Abstract
Teratomas of the testis in post-pubertal patients are histologically diverse tumors that often coexist with other types of germ cell tumors. Using laser capture microdissection and loss of heterozygosity analysis, we investigated the clonality of mature teratoma and its relationship to other components of malignant mixed germ cell tumors to gain potential insight into the histogenetic relationship of teratoma with other germ cell tumor components. All 16 patients had mature teratoma as one component of their mixed germ cell tumors. The other histological subtypes included immature teratoma, seminoma, embryonal carcinoma, yolk sac tumor, and choriocarcinoma. Laser-assisted microdissection was performed on the formalin-fixed, paraffin-embedded tissue. Polymerase chain reaction was used to amplify genomic DNA at specific loci on chromosome 1p36.2 (D1S508), 2q22–32 (D2S156), 9p21–22 (D9S162), 11p13 (D11S903), 12q22–23 (D12S1051), and 18q21 (D18S46). Fourteen of 16 (88%) cases showed allelic loss in one or more components of the mixed germ cell tumors. Fourteen of 16 mature teratomas showed allelic loss in at least one of six microsatellite polymorphic markers analyzed. The frequency of allelic loss in mature teratoma was 50% (7 of 14) with D1S508, 33% (5 of 15) with D2S156, 58% (7 of 12) with D9S162, 43% (6 of 14) with D11S903, 20% (3 of 15) with D12S1051, and 33% (5 of 15) with D18S46. Completely concordant allelic loss patterns between mature teratoma and all of the other germ cell tumor components were seen in 10 of 14 tumors in which mature teratoma showed loss of heterozygosity. Our data support the common clonal origin of mature teratoma with other components of malignant mixed germ cell tumors of the testis.
Germ cell tumor of the testis is the most common solid tumor malignancy in young adult men. 1 Studies show a six- to tenfold increase in germ cell tumors in patients with a first degree relative with germ cell tumor, 2 demonstrating a strong familial and genetic component. Mature teratoma is present in approximately 50% of mixed germ cell tumors (MGCT) but is uncommon as a “pure” germ cell tumor in post-pubertal patients, supporting the belief that teratoma is mostly derived from other forms of invasive germ cell tumor of the testis. Other components frequently seen in MGCT include immature teratoma, seminoma, embryonal carcinoma, yolk sac tumor, and choriocarcinoma.
Teratomas are histologically diverse tumors that contain a variety of tissues, including elements derived from ectoderm (squamous epithelium, neural tissue), mesoderm (muscle, cartilage, bone), and endoderm (intestinal, pancreatic, respiratory). Mature teratomas are usually composed of differentiated tissues from two or three of these germ cell layers. They occur in both children and adults, but the behavior in these groups is different, with the post-pubertal cases having associated metastases but not the pre-pubertal. Studies have shown that 22 to 43% of adult patients with pure teratoma may have metastases during their clinical course 3-5 and there is commonly coexisting intra-tubular germ cell neoplasia of the unclassified type in the testis. 3,6 The metastatic lesions in such cases may be comprised of either pure teratoma or other components of MGCT. 3,5 Regardless of whether these metastatic lesions are the direct metastasis of a primary teratoma or the teratomatous differentiation of a more primitive component after metastasis, it is evident that primary pure teratomas of the testis have metastatic potential, and should be considered malignant. These patients typically do well with complete surgical excision of the metastatic deposits. 7-9
The histogenesis of mature teratoma is uncertain. One hypothesis for the development of teratoma in adults, as mentioned, is differentiation from a non-teratomatous germ cell tumor element, a supposition which explains many of the aforementioned observations regarding teratoma; ie, the association with intra-tubular germ cell neoplasia, the uncommon occurrence of teratoma as a pure tumor, and the occurrence of non-teratomatous metastases in association with “pure” teratoma, which presumably is “pure” by virtue of spontaneous regression of its non-teratomatous precursor. It remains unclear, however, what components of germ cell tumors commonly give rise to teratoma, and there are even limited data regarding the common clonal origin of teratoma and other germ cell tumor components. We therefore investigated, through loss of heterozygosity studies, the relationship of teratoma and associated germ cell tumor components to gain insight into the histogenesis of teratoma.
