Abstract
The majority of trigeminal ganglia (TGs) are latently infected with α-herpesviruses [herpes simplex virus type-1 (HSV-1) and varicella-zoster virus (VZV)]. Whereas HSV-1 periodically reactivates in the TGs, VZV reactivates very rarely. The goal of this study was to determine whether herpesvirus latency is linked to a local immune cell infiltration in human TGs. T cells positive for the CD3 and CD8 markers, and CD68-positive macrophages were found in 30 of 42 examined TGs from 21 healthy individuals. The presence of immune cells correlated constantly with the occurrence of the HSV-1 latency-associated transcript (LAT) and only irregularly with the presence of latent VZV protein. In contrast, uninfected TGs showed no immune cell infiltration. Quantitative RT-PCR revealed that CD8, interferon-γ, tumor necrosis factor-α, IP-10, and RANTES transcripts were significantly induced in TGs latently infected with HSV-1 but not in uninfected TGs. The persisting lymphocytic cell infiltration and the elevated CD8 and cytokine/chemokine expression in the TGs demonstrate for the first time that latent herpesviral infection in humans is accompanied by a chronic inflammatory process at an immunoprivileged site but without any neuronal destruction. The chronic immune response seems to maintain viral latency and influence viral reactivation.
Herpes simplex virus type-1 (HSV-1) typically causes infections of the oral mucosa and establishes a lifelong persistence in the sensory ganglia neurons. Persistence and latency have been demonstrated in human trigeminal, facial, and vestibular ganglia. 1-3 The reactivation of the virus in these ganglia generally causes cranial nerve disorders such as herpes labialis, Bell’s palsy, and vestibular neuritis. A variety of stimuli (UV light, fever, stress, immunosuppression, and other infections) can induce a reactivation. The pathomechanism governing the transition of HSV-1 from latency to reactivation is not yet clear, but T cells are believed to play a crucial role in the interplay between the immune system and latent HSV-1. 4
The cell-mediated immune response is the most important immune defense mechanism against HSV-1 infection. 5 Alterations in the T-cell-mediated immune response, mostly caused by immunosuppression, are of particular relevance for herpesviral reactivation.
The central and peripheral nervous systems (CNS and PNS) are shielded by the blood-brain barrier (BBB) and blood-nerve barrier (BNB). Because of these anatomical barriers, as well as the non-expression of the typical major histocompatibility complex (MHC), the absence of regular lymphoid drainage and the lack of intraneurial lymphatic channels, 6 the CNS and PNS are traditionally considered immunoprivileged organs. However, the CNS permits entry to activated T cells, which play an important role in immunological surveillance of the CNS. 7 This is also thought to be true for the PNS, since the BNB is even more permeable than the BBB. 8 The immunological surveillance mechanism has never been studied at the latency site of herpesviral infection in humans, namely the sensory nerve ganglia. Studies from the HSV-1 mouse model suggested that the immune system provides active surveillance of HSV-1 latently infected neurons 9-13 and that CD8+ T cells play a crucial role in maintaining the virus in a latent state. 12 To test if this situation holds true also in human TGs latently infected with HSV-1 and/or VZV, we investigated whether immune cells are present in relation to latently infected ganglia. The presence of chemokines that might attract immune cells and of cytokines that could affect viral replication was determined by quantitative RT-PCR.
Materials and Methods
The use of autopsy samples for the present study was approved by the Ethics Committee of the Medical Faculty of the Ludwig-Maximilians University of Munich.
TGs on both sides were removed 6 to 24 hours after death from 21 subjects whose ages ranged from 5 weeks to 81 years. The cause of death of the subjects was mainly related to trauma in adults and sudden infant death in newborns (Table 1) ▶ . The subjects neither had lesions suggestive of an active orolabial herpes infection nor a history of cranial nerve disorders.
Table 1.
