Abstract
The molecular pathogenesis of alcoholic liver disease (ALD) is not well understood. Gene expression profiling has the potential to identify new pathways and altered molecules in ALD. Gene expression profiles of ALD in a baboon model and humans were compared using DNA arrays. Reverse transcriptase-polymerase chain reaction and immunohistochemistry were used for downstream analysis of array results. cDNA array analysis revealed differential expression of several novel genes and pathways in addition to genes known to be involved in ALD pathogenesis. Overall gene expression profiles were similar in both species, with a majority of genes involved with fibrogenesis and xenobiotic metabolism, as well as inflammation, oxidant stress, and cell signaling. Genes associated with stellate cell activation (collagens, matrix metalloproteinases, tissue inhibitors of matrix metalloproteinase) were up-regulated in humans. Decreased expression of several metallothioneins was unexpected. Fourteen molecules related to the annexin family were up-regulated, including annexin A1 and A2. Immunofluorescence revealed a marked overexpression of annexin A2 in proliferating bile duct cells, hepatocyte cell surface, and selective co-localization with CD14-positive cells in human ALD. The gene expression profile of ALD is dominated by alcohol metabolism and inflammation and differs from other liver diseases. Annexins may play a role in the progression of fibrosis in ALD.
Alcoholic liver disease (ALD) remains a major cause of mortality and morbidities that include gastrointestinal hemorrhage, liver failure, hepatocellular carcinoma, and the need for liver transplantation. Alcohol has been identified as a direct hepatotoxin and various pathways of pathogenesis have been implicated but their respective contribution to liver injury in humans is not clear. 1 Presently, there is no satisfactory treatment for this disease. Recent studies, mostly in animal models, suggest that alcohol initiates liver injury via endotoxin, 2 oxidative stress, 3 and inflammation. 1 Disease progression involves continuing liver injury, fibrosis, and impaired liver regeneration. It is likely that a more detailed understanding of the pathogenesis will lead ultimately to more effective strategies for prevention and treatment of liver injury. DNA array analysis has provided novel data regarding pathogenesis of human liver disease. Studies of gene expression profiling in humans with different forms of liver cirrhosis (autoimmune hepatitis, primary biliary cirrhosis, primary sclerosing cholangitis, hepatitis C) from our laboratory have identified several novel genes and pathways in liver disease. 4,5 Accordingly, we propose that gene expression profiling will define prominently expressed pathways and new molecular targets in ALD.
The severity of ALD may be increased by co-factors such as nutritional impairment and chronic viral infection. Accordingly, observations made in humans and attributed to the effects of alcohol may be a consequence of other pathological processes seen in alcoholics rather than alcohol itself or liver disease. It may be difficult to properly identify and control for these confounders. An important complementary approach for studies of human liver tissue is to determine the effects of alcohol in an appropriate animal model. Of the various animal models of ALD, the baboon model has been shown to reproduce sequential development of all of the liver lesions observed in human alcoholics without exposure to other hepatotoxins. 6
For this study, the pathogenesis of ALD was studied in the baboon model by comparing intrahepatic gene expression of alcohol-fed and control baboons using cDNA array analysis. The studies were extended to human ALD by comparison of gene expression profiles in nondiseased and cirrhotic liver tissue. The arrays have identified similarities in the overall gene expression profile and differential expression of many genes including a number of annexins and related molecules.
Materials and Methods
Baboon Liver Biopsies
Animal experiments were performed in accordance with local ethical requirements. Animals were fed the Lieber-DeCarli diet, a nutritionally adequate liquid diet in which the control and ethanol animals are fed isocaloric diets in which dextrose is substituted for ethanol as described. 6 Needle liver biopsies were obtained from alcohol-fed animals and relevant pair-fed controls (two biopsies from each animal). The histopathology of the biopsies showed cirrhosis, steatosis, and inflammation of the tissue as previously reported 6 and illustrated in Figure 1 ▶ . The biopsies were snap-frozen, and stored in liquid N2 until extraction of RNA was performed.
Figure 1.

Micronodular cirrhosis, steatosis, and inflammation in alcohol-fed baboon liver. Baboon liver biopsies stained with H&E (A, B) and sirius red (C, D). Marked steatosis (B, D), inflammation (B), and micronodular cirrhosis (D) were evident in the alcohol-fed baboon but absent from control liver (A, C). Original magnifications: ×200 (A–C); ×100 (D).
Human Explant Liver
Human liver samples from seven male patients with advanced ALD (end-stage cirrhosis without viral hepatitis or other defined liver disease; Table 1 ▶ ) and seven nondiseased (donor) livers with normal histopathology, were obtained at the time of liver transplantation according to procedures approved by the Central Sydney Area Health Service (Royal Prince Alfred Hospital Zone) Ethics Committee guidelines. Explant tissues were snap-frozen in liquid N2 and stored at −70°C until further use.
Table 1.
Human Liver ALD Patient Profiles
| Patient | Age | Histopathology |
|---|---|---|
| 1 | 59 | Micronodular cirrhosis, mild steatosis, alpha-1 antitrypsin deficiency, moderate bile duct proliferation, mild hepatic dysplasia |
| 2 | 57 | Micronodular cirrhosis, mild steatosis, focal Mallory’s hyaline, moderate chronic inflammation in fibrous septa |
| 3 | 33 | Micronodular cirrhosis, diffuse steatosis, bile duct proliferation, scattered Mallory’s hyaline, florid ductular proliferation, marked parenchymal iron deposition |
| 4 | 60 | Micronodular cirrhosis, minimal steatosis, chronic inflammation in fibrous septa, florid bile duct proliferation, focal piece-meal necrosis, focal hepatocyte dysplasia |
| 5 | 51 | Micro- and macro-nodular cirrhosis, increased hepatocyte iron staining, mild intracanalicular cholestasis, prominent bile duct proliferation |
| 6 | 52 | Micronodular cirrhosis, bile ductular proliferation, abundant Mallory’s hyaline, mild parenchymal iron |
| 7 | 52 | Micronodular cirrhosis, prominent bile ductular proliferation, focal piece-meal necrosis |
Extraction and Amplification of Baboon RNA, Probe Generation, and Membrane Array Hybridization
Total RNA from biopsies was isolated in the presence of glycogen (250 μg/ml) using Trizol (Life Technologies, Inc., Gaithersburg, MD) as described. 7 The RNA quality was verified by agarose gel electrophoresis and ensuring that the absorbance ratio (A260/280) was 1.8 to 2.1. The amount of RNA from baboon liver biopsies (50 to 80 μg) was insufficient for replicate gene array analysis. Therefore, RNA was amplified using the SMART-LD (switch mechanism at the 5′ end of reverse transcript-long distance) PCR (long distance PCR)-based exponential amplification system (Clontech, Palo Alto, CA) as described. 7 SMART-LD PCR products have been validated for maintenance of gene expression levels after amplification with real-time RT-PCR. 7 The purified samples could be stored for up to 4 weeks at −20°C.
