Abstract
The human endometrium is a dynamic tissue, the proliferative activity of which dramatically changes throughout the menstrual cycle, with exquisite regulation by sex-steroid hormones. Primary endometrial epithelial cells fall into senescence within 2 weeks when cultured on plastic dishes, and more complete understanding of endometrial biology has been delayed because of, in part, a lack of an in vitro culture model for endometrial epithelial cells. Our goal was to establish immortalized human endometrial glandular cells that retain the normal functions and characteristics of the primary cells. Because the Rb/p16 and p53 pathways are known to be critical elements of epithelial senescence in early passages, we used human papillomavirus E6/E7 to target these pathways. The combination of human papillomavirus-16 E6/E7 expression and telomerase activation by the introduction of human telomerase reverse transcriptase (hTERT) led to successful immortalization of the endometrial glandular cells. E6/E7 expression alone was sufficient to extend their life span more than 20 population doublings, but the telomerase activation was further required to enable the cells to pass through the subsequent replicative senescence at 40 population doublings. Isolated immortalized cells contained no chromosomal abnormalities or only nonclonal aberrations, retained responsiveness to sex-steroid hormones, exhibited glandular structure on three-dimensional culture, and lacked transformed phenotypes on soft agar or in nude mice. These findings support the notion that both Rb inactivation/p53 inactivation and telomerase activation are necessary to immortalize endometrial epithelial cells, but additional factors are required for endometrial carcinogenesis. Our established cell lines show great promise for investigation of hormone functions, endometrial biology, and endometrial carcinogenesis.
The human endometrium is a unique tissue characterized by constant and rapid cell proliferation, differentiation, and breakdown during a menstrual cycle. This cyclic change in proliferation is exquisitely regulated by the cooperative actions of estrogen and progesterone, indicating that human endometrium is highly susceptive to sex-steroid hormones and that endometrial glandular epithelial cells may provide a good model with which to study hormone function and regulation. However, in classical tissue culture using plastic or glass dishes, epithelial cells loose their proliferative capacity during ongoing cultivation throughout several days, whereas stromal cells are more easily cultured in the longer term. Most investigators in this field have tried to develop endometrial cell cultures with a mixture of stromal cells and/or organotypic cultures, but both for short-term experiments only. 1-3
The lack of a stable in vitro culture system of endometrial cells also renders studying the molecular carcinogenesis of the endometrium difficult. Most endometrial cancers arise from endometrial glandular cells via the multistep accumulation of abnormalities in oncogenes and tumor suppressor genes, including PTEN, Ras, and p53. 4 These factors are frequently mutated or deregulated in endometrial cancers or even in its precursors. However, in vitro experiments to investigate the role of these factors in endometrial carcinogenesis have been impossible because of the extremely short life span of primary cultured endometrial epithelial cells in vitro.
One major mechanism that accounts for the limited life span of primary cultured cells is telomere-based replicative senescence. Telomeres are the specialized nucleoprotein structures at the ends of chromosome that play pivotal roles in chromosome stability. 5,6 Without new synthesis, telomeres undergo progressive shortening with each cell division, according to Watson’s 7 model. Critically short telomeres arising after a considerable number of cell divisions induce specific signals for cell-cycle arrest via DNA damage check point, resulting in replicative senescence of cells. 8,9 Telomerase is a ribonucleoprotein complex that extends and maintains the telomeres. Activation of this enzyme is therefore required for cells to overcome replicative senescence and obtain the ability to divide without limit. 10,11 Studies of the telomerase enzyme complex have revealed the presence of two major subunits contributing to enzymatic activity—a structural RNA component (hTR) that contains a template region binding to TTAGGG repeats in telomeres 12 and a catalytic subunit with reverse transcriptase activity (hTERT). 13,14 Although hTR is constitutively present in normal and cancer cells, expression of hTERT is almost exclusively limited to cancer cells. 15-18 Introduction of the hTERT gene into telomerase-negative normal cells is sufficient to induce telomerase activity and to immortalize some types of normal cells. 19-21 These findings indicate that hTERT expression is the rate-limiting step in telomerase activity and cellular immortalization.
Although telomere-based replicative senescence is a critical barrier to cell immortalization, some epithelial cells appear to senesce at an earlier stage, at ∼20 population doublings (PDs) in which telomeres do not reach their critical short length. 22,23 Introduction of hTERT into these cells did not result in extension of their life span. 22 This telomere-independent senescence is thought to be controlled by the Rb/p16 pathway, because it can either be prevented by introduction of viral oncoproteins that bind Rb and inhibit its function, such as human papillomavirus (HPV) E7, or a few cells spontaneously escape from senescence with reduced p16 expression because of promoter methylation. 24-26 Based on these findings, we previously reported that both Rb/p16 inactivation and telomerase activity are required to immortalize some types of human epithelial cells. 22 A more recent report 27 demonstrated a two-stage, p16- and p53-dependent senescence mechanism of keratinocytes that limits replicative potential independent of telomere status, suggesting a role of the p53 pathway in early-stage senescence of epithelial cells.
Establishment of immortalized human endometrial epithelial cells will greatly benefit the study of endometrial biology and carcinogenesis. Thus, we attempted to immortalize human endometrial epithelial cells. Because high-risk type HPV E6 and E7 seem to be useful to target both Rb and p53 pathways, we introduced HPV16 E6/E7 and/or hTERT into endometrial glandular cells. We successfully isolated immortalized endometrial epithelial cells expressing HPV 16 E6/E7 and hTERT. Of particular interest is that these cells do not have transformed phenotypes and retain the natural characteristics of endometrial glands without severe chromosomal abnormalities, and thus are potentially useful as an experimental model with which to research hormone functions, implantation, and endometrial carcinogenesis.
Materials and Methods
Isolation of Human Endometrial Glands
Human endometrial tissue samples were obtained from two patients (42 and 52 years of age) undergoing hysterectomy as a treatment for uterine myoma. They had regular menstrual cycles and were in late proliferative phase at the time of operation. Minced endometrial tissue was placed in Dulbecco’s modified Eagle’s medium (DMEM) containing 350 U/ml of deoxyribonuclease I (Takara, Ohstu, Japan) and 180 U/ml of collagenase type 3 (Washington Biochemical Corp., Lakewood, NJ) in plastic dishes and gently shaken for 40 minutes at 37°C. 28,29 Individual glands on the bottom of the dishes were directly picked up one by one under a microscope, collected into Eppendorf tubes, and seeded onto 24-well dishes for subsequent gene transfection by viral vectors.