Tissue Sample Overview Used for IHC and ISH: Presence of LAT Signal, T-Cell Antigen, and VZV Antigen in Human TGs
| Subject | Age/gender | Cause of death | LAT (ISH) positive neurons | CD8 (IHC) positive T cells | VZV 62 (IHC) positive neurons |
|---|---|---|---|---|---|
| 1 | 5 weeks | Sudden infant death | 0 | 0 | 0 |
| 2 | 11/2 mo/f | Sudden infant death | 0 | 0 | 0 |
| 3 | 8 mo/f | Sudden infant death | 0 | 0 | 0 |
| 4 | 9 mo/m | Sudden infant death | 0 | 0 | 5 |
| 5 | 4 yr/m | Car accident | 9 | 64.3 | 4.6 |
| 6 | 5 yr/f | Central paralysis | 0 | 0 | 0 |
| 7 | 7 yr/m | Murder | 0 | 8 | 0 |
| 8 | 8 yr/m | Drowning | 4 | 47.3 | 6.3 |
| 9 | 11 yr/m | Drowning | 6 | 52 | 7.3 |
| 10 | 18 yr/m*† | Drug abuse | 28 | 64 | ‡ |
| 11 | 29 yr/m | Not known | 5 | 36.6 | 0 |
| 12 | 37 yr/m | Suicide | 13 | 43 | 8 |
| 13 | 38 yr/m | Not known | 10 | 32.3 | ‡ |
| 14 | 39 yr/m | Murder | 14 | 30 | 2.5 |
| 15 | 40 yr/f | Drug abuse | 6 | 25 | 0 |
| 16 | 51 yr/m† | Natural death | 23 | 28 | 3 |
| 17 | 59 yr/m*† | Haemorrhage | 8 | 38.3 | 3 |
| 18 | 59 yr/f† | Not known | 16 | 64 | ‡ |
| 19 | 61 yr/m | Murder | 12 | 64.3 | 0 |
| 20 | 78 yr/f† | Suicide | 44 | 37 | ‡ |
| 21 | 81 yr/f | Natural death | 5 | 26.6 | 0 |
*Only paraffin sections available;
†no RNA sample available;
‡positive signal by VZV 62 ISH.
yr, year; mo, month; f, female; m, male.
Ganglia were embedded in Tissue Tek compound (Sakura, Zoeterwoude, The Netherlands) and stored on dry ice at −70°C until use. For paraffin processing the tissue was first fixed in 4% buffered paraformaldehyde for 48 hours. Frozen sections were made of 8-μm thickness and paraffin sections, of 4-μm thickness. The sections were mounted on positively charged slides (SuperFrost/Plus; Menzel, Braunschweig, Germany). Several tissue sections (up to 10) from each TG were stained with hematoxylin and eosin (H&E) for light microscopy examination. Brain paraffin sections from a 40-year-old man who died of herpes encephalitis were used as positive controls for the HSV-1 protein immunohistochemistry.
In Situ Hybridization (ISH)
The probe for the HSV-1 latency-associated transcript (LAT) was generated from the ATD19 plasmid kindly provided by Dr. T. Margolis. 14 The ISH protocol used was described in detail in a previous work. 3 After visualization of the ISH signal by incubation in nitroblue tetrazolium (NBT) and X-phosphate (BCIP 5-bromo-4-chloro-3-indolyl-phophate) staining solution, the sections were washed in PBS, fixed in 0.5% paraformaldehyde for 10 minutes, and immunostained with anti-CD3 or anti-CD68 antibodies.
A oligonucleotide probe was used to detect the VZV transcript 62. 15 The probe was synthesized and labeled with digoxigenin (MWG-Biotech AG, Ebersberg, Germany). In situ hybridization was carried out at 37°C overnight in a formamide-free hybridization buffer. Signal visualization was done as described above.
Immunohistochemistry (IHC)
Immunohistochemical stainings were done with primary antibodies against T-cell markers and an antibody (anti-CD68) that reacts with a lysosomal protein present in macrophages: rabbit anti-human CD3 (1:1000), rabbit anti-human CD68 (1:1000), mouse anti-human CD4 (1:100), and mouse anti-human CD8 (1:80; DAKO, Hamburg, Germany). For the detection of HSV-1- and VZV-specific antigens, the following antibodies were used: rabbit anti-HSV-1 (1:000; DAKO), mouse anti-HSV-1 immediate early protein ICP4 (1:500), mouse anti-HSV-1 immediate early protein ICP0 (1:1000; East Coast Biologics, North Berwick, ME), and mouse anti-VZV protein 62 (1:100; Chemicon, Hofheim, Germany).
Frozen tissue sections were thawed, dried at 37°C for 15 minutes, fixed in acetone for 10 minutes, and then washed in phosphate-buffered saline (PBS). Paraffin sections were dewaxed, rehydrated, and then washed in PBS. For the immunostainings with anti-CD3 and -CD68 antibodies, paraffin sections were incubated with trypsin (Sigma, St. Louis, MO) for 30 minutes. For staining with the mouse anti-ICP4, paraffin sections were heated in citrate buffer (pH 6.0). Frozen and paraffin sections were then sequentially incubated with 3% hydrogen peroxidase for 10 minutes, 5% normal donkey serum (when monoclonal antibodies were used), or 5% normal goat serum (when polyclonal antibodies were used) for 30 minutes. The diluted primary antibodies were applied to the sections and left to incubate overnight at 4°C or for 1 hour at 37°C.