Amplified DNA (500 ng for each ethanol-fed and control baboon) was labeled with α-32P-dATP (10 μCi/μl, 3000 Ci/mmol; NEN Life Sciences Inc., Boston, MA) and Klenow fragment (MBI Fermentas GMBH, St. Leon-Pot, Germany). The probe was enriched for cDNAs spotted on the Atlas human 3.6K arrays using cDNA synthesis (CDS) primer (CDS 1.2I, CDS 1.2II, and CDS 1.2III provided in the Clontech kit) in the same reaction mix. Purified probes at ∼106 cpm/ml were hybridized to the arrays at 65°C overnight. Membranes were washed and exposed to phosphor imaging (Cyclone Phosphor Imager; Packard Instrument Company, Meriden, CT) for 24 to 96 hours. 7 The experiment was performed four times with the same or a different batch of SMART-generated samples.
Data Analysis
DNA-membrane array images were analyzed with AtlasImage (version 2.1, Clontech) software. Further analysis was performed using nonlinear regression of raw gene expression dpm values for control versus experimental samples for each membrane. The predicted value for each gene was calculated from the regression equation and an expression ratio was calculated as an observed:predicted ratio for each gene. 4,7 Genes with expression ratios of 1.5 or more in at least three of the four replicate DNA array experiments and >1.0 in all four experiments were considered to be up-regulated.
RNA Extraction from Human Explants
RNA was extracted with a RNAqueous-Midi kit (Ambion, Austin, TX) following the manufacturer’s instructions (RNAqueous-Midi Instruction Manual). Explant liver tissue (0.15 × g per sample) and nondiseased liver tissue from wedge biopsies were individually homogenized in the guanidine lysis buffer provided in the kit using an Ultra-Turrax T8 tissue homogenizer (IKA Labortechnik, Staufen, Germany). The homogenized tissue was applied to the glass fiber filter (included in the kit), washed extensively with the buffer before eluting RNA in water. The quality of RNA was tested as above and all samples were stored at −70°C.
Hybridization of Cy3/Cy5-Labeled Human RNA to Glass Arrays
Total RNA from the ALD and nondiseased explants were pooled in each group in equal amounts. Pooled RNA (60 μg) from ALD and nondiseased group was directly labeled with either dUTP-Cy5 or dUTP-Cy3 fluorophores, respectively, and 4 μg Oligo-dT (Roche, Basil, Switzerland) using SuperscriptII (Invitrogen Corporation, Rockville, MD) at the time of reverse transcription. Five μl of Lucidea Microarray ScoreCard Spike Mix (Amersham Pharmacia Biotech) was also added to the RNA mix. The RNA mix was denatured at 65°C for 10 minutes and added to 5× first strand buffer (Invitrogen); 400 U SuperScript II; 0.5 mmol/L dATP, dTTP, dGTP; 0.3 mmol/L dCTP (Amersham Pharmacia Biotech); 11 mmol/L DTT; and 16 U RNaseOUT Recombinant Ribonuclease Inhibitor (Invitrogen). Fluorescence-labeled d-UTPs [Cy3 (PA53022) or Cy5 (PA55022) (2.5 nmol), Amersham Pharmacia Biotech)] were added and the reaction incubated at 42°C for 2 hours. An additional 200 U SuperScript II was added to the tube after 1 hour of incubation. Adding 15 μl of fresh 0.1 mol/L NaOH and incubating at 70°C for 10 minutes terminated the reaction. The cDNA mixture was neutralized by adding 15 μl of 0.1 mol/L HCl. The labeled cDNA was purified with a Qia-Quick PCR purification kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. The probe was concentrated using a Microcon YM-30 filter (Millipore, Bedford, MA). Briefly, the two RNA reactions (Cy3 and Cy5) were combined and added to the microcon filter. TE buffer (400 μl) containing 20 μg each of Cot1 human DNA (Invitrogen), poly A RNA (Sigma, St. Louis, MO) and tRNA (Invitrogen) was added to the filter. The probe was concentrated to a final volume of 20 μl.
Probe was denatured in 4.25 μl of 20× standard saline citrate (SSC) and 0.65 μl of 10% sodium dodecyl sulfate for 2 minutes at 99°C. The human 6K cDNA glass microarray (RGHu6K on Telechem SuperAmine slides from The Clive and Vera Ramaciotti Centre for Gene Function Analysis, University of New South Wales, Kensington, Australia) was hybridized with 24.5 μl of the probe mixture in four replicate experiments. The slide was placed in a 50-ml Falcon humidified using 2× SSC and incubated in the dark at 65°C overnight in a Mini Hybridization Oven (ThermoHybaid, Ulm, Germany).
The slide was washed once in 2× SSC, 0.1% sodium dodecyl sulfate for 1 minute to remove the coverslip, once in 1× SSC for 30 seconds, and once in 0.2× SSC for 1 minute. All washing steps were performed at room temperature with gentle rocking. The slides were dried immediately by spinning in a tube at 600 rpm for 3 minutes.
Microarray Image Acquisition and Analysis
The slides were scanned with a GenePix 4000 microarray scanner and images and data were acquired with GenePix Pro 3.0 microarray image acquisition and image analysis software (Axon Instruments, Inc., CA). The scanner was set at 100% laser power with pixel resolution at 10 μm. The photomultiplier tube voltage varied depending on the signal intensity and was adjusted so that the total red intensity of each slide was equal to the total green intensity of that slide. Corrected intensities were calculated for both the red and green channels by subtracting the median measure of background pixel intensities from the median measure of spot pixel intensity for each spot as follows:
 |
 |
Data normalization was performed using the R statistical computing environment 8 using the statistical microarray analysis library. 9 This procedure is specific for normalization of data generated from two-dye experiments. Log ratio (M) and log average intensity (A) values were calculated for each spot as follows:
 |
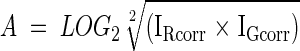 |
Nonparametric, locally weighted regression curves (Lowess function with the f value set at 0.1) were fitted to the data points. Normalization using this technique adjusts the local mean of the log expression ratio to M = 0. Print-tip group Lowess normalization 9,10 was chosen and Lowess curves were fitted to MA data from each print-tip group (ie, each block) separately. Using this normalization method the intensities of the spots within a particular print-tip group are normalized independently of the spots in other print-tip groups. Print-tip group Lowess normalization was chosen because it deals with the intraarray variability and print-tip effects by treating and comparing each block of the array separately, as opposed to global Lowess normalization.