Patients and Methods
Patients
Sixteen men with malignant MGCT of the testis who underwent radical orchiectomy in 2000–2001 were included. All patients had mature teratoma as one component of their germ cell tumor, as well as one or more other components of MGCT. The other components included immature teratoma, seminoma, embryonal carcinoma, yolk sac tumor, and choriocarcinoma. The patients ranged from 16 to 45 years of age, with a mean age of 26 years. The 2002 TNM (tumor, nodes, metastasis) system was used for pathological staging. 10 Nine were stage pT1, six were stage pT2, and one was stage pT3. This research was approved by the Indiana University Institutional Review Board.
Tissue Samples and Microdissection
Histological sections were prepared from formalin-fixed, paraffin-embedded tissue and were stained with hematoxylin and eosin for microscopic evaluation. From these slides, the various components of the mixed germ cell tumors were identified (Figure 1) ▶ . Laser-assisted microdissection of the various components was performed on the unstained sections using a PixCell II Laser Capture Microdissection (LCM) system (Arcturus Engineering, Mountain View, CA), as previously described. 11,12 Approximately 400 to 1000 cells of each component were microdissected from the 5-μm histological sections. Normal tissue from each case was microdissected as a control.
Figure 1.
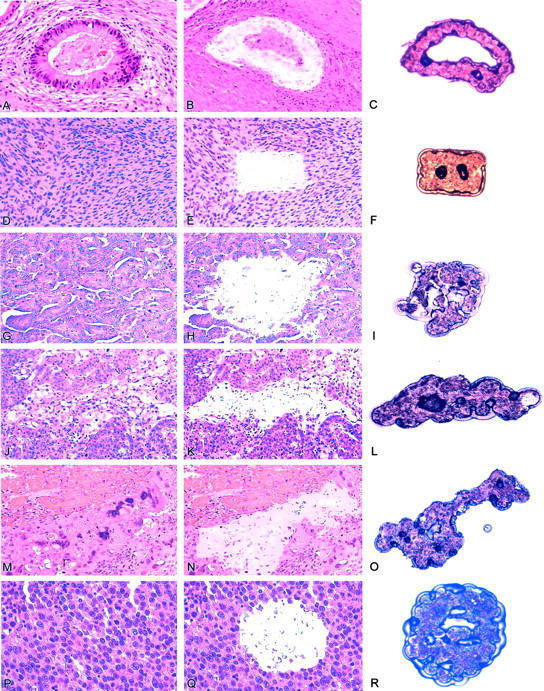
Laser micordissection of tumor from patients with malignant mixed germ cell tumor of the testis. Hematoxylin-eosin-stained sections showed mature teratoma (A), immature teratoma (D), embroynla carcinoma (G), yolk sac tumor (J), choriocarcinoma (M), and seminoma (P) before laser microdissection. Panels B, E, H, K, N, G, showed corresponding tumors after microdissection. Panels C, F, I, L, O, and R showed micordissected tissues ready for DNA analysis.
Amplification of DNA
The dissected cells were de-paraffinized with xylene and ethyl alcohol. Polymerase chain reaction (PCR) was used to amplify genomic DNA at various specific loci on chromosome 1p36.2(D1S508), 2q22–32(D2S156), 9p21–22(D9S162), 11p13(D11S903), 12q22–23(D12S1051), and 18q21(D18S46). Previous studies demonstrated that loss of heterozygosity (LOH) at these loci occur frequently in testicular germ cell tumors, particularly in teratomas. 8,13-21 Polymerase chain reaction amplification and gel electrophoresis were performed as previously described. 22-26 The criterion for allelic loss was complete or nearly complete absence of one allele in tumor DNA. 22-26 Polymerase chain reactions for each polymorphic microsatellite marker were repeated at least twice from the same DNA preparations and the same results were obtained. Results were reported as non-informative when visual inspection could not distinguish two distinct band forms in control DNA following polymerase chain reaction amplification.