Afterward, tissue sections were incubated for 30 minutes in biotinylated donkey anti-mouse IgG antibody (1:500; Dianova, Hamburg, Germany) or biotinylated goat anti-rabbit IgG antibody (1:300; DAKO). The sections were incubated with peroxidase-conjugated streptavidin (DAKO) for 30 minutes, followed by a final wash, and then incubated with diaminobenzidine (DAB) (DAKO) for up to 10 minutes.
Expression of LAT, CD8, and VZV62 antigen was evaluated in TG sections of both sides from each individual. Since LAT ISH gives a very clear signal in the neuronal nucleus, all positive neurons in each tissue section were counted at magnification of ×100. To assess the CD8 expression, three representative fields with the most abundant distribution of CD8+ cells were selected on each section and counted at magnification of ×200. The expression of VZV protein 62 was determined in the same way as CD8. The average number of CD8+ cells and VZV protein 62 neurons is shown in Table 1 ▶ .
Quantitative Real-Time RT-PCR and LAT RT-PCR
RNA was extracted and reverse transcribed as described previously. 3 Primer and TaqMan probes for the chemokines RANTES/CCL5, IP-10/CXCL10, interleukin (IL)-6, and nerve growth factor (NGF) were designed using the primer express software (RANTES: 5′-GAGTATTTCTACACCAGTGGCAAGTG, 3′-TCCCGAACCCATTTCTTCTCT, probe CCCAGCAGTCGTCTTTGTCACCCGA; IP-10: 5′-ATCGAAGGCCATCAAGAATTTACT, 3′-GCTCCCCTCTGGTTTTAAGGA, probe AAAGCAGTTAGCAAGGAAATGTCTAAAAGAT; IL-6: 5′-TCCAGGAGCCCAGCTATGAA, 3′-CCCAGGGAGAAGGCAACTG, probe TCCTTCTCCACAAGCGCCTTCGGT; NGF: 5′-CAGTTTTACCAAGGGAGCAGCTT, 3′-CGCCTGTATGCCGATCAGA, probe CAACATGGACATTACGCTATGCACCTCAGT). Sequences of primers and TaqMan probes for CD8, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α were taken from the literature. 16-18 All these primers were intron-spanning. Cyclophillin D primers and TaqMan probes were purchased from Applied Biosystems (Foster City, CA). The TaqMan PCR was performed using the GeneAmp 5700 Sequence Detection System. Data were analyzed with GeneAmp5700 SDS software. Each measurement was done in triplicate. The y axis in Figure 2 ▶ represents the relative transcript number of the gene of interest compared to the housekeeping gene cyclophillin. The relative transcript number was calculated from the formula 2 − ΔCt x 105, where ΔCt stands for the difference between the threshold cycle number of the housekeeping gene and the respective gene of interest. Data from the TaqMan were assessed statistically using the Mann-Whitney U-test for small sample numbers without standard distribution. The LAT RT-PCR was performed as described in a previous work. 3
Figure 2.
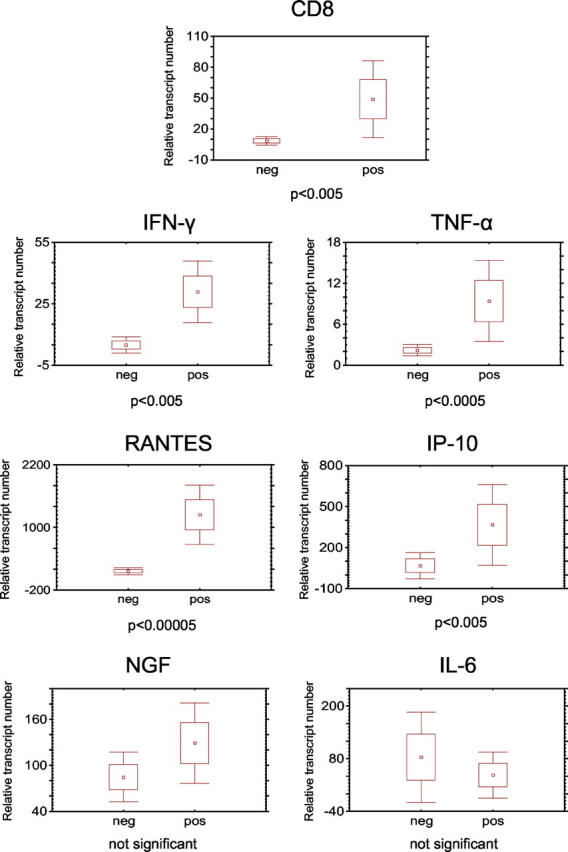
Quantification of CD8, cytokine, and chemokine mRNA expression by real-time RT-PCR. Each diagram shows the comparison of 12 HSV-1 negative TGs (neg) with 15 HSV-1 positive TGs (pos). The y axis represents the relative transcript number of the gene of interest compared to the housekeeping gene. Dots represent mean values, boxes represent SEM, and whiskers represent 1.96 × SEM.