The low-intensity spots were eliminated using the upper 95th percentile of A values of water (negative control) spots on the array as the cut-off threshold. Intensities of gene spots comparable to the intensity of the water spots were considered to represent background signal and were therefore eliminated. Separate cut-off values were determined for each of the four replicate arrays. After normalization and elimination of low-intensity spots, duplicate spots for each gene were averaged. A gene was determined as being differentially expressed if its M value was greater than or equal to 1 or less than or equal to −1, which corresponds to a twofold differential expression ratio, in at least three of four arrays.
Gene Annotation
Genes determined as being differentially expressed were annotated with its SwissProt description, function, and keywords using Database Referencing of Array Genes Online, 11 at: http://pevsnerlab.kennedykrieger.org/dragon.htm. Each differentially expressed gene was annotated. Where feasible, functional categories were allocated as they relate to liver biology from the literature.
Real-Time PCR of Selected Genes
Two sets of cDNAs were synthesized for each RNA sample to serve as replicate. One μg of total RNA was subjected to first-strand cDNA synthesis using Superscript II (Life Technologies) and oligo dT (Roche). The cDNAs were diluted fivefold, stored in small aliquots at −70°C and 1 μl was used for real-time PCR. Real-time RT-PCR was performed and analyzed as described previously. 7,12 Primers for real-time RT-PCR were obtained from previous reports for: frizzled related protein, ubiquitin, 13 glial cell-derived neurotrophic factor, neuromodulin (GAP43), 13 annexin A2 light chain (p11), 14 and cytochrome P450 (CYP) IIE1 (CYP2E1). 7 Other primer sequences are defined below. Annexin A2 (heavy chain p36) forward: 5′ TCTGAGCTAAAAGCTTCCATGAA 3′, reverse: 5′ GGAGTGAAGAGGAAAGGA 3′; annexin A1 (ANXA 1) forward: 5′ CGATCTGAGGACTTTGGTGTGAAT 3′, reverse: 5′ GTTTGCTTGTGGCGCACTT 3′; annexin A7 (ANXA7) forward: 5′ GCTGCCAACTTCGATGCTATAAGA, reverse: 5′ CTGGTTCTCATCACGATTTCCC 3′; aldehyde dehydrogenase 1 forward: 5′ ATGCCGACTTGGACAATGC 3′, reverse: 5′ CCCCTTCTTTCTTCCCACTCTC 3′; interferon γ-induced protein-10 (CXCL10) forward: 5′ TGCTGCCTTATCTTTCT 3′, reverse: 5′ GAAAGCAGTTAGCAAGG 3′. The PCR products were sequence verified.
All real-time RT-PCR studies were performed using duplicate cDNAs from individual samples (n = 8 for human and n = 4 for baboon). The experiment was performed at least twice for each set of cDNA and the number of transcript molecules per μg of RNA was calculated from DNA standards and normalized on ubiquitin data. The data are represented as means ± SD.
Immunofluorescence Staining
Frozen human liver specimens were sectioned at 5 to 10 μm thickness at −17°C, collected onto gelatin/chrome alum slides, and fixed in 100% ethanol (BDH AnalaR, Kilsyth, Australia) for 15 minutes. Slides from −20°C were prewarmed at 37°C for 15 minutes before blocking, staining, and imaging as described previously 15,16 using the reagents listed in Table 2 ▶ . Goat anti-rabbit IgG-Alexa Fluor 594 and goat anti-mouse IgG-Alexa Fluor 488 (Molecular Probes, Eugene, OR) secondary antibodies (1:400 dilution) were used for detection.
Table 2.
Primary Antibodies
| Antigen | Clone/name | Isotype | Supplier | Working dilution |
|---|---|---|---|---|
| Annexin II (H-50) | Rabbit IgG | Santa Cruz, Santa Cruz, CA | 5.0 μg/ml | |
| Annexin II | ZO14 | Mouse IgG1 | Zymed, South San Francisco, CA | 1.7 μg/ml |
| CD34 | My10 | Mouse IgG1 | Becton Dickinson (Franklin Lakes, NJ) | 1:10 |
| Cytokeratin 7 (CK7) | OV-TL 12/30 | Mouse IgG1 | DAKO (A/S, Glostrup Denmark) | 3.5 μg/ml |
| Anti-human c-Kit (CD117) | YB5.B8 | Mouse IgG1 | Pharmingen, Glostrup, CA | 1.7 μg/ml |
| Anti-human FAP | F19 | Mouse IgG | W. Rettig; Levy et. al., 1999 | 1:5 |
| CD14 | UCH-M1 | Mouse IgG2a | Santa Cruz | 2.0 μg/ml |
| Macrophage CD68 | EBM11 | Mouse IgG1 | DAKO | 2.3 μg/ml |
| T cell CD3 | UCHT 1 | Mouse IgG1 | DAKO | 3 μg/ml |
| GFAP | Rabbit IgG | DAKO | 1.0 mg/ml | |
| CD19 | HD37 | Mouse IgG1 | DAKO | 1.7 μg/ml |
| Synaptophysin | Rabbit IgG | Novocastra, Newcastle upon Tyne, UK | 1:40 | |
| Mouse IgG1, control | MOPC-21 | Mouse IgG1 | Sigma, St. Louis, MO | 10 μg/ml |
| Immunoglobulin |
Results
Gene Expression in Baboons and Humans
Probes generated from SMART-amplified biopsy samples from control-fed and ethanol-fed baboons were successfully hybridized to Atlas human 3.6K cDNA arrays (array, Figure 2 ▶ ). Linear regression of raw gene expression values (average of four experiments in the three membranes of the 3.6K set: 1.2I R2 = 0.929, 1.2II R2 = 0.889, 1.2III R2 = 0.861) for control versus ethanol-treated samples revealed that of the 36% of genes detected twofold greater than background, 26% were differentially expressed (288 genes up-regulated and 43 genes down-regulated). Differential expression of CYP2E1 7 and other genes is evident in Figure 2 ▶ .
Figure 2.