Results
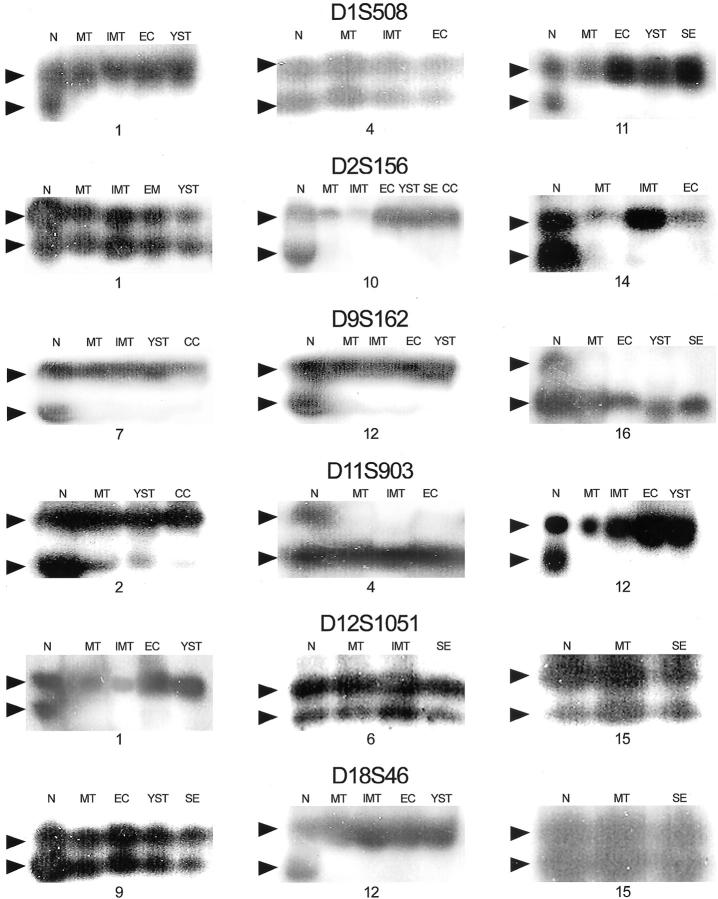
Fourteen of 16 (88%) patients with mixed germ cell tumors showed allelic loss in one or more components (Table 1) ▶ . The number of specific loci lost ranged from one to four. Fourteen of 16 mature teratomas showed allelic loss in at least one of six microsatellite polymorphic markers. Figure 2 ▶ showed representative results of loss of heterozygosity analysis.
Table 1.
Loss of Heterozygosity (LOH) Analysis of Malignant Mixed Germ Cell Tumors of the Testis
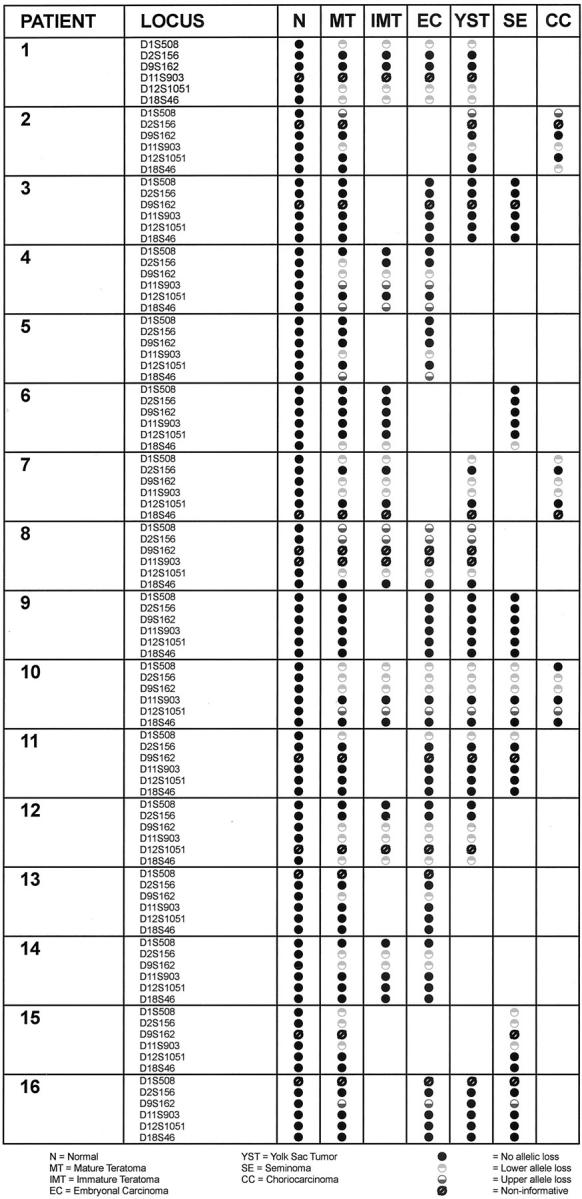
Figure 2.