Vector Construction and Retroviral Transfection of HPV16 E6, E7, and hTERT
pCMSCVpuro comprises the CMV/LTR fusion promoter, packaging signal Psi+, and the multicloning sequence from pCLXSN (Imgenex Corp., San Diego, CA) followed by the PGK-puro cassette and 3′ long terminal repeat of murine embryonic stem cell virus from pMSCVpuro (Clontech, Palo Alto, CA). The Gateway system (Invitrogen, Carlsbad, CA) was used for subcloning genes into the retroviral vectors. The destination vectors pCLXSN-DEST and pCMSCVpuro-DEST were constructed by inserting a modified cassette containing attR sites and ccdB (Invitrogen) between the EcoRI and BglII sites of pCLXSN and pCMSCVpuro, respectively. Cloning of the full-length hTERT cDNA has been described previously. 22 After cloning segments of HPV16 E7 (16E7) and a deletion mutant of HPV16 E6 (16E6SD-D151) 30 into pDONR201 (Invitrogen), these segments were recombined into the retroviral vectors via the LR reaction (Invitrogen) to generate pCMSCVpuro-hTERT, pCMSCVpuro-16E6SDD151, pCLXSN-16E6SDD151, and pCLXSN-16E7. pCLXSN-16E6E7 was constructed by inserting the EcoRI-BamHI segment containing HPV16 E6 and E7 between the EcoRI and BglII sites of pCLXSN. Production of recombinant retroviruses has been described. 31 Briefly, retroviral vector and packaging construct, pCL-10A1, were co-transfected into 293T cells using TransIT-293 (Mirus Co., Madison, WI) according to the manufacturer’s instructions, and the culture fluid was harvested at 48 to 72 hours after transfection. The titer of the recombinant viruses was greater than 5 × 105 drug-resistant colony forming units/ml on HeLa cells. One ml of the culture fluid was added to primary endometrial glandular cells seeded on 24-well dishes in the presence of polybrene (4 μg/ml). After inoculation with viruses, cells were grown without drug selection as mock-infected cells and stopped growing within 2 weeks. For combinations of retroviral infections, cells were first transduced with E6 and/or E7, and then transduced with hTERT.
Cell Culture
Stably transduced endometrial epithelial cells were maintained in DMEM/F12 (1:1) supplemented with 10% fetal bovine serum and ITS (BD Biosciences, Bedford, MA) in an atmosphere of 5% CO2 at 37°C. Three-dimensional culture was performed on Millicell CM filter inserts (Millipore, Bedford, MA) containing Matrigel (Becton Dickinson Labware, Bedford, MA) as described previously. 1 Ishikawa cells and HEC1 cells were provided by Dr. Masato Nishida (Kasumigaura National Hospital, Tsuchiura City, Japan) and Dr. Hiroyuki Kuramoto (Kitasato University, Tokyo, Japan), respectively. C33A cells were obtained from the American Type Culture Collection (Rockville, MD). These cells were grown in DMEM with 10% fetal bovine serum. PDs were calculated as follows: PD = log(number of cells obtained/initial number of cells)/log2.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
The expression of HPV16 E6, E7, and hTERT mRNAs as well as estrogen receptor (ΕR)-α, ER-β, and progesterone receptor (PR) mRNAs was analyzed by RT-PCR amplification. The primer pairs used were 5′ to 3′ and 5′-GCAACAGTTACTGCGACGTG-3′ (forward), 5′-GGACACAGTGGCTTTTGACA-3′ (reverse) for E6, 5′-TTCCCGGGATCCTTATGGTTTCTGAGAACAGAT-3′ (forward) and 5′-TTCCCGGGATCCATGCATGGAGATACACCTACAT-3′ (reverse) for E7, 5′-CGGAAGAGTGTCTGGAGCAA-3′ (forward) and 5′-GGATGAAGCGGAGTCTGGA-3′(reverse) for hTERT, 5′-AGAGATGCTCCATGCCTTTG-3′ (forward), 5′-GCAGACAGGGAGCTGGTTCA-3′ (reverse) for ΕRα, and 5′-TCACATCTGTATGCGGAACC-3′ (forward), 5′-CGTAACACTTCCGAAGTCGG-3′ (reverse) for ERβ, and 5′-AACACGTCAGTGGGCAGATG-3′ (forward), 5′-GCAGCAATAACTTCAGACATC-3′ (reverse) for PR. Total RNA was isolated from the cells using Isogen (Nippon Gene, Tokyo, Japan) according to the manufacturer’s protocol, and cDNA was synthesized from 1 μg of RNA using the RNA PCR kit version 2 (TakaRa, Ohtsu, Japan) with random primers. Typically, 2-μl aliquots of the reverse-transcribed cDNA were amplified by 28 cycles of PCR in 50 μl of 1× buffer [10 mmol/L Tris-HCl (pH 8.3), 2.5 mmol/L MgCl2, and 50 mmol/L KCl] containing 1 mmol/L each of dATP, dCTP, dGTP, and dTTP, 2.5 U of Gene Taq (Nippon Gene), and 0.2 μmol/L primers. Each cycle consisted of denaturation at 94°C for 30 seconds, annealing at 55°C (for HPV16 E6 and E7), 62°C (for PR and ER), and 60°C (for hTERT) for 30 seconds and extension at 72°C for 45 seconds. The PCR products were resolved by electrophoresis in 7% polyacrylamide gels and stained with SYBR green I (FMC BioProducts, Rockland, ME). The efficiency of cDNA synthesis from each sample was estimated by PCR using GAPDH-specific primers as described previously. 21
Western Blot Analysis
Cytoplasmic extracts from cells with extended life span (EM cells) were prepared using the method of Schreiber and colleagues. 32 Briefly, 1 × 106 cells were collected, washed with PBS, and resuspended in 400 μl of buffer containing 10 mmol/L HEPES (pH 7.9), 10 mmol/L KCl, 0.1 mmol/L ethylenediaminetetraacetic acid, 0.1 mmol/L EGTA, 1 mmol/L dithiothreitol, and 0.5 mmol/L phenylmethyl sulfonyl fluoride, and allowed to swell for 15 minutes, after which 25 μl of a 10% solution of Nonidet P-40 (Sigma-Aldrich, St. Louis, MO) is added and the tube is vigorously vortex for 10 seconds. The homogenate is centrifuged at 12,000 rpm for 30 seconds. The supernatant is recovered as cytoplasmic extracts. Then, 50 μg of cytoplasmic extracts were electrophoresed on a sodium dodecyl sulfate-polyacrylamide gel and transferred to polyvinylidene difluoride membranes. Membranes were blocked in TBST (150 mmol/L NaCl, 20 mmol/L Tris-Cl, pH 7.5, 0.1% Tween) containing 5% nonfat dried milk, and then incubated with specific antibody against PTEN (clone 6H2.1; Cascade Bio Science, Winchester, MA), followed by reaction with horseradish peroxidase-linked anti-mouse IgG. Immunoreactive bands were visualized using the ECL detection system (Amersham), as suggested by the manufacturer.
