Abstract
EphA2 is a transmembrane receptor tyrosine kinase that is overexpressed in many carcinomas. Specific targeting of EphA2 with monoclonal antibodies is sufficient to inhibit the growth, migration and invasiveness of aggressive cancers in animal models. Using immunohistochemical analyses, we measured the expression of EphA2 in prostatic adenocarcinoma, high-grade prostatic intraepithelial neoplasia, and adjacent benign prostate tissue from ninety-three radical prostatectomy specimens. These results were related to multiple clinical and pathologicalcharacteristics. The fraction of cells staining positively with EphA2 in benign prostatic epithelium (mean, 12%) was significantly lower than that in high-grade prostatic intraepithelial neoplasia (mean, 67%, P < 0.001) and prostatic adenocarcinoma (mean, 85%, P < 0.001). Moreover, the intensity of EphA2 immunoreactivity in prostatic adenocarcinoma was significantly higher than in benign prostatic tissue (P < 0.001) or high-grade prostatic intraepithelial neoplasia (P < 0.001). Benign prostatic epithelium showed weak or no immunoreactivity for EphA2 in all cases examined. Whereas EphA2 immunoreactivity related to neoplastic transformation, it did not correlate with other clinical and pathological parameters examined. Our data suggest that EphA2 levels increase as prostatic epithelial cells progress toward a more aggressive phenotype. Progressively higher levels of EphA2 in high-grade prostatic intraepithelial neoplasia and prostatic carcinoma are consistent with recent evidence that EphA2 functions as a powerful oncogene. Moreover, the presence of high levels of EphA2 in these cells suggests opportunities for prostate cancer prevention and treatment.
Protein tyrosine kinases (PTK) are generally understood to play important roles in cellular growth and differentiation. 1-5 In particular, elevated PTK activity is frequently associated with cellular transformation and carcinogenesis. 4,6 The PTK family consists of many subtypes based on the sequences of their motifs. The largest family of PTKs, the Eph family of receptor tyrosine kinases, is highly expressed during embryonic development and are expressed in a restricted pattern in adults. 1,5 Much recent interest has focused on one particular Eph kinase, EphA2, which appears to regulate many aspects of adult epithelial cell behavior 7,8
EphA2 has been implicated in carcinogenesis of melanoma, breast, colon, lung, and esophageal cancers. 6,7,9-12 These studies have identified high levels of EphA2 in both tissue and cell models of these diseases. Moreover, ectopic overexpression of EphA2 is sufficient to confer malignant transformation and tumorgenic potential on non-transformed mammary epithelial cells. 12 The potential importance of EphA2 in cancer is also indicated by recent studies, which indicate that specific targeting of EphA2 with monoclonal antibodies is sufficient to inhibit the growth, migration, and invasiveness of aggressive breast and prostate cancer cells. 12,13
While much investigation has focused on EphA2 levels in aggressive cancers, most of these studies have involved a relatively small sample size. We therefore studied EphA2 expression in a relatively large collection of prostate carcinomas. Moreover, we sought to determine whether the expression of EphA2 is increased in pre-malignant conditions such as high-grade prostatic intraepithelial neoplasia (PIN), a putative precursor of prostatic adenocarcinoma.
Materials and Methods
Tissue Specimens
Ninety-three cases of radical retropubic prostatectomy were obtained from the surgical pathology files of Indiana University Medical Center (Table 1) ▶ . These cases were selected as representative of the full spectrum of Gleason grades and pathological stage. None had received hormonal or radiation therapy before surgery. Patients ranged in age from 44 to 77 years (mean, 63 years).
Table 1.
