of non-pregnancy or in utero pregnancy (Figure 2, B and C ▶ , Table 1 ▶ ).
Figure 2.
Expression of trophinin, tastin, and bystin proteins in intact fallopian tube and in fallopian tube with tubal pregnancy. Immunohistochemistry of an intact fallopian tube (A–D) and a fallopian tube with tubal pregnancy (E–K) for trophinin (A, E, J, K), tastin (B, F), and bystin (C, G). Hematoxylin and eosin-stained specimen from a tubal pregnancy (I), and high magnification of insets showing immunohistochemistry for trophinin in the area close to (J) or distant from (K) the chorionic villi. The implantation site in (I) is marked by arrowheads. All photographs except (I) are in the same magnification, and the bar in (K) indicates 100 μm. Bar in (I) indicates 2.0 mm. (cv, chorionic villi; te, tubal epithelia).
The signals for trophinin protein in tubal epithelia adjacent to the implantation site of tubal pregnancies were remarkably higher (Figure 2E) ▶ than those in intact fallopian tubes (Figure 2A ▶ , Table 2 ▶ ). Trophinin proteins were found in the cytoplasm of tubal epithelia with large apical protrusions or pinopodes (Figure 2E) ▶ . Strong signals for tastin and bystin proteins were also seen in tubal epithelia with tubal pregnancies (Figure 2, F and G ▶ , Table 2 ▶ ). The levels of tastin and bystin appear to be elevated in association with ectopic pregnancy compared to those seen in intact fallopian tubes (Tables 1 and 2) ▶ .
Table 2.
Expression of Trophinin, Tastin, and Bystin Proteins in the Fallopian Tubes during Tubal Pregnancy
| Patient no. | Age | Gestational age (day) | Trophinin | Tastin | Bystin |
|---|---|---|---|---|---|
| 1 | 30 | 41 | +++* | +++ | +++ |
| 2 | 36 | 61 | +++ | ++ | +++ |
| 3 | 24 | 122 | +++ | +++ | +++ |
| 4 | 37 | 73 | +++ | +++ | +++ |
| 5 | 35 | 49 | ++ | +++ | ++ |
| 6 | 29 | 56 | +++ | +++ | +++ |
| 7 | 26 | 44 | +++ | +++ | +++ |
| 8 | 35 | 50 | ++ | ++ | ++ |
| 9 | 37 | 51 | +++ | ++ | +++ |
| 10 | 25 | 50 | +++ | +++ | +++ |
| 11 | 40 | 58 | ++ | +++ | ++ |
| 12 | 30 | 48 | + | + | ++ |
| 13 | 36 | 55 | +++ | +++ | +++ |
| 14 | 36 | 58 | ++ | ++ | ++ |
| 15 | 34 | 49 | +++ | ++ | ++ |
| 16 | 29 | 56 | +++ | ++ | + |
| 17 | 37 | 49 | ++ | ++ | +++ |
| 18 | 22 | 47 | +++ | ++ | +++ |
| 19 | 31 | 52 | ++ | +++ | +++ |
| 20 | 34 | 43 | ++ | + | +++ |
| 21 | 24 | 57 | +++ | +++ | +++ |
| 22 | 29 | 71 | +++ | +++ | +++ |
| 23 | 22 | 47 | ++ | +++ | ++ |
*−, +, ++, and +++ show no, minimal, moderate, and maximal immunohistochemical reactivity, respectively.
In addition, we also found that within a specimen of tubal pregnancy (Figure 2I) ▶ , maternal epithelia close to the chorionic villi showed a stronger signal for trophinin (Figure 2J) ▶ than epithelial cells distant from the chorionic villi (Figure 2K) ▶ . Similar observations that strong signals for trophinin were detected in the maternal endometrial glandular epithelial cells adjacent to the chorionic villi were made previously in human placenta from early pregnancy. 15 These results suggest the possibility that an embryonic factor functions locally to induce trophinin expression in maternal cells.