Telomeric Repeat Amplification Protocol (TRAP) Assay
TRAP assays were performed using the TRAPEZE telomerase detection kit (Intergen, Purchase, NY) according to the manufacturer’s protocol.
Immunocytochemistry and Immunohistochemistry
EM cells were cultured on LAB TEK chamber slides (Nalge Nunc International, Naperville, IL) for 24 hours, fixed with methanol, and subjected to immunocytochemistry. Gland-like structures of EM cells grown in Matrigels were picked up by microscopic manipulation, formalin-fixed, and paraffin-embedded, and thin sections were prepared for immunohistochemistry. Antigen retrieval was then performed for 10 minutes in 1× antigen retrieval solution (Biogenex, San Ramon, CA). These samples were washed with PBS and incubated with mouse monoclonal antibody against pan-cytokeratin (4/5/8/10/13/18) (NCL-C11; Novocastra Laboratories, Newcastle, UK) at a 1:10 dilution, vimentin (NCL-VIM, Novocastra Laboratories) at a 1:100 dilution, CD10 (sc-7632; Santa Cruz Biotechnology, Inc, Santa Cruz, CA), and CD34 (NCL-VIM, Novocastra Laboratories) at a 1:50 dilution and control serum for 1 hour at room temperature. After incubation with an anti-mouse secondary antibody, the immune complex was visualized using the ABC-elite kit (Vector Laboratories Inc., Burlingame, CA).
Telomere Length Analysis by Southern Blotting
Telomere length was analyzed by the terminal restriction fragment (TRF) length assay using a TeloTTAGGG Telomere Length Assay kit (Roche). Briefly, genomic DNA was isolated from cells, and then analyzed by Southern blots probed with a telomere-specific probe to visualize the TRF.
β-Gal Assay
The β-gal assay was performed as previously described. 33 Briefly, cells were fixed for 5 minutes at room temperature in 3% formaldehyde followed by incubation at 37°C with senescence-associated β-gal stain solution: 1 mg of 5-bromo-4 chloro-3-indolyl β-d-galactoside (X-Gal) per/ml/40 mmol/L citric acid/sodium phosphate, pH 6.0/5 mmol/L potassium ferrocyanide/5 mmol/L potassium ferricyanide/150 mmol/L NaCl/2 mmol/L MgCl2. After a 6- to 12-hour incubation, positive staining was confirmed by microscopy.
WST-1 Assay
The proliferative activity of cells treated with sex-steroid hormones was examined using the WST-1 assay according to the manufacturer’s protocol (Boehringer Mannheim, Indianapolis, IN). Briefly, the cells were seeded at 2.5 × 103 cells/well in 96-well flat-bottomed plates and incubated with 100 μl of the growth media overnight at 37°C. After starvation with phenol red-free media containing charcoal-treated fetal bovine serum for 24 hours, the cells were treated with 17β-estradiol (E2) or 6α-methyl-17α-hydroxy-progesterone acetate (MPA) at various concentrations for different time periods. A 10-μl aliquot of WST-1 reagent was then added to the media and incubated for 1 to 2 hours at 37°C. The absorbance at wavelengths between 420 to 480 nm was measured using a microplate reader. The relative WST-1 activity was calculated and shown as WST-1 index in which the activity of untreated control samples was normalized to 1.
Anchorage Independence of Growth
A total of 1 × 104 endometrial cancer HEC1 and Ishikawa cells as well as EM-E6E7TERT-1, -2, and -3 cells were seeded onto 6-cm dishes with 0.33% noble agar in DMEM supplemented with 10% fetal calf serum on top of 0.5% base agar in DMEM supplemented with 10% fetal calf serum. Colonies larger than 0.125 mm were counted after incubation for 2 weeks.
Nude Mice Xenograft Experiments
Endometrial cancer HEC1 and Ishikawa ells and as well as EM-E6E7TERT-1, -2, and -3 cells were resuspended in growth media (107/ml) and subcutaneously injected (0.1 ml) at the base of the left flank of female BALB/c nu/nu mice (7 to 9 weeks of age) (SLC, Hamamatsu, Japan). Tumor growth was monitored weekly, and tumor formation was confirmed 60 days after inoculation.
Results
Isolation and Characterization of Human Endometrial Epithelial Cells Expressing HPV16 E6/E7 and hTERT
We hypothesized that the early senescence of endometrial epithelial cells that occurs within a couple of passages in primary culture is because of cell-cycle arrest via the Rb pathway. One way to effectively block the Rb pathway is the use of high-risk type HPV E7, which specifically binds Rb and abrogates its functions. High-risk type HPV E6 that binds p53 and interferes with its normal functions is known to support the various activities of E7. Thus, we sought to introduce HPV 16 E6 and/or E7 genes into primary endometrial epithelial cells, with or without introduction of the hTERT gene. Human endometrial samples in late proliferative phase were collected from the 42- and 52-year-old women with regular menstrual cycle, who underwent hysterectomy as a treatment of uterine myoma. These samples were minced and digested in a collagenase solution, and endometrial glands were then isolated from the stromal cells. The isolated glands were directly collected one by one by microscopic manipulation (Figure 1A) ▶ . Approximately 10 glands were seeded on one well of plastic dishes and infected with retroviral vectors for expression of HPV16 E6, E7 and hTERT in various combinations (Figure 1B) ▶ . To dissect the role of E6, mutant E6 (E6Δ151) in which leucine at amino acid 151 was deleted, was also introduced, instead of wild-type E6. This mutant lacks the transforming ability of E6 but retains the ability to bind and abrogate p53 function. 31 Infection was performed once per each sample isolated from the two patients, and stable infectants were then screened by drug-selection. Introduction of hTERT gene alone failed to generate clones with extended life span. Similarly, transfection of E7 or E6 gene alone was not able to produce such clones. Combinatorial transfection of these genes successfully isolated a total of five independent clones (Figure 1D) ▶ , three of which were transfectants harboring the E6, E7, and hTERT genes (EM-E6/E7/TERT-1, -2, and -3), one harbored the E6Δ151, E7, and hTERT genes (EM-E6Δ151/E7/TERT) and the other contained only the E6 and E7 genes (EM-E6E7). Morphologically, these cells exhibited the round shape typical of epithelial cells (Figure 1, B and C) ▶ . Gene expression in primary glands and isolated clone was analyzed by RT-PCR (Figure 2) ▶ . As expected, EM-E6/E7/TERT clones and EM-E6Δ151/E7/TERT cells expressed E6, E7, and hTERT mRNAs, whereas EM-E6E7 cells expressed only E6 and E7 mRNAs (Figure 2A) ▶ . Primary endometrial glands cultured for retroviral infection were also examined, and no expression of E6/E7 mRNA or hTERT mRNA was detected. Our previous study demonstrated that endometrial glands in proliferative phase exhibit telomerase activity. 28 However, once they are cultured on dishes, telomerase activity rapidly decreases, suggesting that telomerase activity in endometrial glands cannot be maintained in in vitro culture. Steroid hormone receptor mRNA expression was next examined by RT-PCR (Figure 2B) ▶ . All isolated clones expressed estrogen receptor-α (ΕR-α) and progesterone receptor (PR) mRNA, whereas expression of ER-β mRNA was basically negative but faint expression was observed only in EM-E6/E7/hTERT-3 cells. PTEN protein expression was also examined by Western blot analysis (Figure 2C) ▶ . All clones were confirmed to express significant levels of PTEN protein. The TRAP assay revealed that EM-E6/E7/TERT and EM-E6Δ151E7/TERT cells exhibited telomerase activity while EM-E6/E7 cells did not (Figure 2D) ▶ .
