Abstract
Penicillin-"virgin" strains of Enterococcus faecalis collected from a population of individuals with no previous antibiotic exposure were subjected in vitro to penicillin delivered as repeated pulses, stepwise increasing concentrations, or sustained levels of a single concentration. Changes in resistance to penicillin were assessed by determination of MICs, and time-kill studies were performed to evaluate changes in tolerance to the bactericidal effects of penicillin. Isogenic clones, derived from various exposure regimens, which exhibited changes in either resistance or tolerance were further examined for changes in penicillin-binding proteins. Exposure to repeated pulses of penicillin resulted in the development of tolerance to penicillin without changes in the level of resistance. Clones derived from a regimen of stepwise increases in the penicillin concentration acquired both increased penicillin resistance and tolerance. Clones selected after prolonged continuous exposure to a fixed concentration of penicillin displayed minimally increased resistance to penicillin, but they retained the lytic, nontolerant response to the bactericidal effect of penicillin. Clones which acquired tolerance to the bactericidal effect of penicillin without changes in penicillin resistance exhibited a penicillin-binding protein pattern identical to that of the parental strain. Increased labeling of several penicillin-binding proteins accompanied the development of increased penicillin resistance in both penicillin-tolerant and nontolerant strains. Exposure of E. faecalis to penicillin in repeated pulses of brief duration, for prolonged periods at a constant concentration, or in stepwise graded concentrations can result in the selection of clones with increased resistance to the inhibitory or bactericidal effects of penicillin, or both. These observations may be relevant to the selection of dosing regimes for penicillin in the treatment of enterococcal infections, when bactericidal synergism cannot be achieved with penicillin-aminoglycoside combinations.
Full text
PDF

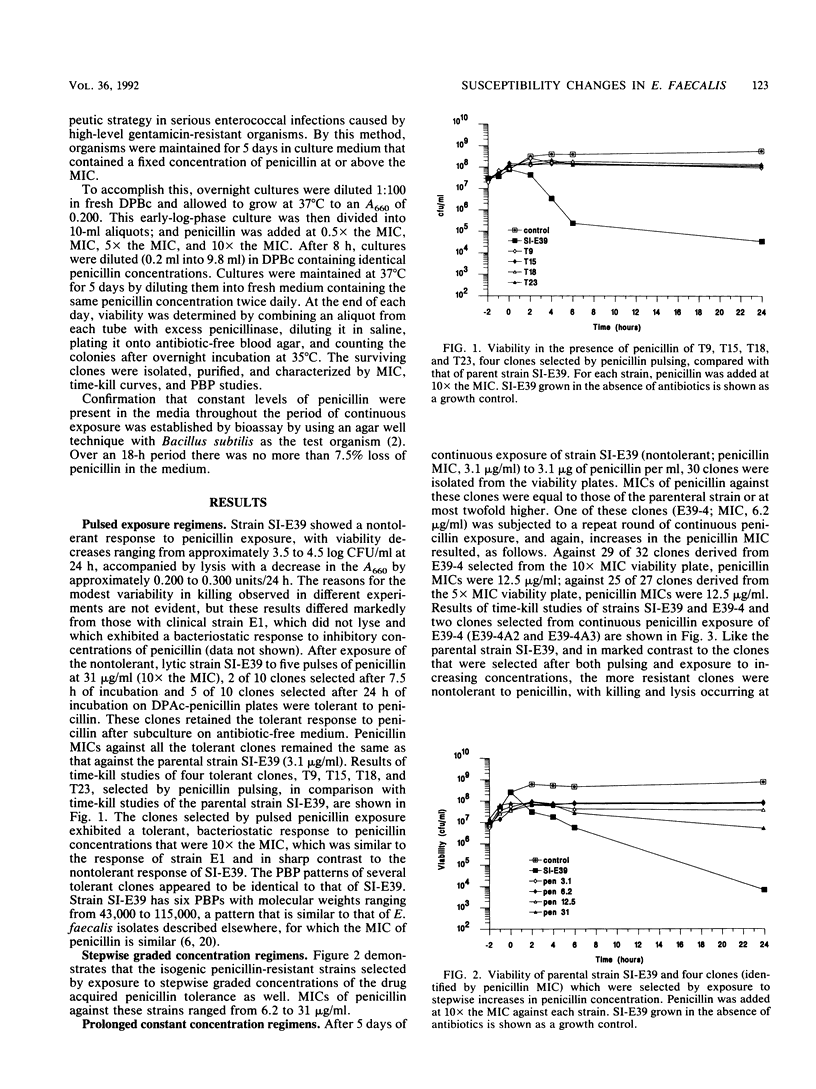
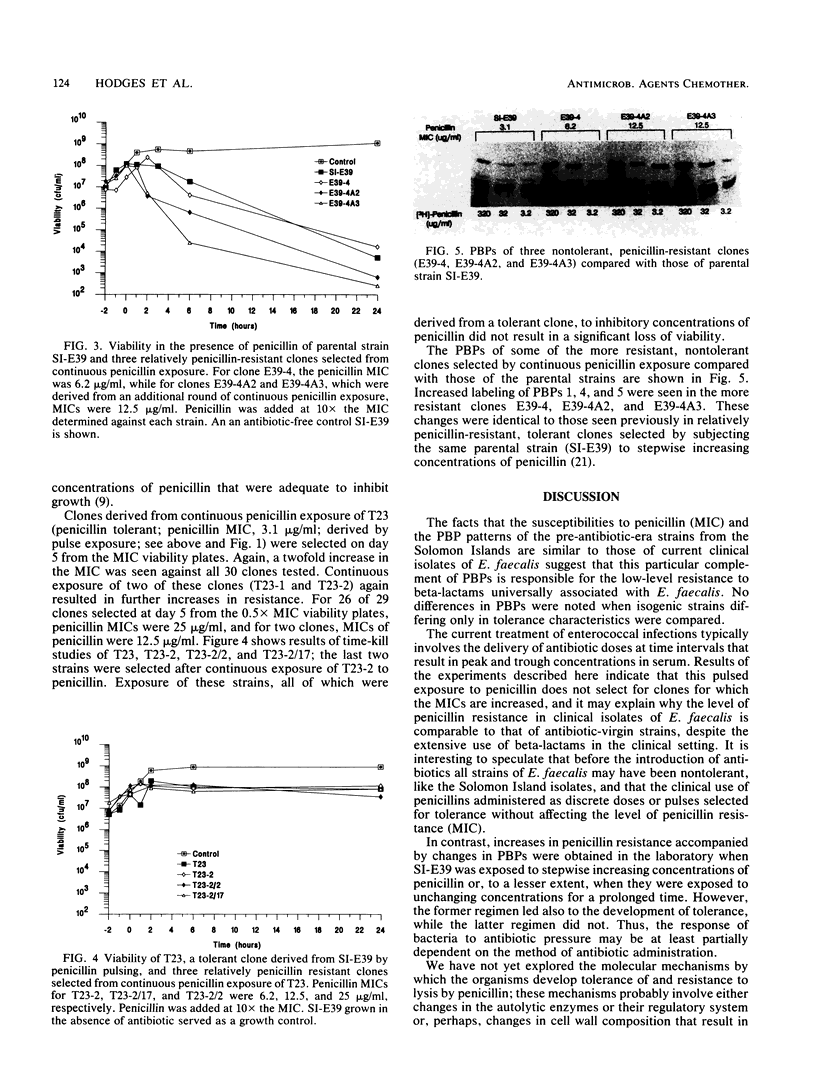