Patient Characteristics
| Patient characteristic | % of Total patients (n = 93) | Mean % of cells staining w/EphA2 antibody (±SD) | Mean EphA2 antibody staining intensity (±SD) |
|---|---|---|---|
| Primary gleason grade | |||
| 2 | 12 | 83 ± 2 | 2.0 ± 0.6 |
| 3 | 43 | 86 ± 10 | 2.3 ± 0.7 |
| 4 | 23 | 84 ± 16 | 2.3 ± 0.7 |
| 5 | 15 | 86 ± 11 | 2.3 ± 0.6 |
| Secondary gleason grade | |||
| 2 | 15 | 82 ± 16 | 2.3 ± 0.5 |
| 3 | 29 | 85 ± 15 | 2.1 ± 0.6 |
| 4 | 35 | 85 ± 9 | 2.3 ± 0.7 |
| 5 | 14 | 88 ± 8 | 2.4 ± 0.8 |
| Gleason sum | |||
| <7 | 28 | 83 ± 12 | 2.2 ± 0.6 |
| 7 | 35 | 85 ± 14 | 2.2 ± 0.7 |
| >7 | 30 | 87 ± 10 | 2.4 ± 0.7 |
| T classification | |||
| T2a | 9 | 89 ± 6 | 2.3 ± 0.5 |
| T2b | 43 | 84 ± 12 | 2.2 ± 0.7 |
| T3a | 27 | 84 ± 15 | 2.2 ± 0.7 |
| T3b | 14 | 63 ± 10 | 2.4 ± 0.6 |
| Lymph node metastasis | |||
| Positive | 13 | 88 ± 9 | 2.3 ± 0.6 |
| Negative | 80 | 84 ± 13 | 2.2 ± 0.7 |
| Extraprostatic extension | |||
| Positive | 53 | 86 ± 11 | 2.3 ± 0.7 |
| Negative | 40 | 84 ± 14 | 2.2 ± 0.7 |
| Surgical margin | |||
| Positive | 50 | 86 ± 11 | 2.1 ± 0.6 |
| Negative | 43 | 84 ± 13 | 2.4 ± 0.7 |
| Vascular invasion | |||
| Positive | 30 | 85 ± 11 | 2.1 ± 0.8 |
| Negative | 63 | 86 ± 13 | 2.3 ± 0.6 |
| Perineural invasion | |||
| Positive | 82 | 82 ± 15 | 2.4 ± 0.5 |
| Negative | 11 | 85 ± 12 | 2.2 ± 0.7 |
| High-Grade PIN | |||
| Positive | 89 | 85 ± 12 | 2.3 ± 0.7 |
| Negative | 4 | 85 ± 9 | 2.0 ± 0.8 |
After surgery, each prostate was weighed, measured, inked, and fixed in 10% formalin for 18 to 24 hours. After fixation, the apex and base were amputated at a thickness of 3 to 4 mm and serially sectioned at 3 mm. The seminal vesicles were sectioned parallel to the junction of the prostate. The remaining prostate was serially sectioned at approximately 3- to 4-mm intervals by a knife perpendicular to the long axis of the gland from the apex of the prostate to the tip of the seminal vesicles. Standard sections, including both peripheral and transitional zone prostate, were prepared for histological examination. Grading of the primary tumor from radical prostatectomy specimens was performed according to the Gleason system. 14,15 The Gleason score ranged from 4 to 10. Pathological stage was performed according to the 1997 TNM (tumor, lymph nodes, and metastasis). 16 Pathological stages were T2a (n = 9 patients), T2b (n = 43), T3a (n = 27), and T3b (n = 14). Thirteen patients had lymph node metastasis at the time of surgery. This research was approved by the Indiana University Institutional Review Board.