Representative results of loss of heterozygosity analysis. DNA was prepared from normal tissue and different components of malignant mixed germ cell tumor, amplified by polymerase chain reaction using polymorphic markers D1S508, D2S156, D9S162, D11S903, D12S1051, and D18D46, and separated by gel electrophoresis. Arrows, allelic bands; N, normal tissue (control); MT, mature teratoma; IMT, immature teratoma; EC, embryonal carcinoma; YST, yolk sac tumor; CC choriocarcinoma, SE, seminoma.
The frequency of allelic loss in mature teratoma was 50% (7 of 14) with D1S508, 33% (5 of 15) with D2S156, 58% (7 of 12) with D9S162, 43% (6 of 14) with D11S903, 20% (3 of 15) with D12S1051, and 33% (5 of 15) with D18S46. Table 2 ▶ shows the LOH rate among different components of MGCT (mature teratoma, immature teratoma, seminoma, embryonal carcinoma, yolk sac tumor, and choriocarcinoma).
Table 2.
Overall Frequency of Loss of Heterozygosity (LOH) in Different Components of Malignant Mixed Germ Cell Tumors of the Testis
| Locus | MT | IMT | EC | YST | SE | CC |
|---|---|---|---|---|---|---|
| D1S508 | 7/14 (50%) | 4/8 (50%) | 4/10 (40%) | 6/9 (66%) | 3/6 (50%) | 2/3 (66%) |
| D2S156 | 5/15 (33%) | 3/8 (38%) | 3/12 (25%) | 2/9 (22%) | 2/7 (29%) | 1/2 (50%) |
| D9S162 | 7/12 (58%) | 5/7 (71%) | 6/9 (66%) | 3/7 (43%) | 2/4 (50%) | 2/3 (66%) |
| D11S903 | 6/14 (43%) | 3/6 (50%) | 3/10 (30%) | 3/8 (38%) | 1/7 (14%) | 2/3 (66%) |
| D12S1051 | 3/15 (20%) | 3/7 (43%) | 3/10 (30%) | 3/9 (33%) | 1/7 (14%) | 1/3 (33%) |
| D18S46 | 5/15 (33%) | 4/7 (57%) | 4/12 (33%) | 2/9 (22%) | 1/7 (14%) | 1/2 (50%) |
N, Normal; EC, Embryonal carcinoma; CC, Choriocarcinoma; MT, Mature teratoma; YST, Yolk sac tumor; IMT, Immature teratoma; SE, Seminoma.
Completely concordant allelic loss patterns between mature teratoma and the other germ cell tumor components were seen in 10 of 14 tumors in which mature teratoma showed LOH. For example, cases 1, 7, 8, 12, and 15 demonstrated LOH at three separate alleles, all of which showed identical loss patterns in all tumor components present. In cases 7, 8, and 12, at least four different components of MGCT were present in the same tumor, and all showed identical allelic loss at all loci.
Only 4 of 14 cases (cases 2, 4, 10, and 16) showed some discordance between allelic loss of heterozygosity patterns. In case 2 (Table 1) ▶ , the choriocarcinoma component showed allelic loss at locus D18S46, but none of the other components did, whereas in case 10, the choriocarcinoma component failed to show the same LOH that was seen in all other tumor components at locus D1S508, although identical allelic patterns were found at the other loci compared to the other components. In case 4, allelic loss was seen in the mature teratoma component at locus D2S156 that was not present in the immature teratoma or the embryonal carcinoma components. And in case 16, there was discordance in allelic loss at locus D9S162 in the yolk sac tumor component. In this case, the embryonal carcinoma and seminoma components shared identical allelic loss when compared with the mature teratomatous component.