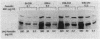
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calderwood S. A., Wennersten C., Moellering R. C., Jr, Kunz L. J., Krogstad D. J. Resistance to six aminoglycosidic aminocyclitol antibiotics among enterococci: prevalence, evolution, and relationship to synergism with penicillin. Antimicrob Agents Chemother. 1977 Sep;12(3):401–405. doi: 10.1128/aac.12.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana R., Cerini R., Longoni P., Grossato A., Canepari P. Identification of a streptococcal penicillin-binding protein that reacts very slowly with penicillin. J Bacteriol. 1983 Sep;155(3):1343–1350. doi: 10.1128/jb.155.3.1343-1350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner P., Smith D. H., Beer H., Moellering R. C., Jr Recovery of resistance (R) factors from a drug-free community. Lancet. 1969 Oct 11;2(7624):774–776. doi: 10.1016/s0140-6736(69)90482-6. [DOI] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Liu F. Y. Binding of beta-lactam antibiotics to penicillin-binding proteins of Staphylococcus aureus and Streptococcus faecalis: relation to antibacterial activity. Antimicrob Agents Chemother. 1980 Nov;18(5):834–836. doi: 10.1128/aac.18.5.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwick H. J., Kalmanson G. M., Guze L. B. In vitro activity of ampicillin or vancomycin combined with gentamicin or streptomycin against enterococci. Antimicrob Agents Chemother. 1973 Oct;4(4):383–387. doi: 10.1128/aac.4.4.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindes R. G., Willey S. H., Eliopoulos G. M., Rice L. B., Eliopoulos C. T., Murray B. E., Moellering R. C., Jr Treatment of experimental endocarditis caused by a beta-lactamase-producing strain of Enterococcus faecalis with high-level resistance to gentamicin. Antimicrob Agents Chemother. 1989 Jul;33(7):1019–1022. doi: 10.1128/aac.33.7.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karchmer A. W., Moellering R. C., Jr, Watson B. K. Susceptibility of various serogroups of streptococci to clindamycin and lincomycin. Antimicrob Agents Chemother. 1975 Feb;7(2):164–167. doi: 10.1128/aac.7.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mederski-Samoraj B. D., Murray B. E. High-level resistance to gentamicin in clinical isolates of enterococci. J Infect Dis. 1983 Apr;147(4):751–757. doi: 10.1093/infdis/147.4.751. [DOI] [PubMed] [Google Scholar]