Murine Monoclonal Antibody to Human EphA2 Protein
Murine monoclonal antibody specific to EphA2 was generated by immunizing mouse with purified, recombinant human EphA2 protein. A plasmid encoding a fusion protein of the extracellular domain of EphA2 linked to immunoglobulin chain was used as the immunogen. The antisera was affinity purified. The specificity of the purified IgG antibody was confirmed by immunoprecipitation, ELISA, BiaCore, and functional activation assays (ie, increased EphA2 phosphotyrosine content) (MedImmune Inc, Gaithersburg, MD). In addition, fluorescence microscopy study revealed the overexpression of EphA2 protein in a right pattern and in the right cellular location in the EphA2-overexpressing cells but not EphA2-deficient cells. 17-19
Immunohistochemistry
Serial 5-μm-thick sections of formalin-fixed slices of radical prostatectomy specimens were used for the studies. Tissue blocks that contained the maximum amount of tumor and highest Gleason score were selected. One representative slide from each case was analyzed. We recognized the limitation of sample variation. Slides were deparaffinized in xylene twice for 5 minutes and rehydrated through graded ethanols to distilled water. Antigen retrieval was carried out by heating sections in 1 mmol/L ethylene diaminetetraacetic acid (EDTA) (pH 8.0) for 30 minutes. Endogenous peroxidase activity was inactivated by incubation in 3% H2O2 for 15 minutes. Non-specific binding sites were blocked using Protein Block (DAKO, Carpinteria, CA) for 20 minutes. Tissue sections were then incubated with the mouse monoclonal antibody against human EphA2 (IgG1, 1:100 dilution; MedImmune, Inc.) overnight at room temperature, followed by biotinylated secondary antibody (DAKO) and peroxidase-labeled streptavidin. 3,3-diaminobenzidine was used as the chromogen in the presence of hydrogen peroxide. Positive controls consisted of human prostate cancer samples that had been shown to express EphA2 and PC-3 cells. 20 LnCaP cells were used as negative control. 20 As an additional parallel negative control, negative controls were performed using blocking serum in place of primary antibody.
Evaluation of EphA2 Expression
The extent and intensity of staining were evaluated in benign epithelium, high-grade PIN and adenocarcinoma from the same slide for each case. Microscopic fields with the highest degree of immunoreactivity were chosen for analysis. At least 1000 cells were analyzed in each case. The percentage of cells exhibiting staining in each case was evaluated semiquantitatively on a 5% incremental scale ranging from 0 to 95%. A numeric intensity score is set from 0 to 3 (0, no staining; 1, weak staining; 2, moderate staining; and 3, strong staining). These methods follow our previous work. 21,22
Statistical Analysis
The mean percentage of immunoreactive cells in benign epithelium, high-grade PIN and adenocarcinoma were compared using the Wilcoxon paired signed rank test. The intensity of staining for EphA2 in benign epithelium, high-grade PIN, and adenocarcinoma were compared using Cochran-Mantel-Haenszel tests for correlated ordered categorical data. A P value <0.05 was considered significant, and all P values were two-sided.
Results
EphA2 immunoreactivity readily distinguished neoplastic prostatic epithelial cells from their normal counterparts (Figure 1) ▶ . EphA2 immunoreactivity was observed in all cases of high-grade PIN and cancers, whereas benign epithelial cells (from both peripheral and transitional zone) showed weak or no EphA2 immunoreactivity (Figure 1 ▶ ; Table 2 and 3 ▶ ). Benign epithelium from both atrophic glands and benign prostatic hyperplasia showed weak or no EphA2 immunostaining. EphA2 expression (both the mean percentage of immunoreactive cells and staining intensity) was increased in both high-grade PIN (P < 0.001) and cancers (P < 0.001) relative to benign epithelial cells (Tables 2 and 3) ▶ . Similarly, EphA2 immunoreactivity (both the mean percentage of immunoreactive cells and staining intensity) was increased in prostatic carcinomas compared with high-grade PIN (P < 0.001) (Tables 2 and 3) ▶ . In contrast, no EphA2 immunoreactivity was observed in tumor-proximal stromal or endothelial cells.
Figure 1.