Only two of 16 tumors (cases 3 and 9) did not show loss of heterozygosity in the mature teratoma component in at least one of the six loci. Both of these cases also lacked allelic loss in the other components of the MGCT at all six DNA loci.
Discussion
This study provides molecular evidence for the common clonal origin of mature teratoma with other components of malignant mixed germ cell tumors of the testis. Our data show complete concordance of allelic loss at six different chromosome microsatellite loci between mature teratoma and other germ cell tumor components in the majority of cases. These data supports that mature teratoma arises from the same progenitor cell as other components of MGCT and provides additional support for a pathogenetic model of adult teratoma of the testis that hypothesizes its usual derivation from other invasive germ cell tumor components.
Other recent evidence also supports a common clonal origin of MGCT. Rothe et al 19 analyzed 76 testicular germ cell tumors for LOH, of which 56 were pure tumors and 20 were comprised of two or more distinct germ cell tumor components. Of the 20 MGCT, 11 showed similar LOH patterns between the various components. A study using in situ hybridization for centromeric regions of chromosome 15 showed evidence of clonality among the different components of germ cell tumors, including the in situ and teratoma component. 27 de Graaff et al 28 separately karyotyped the two components, mature teratoma and choriocarcinoma, in a single case, and found that choriocarcinoma and mature teratoma showed almost identical patterns, with numerous complex chromosomal abnormalities. Mature teratoma and choriocarcinoma also showed a similar DNA aneuploid peak. 28 Previous studies also showed that teratoma was aneuploid, further supporting its malignant nature. 29
The selective gain and loss of genetic materials is important in the development of testicular cancer. 30 Testicular mixed germ cell tumors likely result from activation of oncogenes and inactivation of tumor suppressor genes. The inactivation of tumor suppressor genes is demonstrated by LOH at specific loci. 19 A common chromosomal abnormality is gain of 12p, most often in the form of isochromosome 12p. 30-32 The gain of chromosomal material (oncogenes) and loss of tumor suppressor genes, demonstrated by loss of heterozygosity, 18,32 represent a multi-step process which leads to malignant transformation and cancer progression. LOH of several DNA loci is frequent in testicular germ cell tumors, including chromosome 2q, 11p, 12p, and 18q. 30 In addition, transformation to somatic-type malignancies have been shown in metastatic mature teratomas. 33
Studies show that at 18q21.3, the DCC gene (deleted in colon cancer), is frequently lost in testicular germ cell tumors. Allelic loss of this chromosome region occurs in all histological subsets of germ cell tumors and may represent early genetic alterations in the development of germ cell tumors. 18 Peng et al 18 demonstrated LOH at 18q21.3 in up to 55% of informative cases. We found LOH in 33% of informative cases at this locus. LOH has also frequently been described at chromosome11p13, 18,33,34 which appears to be in close proximity to the WT-1 gene. Previous studies showed LOH at this locus in 27% of informative cases of germ cell tumors. 18 Our study showed a 43% LOH at this locus.
van Echten et al 8 studied 12 patients with primary non-seminomatous germ cell tumors and post-chemotherapy residual mature teratoma and found several chromosomal abnormalities that differed between residual mature teratoma after chemotherapy and the original primary non-seminomatous germ cell tumors. However, in a subsequent larger study, the primary non-seminomatous germ cell tumors and residual mature teratoma showed comparable patterns of chromosomal abnormalities. 13
In the current study, the number of components present in addition to mature teratoma ranged from one to five (immature teratoma, embryonal carcinoma, yolk sac tumor, seminoma, and choriocarcinoma). We found complete concordance of allelic loss in 10 of 14 cases when comparing mature teratoma with all other coexistent components of MGCT at six specific DNA loci used for this study. Of the 14 cases, only four showed discordance of allelic loss (cases 2, 4, 10, and 16) (Table 1) ▶ . In case 2, the choriocarcinoma component showed allelic loss at locus D18S46 that was not present in the mature teratoma or associated yolk sac tumor components, although all other loci in these three components had identical patterns. In case 4, allelic loss was seen in the mature teratoma component at locus D2S156 that was not present in the immature teratoma or the embryonal carcinoma components. However, concordance of allelic loss was present in loci D9S162, D11S903, and D18S46. In case 10, there was only one component at one locus that differed from the allelic loss seen with the mature teratoma. This was seen in the D1S508 locus of the choriocarcinoma component. Other than this component, there was complete concordance of allelic loss in the loci (D1S508, D2S156, D9S162, and D12S1051) involving all components of MGCT, including mature teratoma, immature teratoma, embryonal carcinoma, yolk sac tumor, and choriocarcinoma. In case 16, there was discordance in allelic loss only with locus D9S162 (yolk sac tumor). The embryonal carcinoma and seminoma components shared identical allelic loss of this locus with the mature teratomatous component.