- Moellering R. C., Jr, Korzeniowski O. M., Sande M. A., Wennersten C. B. Species-specific resistance to antimocrobial synergism in Streptococcus faecium and Streptococcus faecalis. J Infect Dis. 1979 Aug;140(2):203–208. doi: 10.1093/infdis/140.2.203. [DOI] [PubMed] [Google Scholar]
- Moellering R. C., Jr, Wennersten C., Weinberg A. N. Studies on antibiotic synergism against enterococci. I. Bacteriologic studies. J Lab Clin Med. 1971 May;77(5):821–828. [PubMed] [Google Scholar]
- Murray B. E., Mederski-Samaroj B. Transferable beta-lactamase. A new mechanism for in vitro penicillin resistance in Streptococcus faecalis. J Clin Invest. 1983 Sep;72(3):1168–1171. doi: 10.1172/JCI111042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch G. A., Krogstad D. J. Antibiotic-induced lysis of enterococci. J Clin Invest. 1981 Sep;68(3):639–645. doi: 10.1172/JCI110298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauvin C., Eliopoulos G. M., Willey S., Wennersten C., Moellering R. C., Jr Continuous-infusion ampicillin therapy of enterococcal endocarditis in rats. Antimicrob Agents Chemother. 1987 Feb;31(2):139–143. doi: 10.1128/aac.31.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uttley A. H., Collins C. H., Naidoo J., George R. C. Vancomycin-resistant enterococci. Lancet. 1988 Jan 2;1(8575-6):57–58. doi: 10.1016/s0140-6736(88)91037-9. [DOI] [PubMed] [Google Scholar]
- Williamson R., Gutmann L., Horaud T., Delbos F., Acar J. F. Use of penicillin-binding proteins for the identification of enterococci. J Gen Microbiol. 1986 Jul;132(7):1929–1937. doi: 10.1099/00221287-132-7-1929. [DOI] [PubMed] [Google Scholar]
- al-Obeid S., Gutmann L., Williamson R. Modification of penicillin-binding proteins of penicillin-resistant mutants of different species of enterococci. J Antimicrob Chemother. 1990 Nov;26(5):613–618. doi: 10.1093/jac/26.5.613. [DOI] [PubMed] [Google Scholar]