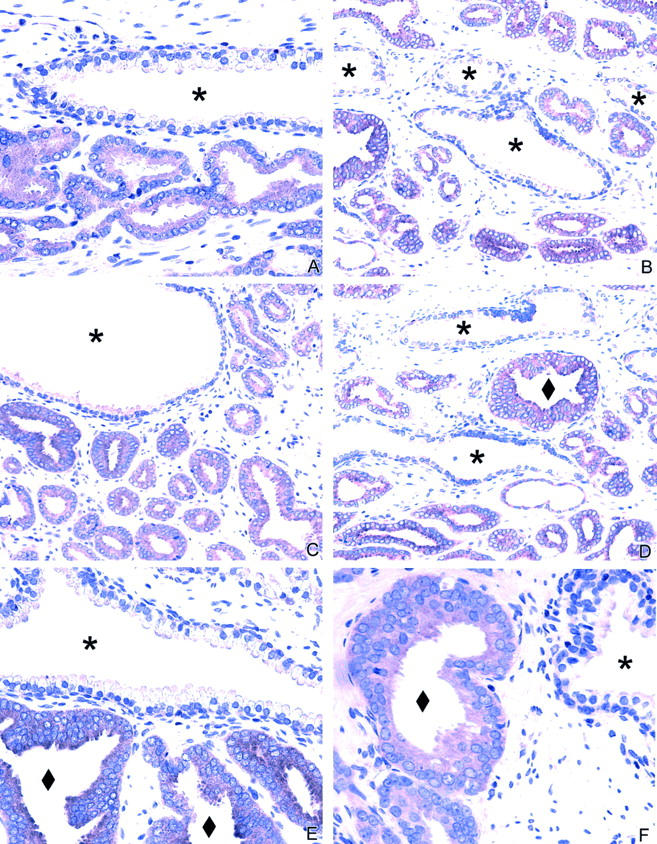
EphA2 immunohistochemistry in the prostate. A to D: Normal prostate glands show no or minimal staining in the secretory cells lining the lumen of the gland. Adjacent cancer cells show strong expression. E to F: EphA2 expression in high-grade prostatic intraepithelial neoplasia (PIN). The adjacent normal gland showed no or minimal EphA2 immunoreactivity. ♦, PIN; *, adjacent normal glands.
Table 2.
Intensity of EphA2 Antibody Staining of Cells in Prostate Tissues in 93 Radical Prostatectomy Specimens
| Cell type | Staining Intensity Grade | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Benign epithelium | 31 (33%) | 61 (66%) | 1 (1%) | 0 (0%) |
| High-Grade PIN* | 0 (0%) | 20 (22%) | 68 (73%) | 5 (5%) |
| Adenocarcinoma* † | 0 (0%) | 12 (13%) | 47 (50%) | 34 (37%) |
*Indicates percentage of staining intensity was statistically higher compared to that of the normal cells with a P value <0.001 using a Wilcoxon paired signed rank test.
†The staining intensity was significantly higher compared to high-grade PIN (P < 0.001, Cochran-Mantel-Henszel test).
After finding high levels of EphA2 in neoplastic cells, we asked whether the level of EphA2 expression related to disease type. In the high-grade PIN group, 22% showed grade 1 staining intensity, 73% showed grade 2 staining intensity, and 5% showed grade 3 staining intensity (Table 2) ▶ . In the adenocarcinoma group, 13% of cases showed grade 1 staining intensity, 50% showed grade 2 staining intensity, and 37% showed grade 3 staining intensity. In contrast, the normal epithelium group showed grade 1 stain in 66% of the cases, and the remainder showed no immunoreactivity for EphA2 protein (grade 0 staining intensity) (Table 2) ▶ . The mean percentage of EphA2 immunoreactive cells was 12% in normal epithelial cells, 67% in high-grade PIN, and 85% in prostatic adenocarcinoma (Table 3) ▶ .
Table 3.
EphA2 Immunoreactivity of Cells in Prostate Tissues in Radical Prostatectomy Specimens
| Cell type | Mean % of cells staining ± SD | Range (%) |
|---|---|---|
| Normal cells | 12 ± 17 | 0–90 |
| High-Grade PIN* | 67 ± 18 | 5–95 |
| Adenocarcinoma* † | 85 ± 12 | 30–95 |
*Indicates percentage of staining statistically higher compared to that of the normal cells with a P value <0.001 using a Wilcoxon paired signed rank test.
†The percentage of staining was statistically higher compared to high-grade PIN (P < 0.001).
Whereas high levels of EphA2 could distinguish neoplastic from benign prostatic epithelial cells, EphA2 did not relate to other clinical and pathological parameters. For example, high levels of EphA2 were observed in most prostatic carcinomas and did not relate to age, Gleason grade, pathological stage, lymph node metastasis, extraprostatic extension, surgical margins, vascular invasion, perineural invasion, or the presence of high-grade PIN (Table 1) ▶ .