It is interesting that in the four cases with some discordance, identical allelic loss was still found at other DNA loci. It is possible that the admixture of a small amount of normal tissue with the malignant cells of interest may contribute to the bi-allelic patterns seen in some tumor components. Tumor subtypes may have an integral, non-neoplastic stromal component that contributes to such falsely negative results.
The histogenesis of adult male germ cell tumor is complex. It is thought that adult male germ cell tumors arise from a precursor lesion, intra-tubular germ cell neoplasia, unclassified (IGCNU). It is felt that most often IGCNU gives rise to seminoma, which can progress to other components of MGCT, and ultimately mature teratoma. 34 Rarely, the intra-tubular component may progress directly to various germ cell tumor types such as yolk sac tumor and embryonal carcinoma. Our study sought specifically to address the question of a common clonal origin of mature teratoma with other components of MGCT. Although this study was not specifically designed to address the histogenesis of MGCT, the close relationship of LOH patterns in teratoma with other components of MGCTs is consistent with the derivation of teratoma from either a common progenitor cell or, further down the pathway, from an invasive malignant germ cell tumor. This would account for the similar LOH patterns in mature teratoma and the other components, as well as explain why mature teratoma is uncommon as a pure tumor in the post-pubertal testis. This would also explain the common association of mature teratoma with intra-tubular germ cell neoplasia, and the frequent occurrence of non-teratomatous metastases with pure mature teratoma of the adult testis. Presumably such cases represent tumors with regression of the non-teratomatous component in the testis. To specifically address the question of the MGCT histogenesis, future studies could look specifically at the LOH patterns of IGCNU and seminoma, and the relationship to other components of MGCTs. Our data support the common clonal origin of mature teratoma with other components of a mixed germ cell tumor in the majority of testicular cancer patients.
Footnotes
Address reprint requests to Liang Cheng, M.D., Department of Pathology and Laboratory Medicine, Indiana University Medical Center, University Hospital 3465, 550 North University Blvd., Indianapolis, IN 46202. E-mail: lcheng@iupui.edu.
References
- 1.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun M: Cancer statistics, 2003. CA Cancer J Clin 2003, 53:5-26 [DOI] [PubMed] [Google Scholar]
- 2.Forman D, Oliver RTD, Brett AR, Marsh SGE, Moses JH, Bodmer JG, Chilvers CED, Pike MC: Familial testicular cancer: a report of the UK family register, estimation of risk, and an HLA 1 sib-pair analysis. Br J Urol 1992, 65:255-262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simmonds PD, Lee AHS, Theaker JM, Tung K, Smart CJ, Mead GM: Primary pure teratoma of the testis. J Urol 1996, 155:939-942 [PubMed] [Google Scholar]
- 4.Heidenreich A, Moul JW, McLeod DG, Mostofi FK, Engelmann UH: The role of retroperitoneal lymphadenectomy in mature teratoma of the testis. J Urol 1997, 157:160-163 [PubMed] [Google Scholar]