Differential expression of genes in the baboon model of ALD. Human cDNA array (1.2II) hybridized with SMART amplified baboon liver cDNA from control (A) and ALD (B). Arrows point to differential expression of some genes belonging to various categories. Up-regulated: (a) plasma glutathione peroxidase precursor (GPXP) (c) cytochrome P450 IIE1 (CYP2E1) (d) cytochrome P450 IIIA (CYP3A3, 3A4, 3A5, 3A7) (e), annexin I (ANXA1) (f) annexin II (ANXA2) (g) membrane-associated phospholipase A2 (PLA2G2A) (h) phosphatidylethanolamine-binding protein (PBP) (i) platelet-derived growth factor receptor β-like tumor suppressor (PRLTS) (j) fibrinogen G gamma polypeptide (k) mitochondrial acetyl-CoA acetyltransferase precursor (l) mitochondrial ATP synthase α-chain precursor (m) lipoprotein-associated coagulation inhibitor (n) fibrinogen B β-polypeptide. Down-regulated: (b) Metallothioneins (MT1H, MT-0, MT-1L, MT 1R, MT2).
Differential expression of genes in human ALD was evident as shown in the raw array image (Figure 3) ▶ . Normalization of array data using print-tip group Lowess minimized the ratio bias generated from variable intensities across individual slides (Figure 4) ▶ . Of the 57.5% detected genes, 8% (206 genes up-regulated and 76 genes down-regulated) were found to be differentially expressed after normalization and further analysis.
Figure 3.

Differential expression of genes in human ALD. A typical Axon GenePix image of a human 6K cDNA array (nonnormalized) hybridized with ALD (Cy5, red) and nondiseased (Cy3, green) probes is shown. Each gene is represented as duplicate spots on this array, the red spots represent up-regulation and green spots represent down-regulation of gene expression in human ALD. Arrows point to differential expression in ALD of some genes belonging to various categories. Up-regulated: a, CCL2, monocyte chemotactic protein 1; b, immunoglobulin J chain; c, lumican; d, immunoglobulin lambda light chain; f, TIMP2; g, MHC class II DQ-β associated with DR2, DQw1 protein; h, interleukin 7 receptor; i, neuromedin B; j, annexin I (lipocortin I); l, calgizzarin; n, insulin-like growth factor-binding protein 5; o, superoxide dismutase 3. Down-regulated: e, metallothionein 1L; k, contactin 1; m, binding regulatory factor; p, Bcl-XL.
Figure 4.
Print-tip group Lowess function reduces intraslide intensity-dependent ratio variability. Box-whisker plots of a typical array without normalization (A), and print-tip group global Lowess normalization. 9,10 (B). M is defined in Materials and Methods.
The differentially expressed genes were assigned a broad category based on their biological functions or cellular location. A comparative global hepatic gene expression profile was similar in the baboon and human (Figure 5) ▶ . A high proportion of genes, from both baboons and humans, were found in the following categories: fibrosis/extracellular matrix (ECM), metabolism, immune system, transcription, and cell signaling. Many genes appeared in the fibrosis/ECM category in both the baboon (19%) and human (24%) arrays. A comparatively large proportion of genes belong to the immune system (14%) or nuclear/transcription (12%) category in advanced human ALD, whereas the trafficking (9%), signaling (8%), and neural (6%) categories were more prominent in the baboon. The proportion of genes in the metabolism category was nearly double in the baboons (24%) as in the humans (12%). The complete lists of differentially expressed genes from the baboon and human arrays with Unigene and GenBank numbers is provided as supplementary material for placement on the journal web site. (These supplemental data are currently available on the following nonlinked web site: http://www.centenary.usyd.edu.au/pubs/additions/ALD/data.html).
Figure 5.
Global gene expression profile was similar in the baboon model and human ALD. Comparison of categories of genes with differential expression in the baboon □ and human  ALD. It depicts an overall similarity in the global gene expression profile and highlights the predominance of metabolism genes in the baboon with ongoing alcohol ingestion compared to abstinent humans. Each category is represented as a percentage of the total number of differentially expressed genes.
ALD. It depicts an overall similarity in the global gene expression profile and highlights the predominance of metabolism genes in the baboon with ongoing alcohol ingestion compared to abstinent humans. Each category is represented as a percentage of the total number of differentially expressed genes.
Selected differentially expressed genes from the most prominent categories with respect to disease pathology (fibrosis, energy metabolism, oxidant stress, immune related, trafficking, and neural), are listed for baboons (Table 3) ▶ and humans (Table 4) ▶ . A number of the differentially expressed molecules are known to be implicated in the progression of liver fibrosis in response to injury by various toxins, including alcohol, for example, CYP2E1, Bcl-xL, superoxide dismutase, and glutathione S-transferase.
Table 3.