Results
LAT in Situ Hybridization and Immunohistochemical Findings
Using ISH we detected LAT in the TGs on both sides in 15 of 21 individuals. The negative finding in six individuals was verified with the more sensitive LAT RT-PCR. IHC was performed on consecutive sections using antibodies to T-cell markers. CD3+ T cells were found in both TGs of the 15 LAT-positive individuals. The vast majority of T cells in the TGs were CD8+. Only a few CD4+ T cells were present among the lymphocytic infiltrates and scattered among the nervous tissue fibers (Figure 1f) ▶ .
Figure 1.
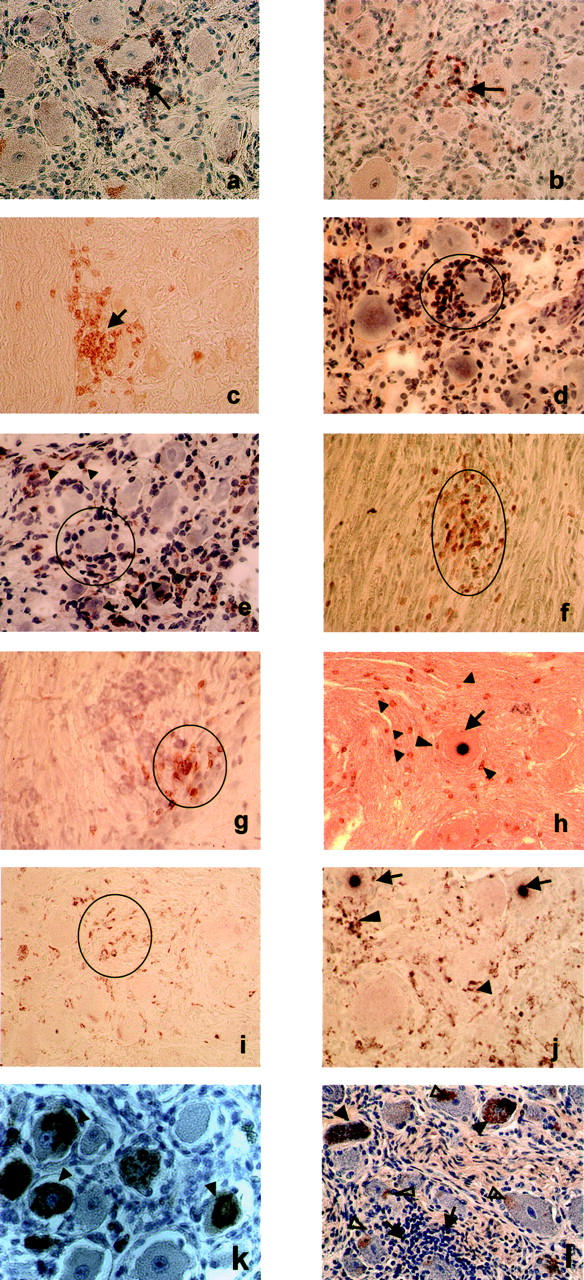
Representative photomicrographs of tissue sections from human TGs latently infected with HSV-1 and/or VZV, which were stained by IHC with anti-CD3, anti-CD8, and anti-CD68 antibodies. Neuron encircled by several rows of CD3-positive T cells (arrow, a) and clusters of CD3-positive T cells among ganglionic neurons (arrow, b). Infiltrating T cells stained positive with anti-CD8 antibodies. The pattern of staining was similar to that of CD3-positive T cells. CD8-positive T cells surrounding single neurons (arrow, c; encircled, d). Only a few T cells stained positive with anti-CD4 antibodies (encircled, e; arrowheads point to CD4-positive cells scattered among the ganglionic neurons). T cells were also present as clusters among nerve fibers: CD3-positive cells in a sagittal section (encircled, f) and CD8-positive cells in a transverse section of nerve fibers (encircled, g). When LAT ISH was combined with IHC (h) some CD3-positive T cells (arrowheads) were found occasionally around LAT-positive neurons (arrows). Single cells or clusters of cells among the neurons showed CD68 positivity (encircled, i). Small clusters of CD68-positive macrophages (encircled, j) were found in the vicinity of LAT-positive neurons (arrows). Neurons positive for VZV-protein 62 (k, arrowheads) from a child who died of sudden infant death and who showed no HSV-1 and no T-cell infiltration. Neurons positive for VZV-protein 62 (l, filled arrowheads) from a young man who was latently infected with HSV-1 and showed T-cell infiltrates (arrows, l) around VZV-free neurons. Some neurons showed brown lipofuscin granules near the plasma membrane (open arrowheads, l) distinguishable from the VZV protein that usually fills up the whole cytoplasm. Photomicrographs of a to c, f, and h to l were taken from paraffin and photomicrographs of d, e, g from frozen tissue sections. Magnification, ×400. Tissue sections from a, b, d, e, g, k, and l were slightly counterstained with hematoxylin.