- 5.Leibovitch I, Foster RS, Ulbright TU, Donohue JP: Adult primary pure teratoma of the testis: the Indiana experience. Cancer 1995, 75:2244-2250 [DOI] [PubMed] [Google Scholar]
- 6.Manivel JC, Reinberg Y, Niehans G, Fraley EE: Intratubular germ cell neoplasia in testicular teratomas and epidermoid cysts: correlation with prognosis and possible biologic significance. Cancer 1989, 64:715-720 [DOI] [PubMed] [Google Scholar]
- 7.Tongaonkar HB, Deshmane VH, Dalal AV, Kulkarni JN, Kamat MR: Growing teratoma syndrome. J Surg Oncol 1994, 55:56-60 [DOI] [PubMed] [Google Scholar]
- 8.van Echten J, Sleijfer DT, Wiersema J, Koops HS, Oosterhuis JW, de Jong B: Cytogenetics of primary testicular nonseminoma, residual mature teratoma, and growing teratoma lesion in individual patients. Cancer Genet Cytogenet 1997, 96:1-6 [DOI] [PubMed] [Google Scholar]
- 9.Logothetis C, Samuels M, Trindade A, Johnson D: The growing teratoma syndrome. Cancer 1982, 50:1629-1634 [DOI] [PubMed] [Google Scholar]
- 10.Greene FL, Page DL, Flemming ID, Fritz AG, Balch CM, Haller DG, Morrow M: AJCC Cancer Staging Manual, ed 6 2002. Springer-Verlag New York
- 11.Emmert-Buck MR, Bonner RF, Smith PD, Chuaqui RF, Zhuang Z, Goldstein S, Liotta LA: Laser capture microdissection. Science 1997, 274:998-1001 [DOI] [PubMed] [Google Scholar]
- 12.Bonner RF, Emmert-Buck M, Cole K, Pohida T, Chuaqui R, Goldstein S, Liotta LA: Laser capture microdissection: molecular analysis of tissue. Science 1997, 278:1481-1483 [DOI] [PubMed] [Google Scholar]
- 13.van Echten J, van der Vloedt WS, van de Pol M, Dam A, te Meerman GJ, Koops HS, Sleifer DT, Oosterhuis JW, de Jong B: Comparison of the chromosomal pattern of primary testicular nonseminomas and residual mature teratomas after chemotherapy. Cancer Genet Cytogenet 1997, 99:59-97 [DOI] [PubMed] [Google Scholar]
- 14.Murty VVVS, Houldsworth J, Baldwin S, Reuter V, Hunziker W, Besmer P, Bosl G, Chaganti RSK: Allelic deletions in the long arm of chromosome 12 identify sites of candidate tumor suppressor genes in male germ cell tumors. Proc Natl Acad Sci USA 1992, 89:11006-11010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murty VVVS, Bosl GJ, Houldsworth J, Meyers M, Mukherjee AB, Reuter V, Chaganti RSK: Allelic loss and somatic differentiation of human male germ cell tumors. Oncogene 1994, 9:2245-2251 [PubMed] [Google Scholar]
- 16.Murty VVVS, Renault B, Falk CT, Bosl GJ, Kucherlapati R, Chaganti RSK: Physical mapping of a commonly deleted region, the site of a candidate tumor suppressor gene, at 12q22 in human male germ cell tumors. Genomics 1996, 35:562-570 [DOI] [PubMed] [Google Scholar]
- 17.Brandli DW, Ulbright TM, Foster RS, Cummings OW, Zhang S, Sweeney CJ, Eble JN, Cheng L: Stroma adjacent to metastatic mature teratoma after chemotherapy for testicular germ cell tumor is derived from the same progenitor cells as the teratoma Cancer Res , 63:6063-6068 [PubMed] [Google Scholar]
- 18.Peng H, Bailey D, Bronson D, Goss PE, Hogg D: Loss of heterozygosity of tumor suppressor genes in testis cancer. Cancer Res 1995, 55:2871-2875 [PubMed] [Google Scholar]
- 19.Rothe M, Albers P, Wernert N: Loss of heterozygosity, differentiation, and clonality in microdissected male germ cell tumours. J Pathol 1999, 188:389-394 [DOI] [PubMed] [Google Scholar]