Selected Differentially Expressed Genes in the Baboon Model of ALD
| Genes | GenBank ID | Mean expression ratio |
|---|---|---|
| Fibrosis/ECM | ||
| Annexin II (ANX2); lipocortin II; calpactin I heavy subunit | D00017 | 5.1 |
| Astrocyte glial fibrillary acidic protein (GFAP) | J04569 | 1.8 |
| Calgizzarin; S-100 calcium-binding protein A11 (S100A11) protein | D38583 | 1.7 |
| Calpactin I light chain; annexin II ligand | M81457 | 1.6 |
| Follistatin 1 and 2 (FS) (Activin-binding protein) | M19481 | 1.5 |
| PLA2; phospholipase A2*† | M86400 | 1.7 |
| Plasminogen precursor (PLG) | X05199 | 1.5 |
| Platelet-derived growth factor (PDGF) receptor beta-like tumor suppressor (PRLTS) | D37965 | 2.6 |
| Immune | ||
| Annexin XI (Calcyclin-associated annexin 50) (CAP-50) | L19605 | 1.5 |
| CCL2; monocyte chemotactic protein 1 (MCP1) | M24545 | 1.5 |
| CD11a; integrin alpha L (ITGAL) | Y00796 | 2.5 |
| CD80; CD28 ligand; T-lymphocyte activation CD80 antigen | M27533 | 1.9 |
| Complement 3 (C3)*‡ | K02765 | 6.5 |
| CXCL10; IP10; interferon gamma-induced protein; SCYB10 | X02530 | 2.2 |
| Granulocyte colony-stimulating factor (G-CSF); pluripoietin | X03438 | 1.8 |
| Lipopolysaccharide-binding protein (LBP) | M35533 | 2.2 |
| Trafficking | ||
| Annexin I (ANX1) | X05908 | 2.0 |
| Cellular retinol-binding protein I (RBP1; CRBP1) | M11433 | 1.5 |
| Clathrin assembly protein lymphoid myeloid leukemia (CALM) | U45976 | 1.8 |
| Clathrin coat assembly protein AP50; KIAA0109 | D63475 | 1.8 |
| Clathrin heavy subunit 1 (CLH-17); KIAA0034 | D21260 | 1.9 |
| Clathrin light chain A (Brain and Lymphocyte LCA) | M20472 | 1.8 |
| EDF-1 protein | D63475 | 2.4 |
| Lysosomal protective protein; cathepsin A; carboxypeptidase C; PPGB | M22960 | 1.6 |
| Metabolism | ||
| Known alcohol metabolism enzymes | ||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA reductase; HMGCR) | M11058 | 1.6 |
| Alcohol dehydrogenase alpha subunit *§ | M12271 | 7.4 |
| Aldehyde dehydrogenase (ALDH6) | U07919 | 1.7 |
| Aldehyde dehydrogenase 5 (ALDH5; ALDHx) | M63967 | 1.5 |
| Cytochrome P450 IIE1 (CYP2E1) | J02625 | 2.8 |
| Cytochrome P450 IIIA3 (CYP3A3)*¶ | M13785 | 3.8 |
| Glutathione peroxidase (GSHPX1; GPX1)*∥ | Y00483 | 2.0 |
| Glutathione S-transferase A1 (GTH1; GSTA1); HA subunit 1; GST-epsilon** | M25627 | 2.1 |
| Other metabolism genes | ||
| Apolipoprotein E (APOE) | M12529 | 2.5 |
| Cholesteryl ester transfer protein (CETP); lipid transfer protein I | M30185 | 1.7 |
| Cytochrome c oxidase polypeptide Vb (COX5B), mitochondrial*†† | M19961 | 2.2 |
| Cytoplasmic hydroxymethylglutaryl-CoA synthase | X66435 | 1.8 |
| Hepatic triglyceride lipase (HTGL) | X07228 | 2.6 |
| Hydroxyacyl-CoA dehydrogenase; 3-ketoacyl-CoA thiolase; enoyl-CoA hydratase beta subunit | D16481 | 1.7 |
| Mitochondrial acetyl-CoA acetyltransferase | D90228 | 2.9 |
| Mitochondrial ATP synthase alpha chain; (ATP5A1; ATPM; hATP1) ATP5A1 | D14710 | 3.0 |
| Mitochondrial hydroxymethylglutaryl-CoA synthase | X83618 | 2.7 |
| NADH-cytochrome B5 reductase | Y09501 | 1.6 |
| Cell signaling | ||
| Calmodulin | M27319 | 1.6 |
| Calmodulin-related protein NB-1; calmodulin-like protein (CLP) | M58026 | 1.6 |
| Stress | ||
| C-reactive protein | X56692 | 2.0 |
| MT1H (Methallothionein-IH)*‡‡ | X64177 | 0.5 |
| Protein synthesis/degradation | ||
| 23-kDa highly basic protein; 60S ribosomal protein L13A (RPL13A)*§§ | X56932 | 2.3 |
| DNA-directed RNA polymerase II 14.4-kDa polypeptide; RPB6*¶¶ | Z27113 | 1.7 |
*Upregulated isoforms/family members of the gene.
†Lysosomal phospholipase A2; membrane-associated phospholipase A2;PLA2G2A; PLA2B; RASFA.
‡Complement component 1r (C1R); complement component 1s (C1S); Complement factor B.
§Alcohol dehydrogenase2; alcohol dehydrogenase 3.
¶CYP3A4; CYP3A5; CYP3A7; CYP51 + CYP51P1 + CYP51P2; CYP1A1 + CYP1A2; CYP2B6 + CYP2B3; CYP4A11; CYP4B1; CYP4F2 + CYP4F3; CYP7A1; CYP17A1; CYP27A.
∥Plasma glutathione peroxidase (GPXP; GPX3); GSHPX-GI; glutathione peroxidase-related protein 2 (GPRP).
**Glutathione S-transferase Mu 5 (GSTM5-5) (GST Class-MU).
††Cytochrome c oxidase (COX) polypeptide VIb (COX6B); COX6C; COX7A, liver; COX7C; COX8, liver/heart.
‡‡Metallothionein isoform 1L (MT-1L) + metallothionein isoform 1R + MT2 (methallothionein-II); metallothinein-0,
§§40S ribosomal protein S9; 60S Acidic ribosomal protein P1; 60S ribosomal protein L14 (RPL14); CAG-ISL 7; 60S ribosomal protein L22 (RPL22); 60S ribosomal protein L37 (RPL37).
¶¶DNA directed RNA polymerase II 14.5-kDa polypeptide; RPB9; RPB14.5; DNA-directed RNA polymerase II 23 KD polypeptide (RPB25) (XAP4) (RPB5).
Table 4.