Discussion
In this study, we found that expression of EphA2 is significantly increased in both high-grade PIN and prostatic adenocarcinomas as compared to benign prostatic epithelium. These findings suggest that EphA2 overexpression may arise early in the course of prostate cancer progression. Moreover, our findings suggest that EphA2 may provide an opportunity for prostate cancer prevention or disease management.
High levels of EphA2 have been reported in other cancers including melanoma, breast, lung, colon, and esophageal cancers. 6,7,9-12 Our data are unique in part because they provide the first indication that high levels of EphA2 are found early in disease progression. For example, pre-malignant conditions of the prostate such as high-grade PIN overexpress EphA2. This suggests that EphA2 may play an important role in the early stage of prostatic carcinogenesis. This hypothesis is supported by evidence that overexpression of EphA2 is sufficient to induce malignant transformation of non-transformed mammary epithelial cells. 12,13 It will be interesting to determine whether high levels of EphA2 are also found in pre-malignant conditions of other cancers as well (eg, ductal carcinoma in situ). Taken together, EphA2 may be an important component during the early carcinogenesis of many human cancers.
Overexpression of several tyrosine kinases including fibroblast growth factor, epidermal growth factor, and focal adhesion kinase has been reported in prostate cancer. 1,23-25 However, these PTKs are generally found to be sporadically overexpressed in prostate cancer. Multiple and different lines of evidence suggest Eph family members contribute to the high tyrosine phosphorylation levels in prostate cancer. 20 We found that EphA2 is overexpressed in both high-grade PIN and prostatic adenocarcinoma. Although information about the expression and regulation of other Eph family members in prostate cancer is rather limited, Robinson et al 26 showed that EphA1 and htk are expressed in some prostatic cancer cells. Members of the Eph family have been shown to have contrasting effects on cell proliferation and tumorigenic potential in cell lines of different origins. For example, EHK-1 and CEK8 have been linked to tumorigenesis of glioma. However, EphA5 has no implication in promotion of cell proliferation in human glioblastoma cell line. Other data showed that EphA3 and EphB1 have no oncogenic effect.
In addition to its overexpression, the EphA2 in malignant cells generally functions differently than the EphA2 in non-transformed cells. For example, defective cell-cell contacts decrease the ability of EphA2 to interact with its ligands, which are located on apposing cells. 27 This observation is interesting in light of a recent report, which showed that overexpression of EphA2 in non-transformed mammary epithelial cells (MCF-10A) is sufficient to destabilize cell-cell contact. Thus, the high levels of EphA2 in high-grade PIN and prostate cancer cells likely do not interact efficiently with its ligand.
These changes in EphA2-ligand binding are important because ligand binding negatively regulates tumor cell growth and invasiveness and can reverse the malignant phenotypes of highly aggressive tumor cells. 12,13,27,28 Moreover, much recent investigation has sought to exploit these changes in EphA2 function by using monoclonal antibodies to mimic the actions of the endogenous ligands of EphA2. In this light, our present findings are notable, in part, because they suggest that antibody-based targeting of EphA2 could also have utility for blocking the progression of PIN toward malignant disease.
The biological role and regulation of EphA2 expression in prostate cancer is unclear. Overexpression of EphA2 in high-grade PIN and prostatic adenocarcinoma are consistent with emerging evidence that EphA2 may be involved in the process of prostatic carcinogenesis. Further studies on the regulation of the EphA2 signaling transduction pathway may provide insights leading to approaches to prostate cancer prevention and treatment.
Figure 2.