Figure 1.
Isolation of endometrial epithelial cell clones with extended life span by the transfection of HPV16 E6, E7, and hTERT. A: Purified endometrial glands isolated from endometrial tissues. Endometrial tissues were minced and digested with a collagenase-based solution to isolate endometrial glands from stromal cells. Endometrial glands in the above solution were directly picked up one by one by microscopic manipulation. B and C: Morphology of isolated clones (EM-E6/E7/TERT-2 cells) cultured on plastic dishes. D: Transfected genes and the number of isolated clones with extended life span. Patient characteristics and the names of isolated clones are shown. Original magnifications: ×100 (B); ×400 (C).
Figure 2.
Analysis of gene expression and telomerase activity in EM cells. A: Expression of HPV E6, E7, and hTERT mRNAs was examined by RT-PCR in primary endometrial glands isolated from the two patients and a series of EM cells. B: Expression of steroid receptor mRNAs was analyzed by RT-PCR in EM cells. C: Expression of PTEN protein in EM cells. Western blot analysis was performed using cytoplasmic extracts from EM cells. A172 cells, homozygously deleted at the PTEN locus, are used as negative control, while PTEN-positive MCF-7 cells are used as positive control. D: Telomerase activity in EM cells was examined by TRAP assay. IC, internal control to normalize the efficiency of PCR amplification.
We next sought to verify the epithelial origin of these clones by immunocytochemistry. As shown in Figure 3 ▶ , these cells were positive for pan-cytokeratin and vimentin, but not for stromal marker, CD10, or vascular endothelial marker, CD34. Thus, these clones were most likely derived from endometrial epithelial cells, but not from other sources such as fibroblasts or endothelial cells.
Figure 3.

Immunocytochemical analysis of EM-E6/E7/TERT cells. EM-E6/E7/TERT-3 cells were immunocytochemically analyzed for the expression of cytokeratin, vimentin, CD-10, and CD34. Pan-cytokeratin was basically positive in these cells, but staining intensity was varied among cells. Vimentin was strongly positive, whereas CD10 and CD34 were negative. CK, pan-cytokeratin; VIM, vimentin.
Karyotype analyses were performed at PD 20 to 32 for each clone (Table 1) ▶ and revealed that EM-E6/E7/TERT-1 and -3 cells had completely normal karyotypes whereas EM-E6/E7/TERT-2 and EM-E6Δ151/E7/TERT had basically diploid normal chromosome number but also contained several nonclonal aberrations. In contrast, EM-E6E7 cells exhibited abnormal chromosome numbers, varying between 43 to 47 at PD 31 when telomere length was 5 to 6 kb.
Table 1.
Karyotype Analyses of EM Cells
| Cells | Karyotype | |
|---|---|---|
| EM-E6/E7/TERT-1 | 46, XX | |
| EM-E6/E7/TERT-2 | 46, XX | dup(3) (q21q25), add(8) (q24), add(14) (p11), add(10) (p13), i(22) (q10) |
| 44-46, XX | -14 | |
| EM-E6/E7/TERT-3 | 46, XX | |
| EM-E6Δ151/E7/TERT | 46, XX | |
| 46, XX | -8, add(11) (p15), +mar1, del(11) (p11) | |
| EM-E6/E7 | 43-47, XX | add(1) (q11), -14, dic(14;17)(p11;p11.2) |
Roles of E6/E7 and hTERT in Growth and Life Span of Human Endometrial Epithelial Cells
All EM cell lineages grew more than PD 20 without any morphological changes. Even EM-E6/E7, which lacked hTERT expression, was able to divide throughout this period, suggesting that expression of E6/E7 allows endometrial epithelial cells to pass through early senescence, without the absolute requirement for hTERT expression. The growth of these cells was further monitored and thereafter we found changes in the growth pattern between cells with and without hTERT expression. The growth pattern of EM-E6/E7/TERT-1, -2, and EM-E6/E7 derived from the same patient is shown in Figure 4 ▶ . EM-E6/E7/TERT-1 and -2 passed through PD 40 and finally grew more than PD 100. In contrast, EM-E6E7 cells stopped dividing at PD 40 with morphological changes producing enlarged and flat cells (Figure 5) ▶ . The growth of EM-E6/E7/TERT-3 and EM-E6Δ151/E7/TERT cells derived from another patient was basically similar to that of EM-E6/E7/TERT-1, and -2 cells.
Figure 4.
Proliferative life span of EM cells. The growth characteristics of the EM cells are represented as a growth curve. PD, population doubling.
Figure 5.

Morphological changes and β-gal staining of EM cells. Morphology of EM-E6/E7/TERT-2 cells (A) at PD 51 and EM-E6/E7 cells (B) at PD 40. β-gal expression was observed in EM-E6/E7 cells (D) whereas no staining was detected in EM-E6/E7/TERT cells (C).
To characterize the growth arrest observed in EM-E6/E7 cells, β-gal assay was performed. The EM-E6/E7 cells at PD 40 were found to be positive for β-gal expression, whereas EM-E6/E7/TERT cells that grew more than PD 50 were not, suggesting that the EM-E6/E7 cells fell into growth arrest indistinguishable from senescence at this point (Figure 5) ▶ .
The telomere length was then examined by Southern blot analysis using telomere probes (Figure 6) ▶ . The telomere length of the EM-E6/E7/TERT-1, -2, and -3 cells was ∼11 kb, 11 kb, and 8 kb, respectively at ∼PD 20, whereas that of EM-E6/E7 cells was 6 to 7 kb. With the progression of PD, the telomere length of the EM-E6/E/7TERT cells never shortened, and particularly interesting is that EM-E6Δ151/E7/TERT cells had elongated telomeres. In contrast, the telomere length of the EM-E6E7 cells progressively shortened and reached ∼5.5 kb at PD 40. These findings suggest that telomere-based replicative senescence occurred at PD 40 in EM-E6E7 cells, and that EM cells with hTERT expression overcame this senescence, probably through the maintenance of telomere length.