Expression of Trophinin Transcripts by Maternal Cells in Tubal Pregnancy
To confirm the results obtained by immunohistochemistry, we compared levels of trophinin, tastin, and bystin transcripts in non-pregnancy, in utero pregnancy and tubal pregnancy by in situ hybridization. Thus we examined three intact fallopian tubes from patients unrelated to tubal pregnancy and three fallopian tubes from tubal pregnancies. The results showed that levels of trophinin transcripts in the fallopian tube epithelia from tubal pregnancies were higher than those seen in intact fallopian tubes from in utero pregnancies or non-pregnancies (Figure 3A and G) ▶ . We also observed that signals for trophinin transcripts are strong in the maternal epithelia adjacent to the chorionic villi, whereas epithelia far from the chorionic villi barely express trophinin transcripts (data not shown). These observations suggest that trophinin expression by maternal cells is induced by tubal pregnancy. By contrast, although the expression levels of tastin and bystin mRNAs were slightly increased in tubal pregnancy (Figure 3, B, C, H, and I) ▶ , these differences in tastin and bystin transcripts between intact fallopian tubes and the fallopian tubes from tubal pregnancies were not as significant as those observed for trophinin.
Figure 3.
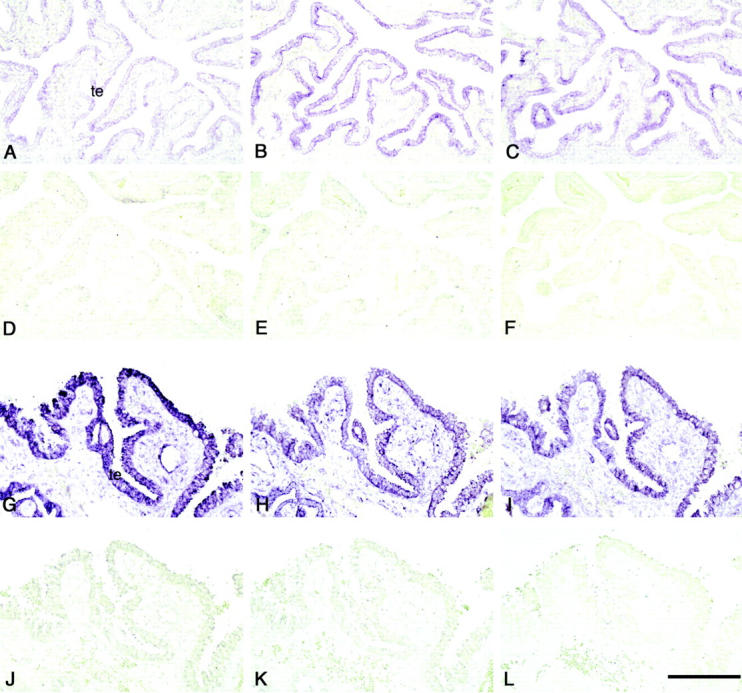
Expression of trophinin, tastin, and bystin transcripts in intact fallopian tube and in that with tubal pregnancy. In situ hybridization of an intact fallopian tube (A–F), and a fallopian tube with tubal pregnancy (G–L) for trophinin (A and G, antisense; D and J, sense), tastin (B and H, antisense; E and K, sense), and bystin (C and I, antisense; F and L, sense) using digoxigenin-labeled RNA probes. All photographs presented are in the same magnification, and the bar in (L) indicates 200 μm. (te, tubal epithelia).
Induction of Trophinin by hCG in Fallopian Tubal Epithelial Cells
Trophinin was barely detectable in intact fallopian tubes, regardless of whether they were derived from pregnant or non-pregnant patients (Figure 2A ▶ , Table 1 ▶ ). This observation suggests that steroid hormones associated with pregnancy are unlikely to promote strong trophinin expression in the tubal epithelial cells.