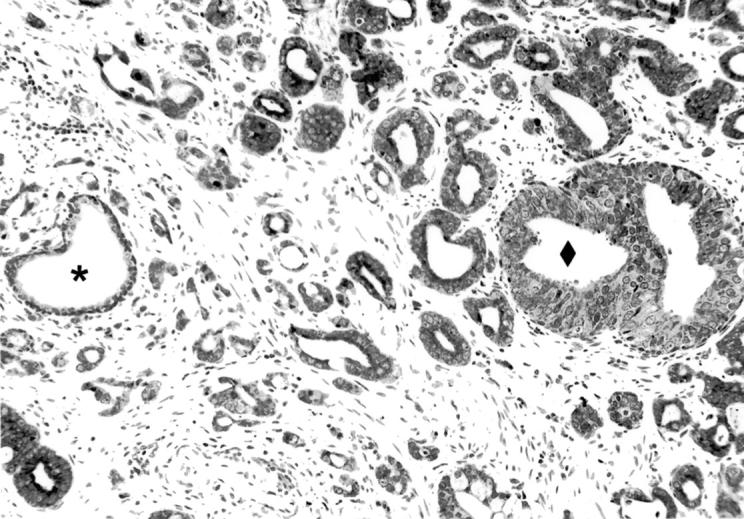
Comparison of EphA2 tyrosine kinase expression in benign epithelium, high-grade PIN, and prostatic adenocarcinoma. ♦, PIN; *, adjacent normal glands.
Footnotes
Address reprint requests to Liang Cheng, M.D., Department of Pathology and Laboratory Medicine, Indiana University Medical Center, University Hospital 3465, 550 North University Boulevard, Indianapolis, IN 46202. E-mail: lcheng@iupui.edu.
Guangyuan Zeng and Zhiqiang Hu contributed equally to this study.
References
- 1.Ullrich A, Schlessinger J: Signal transduction by receptors with tyrosine kinase activity. Cell 1990, 61:203-212 [DOI] [PubMed] [Google Scholar]
- 2.Hunter T: Protein modification: phosphorylation on tyrosine residues. Curr Opin Cell Biol 1989, 6:1168-1181 [DOI] [PubMed] [Google Scholar]
- 3.Hunter T, Lindberg RA, Middlemas DS, Tracy S, van der Geer P: Receptor protein tyrosine kinases and phosphatases. Cold Spring Harb Symp Quant Biol 1992, 57:25-41 [DOI] [PubMed] [Google Scholar]
- 4.Cantley LC, Auger KR, Carpenter C, Duckworth B, Graziani A, Kapeller R, Soltoff S: Oncogenes and signal transduction. Cell 1991, 64:281-302 [DOI] [PubMed] [Google Scholar]
- 5.Schlessinger J, Ullrich A: Growth factor signaling by receptor tyrosine kinases. Neuron 1992, 9:383-391 [DOI] [PubMed] [Google Scholar]
- 6.Easty DJ, Herlyn M, Bennett DC: Abnormal protein tyrosine kinase gene expression during melanoma progression and metastasis. Int J Cancer 1995, 60:129-136 [DOI] [PubMed] [Google Scholar]
- 7.Andres AC, Reid HH, Zurcher G, Blaschke RJ, Albrecht D, Ziemiecki A: Expression of two novel eph-related receptor protein tyrosine kinases in mammary gland development and carcinogenesis. Oncogene 1994, 9:1461-1467 [PubMed] [Google Scholar]
- 8.Pasquale EB: The Eph family or receptors. Curr Opin Cell Biol 1997, 9:608-615 [DOI] [PubMed] [Google Scholar]
- 9.Nemoto T, Ohashi K, Akashi T, Johnson JD, Hirokawa K: Overexpression of protein tyrosine kinases in human esophageal cancer. Pathobiology 1997, 65:195-203 [DOI] [PubMed] [Google Scholar]
- 10.Kinch MS, Moore M, Harpole DH: Predictive value of the EphA2 receptor tyrosine kinase in lung cancer recurrence and survival. Clin Cancer Res 2003, 9:613-618 [PubMed] [Google Scholar]
- 11.Miyazaki T, Kato H, Fukuchi M, Nakajima M, Kuwano H: EphA2 overexpression correlates with poor prognosis in esophageal squamous cell carcinoma. Int J Cancer 2003, 103:657-663 [DOI] [PubMed] [Google Scholar]
- 12.Zelinski DP, Zantek ND, Stewart JC, Irizarry AR, Kinch MS: EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res 2001, 61:2301-2306 [PubMed] [Google Scholar]
- 13.Zelinski DP, Zantek ND, Walker-Daniels J, Peters MA, Taparowsky EJ, Kinch MS: Estrogen and Myc negatively regulate expression of the EphA2 tyrosine kinase. J Cell Biochem 2002, 85:714-720 [DOI] [PubMed] [Google Scholar]
- 14.Bostwick DG: Neoplasms of the prostate. Bostwick DG Eble JN eds. Urologic Surgical Pathology. 1997:pp 343-422 Mosby, St. Louis