The intensity of the T-cell infiltrates varied among the individuals (Table 1) ▶ . There were occasionally a very few CD8+ T cells in the interstitial tissue of the non-infected TGs. The pattern of expression of the CD68 marker was also assessed. LAT-positive TGs showed a strong staining with an obvious increase in number of the CD68+ cells (Figure 1, i and j) ▶ . In contrast, the LAT-negative TGs barely stained for the CD68 marker. When LAT ISH was combined with CD3 IHC staining, CD3-positive cells generally occurred in the near vicinity of the LAT-positive neurons or occasionally surrounded them (Figure 1h) ▶ . The same pattern of staining was observed when LAT ISH was combined with CD68 IHC. Some of the LAT-positive neurons were surrounded by agglomerates of CD68-positive cells (Figure 1j) ▶ .
To exclude the possibility that the presence of T cells was due to reactivation of HSV-1, tissue sections from all individuals were immunostained with a polyclonal HSV-1 antibody that merely interacts with late viral proteins. None of the tissue sections showed a positive staining with the HSV-1 antibody, although an intensive staining was present in the brain sections of a subject who had died of HSV-1 encephalitis (data not shown). None of the tissue sections showed a positive staining for the immediate early genes ICP0 and ICP4, whereas brain sections of an HSV-1 encephalitis case showed prominent staining (data not shown).
Positive staining for VZV-protein 62 was found in eight of 21 cases (Figure 1, k and l) ▶ . Four more subjects were found to be positive by VZV62 ISH (subjects 10, 13, 18, 20). All in all, positivity for latent VZV was found in 12 of 21 subjects. Eleven of these 12 individuals had been latently co-infected with HSV-1 and VZV, and showed abundant T-cell infiltrations. No T cells were found in one of the subjects positive for VZV protein 62 but negative for HSV-1 (Figure 1k) ▶ . In contrast, the TGs of four individuals who were latently infected only by HSV-1 were found to be infiltrated by abundant CD8+ T cells (Figure 1a ▶ was taken from the TG of subject 19). These findings show that the presence of T cells correlated constantly with the presence of latent HSV-1 and only irregularly with the presence of latent VZV.
Systematic examination by light microscopy showed that VZV-positive neurons were not surrounded by lymphocytic cells (Figure 1l) ▶ .
Histological examination of H&E-stained sections from the TGs tested revealed no neuronal damage. Four selected specimens with prominent inflammatory infiltrates were also tested for the presence of apoptotic cells. Only a very few apoptotic cells were detected and identified to be satellite or immune cells in these TGs (unpublished observation I. Paripovic).
Quantitative Real-Time RT-PCR
The expression of IFN-γ, TNF-α, IL-6, RANTES, IP-10, and NGF was compared by quantitative RT-PCR in 15 TGs of nine individuals who had been latently infected with HSV-1 and 12 TGs of six individuals negative for HSV-1 (Figure 2) ▶ . There was a significant induction of CD8 (P < 0.005), IFN-γ (P < 0.005), TNF-α (P < 0.0005), RANTES (P < 0.00005), and IP-10 (P < 0.005) in HSV-1 latently infected TGs versus HSV-1 free TGs. There was no difference in NGF and IL-6 expression between infected and uninfected TGs. IL-6 was elevated in one latently infected and in one uninfected TG.