- 20.Lothe RA, Peltomaki P, Tommerup N, Fossa SD, Stenwig AE, Borresen AL, Nesland JM: Molecular genetic changes in human male germ cell tumors. Lab Invest 1995, 73:606-614 [PubMed] [Google Scholar]
- 21.Faulkner S, Leigh D, Oosterhuis J, Roelofs H, Looijenga L, Friedlander M: Allelic losses in carcinoma in situ and testicular germ cell tumours of adolescents and adults: evidence suggestive of the linear progression model. Br J Cancer 2000, 83:729-736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng L, Shan A, Cheville JC, Qian J, Bostwick DG: Atypical adenomatous hyperplasia of the prostate: a premalignant lesion? Cancer Res 1998, 58:389-391 [PubMed] [Google Scholar]
- 23.Cheng L, Song SY, Pretlow TG, Abdul-Karim FW, Kung HJ, Dawson DV, Park WS, Moon W, Tsai ML, Linehan M, Emmert-Buck MR, Liotta LA, Zhuang Z: Evidence of independent origin of multiple tumors from prostate cancer patients. J Natl Cancer Inst 1998, 90:233-237 [DOI] [PubMed] [Google Scholar]
- 24.Cheng L, Gu J, Eble JN, Bostwick DG, Younger C, MacLennan GT, Abdul-Karim FW, Geary WA, Koch MO, Zhang S, Ulbright TM: Molecular genetic evidence for different clonal origin of components of human renal angiomyolipomas. Am J Surg Pathol 2001, 25:1231-1236 [DOI] [PubMed] [Google Scholar]
- 25.Cheng L, Bostwick DG, Li G, Wang Q, Hu N, Vortmeyer AO, Zhuang Z: Allelic imbalance in the clonal evolution of prostate carcinoma. Cancer 1999, 85:2017-2022 [DOI] [PubMed] [Google Scholar]
- 26.Gu J, Roth L, Younger C, Michael H, Abdul-Karim F, Zhang S, Ulbright TM, Eble JN, Cheng L: Molecular evidence for independent origin of extra-ovarian papillary serous tumors of low malignant potential. J Natl Cancer Inst 2001, 93:1147-1152 [DOI] [PubMed] [Google Scholar]
- 27.Gillis AJ, Looijenga LH, de Jong B, Oosterhuis JW: Clonality of combine testicular germ cell tumors of adults. Lab Invest 1994, 71:874-878 [PubMed] [Google Scholar]
- 28.de Graaff WE, Oosterhuis JW, de Jong B, van Echten-Arends J, Wiersema-Buist J, Koops HS, Sleijfer DT: Cytogenetic analysis of the mature teratoma and the choriocarcinoma component of a testicular mixed nonseminomatous germ cell tumor. Cancer Genet Cytogenet 1992, 61:67-73 [DOI] [PubMed] [Google Scholar]
- 29.Sella A, Naggar J, Dexeus F, Amato R, Lee J, Finn L, Logothetis C: Evidence of malignant features in histologically mature teratoma. J Urol 1991, 146:1025-1028 [DOI] [PubMed] [Google Scholar]
- 30.Looijenga LHJ, Oosterhuis JW: Pathogenesis of testicular germ cell tumours. Rev Reprod 1999, 4:90-100 [DOI] [PubMed] [Google Scholar]
- 31.Lothe RA, Fossa SD, Stenwig AE, Nakamura Y, White R, Borresen A-L, Brogger A: Loss of 3p and 11p alleles is associated with testicular cancer tumors. Genomics 1989, 5:134-138 [DOI] [PubMed] [Google Scholar]
- 32.Chaganti RSK, Houldsworth J: Genetics and biology of adult human male germ cell tumors. Cancer Res 2000, 60:1475-1482 [PubMed] [Google Scholar]
- 33.Motzer RJ, Amsterdam A, Prieto V, Sheinfeld J, Murty VVVS, Mazumdar M, Bosl GJ, Chaganti RSK, Reuter V: Teratoma with malignant transformation: diverse malignant histologies arising in men with germ cell tumors. J Urol 1998, 159:133-138 [DOI] [PubMed] [Google Scholar]
- 34.Ulbright TU, Amin MB, Young RH: Tumors of the Testis, Adnexa, Spermatic cord, and Scrotum, 3rd series fascicle. 1999:pp 41-58 Armed Forces Institute of Pathology Washington DC