Figure 6.
Southern blot analysis for telomere length in EM cells. DNA extracted from EM cells was examined by Southern blot analysis using telomere-specific probes. Size standard is indicated at the left. PD, population doubling.
Three-Dimensional Culture of EM Cells
EM cells cultured on plastic dishes maintained the round shape typical of epithelial cells. However, on plastic dishes, they failed to compose gland-like structures, the natural characteristics of glandular cells. We therefore examined the ability of EM cells to reproduce the morphological characteristics of glands using the support of an extracellular matrix. EM cells were grown on Matrigel-coated Millicell CM filters. When grown on top of the Matrigel, the cells rapidly expanded on the surface of the Matrigel, exhibiting tight lateral junctions, resulting in cluster formation with a mesh-like network (Fig. 7A, 1) ▶ . When cells were grown inside the Matrigel, they formed colonies with spherical structures (Fig. 7A; 2 and 3) ▶ . To further examine the morphological characteristics of these colonies, each spherical colony was picked up by microscopic manipulation, fixed, and histologically examined. Hematoxylin and eosin staining of sections revealed gland-like structures, in which Matrigel clumps were coated with a cell monolayer (Figure 7B) ▶ . Some cells were found in the center of this structure, reminiscence of stromal or mesenchymal-like cells. However, immunohistochemical analysis revealed that CD-10 was negative in these cells while pan-cytokeratin and vimentin were positive (Figure 7C) ▶ . Although primary endometrial epithelial cells cultured in the same conditions are propagated for only a finite period (usually 2 to 3 weeks), the EM cells were able to survive up to confluence in Matrigel culture, and this was repeated by at least 10 passages as we confirmed.
Figure 7.
Three-dimensional culture of EM cells. EM-E6/E7/TERT-2 cells were cultured on Matrigel in Millicell CM filter inserts. The cells rapidly expanded on the surface of the Matrigel exhibiting tight lateral junctions and forming a mesh-like network (A1), while they grew forming gland-like colonies inside the Matrigel (A2 and A3). Colonies were picked up by microscopic manipulation, collected, and then fixed. The histological sections were prepared and stained with H&E (B). These sections were also immunostained with pan-cytokeratin (CK), vimentin (VIM), CD-10, and CD-34 (C). Original magnifications, ×200 (B and C).
Responsiveness of EM Cells to Sex-Steroid Hormones
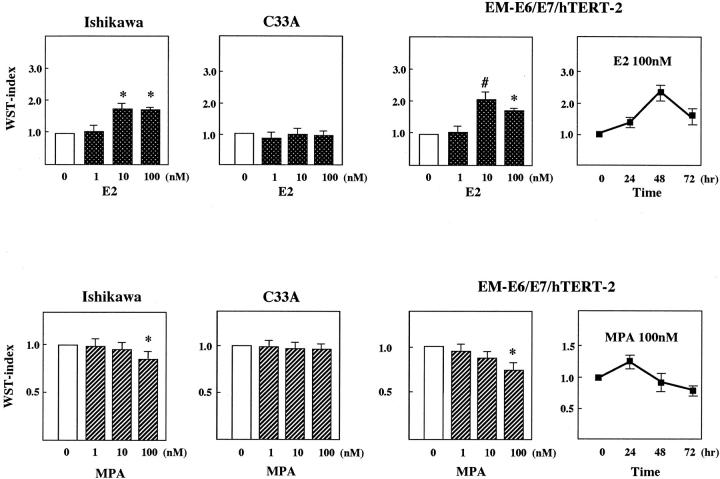
We next examined the responsiveness of EM cells to sex-steroid hormones. EM-E6/E7/TERT-2 cells were incubated with phenol red-free media with charcoal-treated fetal calf serum for 24 hours and then treated with 17β-estradiol (E2) at 1 to 100 nmol/L for different time periods. Cell growth was examined by WST-1 assays. ER- and PR-positive Ishikawa cells and C33 A cells lacking them were used as positive and negative controls (Figure 8) ▶ . Treatment with E2 at 10 to 100 nmol/L promoted cell growth by approximately twofold of WST-1 activity in Ishikawa and EM-E6/E7/TERT-2 cells. Time-course experiments using EM-E6/E7/TERT-2 cells revealed that the effect of E2 appeared at 24 hours and peaked at 48 hours with a gradual decrease to 72 hours in this assay system. The cells were then treated with progesterone (medroxyprogesterone acetate: MPA) in a similar manner. MPA at 100 nmol/L significantly inhibited growth of Ishikawa and EM-E6/E7/TERT-2 cells. Of particular interest is the time-dependent effect of MPA in EM-E6/E7/TERT-2 cells; it transiently activated cell growth in a short period within 24 hours, thereafter inhibiting growth with maximal inhibition at 72 hours after the treatment. Thus, the effect of MPA was biphasic. Similar findings were also observed in EM-E6/E7TERT-1 or -3 cells (data not shown). No regulation was observed in C33A cells in response to E2 or MPA stimulation. These findings suggest that the immortalized EM cells preserved their responsiveness to sex-steroid hormones.
Figure 8.
Responsiveness of EM cells to sex-steroid hormones. Responsiveness of EM-cells to estrogen or progesterone was examined by WST-1 assay. ER- and PR-positive Ishikawa cells or C33A cells lacking both receptor expression were used as positive and negative controls, respectively. EM-E6/E7/TERT-2 cells as well as Ishikawa and C33A cells were seeded, cultured in phenol red-free media with charcoal-treated serum for 24 hours, and treated with E2 or MPA for 48 hours at various concentrations. WST-1 assays were then performed. The WST index indicates the relative proliferative activity in which absorbance of the untreated samples was normalized to 1.0. Ishikawa and EM-E6/E7/TERT-2 cells exhibited growth stimulation in response to E2 at 10 to 100 nmol/L, whereas growth inhibition was observed by the treatment with MPA at 100 nmol/L. No regulation was found in C33 cells. Time course experiments of EM-E6/E7/TERT-2 cells with E2 or MPA are also shown. Results are an average of six wells in each sample. Bars, ±SD. #, P < 0.01; *, P < 0.001, compared with untreated control samples.