Discussion
The persisting lymphocytic cell infiltration (CD8+ T cells, CD68+ macrophages) and the elevated levels of cytokine transcripts, which affect viral replication (IFN-γ, TNF-α), and chemokines, which attract immune cells (IP-10, RANTES) in human TGs, demonstrate for the first time that latent herpesviral infection is accompanied by a chronic inflammatory process at an immunoprivileged site but without any neuronal destruction.
Our findings are consistent with animal experiments showing that latent HSV-1 infection induces a chronic immune response. 9-13 It has been hypothesized that this may be the consequence of low-level expression of immediate early (IE) and early (E) viral genes during latency. 19 More recently, it was found that only a very few neurons in latently infected murine sensory ganglia expressed high levels of HSV-1 lytic cycle transcripts, and these neurons were surrounded by lymphocytic cells. 20 We could not demonstrate expression of the HSV-1 IE proteins ICP0 and ICP4 in the latently infected TGs by means of immunohistochemistry. However, the CD8+ T cells might have been retained in the TGs by persisting MHC class I viral peptide complexes that were below our detection limits. This is supported by the finding of inducible MHC class I expression in neurons. 21 The newly described anti-LAT transcript is capable of encoding a protein with antigenic potential and could be involved in the inflammatory process. 22 Viral antigens could possibly be presented to the CD8+ T cells by infected neuronal cells or professional antigen-presenting cells (APCs) during the process of cross-presentation. 23
Contrary to animal models, human TGs are frequently dually infected with HSV-1 and VZV. 24 Since VZV IE proteins are produced during VZV latency, 25,26 one can speculate that VZV proteins are the antigenic factor attracting T cells to the TGs. In our study inflammatory cell infiltration was not detected in the TGs of an individual infected only with VZV, but it was abundantly present in the TGs latently infected only with HSV-1. Mahalingam and colleagues 26 did not observe inflammatory infiltrates in multiple human ganglia that expressed immediate early protein 63 of VZV. Moreover, reports from the VZV animal model did not mention occurrence of lymphocytic infiltration.
A possible explanation for why HSV-1 rather than VZV latency induces chronic inflammation might be connected with the frequent reactivation of HSV-1. It is essential that the host tightly controls HSV-1 latency. Infiltrates in HSV-1 positive cases could be left from previous episodes of reactivation and inflammation.
CD8+ T cells are able to control virus infections via non-cytolytic mechanisms involving cytokines. 27 Such a mechanism is likely to occur in response to persistent viruses prone to reactivate frequently, because it provides a survival advantage for both host and virus. In our study we found that the level of IFN-γ and TNF-α transcripts were elevated in latently infected but not in uninfected TGs. It has been demonstrated that TNF-α synergizes with IFN-γ to block HSV-1 replication in vitro and in vivo. 28,29 Further, animal models have shown that prevention of HSV-1 reactivation from latency by CD8+ is mediated at least in part by IFN-γ without destroying the neurons. 30,31 Very high levels of RANTES in the latently infected TGs suggest that it is a key molecule in the recruitment of immune cells to the site of infection. This finding agrees with in vitro studies demonstrating that HSV-1 selectively induced expression of RANTES in macrophages. 32 High levels of RANTES and IP-10 have also been detected in human microglia cells during nonproductive HSV-1 infection. 33 The authors of the study demonstrated that IP-10 possesses direct antiviral activity in human neurons.
Persisting CD8+ T cells might interact with macrophages or directly with infected neurons. The latter is supported by a recent study on the HSV-1 mouse model. 34 Using in situ tetramer staining, the authors observed TCR polarization on many CD8+ T cells near the T-cell-neuron junction. The interaction of CD8+ T cells with macrophages or neurons most likely depends on low-level expression of viral antigens during latency; as a consequence, cytokines and chemokines are produced, which can inhibit viral replication and spread.
The immune cells (CD8+ T cells and CD68+ macrophages) inside the HSV-1 latently infected TGs and the increased cytokine levels that affect HSV-1 replication (IFN-γ, TNF-α, IP-10) might provide the morphological background for the clinical observation that immunosuppression can cause viral replication in the cranial nerve ganglia.
Acknowledgments
We thank Dr. T. Margolis for providing the ATD 19 plasmid, Dr. H. Lassmann for providing tissue sections from an HSV-1 encephalitis patient, and Judy Benson for carefully reading and editing the manuscript.
Footnotes
Address reprint requests to Diethilde Theil, Klinikum Grosshadern, Marchioninistrasse 23, 81377 Munich, Germany. E-mail: dtheil@brain.nefo.med.uni-muenchen.de.