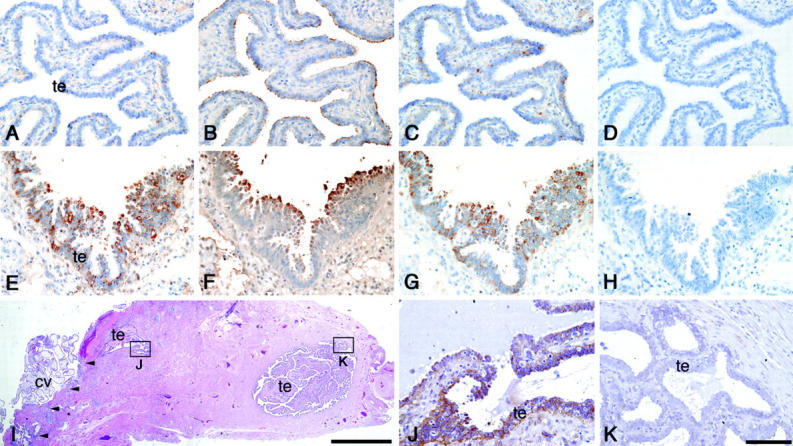
Since it is known that the trophectoderm cells of pre-implantation embryo and chorionic villi trophoblasts secrete hCG, 21-24 we determined if hCG induces trophinin expression by maternal cells. On incubation of explants of the intact fallopian tube with hCG at 100 IU/ml for 24 hours, levels of trophinin proteins were significantly increased (Figure 4, A and E) ▶ . No differences were found in the response to hCG among fallopian tubal explants regardless of whether they were derived from the region close to or far from the uterus. The explants incubated with lower concentrations of hCG (10 IU/ml) also showed elevation of trophinin protein, but at lower levels than at 100 IU/ml (data not shown). On the other hand, significant amounts of tastin and bystin were expressed in intact fallopian tubes (Figure 4, B and C) ▶ , and their levels were not significantly altered by incubation with hCG (Figure 4, F and G) ▶ .
Figure 4.
Comparison of trophinin, tastin, and bystin proteins in explants of intact fallopian tube with or without incubation with hCG. Explants were cultured for 24 hours without hCG (A–D) or with 100 IU/ml hCG (E–H), and were immunostained for trophinin (A, E), tastin (B, F), bystin (C, G), and second antibody alone (D, H). All photographs presented are in the same magnification, and the bar in (H) indicates 50 μm. (te, tubal epithelia).
To examine whether hCG induces the expression of trophinin at the transcriptional level, we determined the quantity of trophinin mRNA in the explants of intact fallopian tube by a real-time RT-PCR method. Thus the fallopian tube specimens isolated from two patients unrelated to the ectopic pregnancy were incubated with hCG, and the quantities of trophinin mRNA were compared with those without hCG. In both experiments, trophinin transcripts in the explants increased in a dose-dependent manner of hCG (Table 3) ▶ . These results strongly suggest that hCG induces the expression of trophinin gene by the fallopian tube epithelia.
Table 3.
Quantitative Analysis of Trophinin mRNA in Explants of the Intact Fallopian Tube Incubated with hCG
| Incubation for 6 hours | Incubation for 24 hours | |||||
|---|---|---|---|---|---|---|
| 0* | 10 | 100 | 0 | 10 | 100 | |
| Experiment 1† | 15.8‡ | 35.4 | 431.2 | 1.1 | 31.2 | 38.7 |
| Experiment 2 | 2.3 | 4.1 | 7.5 | 3.4 | 9.8 | 27.6 |
*, Concentration of hCG (IU/ml).
†, Experiments 1 and 2 were conducted using fallopian tubal explants obtained from each different individual who underwent hysterectomy.
‡, Trophinin:GAPDH mRNA ratios multiplied by 100 are indicated.