- 15.Gleason DF, Mellinger GT: Prediction of prognosis for prostatic adenocarcinoma by combined histologic grading and clinical stage. J Urol 1974, 111:58-64 [DOI] [PubMed] [Google Scholar]
- 16.Fleming ID, Cooper JS, Henson DE, Hutter RVP, Kennedy BJ, Murphy GP, O’Sullivan B, Sobin LH, Yarbro JW: AJCC Cancer Staging Manual 1997:pp 219-224 Raven and Lippincott, Philadelphia
- 17.Carles-Kinch K, Kilpatrick KE, Stewart JC, Kinch MS: Antibody targeting of the EphA2 tyrosine kinase inhibits malignant cell behavior. Cancer Res 2002, 62:2840-2847 [PubMed] [Google Scholar]
- 18.Kinch MS, Moore MB, Harpole DH: Predictive value of the EphA2 receptor tyrosine kinase in lung cancer recurrence and survival. Clin Cancer Res 2003, 9:613-618 [PubMed] [Google Scholar]
- 19.Miao H, Burnett E, Kinch M, Simon E, Wang B: Activation of EphA2 kinase suppresses integrin function and causes focal-adhesion-kinase dephosphorylation. Nat Cell Biol 2000, 2:62-69 [DOI] [PubMed] [Google Scholar]
- 20.Walker-Daniels J, Coffman K, Azimi M, Rhim JS, Bostwick DG, Snyder P, Kerns BJ, Waters DJ, Kinch MS: Overexpression of the EphA2 tyrosine kinase in prostate cancer. Prostate 1999, 41:275-280 [DOI] [PubMed] [Google Scholar]
- 21.Jiang J, Neubauer BL, Graff JR, Chedid M, Thomas JE, Roehm NW, Zhang S, Eckert GJ, Koch MO, Eble JN, Cheng L: Expression of group IIA secretory phospholipase A2 is elevated in prostatic intraepithelial neoplasia and adenocarcinoma. Am J Pathol 2002, 160:667-671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng L, Nagabhushan M, Pretlow TP, Amini SB, Pretlow TG: E-cadherin expression in primary and metastatic prostate cancer. Am J Pathol 1996, 148:1375-1380 [PMC free article] [PubMed] [Google Scholar]
- 23.Visakorpi T, Kallioniemi OP, Koivula T, Harvey J, Isola J: Expression of epidermal growth factor receptor and ERBB2 (HER-2/Neu) oncoprotein in prostatic carcinomas. Mod Pathol 1992, 5:643-648 [PubMed] [Google Scholar]
- 24.Tremblay L, Hauck W, Aprikian AG, Begin LR, Chapdelaine A, Chevalier S: Focal adhesion kinase (pp125FAK) expression, activation and association with paxillin and p50CSK in human metastatic prostate carcinoma. Int J Cancer 1996, 68:164-171 [DOI] [PubMed] [Google Scholar]
- 25.Kondapaka BS, Reddy KB: Tyrosine kinase inhibitor as a novel signal transduction and antiproliferative agent: prostate cancer. Mol Cell Endocrinol 1996, 117:53-58 [DOI] [PubMed] [Google Scholar]
- 26.Robinson D, He F, Pretlow T, Kung HJ: A tyrosine kinase profile of prostate carcinoma. Proc Natl Acad Sci USA 1996, 93:5958-5962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zantek ND, Azimi M, Fedor-Chaiken M, Wang B, Brackenbury R, Kinch MS: E-cadherin regulates the function of the EphA2 receptor tyrosine kinase. Cell Growth Differ 1999, 10:629-638 [PubMed] [Google Scholar]
- 28.Kinch MS, Clark GJ, Der CJ, Burridge K: Tyrosine phosphorylation regulates the adhesions of ras-transformed breast epithelia. J Cell Biol 1995, 130:461-471 [DOI] [PMC free article] [PubMed] [Google Scholar]