EM Cells Do Not Have Transformed Phenotypes
Although EM-cells exhibited the characteristics of glands in Matrigel culture and sustained the responsiveness to sex-steroid hormones, these cells obtained unlimited replicative capacity more than PD 100 and it was unclear whether these cells had transformed phenotypes. We thus sought to examine the growth properties of EM cells on soft agar or in nude mice. A total of 104 EM-E6/E7/TERT-1, -2, and -3 cells were seeded in soft agar on 6-cm dishes and colonies with diameters greater than 0.125 mm counted 14 days after seeding. Endometrial cancer HEC-1 and Ishikawa cells were similarly examined as positive controls. Although HEC-1 and Ishikawa cells formed distinct colonies (mean colony number, 116 ± 13.9 and 287 ± 23.9, respectively), EM-E6E7TERT cells did not (Figure 9) ▶ . Next, we evaluated tumorigenicity using nude mice. EM-E6/E7/TERT-1, -2, and -3 cells (106) as well as Ishikawa cells were subcutaneously injected into five nude mice and tumor formation then monitored 60 days after injection. Tumors formed in all of the mice injected with Ishikawa cells, whereas no tumor formed in mice injected with EM cells (Figure 9) ▶ . These findings suggest that EM cells did not obtain transformed phenotypes.
Figure 9.
Analyses of transformed phenotypes. A and B: Colony formation assay of endometrial cancer HEC-1 cells (A) and EM-E6/E7/TERT-1 cells (B) on soft agar. A total of 104 cells were seeded on soft agar on 6-cm dishes and colonies with diameters greater than 0.125 mm were counted 14 days after seeding. C and D: Tumor formation assay of endometrial cancer Ishikawa cells (C) and EM-E6/E7/TERT-1 cells (D) in nude mice. A total of 106 cells were subcutaneously injected into nude mice and tumor formation monitored 60 days after injection. Arrows indicate a tumor on the left flank of a nude mouse.
Discussion
In vitro culture of human endometrial cells has long been attempted by researchers keen to study endometrial function and biology as well as molecular carcinogenesis of the endometrium. Despite these efforts, stable cell lines of endometrial glandular cells have never previously been established. Although stromal cells can be cultured in vitro to some extent, glandular epithelial cells are hard to propagate on plastic dishes. In vitro experiments using epithelial cells have therefore been performed only with short-term culture for 1 or 2 weeks. The fact that primary endometrial epithelial cells cease proliferation in vitro in such a short period indicates the involvement of telomere-independent senescence mechanisms. This is supported by our data that: 1) introduction of hTERT alone failed to isolate clones that overcame this stage, 2) all of the clones that overcame this stage expressed HPV E6 and E7, and 3) one clone expressing only E6/E7 but not hTERT (EM-E6E7) was able to overcome this early senescence. These facts suggest that EPV E6/E7, but not hTERT, are the critical determinants of life span of endometrial epithelial cells at this stage.
What are the molecular mechanisms by which HPVE6 and E7 enable endometrial epithelial cells to overcome this early senescence? Rb-mediated cell-cycle control mechanisms have been demonstrated as a major event toward immortalization of epithelial cells, 22,34 and blockage of the Rb pathway by HPV E7 is likely to play a critical role in extended life span. However, E7 alone seems to be insufficient, because introduction of E7 alone failed to isolate any clones with extended life span. What is the role of E6 in overcoming early senescence? It is possible that abrogation of p53 function by E6 may cooperatively act to block the Rb pathway. A recent study demonstrated that activated E2F released by E7-induced Rb inactivation up-regulates p14ARF expression, which relocalizes MDM2 to the nucleolus and inhibits the ability of MDM2 to degrade p53. 35-37 Eventually, HPV E7 enhances p53 function via induction of p14 ARF, which may induce apoptosis in some cell types. Abrogation of p53 function by E6 may block these adverse effects of E7. As shown in the present study, an E6 mutant (E6Δ151) lacking the transforming ability of E6 but retaining the ability to target p53 for degradation had a similar effect to wild-type E6, suggesting that p53 inactivation is the major function of E6 to support E7. Based on these findings, we propose that two critical pathways, the Rb and p53 pathways, are involved in early senescence of endometrial glandular cells. This is consistent with the recent observation that a two-stage, p16- and p53-dependent senescence limits the replicative potential of keratinocytes. 27 Thus, the mechanisms of telomere-independent senescence may be basically common among epithelial cells. The requirement for both Rb and p53 inactivation to bypassing senescence is reminiscent of the roles of SV40 large T (LT). Generally, normal cells can bypass senescence through inactivation of Rb and p53 by SV40 LT, 38 and HPV E6/E7 may have a similar function. However, SV40 LT-introduced cells frequently have severe genetic abnormalities including aneuploidy and lose the normal structural and functional characteristics of the original cells. 39 In contrast, our E6/E7 system appears to minimize the genetic abnormalities, successfully isolating EM-clones with normal karyotypes (EM-E6/E7/TERT-1 and -3) and therefore has the great advantage of establishing immortalized cell lines that sustain the natural features of the primary cells.
The failure of E6/E7-expressing clones (EM-E6/E7) to overcome the second senescence is obviously because of the lack of hTERT expression. The telomere length of the EM-E6/E7 cells finally reached ∼5 to 6 kb at the time of senescence, whereas the other clones expressing hTERT maintained telomere length and overcame the second senescence. We thus conclude that endometrial glandular cells have multistep senescence programs that can be overcome by both Rb/p53-inactivaiton and telomerase activation. Telomerase activation seems to be necessary not only to overcome the second senescence, but also to maintain chromosome integrity because EM-E6/E7 cells lacking telomerase activity had severe chromosome abnormalities.
Several previous studies have demonstrated that HPV E6 can activate telomerase. 40 The molecular mechanisms of this activation are complex and our previous study showed that E6 activates the hTERT promoter in a c-Myc-independent manner. 41 It is known that HPV E6 and E7 can immortalize certain types of normal cells, in which E6 substitutes for telomerase to overcome telomere-dependent senescence 22 cells. However, we confirmed that HPV E6 failed to activate telomerase in endometrial epithelial cells as shown in EM-E6/E7 cells that lack detectable telomerase activity. Thus, the immortalization by HPV E6 and E7 appears to be a cell type-specific phenomenon, and only special cell types that allow telomerase activation by E6 may conform to the above model.
Our immortal endometrial epithelial cells may prove useful for various experimental models. EM cells maintain responsiveness to sex steroids, as estrogen activated growth of EM cells, while progesterone inhibited it, which mimics the physiological roles of estrogen and progesterone in endometrium. The growth inhibition by progesterone is of particular interest, considering the role of progesterone in endometrial biology. Progesterone inhibits growth or promotes differentiation of endometrial epithelial cells in the secretory phase of the menstrual cycle. It has widely been used as an anti-cancer drug for endometrial cancers. 42 Various signaling pathways such as the MAP kinase pathway, are activated on short-term exposure to progesterone, resulting in transient proliferation of breast cancer cells, 43 while progesterone induces p21 expression in the long term, associated with cell growth inhibition. 44,45 EM cells were able to reproduce this biphasic pattern of progesterone action, as described in Figure 8 ▶ , and will therefore be a desirable model with which to research signaling pathways or the functions of progesterone.