Discussion
In this study, we found that trophinin, tastin, and bystin are expressed strongly in the trophoblasts and in maternal epithelia during ectopic tubal pregnancy (Figures 1, 2, and 3 ▶ , Table 2 ▶ ). Expression patterns of these molecules in tubal pregnancy are similar to those seen in placenta during in utero pregnancy. 15 Thus the trophoblasts of chorionic villi and the maternal epithelia adjacent to chorionic villi strongly express trophinin, tastin, and bystin. One intriguing finding in this study is that the intact fallopian tubes isolated from women with in utero pregnancy express negligible levels of trophinin (Table 1) ▶ . This finding indicates that steroid hormones alone cannot induce strong trophinin expression in humans. Furthermore, the results suggest the existence of a locally acting embryonic factor inducing trophinin expression by maternal cells (Figure 2, I to K) ▶ . Thus trophinin expression by tubal epithelial cells depends on the presence of an embryo. This is in sharp contrast to mouse trophinin, whose expression is induced by the ovarian hormone, estrogen, independent of the embryo. 16,17
In this study, we showed that hCG induces the levels of trophinin transcripts in the fallopian tube (Table 3) ▶ . This must lead to an elevation of trophinin protein, which was detected by immunohistochemistry (Figure 4) ▶ . In preimplantation stage human embryos, hCG-β transcripts are detected as early as the two-cell stage, 21 and preimplantation stage blastocysts secrete hCG. 22-24 Thus, it is likely that a human blastocyst secretes hCG before and during implantation. Given that trophinin mediates cell adhesion by homophilic binding, 11 evidence collectively suggests that hCG-β secreted from a blastocyst induces trophinin expression by the maternal epithelium, resulting in trophinin-mediated homophilic binding between the embryo and maternal epithelium.
Using fallopian tubal explants, we found that high concentrations (100 IU/ml) of hCG induce trophinin expression in epithelial cells (Figure 4 ▶ , Table 3 ▶ ). This concentration of hCG has physiological relevance if local concentrations of hCG are considered. In pregnant women 6 to 12 days after ovulation the first sign of pregnancy is an hCG concentration of greater than 0.325 mIU/ml in the urine on the day of blastocyst implantation. 25 Urine from non-pregnant women shows no detectable levels of hCG, indicating that elevated hCG in urine is derived from an implanting blastocyst. As the concentration of hCG in the urine and plasma is similar and the plasma volume of a 50-kg woman is 1900 ml, 26 the total amount of hCG produced by the blastocyst is calculated to be 617.5 mIU (= 0.325 mIU/ml × 1900 ml). Estimating that local volume surrounding the blastcyst in the fallopian tube ranges from 1 μl to 10 μl, the intraluminal concentration of hCG around the blastocyst is estimated to be 617.5 ∼ 61.8 IU/ml. Thus the maternal epithelia adjacent to an implanting embryo is likely to be exposed to high concentrations of hCG.
The timing and quantity of hCG produced by the embryo are considered key factors in determining whether a human pregnancy succeeds or fails. 27 Statistics of assisted reproduction show that the rate of gestation increases substantially when several embryos of the two-to-eight-cell stage are transferred into the uterus. 28 The experience of assisted reproduction also suggests that the quality of the oocyte largely determines the success of implantation. 29 In light of present findings, oocyte quality may be closely related to the activity of hCG secretion.
It is well known that tubal pregnancy frequently occurs in chronic salpingitis or salpingitis isthmica nodosa. 30 Since these conditions are associated with inflammation, it is likely that inflammatory destruction of the fallopian tube delays migration of blastocyst within the fallopian tube, thus causing the elevation of intraluminal concentrations of hCG. Such delays will also allow time for the blastocyst to interact with tubal epithelial cells. It is also possible that signal transduction through the luteinizing hormone receptor triggered by hCG binding is coupled to downstream signal transduction of inflammatory cytokines. 31,32 Thus cells undergoing inflammation may be more susceptible to stimulation by hCG.
Evidence suggests that there is cross-talk between the mouse blastocyst and uterus to ensure successful implantation. 33 Thus spaciotemporal actions of steroid hormones, various growth factors, and cytokines are thought to play important roles to prepare the receptive uterus for implantation. However, cellular interactions culminating in implantation and placentation vary significantly among mammalian species. 34,35 We found significant differences in the expression pattern and in vivo function of human and mouse trophinins. 16,17 Trophinin is strongly expressed in trophoblasts in the human placenta during early stage of pregnancy. 15 By contrast, trophinin is not expressed in trophoblastic cells during and after implantation in the mouse. 16 Lack of trophinin has no obvious effect on mouse blastocyst implantation. 17
Genes encoding CG-β are found only in primates. 36,37 Nucleotide sequences of CG-β genes show significant diversion among primate species, and human CG-β diverged further from those found in non-human primates. 37-39 Considering these characteristics of human CG-β and the present findings of the activity of hCG on human trophinin gene expression (Table 3) ▶ , it is likely that trophinin function in embryo implantation is unique to humans. If this is the case, then the activity of hCG on trophinin gene expression may form the basis of ectopic pregnancy, a condition unique to humans. Recently, possible involvement of hCG in tubal pregnancy was suggested based on the finding of hCG expression by the decidualized endometrium. 40 Further studies are needed to determine the role of hCG on the pathogeneisis of tubal pregnancy. Nonetheless, information obtained by studies on trophinin and CG-β will provide us with a clue for early detection and prevention of ectopic pregnancy.