Selected Differentially Expressed Genes in Human ALD
| Genes | GenBank ID | Mean expression ratio |
|---|---|---|
| Fibrosis/ECM | ||
| Actin, alpha 2, smooth muscle, aorta | AA634006 | 2.7 |
| Alpha-platelet-derived growth factor receptor | H23235 | 5.5 |
| Annexin VIII | AA235002 | 5.8 |
| Basic fibroblast growth factor (bFGF) receptor (shorter form) | R54846 | 3.2 |
| Calgizzarin, complete cds | AA464731 | 2.7 |
| Collagen, type IV, alpha 1*† | AA150402 | 2.0 |
| Connective tissue growth factor | AA598794 | 3.7 |
| Endothelin 1 {alternative products} | H11003 | 2.8 |
| Fibroblast growth factor receptor 2, keratinocyte growth factor receptor*‡ | AA443093 | 3.7 |
| Integrin alpha-3 subunit*§ | AA424695 | 4.9 |
| Jagged 1 (HJ1) mRNA | R70685 | 4.5 |
| Laminin B1 chain*¶ | AA446251 | 2.4 |
| Matrix metalloproteinase 2 (gelatinase A, 72kD gelatinase, 72kD type IV collagenase)*∥ | AA936799 | 4.5 |
| Tissue inhibitor of metalloproteinase 2** | AA486280 | 3.6 |
| Transforming growth factor, beta receptor II (70-80kD) | AA487034 | 2.1 |
| Vimentin | AA486321 | 4.2 |
| Immune | ||
| CCL2; small inducible cytokine A2 (monocyte chemotactic protein 1) | AA425102 | 3.0 |
| CD44 antigen (cell adhesion molecule) | AA283090 | 2.6 |
| CD48 antigen (B-cell membrane protein) | R05416 | 2.5 |
| CD69 antigen (early T cell activation antigen) | AA279755 | 4.5 |
| Class II histocompatibility antigen, M alpha chain precursor*†† | H42728 | 2.3 |
| Complement component 7 | AA598478 | 5.5 |
| Interleukin 10 receptor | AA437226 | 2.3 |
| Interleukin 7 receptor | AA485865 | 3.9 |
| Trafficking | ||
| Alpha-2-HS-glycoprotein alpha and beta chain | R92227 | 2.1 |
| Annexin I (lipocortin I) | H63077 | 4.7 |
| Annexin IV (placental anticoagulant protein II) | AA419015 | 3.0 |
| Annexin XIII | AA235002 | 5.0 |
| Metabolism | ||
| Aldehyde dehydrogenase 1, soluble | AA664101 | 3.0 |
| Cytochrome P450 IB1 (dioxin-inducible)*‡‡ | AA448157 | 3.0 |
| Glutathione S-transferase A3*§§ | N30096 | 4.3 |
| Cell signalling | ||
| CGRP type 1 receptor (clone HSNME29) | AA757351 | 2.9 |
| Endothelial differentiation protein (edg-1) | R20666 | 2.0 |
| Frezzled (fre) | W58032 | 2.7 |
| Stress | ||
| Metallothioneins*¶¶ | H77597 | 0.2 |
*Upregulated isoforms/family members of the gene.
†Alpha-2 collagen type VI; alpha-3 collagen type VI; alpha 2 collagen type IV.
‡Fibroblast growth factor receptor 3 (achondroplasia, thanatophoric dwarfism).
§Integrin, alpha V (vitronectin receptor, alpha polypeptide, antigen CD51).
¶Laminin, alpha 2 (merosin, congenital muscular dystrophy).
∥Matrix metalloproteinase 7 (matrilysin, uterine).
**Tissue inhibitor of metalloproteinase 3 (Sorsby fundus dystrophy, pseudoinflammatory).
††Class II histocompatibility antigen, DR alpha; DN alpha; DP beta 1; DQ beta 1; DR beta 5; DQ alpha; DQ beta associated with DR2, DQw1.
‡‡Cytochrome P450, subfamily IIA (phenobarbital-inducible), polypeptide 6 (CYP2A6); CYP3A7.
§§Glutathione S-transferase pi-1.
¶¶Metallothionein (MT)I-F gene; metallothionein 1L; metallothionein I-B gene; metallothionein-Ie gene (hMT-Ie).
Genes up-regulated specifically in the baboon from the cytochrome P450 gene family involved in xenobiotic metabolism were CYP2E1, CYP1A1, CYP3A, 3-7 CYP4A11, CYP4B1, CYP4F2, CYP7A1, CYP17A1, and CYP27A (Table 3) ▶ . In human, CYP1B1, CYP2A6, and CYP3A7 were up-regulated and CYP2E1 and cytochrome P450-aromatic compound-inducible were down-regulated.
A number of genes that have not been previously implicated in ALD were differentially expressed in the baboon model. These included endothelial differentiation factor1 (EDF1), MHC class II molecules, CD44, CD48, CD69, lumican, matrix Gla protein, ankyrin B, and ankyrin G. In addition, a number of kinases including PI 3K, 14-3-3 protein β/α, 14-3-3 protein τ and 14-3-3 protein ζ/δ were overexpressed.
A number of differentially expressed genes were common in both baboons and humans (Table 5) ▶ . The expression of genes in the two species was concordant in some cases but was discordant in others.
Table 5.
Differentially Expressed Genes Common in Both the Baboon Model and Human ALD
| Gene | Category | Mean expression ratio | |
|---|---|---|---|
| Baboon | Human | ||
| (A) Genes up-regulated in both baboon and human | |||
| 14-3-3 Protein zeta/delta (PKC inhibitor protein-1) | Immune | 1.5 | 2.6 |
| Adipophilin | Metabolism | 1.6 | 2.5 |
| Aortic carboxypeptidase-like protein ACLP (AEBP1) | Trafficking | 1.6 | 5.7 |
| Alpha-2-HS-glycoprotein alpha and beta chain | Fibrosis/ECM | 2.1 | 2.2 |
| Annexin I (lipocortin I) | Trafficking | 2.0 | 4.7 |
| Calgizzarin; S-100 calcium-binding protein A11 (S100A11) protein | Fibrosis/ECM | 1.5 | 2.7 |
| Calpain 2 large (catalytic) subunit; M-type calcium-activated neutral proteinase (CANP) | Fibrosis/ECM | 1.5 | 2.8 |
| Carbamoyl-phosphate synthetase 1, mitochondrial | Metabolism | 1.5 | 2.9 |
| CCL2; monocyte chemotactic protein 1 (MCP1) | Immune | 1.6 | 3.0 |
| Coagulation factor II (thrombin) receptor | Fibrosis/ECM | 1.5 | 2.3 |
| Cytochrome P450, subfamily IIIA, polypeptide 7 (CYP3A7) | Metabolism | 2.7 | 4.5 |
| Guanine nucleotide binding protein; transducin beta-1 subunit | Signaling | 2.3 | 2.7 |
| Inhibitor of DNA binding 3 (HLH1R2) | Transcription/Nuclear | 2.4 | 2.5 |
| Lumican | Fibrosis/ECM | 2.3 | 6.8 |
| Matrix gla-protein (MGP) | Fibrosis/ECM | 3.6 | 4.9 |
| Msg1-related gene 1 (mrg1) | Signaling | 1.7 | 3.8 |
| Phospholipase A2 (PLA2) | Fibrosis/ECM | 1.5 | 2.6 |
| Regulator of g-protein signaling 5 (RGS5) (RGP5) | Signaling | 1.5 | 3.4 |
| Thymosin beta 4; FX | Fibrosis/ECM | 1.8 | 2.3 |
| Thymosin beta-10 (TMSB10; THYB10); PTMB10 | Fibrosis/ECM | 1.9 | 2.4 |
| (B) Genes downregulated in both baboon and human | |||
| Glutaredoxin (thioltransferase) | Stress | 0.5 | 0.5 |
| MT-1L (methallothionein-IL) | Stress | 0.5 | 0.3 |
| Superoxide dismutase 1 (SOD1) | Stress | 0.5 | 0.5 |
| (C) Genes discordant in the two species | |||
| C-reactive protein | Immune | 1.8 | 0.2 |
| Cytochrome P450 IIE1 (CYP2E1) | Metabolism | 1.9 | 0.7 |
| farnesyl pyrophosphate synthetase | Metabolism | 2.8 | 0.5 |
| Hepatocyte growth factor-like protein; macrophage-stimulating protein (MSP) | Immune | 1.5 | 0.5 |
| Lipopolysaccharide-binding protein (LBP) | Immune | 1.8 | 0.3 |
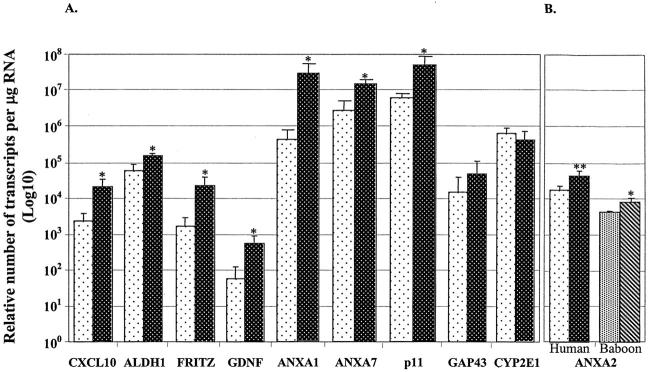
Real-time PCR analysis of the selected genes, CXCL10, glial cell-derived neurotrophic factor, frizzled-related protein, ANXA1, ANXA7, p11, and aldehyde dehydrogenase 1, confirmed the findings of the array results in human ALD (Figure 6) ▶ . Real-time RT-PCR of the baboon samples confirmed up- and down-regulation of these selected genes similar to array results (data not shown).