Supported by the “Förderprogramm für Forschung und Lehre der Medizinischen Fakultät der Ludwig-Maximilians Universität” (FöFoLe Reg.-Nr.180 and 257, and by the DFG (SFB 571).
T. D. and D. T. contributed equally to this work.
References
- 1.Croen DK, Ostrove JM, Dragovic LJ, Smialek JE, Straus SE: Latent herpes simplex virus in human trigeminal ganglia: detection of an immediate early gene ’antisense’ transcript by in situ hybridisation. N Engl J Med 1987, 317:1427-1432 [DOI] [PubMed] [Google Scholar]
- 2.Furuta Y, Takasu T, Sato KC, Fukuda S, Inuyama Y, Nagashima K: Latent herpes virus type 1 in human geniculate ganglia. Acta Neuropathol (Berl) 1992, 84:39-44 [DOI] [PubMed] [Google Scholar]
- 3.Theil D, Arbusow V, Derfuss T, Strupp M, Pfeiffer M, Mascolo A, Brandt T: Prevalence of HSV-1 LAT in human trigeminal, geniculate, and vestibular ganglia and its implication for cranial nerve syndromes. Brain Pathol 2001, 11:408-413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simmons A, Tscharke D, Speck P: The role of immune mechanisms in control of herpes simplex virus infection of the peripheral nervous system. Curr Top Microbiol Immunol 1992, 179:31-56 [DOI] [PubMed] [Google Scholar]
- 5.Mester JC, Rouse BT: The mouse model and understanding immunity to herpes simplex virus. Rev Infect Dis 1991, 13:935-945 [DOI] [PubMed] [Google Scholar]
- 6.Dyck PJ, Giannini C, Lais A: Pathological alterations of nerves. Dyck PJ Thomas PK Griffin JW Low PA Poduslo JF eds. Peripheral Neuropathy. 1993:pp 517-559 WB Saunders Co. Philadelphia
- 7.Hickey WF: Basic principles of immunological surveillance of the normal central nervous system. Glia 2001, 36:118-124 [DOI] [PubMed] [Google Scholar]
- 8.Allt G, Lawrenson JG: The blood-nerve barrier: enzymes, transporters and receptors: a comparison with the blood-brain barrier. Brain Res Bull 2000, 52:1-12 [DOI] [PubMed] [Google Scholar]
- 9.Cantin EM, Hinton DR, Chen J, Openshaw H: Gamma interferon expression during acute and latent nervous system infection by herpes simplex virus type 1. J Virol 1995, 69:4898-4905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimeld C, Whiteland JL, Nicholls SM, Grinfeld E, Easty DL, Gao H, Hill T: Immune cell infiltration and persistence in the mouse trigeminal ganglion after infection of the cornea with herpes simplex virus type 1. J Neuroimmunol 1995, 61:7-16 [DOI] [PubMed] [Google Scholar]
- 11.Halford WP, Gebhardt BM, Carr DJ: Persistent cytokine expression in trigeminal ganglion latently infected with herpes simplex virus type 1. J Immunol 1996, 157:3542-3549 [PubMed] [Google Scholar]
- 12.Liu T, Tang Q, Hendricks RL: Inflammatory infiltration of the trigeminal ganglion after herpes simplex virus type 1 corneal infection. J Virol 1996, 70:264-271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen S-H, Graber DA, Schaffer PA, Knipe DM, Coen DM: Persistent elevated expression of cytokine transcripts in ganglia latently infected with herpes simplex virus in the absence of ganglionic replication or reactivation. Virology 2000, 278:207-216 [DOI] [PubMed] [Google Scholar]
- 14.Ellison AR, Yang L, Voytek C, Margolis T: Establishment of latent herpes simplex virus type 1 infection in resistant, sensitive, and immunodeficient mouse strains. Virology 2000, 268:17-28 [DOI] [PubMed] [Google Scholar]
- 15.Kennedy PGE, Grinfeld E, Bell EJ: Varicella-zoster virus gene expression in latently infected and explanted human ganglia. J Virol 2000, 74:11893-11898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naoe M, Marumoto R, Ishizaki Y, Ogawa Y, Nakagami Y, Yoshida H: Correlation between major histocompatibility complex class I molecules and CD8+ T lymphocytes in prostate, and quantification of CD8 and interferon-γ mRNA in prostate tissue specimens. BJU Int 2002, 90:748-753 [DOI] [PubMed] [Google Scholar]
- 17.Brink N, Szamel M, Young AR, Wittern KP, Bergemann J: Comparative quantification of IL-1β, IL-10, IL-10r, TNFα, and IL-7 mRNA levels in UV-irradiated human skin in vivo. Inflamm Res 2000, 49:290-296 [DOI] [PubMed] [Google Scholar]