Acknowledgments
We thank Dr. Koji Yoshinaga, National Institute of Child Health and Human Development, National Institutes of Health, for his helpful suggestions and discussions, and Ms. Akiko Ishida, Department of Pathology, Shinshu University School of Medicine, for her technical assistance.
Footnotes
Address reprint requests to Jun Nakayama, Department of Pathology, Shinshu University School of Medicine, Matsumoto 390-8621, Japan, E-mail: jun@hsp.md.shinshu-u.ac.jp; or Michiko N. Fukuda, The Burnham Institute, 10901 North Torrey Pines Road, La Jolla, CA 92037. E-mail: michiko@burnham-inst.org.
Supported by NIH grant HD34108 and grant from Kyowa Medex (to M.N.F.), NIH grant HD29964 (to A.T.F.), Grant-in-Aid for Scientific Research B-15390115 from the Japan Society for the Promotion of Science (to J.N.), and Keio University Special Grant-in-Aid for Innovative Collaborative Research Projects (to S.N.).
References
- 1.Ectopic pregnancy—United States, 1990–1992. MMWR Morb Mortal Wkly Rep 1995, 44:46-48 [PubMed] [Google Scholar]
- 2.Brenner PF, Roy S, Mishell DR, Jr: Ectopic pregnancy: a study of 300 consecutive surgically treated cases. JAMA 1980, 243:673-676 [DOI] [PubMed] [Google Scholar]
- 3.Orsini MW, McLaren A: Loss of the zona pellucida in mice, and the effect of tubal ligation and ovariectomy. J Reprod Fertil 1967, 13:485-499 [DOI] [PubMed] [Google Scholar]
- 4.Tutton DA, Carr DH: The fate of trophoblast retained within the oviduct in the mouse. Gynecol Obstet Invest 1984, 17:18-24 [DOI] [PubMed] [Google Scholar]
- 5.Pauerstein CJ, Eddy CA, Koong MK, Moore GD: Rabbit endosalpinx suppresses ectopic implantation. Fertil Steril 1990, 54:522-526 [PubMed] [Google Scholar]
- 6.Croxatto HB, Ortiz ME, Diaz S, Hess R, Balmaceda J, Croxatto HD: Studies on the duration of egg transport by the human oviduct: II. ovum location at various intervals following luteinizing hormone peak. Am J Obstet Gynecol 1978, 132:629-634 [DOI] [PubMed] [Google Scholar]
- 7.Aplin JD: The cell biological basis of human implantation. Baillieres Clin Obstet Gynaecol 2000, 14:757-764 [DOI] [PubMed] [Google Scholar]
- 8.Lessey BA: Endometrial receptivity and the window of implantation. Baillieres Clin Obstet Gynaecol 2000, 14:775-788 [DOI] [PubMed] [Google Scholar]
- 9.Kliman HJ, Coutifaris C, Babalola GO, Soto EA, Kao L-C, Queenan JT, Jr, Feinberg RF, Strauss JF, III: The human trophoblast: homotypic and heterotypic cell-cell interactions. Yoshinaga K Mori T eds. Progress in Clinical and Biological Research 294: Development of Preimplantation Embryos and Their Environment. 1989:pp 425-434 Alan R Liss, New York [PubMed]
- 10.Sulz L, Valenzuela JP, Salvatierra AM, Ortiz ME, Croxatto HB: The expression of αv and β3 integrin subunits in the normal human fallopian tube epithelium suggests the occurrence of a tubal implantation window. Hum Reprod 1998, 13:2916-2920 [DOI] [PubMed] [Google Scholar]
- 11.Fukuda MN, Sato T, Nakayama J, Klier G, Mikami M, Aoki D, Nozawa S: Trophinin and tastin, a novel cell adhesion molecule complex with potential involvement in embryo implantation. Genes Dev 1995, 9:1199-1210 [DOI] [PubMed] [Google Scholar]