EM cells will be available for organotypic culture or mice transplantation systems, which might be useful as implantation model. Alternatively, these cells may be used to establish a carcinogenesis model. As shown in the present study, these cells do not have transformed phenotypes. What factors are required for transformation of these cells? One of the major factors involved in endometrial carcinogenesis is PTEN, which is frequently mutated in endometrial cancers. 4,46 We have confirmed no PTEN mutation in a series of EM cells (data not shown). Therefore, we are currently targeting PTEN in EM cells by the introduction of dominant-negative PTEN or siRNA techniques to define the genetic elements required for transformation.
In summary, we successfully immortalized human endometrial glandular cells by the introduction of HPV E6/E7 and hTERT. Our data show that endometrial glandular cells require Rb/p53 inactivation and telomerase activation for immortalization. The lack of transformed phenotypes of these cells supports the notion that additional factors are required for complete endometrial carcinogenesis. Our system to introduce HPV E6/E7 and hTERT provides the potential to generate relatively normal immortalized cells for research endeavors that have been limited so far by a limited life span of primary cells. We anticipate that our established lines will be useful in various experimental models with which to investigate endometrial biology and carcinogenesis.
Acknowledgments
We thank Dr. Hironori Tashiro, Department of Obstetrics and Gynecology, Kumamoto University, Japan, for critical discussion and advice.
Footnotes
Address reprint requests to Satoru Kyo, Department of Obstetrics, Kanazawa University, 13-1 Takaramachi, Kanazawa, Ishikawa 920-8641, Japan. E-mail: satoruky@med.kanazawa-u.ac.jp.
Supported in part by a grant-in-aid for the Second Term Comprehensive 10-Year Strategy for Cancer Control from the Ministry of Health and Welfare, Japan.
References
- 1.Arnold JT, Kaufman DG, Seppala M, Lessey BA: Endometrial stromal cells regulate epithelial cell growth in vitro: a new co-culture model. Hum Reprod 2001, 16:836-845 [DOI] [PubMed] [Google Scholar]
- 2.Classen-Linke I, Kusche M, Beier HM: Establishment of a human endometrial cell culture system and characterization of its polarized hormone responsive epithelial cells. Cell Tissue Res 1997, 287:171-185 [DOI] [PubMed] [Google Scholar]
- 3.Akoum Ali, Doillon CJ, Koutsilieris M, Dompierre L, Maheux R, Villeneuve M, Bergeron J, Lemay A: Human endometrial cells cultured in a type I collagen gel. J Reprod Med 1996, 41:555-561 [PubMed] [Google Scholar]
- 4.Inoue M: Current molecular aspects of the carcinogenesis of the uterine endometrium. Int J Gynecol Cancer 2001, 11:339-348 [DOI] [PubMed] [Google Scholar]
- 5.Harley CB, Futcher AB, Greider CW: Telomeres shorten during ageing of human fibroblasts. Nature 1990, 345:458-460 [DOI] [PubMed] [Google Scholar]
- 6.Greider CW: Chromosome first aid. Cell 1991, 67:645-647 [DOI] [PubMed] [Google Scholar]
- 7.Watson JD: Origin of concatemeric T7 DNA. Nature (Lond) 1972, 239:197-201 [DOI] [PubMed] [Google Scholar]
- 8.Saretzki G, Sitte N, Merkel U, Wurm E, von Zglinicki T: Telomere shortening triggers a p53-dependent cell cycle arrest via accumulation of G-rich single stranded DNA fragments. Oncogenes 1991, 18:5148-5158 [DOI] [PubMed] [Google Scholar]
- 9.Allsopp RC, Vaziri H, Patterson C: Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci USA 1992, 89:10114-10118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Counter CM, Avilion AA, LeFeuvre CE, Stewart NG, Greider CW, Harley CB, Bacchetti S: Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity EMBO J 1992, 11:1921-1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greider CW, Blackburn EH: A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature 1989, 337:331-337 [DOI] [PubMed] [Google Scholar]
- 12.Feng J, Funk WD, Wang SS, Weinrich AAA, Chiu CP, Adams RR, Chang E, Allsopp RC, Yu J, Le S, West MD, Harley CB, Andrew WH, Greider CW, Villeponteau B: The RNA component of human telomerase. Science (Washington DC) 1995, 269:1236-1241 [DOI] [PubMed] [Google Scholar]
- 13.Meyerson M, Counter CM, Eaton EN, Ellisen LW, Steiner P, Caddle SD, Ziaugra L, Beijersbergen RL, Davidoff MJ, Liu Q, Bacchetti S, Haber DA, Weinberg RA: hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell 1997, 90:785-795 [DOI] [PubMed] [Google Scholar]
- 14.Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, Harley CB, Cech TR: Telomerase catalytic subunit homologs from fission yeast and human. Science 1997, 277:955-959 [DOI] [PubMed] [Google Scholar]
- 15.Ito H, Kyo S, Kanaya T, Takakura M, Inoue M, Namiki M: Expression of human telomerase subunits and correlation with telomerase activity in urothelial cancer. Clin Cancer Res 1998, 4:1603-1608 [PubMed] [Google Scholar]
- 16.Kanaya T, Kyo S, Takakura M, Ito H, Namiki M, Inoue M: hTERT is a critical determinant of telomerase activity in renal-cell carcinoma. Int J Cancer 1998, 78:539-543 [DOI] [PubMed] [Google Scholar]
- 17.Takakura M, Kyo S, Kanaya T, Tanaka M, Inoue M: Expression of human telomerase subunits and correlation with telomerase activity in cervical cancer. Cancer Res 1998, 58:1558-1561 [PubMed] [Google Scholar]
- 18.Kyo S, Kanaya T, Takakura M, Tanaka M, Yamashita A, Inoue H, Inoue M: Expression of human telomerase subunits in ovarian malignant, borderline and benign tumors. Int J Cancer 1999, 80:804-809 [DOI] [PubMed] [Google Scholar]
- 19.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE: Extension of life-span by introduction of telomerase into normal human cells. Science 1998, 279:349-352 [DOI] [PubMed] [Google Scholar]
- 20.Weinrich SL, Pruzan R, Ma L, Ouellette M, Tesmer VM, Holt SE, Bodnar AG, Lichtsteiner S, Kim NW, Trager JB, Taylor RD, Carlos R, Andrews WH, Wright WE, Shay JW, Harley CB, Morin GB: Reconstitution of human telomerase with template RNA component hTERC and the catalytic protein subunit hTERT. Nat Genet 1997, 17:498-502 [DOI] [PubMed] [Google Scholar]