- 18.Yin JL, Shackel NA, Zekry A, McGuinness PH, Richards C, Putten KV, McCaughan GW, Eris JM, Bishop GA: Real-time reverse transcriptase-polymerase chain reaction (RT-PCR) for measurement of cytokine and growth factor mRNA expression with fluorogenic probes or SYBR green I. Immunol Cell Biol 2001, 79:213-221 [DOI] [PubMed] [Google Scholar]
- 19.Kramer MF, Chen S-H, Knipe DM, Coen DM: Accumulation of viral transcripts and DNA during establishment of latency by herpes simplex virus. J Virol 1998, 72:1177-1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feldman LT, Ellison AR, Voytek CC, Yang L, Krause P, Margolis TP: Spontaneous molecular reactivation of herpes simplex virus type 1 latency in mice. Proc Natl Acad Sci USA 2002, 99:978-983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neumann H, Cavalie A, Jenne DE, Wekerle H: Induction of MHC class I genes in neurons. Science 1995, 269:549-552 [DOI] [PubMed] [Google Scholar]
- 22.Perng GC, Maguen B, Jin L, Mott KR, Kurylo J, BenMohamed L, Yukht A, Osorio N, Nesburn AB, Henderson G, Inman M, Jones C, Wechsler SL: A novel herpes simplex virus type 1 transcript (AL-RNA) antisense to the 5′ end of the latency-associated transcript produces a protein in infected rabbits. J Virol 2002, 76:8003-8010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heath WR, Carbone FR: Cross-presentation in viral immunity and self-tolerance. Nat Rev Immunol 2001, 1:126-134 [DOI] [PubMed] [Google Scholar]
- 24.Theil D, Derfuss T, Strupp M, Gilden DH, Arbusow V, Brandt T: Cranial nerve palsies: herpes simplex virus type 1 and varicella-zoster virus latency. Ann Neurol 2002, 51:273. [DOI] [PubMed] [Google Scholar]
- 25.Lungu O, Panagiotidis CA, Annunziato PW, Gershon AA, Silverstein SJ: Aberrant intracellular localization of varicella-zoster virus regulatory proteins during latency. Proc Natl Acad Sci USA 1998, 95:7080-7085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahalingam R, Wellish M, Cohrs R, Debrus S, Piette J, Rentier B, Gilden DH: Expression of protein encoded by varicella-zoster virus open reading frame 63 in latently infected human ganglionic neurons. Proc Natl Acad Sci USA 1996, 93:2122-2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guidotti LG, Chisari FV: Noncytolytic control of viral infections by the innate and adaptive immune response. Annu Rev Immunol 2001, 19:65-91 [DOI] [PubMed] [Google Scholar]
- 28.Feduchi E, Alonso MA, Carrasco L: Human gamma interferon and tumor necrosis factor exert a synergistic blockade on the replication of herpes simplex virus. J Virol 1989, 63:1354-1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paludan SR, Mogensen SC: Virus-cell interactions regulating induction of tumor necrosis factor α production in macrophages infected with herpes simplex virus. J Virol 2001, 75:10170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu T, Khanna KM, Chen XP, Fink DJ, Hendricks LR: CD8+ T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J Exp Med 2000, 191:1459-1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu T, Khanna KM, Carriere BN, Hendricks RL: Gamma interferon can prevent herpes simplex virus type 1 reactivation from latency in sensory neurons. J Virol 2001, 75:11178-11184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melchjorsen J, Pedersen FS, Mogensen SC, Paludan SR: Herpes simplex virus selectively induces expression of the CC chemokine RANTES/CCL5 in macrophages through a mechanism dependent on PKR and ICP0. J Virol 2002, 76:2780-2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lokensgard JR, Hu S, Sheng W, van Oijen M, Cox D, Cheeran MC, Peterson PK: Robust expression of TNF-α, IL-1β, RANTES, and IP-10 by human microglial cells during nonproductive infection with herpes simplex virus. J Neurovirol 2001, 7:208-219 [DOI] [PubMed] [Google Scholar]
- 34.Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL: Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity 2003, 18:593-603 [DOI] [PMC free article] [PubMed] [Google Scholar]