- 12.Suzuki N, Zara J, Sato T, Ong E, Bakhiet N, Oshima RG, Watson KL, Fukuda MN: A cytoplasmic protein, bystin, interacts with trophinin, tastin, and cytokeratin and may be involved in trophinin-mediated cell adhesion between trophoblast and endometrial epithelial cells. Proc Natl Acad Sci USA 1998, 95:5027-5032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuda MN, Nozawa S: Trophinin, tastin, and bystin: a complex mediating unique attachment between trophoblastic and endometrial epithelial cells at their respective apical cell membranes. Semin Reprod Endocrinol 1999, 17:229-234 [DOI] [PubMed] [Google Scholar]
- 14.Fukuda MN, Nadano D, Suzuki N, Nakayama J: Potential involvement of trophinin, bystin, and tastin in embryo implantation. Carson DD eds. Embryo Implantation: Molecular, Cellular, and Clinical Aspects. 1999:pp 132-140 Springer-Verlag, New York
- 15.Suzuki N, Nakayama J, Shih IM, Aoki D, Nozawa S, Fukuda MN: Expression of trophinin, tastin, and bystin by trophoblast and endometrial cells in human placenta. Biol Reprod 1999, 60:621-627 [DOI] [PubMed] [Google Scholar]
- 16.Suzuki N, Nadano D, Paria BC, Kupriyanov S, Sugihara K, Fukuda MN: Trophinin expression in the mouse uterus coincides with implantation and is hormonally regulated but not induced by implanting blastocysts. Endocrinology 2000, 141:4247-4254 [DOI] [PubMed] [Google Scholar]
- 17.Nadano D, Sugihara K, Paria BC, Saburi S, Copeland NG, Gilbert DJ, Jenkins NA, Nakayama J, Fukuda MN: Significant differences between mouse and human trophinins are revealed by their expression patterns and targeted disruption of mouse trophinin gene. Biol Reprod 2002, 66:313-321 [DOI] [PubMed] [Google Scholar]
- 18.Ishihara K, Kurihara M, Goso Y, Urata T, Ota H, Katsuyama T, Hotta K: Peripheral α-linked N-acetylglucosamine on the carbohydrate moiety of mucin derived from mammalian gastric gland mucous cells: epitope recognized by a newly characterized monoclonal antibody. Biochem J 1996, 318:409-416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawakami M, Nakayama J: Enhanced expression of prostate-specific membrane antigen gene in prostate cancer as revealed by in situ hybridization. Cancer Res 1997, 57:2321-2324 [PubMed] [Google Scholar]
- 20.Shimizu F, Nakayama J, Ishizone S, Zhang MX, Kawakubo M, Ota H, Sugiyama A, Kawasaki S, Fukuda M, Katsuyama T: Usefulness of the real-time reverse transcription-polymerase chain reaction assay targeted to α1,4-N-acetylglucosaminyltransferase for the detection of gastric cancer. Lab Invest 2003, 83:187-197 [DOI] [PubMed] [Google Scholar]
- 21.Jurisicova A, Antenos M, Kapasi K, Meriano J, Casper RF: Variability in the expression of trophectodermal markers β-human chorionic gonadotrophin, human leukocyte antigen-G, and pregnancy-specific β-1 glycoprotein by the human blastocyst. Hum Reprod 1999, 14:1852-1858 [DOI] [PubMed] [Google Scholar]
- 22.Dokras A, Sargent IL, Gardner RL, Barlow DH: Human trophectoderm biopsy and secretion of chorionic gonadotrophin. Hum Reprod 1991, 6:1453-1459 [DOI] [PubMed] [Google Scholar]
- 23.Dimitriadou F, Phocas I, Mantzavinos T, Sarandakou A, Rizos D, Zourlas PA: Discordant secretion of pregnancy-specific β1-glycoprotein and human chorionic gonadotropin by human pre-embryos cultured in vitro. Fertil Steril 1992, 57:631-636 [DOI] [PubMed] [Google Scholar]