Figure 6.
Confirmation of differential expression of selected genes in human ALD using real-time RT-PCR. A: Significant up-regulation in human ALD of transcripts for CXCL-10, aldehyde dehydrogenase1, frizzled-related protein, ANXA1, ANXA7, p11, and glial cell-derived neurotrophic factor. Up-regulation of GAP43 (neuromodulin) and down-regulation of CYP2E1 did not reach significant levels. Glial cell-derived neurotrophic factor and neuromodulin genes were examined because they are up-regulated in other forms of liver cirrhosis. 4,13 B: Annexin A2 was significantly up-regulated in the baboon (n = 4) and in human (n = 8) ALD. The data represents means ± SD (*, P = 0.02; **, P = 0.04). □, Nondiseased;  , ALD (human);
, ALD (human);  , nondiseased;
, nondiseased;  , ALD (baboon).
, ALD (baboon).
Annexin A2 was differentially expressed in ALD in all four baboon arrays (5.1-fold mean increase). We also identified several other differentially expressed molecules in the baboon and humans that are related to or associated with annexin A2 (Table 6 ▶ , Figure 6 ▶ ). Significant increases in the expression of annexin A2 (mRNA molecules per μg of RNA) were shown by real-time RT-PCR in both baboon (2.2-fold, P = 0.02) and human (twofold, P = 0.04) liver (Figure 6) ▶ . Annexin A2 protein was found at the portal-parenchymal interface and hepatic parenchyma. A marked increase in annexin A2 expression was evident on the proliferating bile duct cells and hepatocyte cell surface in human ALD (Figure 7A, a to d) ▶ . Some small liver cells, also positive for annexin A2, were in greater abundance in ALD than in nondiseased tissue (Figure 7A, d) ▶ . Dual staining with cytokeratin 7, a proliferating bile duct cell marker, confirmed the co-localization of annexin A2 in the proliferating bile duct cells (Figure 7B, a to h) ▶ .
Table 6.
Molecules Differentially Regulated and Associated with/Related to Annexin A2
| Gene name | Expression ratio | |
|---|---|---|
| Baboon | Human | |
| Annexin A1 | 2.0 | 2.3 |
| Annexin A2 | 5.1 | n |
| Annexin A4 | 1.4 | 2.3 |
| Annexin A7 | 1.5 | n |
| Annexin A8 | n | 3.0 |
| Annexin A11 | 1.5 | n |
| Annexin A13 | n | 2.4 |
| Calgizzarin S100A11 | 1.7 | 1.5 |
| Calpactin light chain (p11) | 1.6 | n |
| Calpain 2 large subunit (CANP) | 2.0 | n |
| F-actin | 1.6 | n |
| Plasminogen | 1.6 | n |
| Phospholipase A2 | 2.4 | 2.7 |
| RAN GTPase activating protein | 1.6 | 0.3 |
| Tissue plasminogen activator (tPA) | n | 1.9 |
n, not differentially expressed.
Figure 7.

A: Increased expression of annexin A2 protein in human ALD. Increased annexin A2 expression (red) in the portal rim and portal-parenchymal interface (a), hepatic parenchyma cell surface (b), proliferating bile duct cells in the portal tract (c, g), and small cells (arrows, d and h) in human ALD (a–d) as compared to nondiseased (e–h) liver. B: Annexin A2 in ALD and nondiseased liver co-localized with cytokeratin 7. Cytokeratin 7 (red) identified proliferating bile duct cells, which were all immunopositive for annexin A2 (green) (a–h). Single color [annexin A2, green (a, e); cytokeratin 7, red (b, f)] red-green overlay (c, g) and three-color overlay (d, h) with nuclei stained blue by DAPI are depicted. B, Proliferating bile duct cells; H, hepatic parenchyma/lobule; I, portal/parenchymal interface; M, macrophage; PR, portal rim; PT, portal triad; S, septum. Red, Alexa Fluor 594; green, Alexa Fluor 488. Original magnifications, ×400.
It proved difficult to fully characterize annexin A2-positive small cells despite dual staining with various markers. Some annexin A2-positive small cells in ALD were identified as bearing the monocyte/macrophage marker CD14 in the portal-parenchymal interface (Figure 8A, a to d) ▶ and hepatocyte parenchymal region (Figure 8A, e to h) ▶ . Dual staining with the T-cell marker CD3 identified very few double-positive cells in the hepatic parenchyma of the nondiseased tissue (Figure 8B, a to d) ▶ . However, annexin A2 did not co-localize with the macrophage-specific antigen CD68 (supplementary Figure 1A, a to h ▶ ) and glial fibrillary acidic protein (supplementary Figure 1B, a to d ▶ ).