- 21.Nakayama J, Tahara H, Tahara E, Saito M, Ito K, Nakamura H, Nakanishi T, Tahara E, Ide T, Ishikawa F: Telomerase activation by hTRT in human normal fibroblasts and hepatocellular carcinomas. Nat Genet 1998, 18:65-68 [DOI] [PubMed] [Google Scholar]
- 22.Kiyono T, Foster SA, Koop JI, McDougall JK, Galloway DA, Klingelhutz AJ: Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature 1998, 396:84-88 [DOI] [PubMed] [Google Scholar]
- 23.Kang MK, Guo W, Park NH: Replicative senescence of normal human oral keratinocytes is associated with the loss of telomerase activity without shortening of telomeres. Cell Growth Differ 1998, 9:85-95 [PubMed] [Google Scholar]
- 24.Foster SA, Galloway DA: Human papillomavirus type 16 E7 alleviates a proliferation block in early passage human mammary epithelial cells. Oncogene 1996, 12:1773-1779 [PubMed] [Google Scholar]
- 25.Foster SA, Wong DJ, Barrett MT, Galloway DA: Inactivation of p16 in human mammary epithelial cells by CpG island methylation. Mol Cell Biol 1998, 18:1793-1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brenner AJ, Stampfer MR, Aldaz CM: Increased p16 expression with first senescence arrest in human mammary epithelial cells and extended growth capacity with p16 inactivation. Oncogene 1998, 17:199-205 [DOI] [PubMed] [Google Scholar]
- 27.Rheinwald JG, Hahn WC, Ramsey MR, Wu JY, Guo Z, Tsao H, De Luca M, Catricala C, O’Toole KM: A two-stage, p16(INK4A)- and p53-dependent keratinocyte senescence mechanism that limits replicative potential independent of telomere status. Mol Cell Biol 2002, 14:5157-5172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka M, Kyo S, Takakura M, Kanaya T, Sagawa T, Yamashita K, Inoue M: Expression of telomerase activity in human endometrium is localized to epithelial glandular cells and regulated in a menstrual phase-dependent manner correlated with cell proliferation. Am J Pathol 1998, 153:1985-1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka M, Kyo S, Kanaya T, Yatabe N, Nakamura M, Maida Y, Okabe M, Inoue M: Evidence of the monoclonal composition of human endometrial epithelial glands and mosaic pattern of clonal distribution in luminal epithelium. Am J Pathol 2003, 163:295-301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiyono T, Hiraiwa A, Fujita M, Hayashi Y, Akiyama T, Ishibashi M: Binding of high-risk human papillomavirus E6 oncoproteins to the human homologue of the Drosophila discs large tumor suppressor protein. Proc Natl Acad Sci USA 1997, 94:11612-11616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naviaux RK, Costanzi E, Haas M, Verma IM: The pCL vector system: rapid production of helper-free, high-titer, recombinant retroviruses. J Virol 1996, 70:5701-5705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schreiber E, Matthias P, Muller MM, Schaffner W: Rapid detection of octamer binding proteins with ‘mini-extracts,’ prepared from a small number of cells. Nucleic Acids Res 1989, 17:6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, Peacocke M, Campisi J: A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Nat Acad Sci USA 1995, 92:9363-9367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA, Louis DN, Li FP, Rheinwald JG: Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol Cell Biol 2000, 20:1436-1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bates S, Phillips AC, Clark PA, Stott F, Peters G, Ludwig RL, Vousden KH: p14ARF links the tumour suppressors RB and p53. Nature 1998, 395:124-125 [DOI] [PubMed] [Google Scholar]
- 36.Palmero I, Pantoja C, Serrano M: Nature p19ARF links the tumour suppressor p53 to Ras. Nature 1998, 395:125-126 [DOI] [PubMed] [Google Scholar]
- 37.Weber JD, Taylor LJ, Roussel MF, Sherr CJ, Bar-Sagi D: Nucleolar Arf sequesters Mdm2 and activates p53. Nat Cell Biol 1999, 1:20-26 [DOI] [PubMed] [Google Scholar]
- 38.Wright WE, Pereira-Smith OM, Shay JW: Reversible cellular senescence: implications for immortalization of normal human diploid fibroblasts. Mol Cell Biol 1989, 7:3088-3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ray AF: Simian virus 40 large T antigen induces chromosome damage that precedes and coincides with complete neoplastic transformation. Barbanti-Bridano G Bendinelli M Frieman H eds. DNA Tumor Virus. 1995:pp 15-26 Plenum, New York
- 40.Klingelhutz AJ, Foster SA, McDougall JK: Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature 1996, 380:79-82 [DOI] [PubMed] [Google Scholar]
- 41.Oh ST, Kyo S, Laimins LA: Telomerase activation by human papillomavirus type 16 E6 protein: induction of human telomerase reverse transcriptase expression through Myc and GC-rich Sp1 binding sites. J Virol 2001, 75:5559-5566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henderson BE, Ross RK, Pike MC: Hormonal chemoprevention of cancer in women. Science 1993, 259:633-638 [DOI] [PubMed] [Google Scholar]
- 43.Migliaccio A, Piccolo D, Castoria G, Di, Domenico M, Bilancio A, Lombardi M, Gong W, Beato M, Auricchio F: Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. EMBO J 1998, 17:2008-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Groshong SD, Owen GI, Grimison B, Schauer IE, Todd MC, Langan TA, Sclafani RA, Lange CA, Horwitz KB: Biphasic regulation of breast cancer cell growth by progesterone: role of the cyclin-dependent kinase inhibitors, p21 and p27(Kip1). Mol Endocrinol 1997, 11:1593-1607 [DOI] [PubMed] [Google Scholar]
- 45.Wang Z, Kyo S, Takakura M, Tanaka M, Yatabe N, Maida Y, Fujiwara M, Hayakawa J, Ohmichi M, Koike K, Inoue M: Progesterone regulates human telomerase reverse transcriptase gene expression via activation of mitogen-activated protein kinase signaling pathway. Cancer Res 2000, 60:5376-5381 [PubMed] [Google Scholar]
- 46.Tashiro H, Blazes MS, Wu R, Cho KR, Bose S, Wang SI, Li J, Parsons R, Ellenson LH: Mutations in PTEN are frequent in endometrial carcinoma but rare in other common gynecological malignancies. Cancer Res 1997, 57:3935-3940 [PubMed] [Google Scholar]