- 24.Lopata A, Oliva K, Stanton PG, Robertson DM: Analysis of chorionic gonadotrophin secreted by cultured human blastocysts. Mol Hum Reprod 1997, 3:517-521 [DOI] [PubMed] [Google Scholar]
- 25.Wilcox AJ, Baird DD, Weinberg CR: Time of implantation of the conceptus and loss of pregnancy. N Engl J Med 1999, 340:1796-1799 [DOI] [PubMed] [Google Scholar]
- 26.Wintrobe MM: Calculations and determination of red cell, plasma, and total blood volumes in adult men and women. Clinical Hematology. 1974:pp 1792 Lea & Febiger, London
- 27.Heam JP, Webley GE, Gildley-Baird AA: Chorionic gonadotropin and embryo-maternal recognition during peri-implantation period in primates. Reprod Fertil 1991, 92:497-507 [DOI] [PubMed] [Google Scholar]
- 28.Norwitz ER, Schust DJ, Fisher SJ: Implantation and the survival of early pregnancy. N Engl J Med 2001, 345:1400-1408 [DOI] [PubMed] [Google Scholar]
- 29.Sauer MV, Paulson RJ, Lobo RA: Reversing the natural decline in human fertility: an extended clinical trial of oocyte donation to women of advanced reproductive age. JAMA 1992, 268:1275-1279 [DOI] [PubMed] [Google Scholar]
- 30.Green LK, Kott ML: Histopathologic findings in ectopic tubal pregnancy. Int J Gynecol Pathol 1989, 8:255-262 [DOI] [PubMed] [Google Scholar]
- 31.Dufau ML: The luteinizing hormone receptor. Annu Rev Physiol 1998, 60:461-496 [DOI] [PubMed] [Google Scholar]
- 32.McFarland KC, Sprengel R, Phillips HS, Kohler M, Rosemblit N, Nikolics K, Segaloff DL, Seeburg PH: Lutropin-choriogonadotropin receptor: an unusual member of the G protein-coupled receptor family. Science 1989, 245:494-499 [DOI] [PubMed] [Google Scholar]
- 33.Paria BC, Reese J, Das SK, Dey SK: Deciphering the cross-talk of implantation: advances and challenges. Science 2002, 296:2185-2188 [DOI] [PubMed] [Google Scholar]
- 34.Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, Yoshinaga K: Embryo implantation. Dev Biol 2000, 223:217-237 [DOI] [PubMed] [Google Scholar]
- 35.Enders AC, Lantz KC, Peterson PE, Hendrickx AG: From blastocyst to placenta: the morphology of implantation in the baboon. Hum Reprod 1997, 3:561-573 [DOI] [PubMed] [Google Scholar]
- 36.Pierce JG, Parsons TF: Glycoprotein hormones: structure and function. Annu Rev Biochem 1981, 50:465-495 [DOI] [PubMed] [Google Scholar]
- 37.Maston GA, Ruvolo M: Chorionic gonadotropin has a recent origin within primates and an evolutionary history of selection. Mol Biol Evol 2002, 19:320-335 [DOI] [PubMed] [Google Scholar]
- 38.Talmadge K, Vamvakopoulos NC, Fiddes JC: Evolution of the genes for the β subunits of human chorionic gonadotropin and luteinizing hormone. Nature 1984, 307:37-40 [DOI] [PubMed] [Google Scholar]
- 39.Fiddes JC, Goodman HM: The cDNA for the β-subunit of human chorionic gonadotropin suggests evolution of a gene by readthrough into the 3′-untranslated region. Nature 1980, 286:684-687 [DOI] [PubMed] [Google Scholar]
- 40.Zimmermann G, Baier D, Majer J, Alexander H: Expression of beta hCG and alpha CG mRNA and hCG hormone in human decidual tissue in patients during tubal pregnancy. Mol Hum Reprod 2003, 9:81-89 [DOI] [PubMed] [Google Scholar]