Figure 8.
A: Annexin A2 co-localized with some CD14-positive cells. Some cells were double-positive for annexin A2 (green) and monocyte/macrophage marker CD14 (red) in the septa and portal-parenchymal interface (a–h) and hepatic parenchyma (e–h) in ALD liver. Single-color (annexin, a and e; CD14, b and f); red-green overlay (c and g); and three-color overlay with nuclei stained blue by DAPI (d and h) are depicted. B: Annexin A2 co-localized with some CD3-positive cells. Annexin A2 (green) and the pan T-cell marker CD3 (red) co-localized in a few cells (arrows) in the hepatic parenchyma of nondiseased liver (a–d). Single-color (annexin, a; CD3, b); red-green overlay (c); and three-color overlay with nuclei stained blue by DAPI (d) are depicted. H, hepatic parenchyma; I, portal/parenchymal interface; M, monocyte/macrophage. Red, Alexa Fluor 594; green, Alexa Fluor 488. Original magnifications: ×400 (CD3); ×1000 (CD14).
No co-localization was observed with the stem cell markers CD117 (c-kit) and CD34; a B-cell marker (CD 19); a marker for quiescent stellate cells (synaptophysin); or for activated stellate cells 15 fibroblast activation protein (data not shown).
Discussion
The study of global intrahepatic gene expression by cDNA array analysis is a new approach to investigate the pathogenesis of ALD. We have shown that the global gene expression profile of the Lieber-DeCarli baboon model of ALD is similar to that of human ALD. We have also identified a number of novel molecular pathways that are altered in this disease. Energy metabolizing genes were the predominant class of differentially expressed genes. Other differentially expressed genes were involved with metabolism, fibrosis, proliferation, regeneration, immune system, oxidant stress, inflammation, apoptosis, as well as genes of the annexin family and associated pathways. This contrasts with observations in primary biliary cirrhosis and hepatitis B- and C-related cirrhosis in which pro- and anti-inflammatory genes were the most prominent category identified. 4,5,13,17,18 Differential expression of selected genes observed in the arrays was confirmed with real-time RT-PCR and immunofluorescence.
Gene expression data from the baboon ALD model is sparse in literature, however, this study has identified a number of differentially expressed genes previously reported in humans and other animal models of ALD including CYP2E1, Bcl-xL, 19 lipopolysaccharide-binding protein, 20 MCP1, 21 matrix metalloproteinases (MMPs), and tissue inhibitors of matrix metalloproteinase (TIMPs), 22,23 ADH, 24 gamma-aminobutyric acid receptor, 25,26 plasminogen activator (PA), 27 and collagens. 28,29 These indicate that the array methodology generated data consistent with published literature. Differential expression of CYP2E1 (Figure 2) ▶ in the baboon is of particular interest because previous studies have demonstrated induction of CYP2E1 after chronic alcohol administration in several animal models. 30 Up-regulation of CYP2E1 in human ALD was not expected as these patients had been abstinent in the period leading up to liver transplantation and in experimental animals, induction of CYP2E1 disappears within 3 days of alcohol withdrawal. 31 Thus the effects of recent alcohol consumption were more evident in the baboon model because the samples were obtained during ongoing alcohol treatment. In this model, genes involved in alcohol metabolism were prominently differentially expressed to a greater extent than in the samples obtained from abstinent humans with advanced disease.
Metabolism
Many differentially expressed genes associated with alcohol and xenobiotic metabolism were detected. Indeed, this category was the predominant category of differential expression in the baboon samples that had been collected soon after exposure to ethanol (Figure 5) ▶ . The principal CYP component of the microsomal ethanol-oxidizing system is CYP2E1, but up-regulation of other CYPs have been reported after ethanol administration including CYP2A1, CYP1A2, CYP3A3, and CYP3A4. 32,33 The spectrum of CYP induction/regulation by ethanol observed in the present study extends previous reports. This finding might explain how knock-out of a single CYP (CYPE1) failed to prevent ALD. 34 The extent to which alcohol-metabolizing enzymes other than CYPs may be inducible by chronic ethanol consumption has been controversial. In this study, several genes relevant to alcohol metabolism were identified as differentially expressed (Table 3) ▶ . The present data suggest that the biological response to chronic alcohol consumption is complex and involves multiple CYPs as well as other pathways.
Oxidant Stress
Oxidant stress is an important component of alcohol hepatotoxicity. 35 Several genes involved with the glutathione component of oxidant stress were differentially regulated in both humans and baboons. A strong correlation between reduced superoxide dismutase (SOD) protein expression and advanced fibrosis has also been detected in ALD. 36 We found the cytosolic SOD1 to be down-regulated in baboon and human liver and the extracellular SOD3 to be up-regulated in the human. The differential regulation of SODs in our study provides further evidence for their role in ALD, and suggests that the inducible Mn-SOD enzyme may be involved in recovery and cell protection in ALD as previously proposed. 36
An interesting novel finding was the down-regulation of six of the seven isoforms of metallothionein-1 in human ALD cirrhotic liver (metallothionein-1G, -1H (-0), -1F, -1L (-1X), -1B, -1E). Metallothionein-1L was down-regulated in the baboon. Metallothioneins have recently been shown to protect liver from oxidative injury induced by alcohol. 37 This study suggests that down-regulation of metallothioneins contributes to progression of ALD.
Fibrosis and ECM
Genes associated with fibrosis were the most prominent category of differentially expressed genes in human ALD (Figure 5) ▶ , consistent with the fibrotic nature of ALD. However, the genes identified as differentially expressed were not specific to alcohol and have been reported in primary biliary cirrhosis 4 and other forms of liver cirrhosis. 5,13,17 Consistent with previous findings of stellate cell activation in hepatic fibrosis, 38 we identified a number of molecules associated with stellate cell biology. In particular, markers of activation (endothelin-1), chemotaxis [CCL2 (monocyte chemotactic protein-1)] and matrix synthesis (transforming growth factor-β receptor II, latent transforming growth factor-β-binding protein 2, connective tissue growth factor, platelet-derived growth factor receptor, fibroblast growth factor receptors 2 and 3, insulin-like growth factor binding proteins 1 and 5) were identified. Haptoglobin and complement 3 transcripts were also up-regulated in the baboon model. Increased haptoglobin 39 and complement 3 have been reported in serum after an acute dose of alcohol and in ALD. 40 The present study is the first demonstration of differential expression at the mRNA level of haptoglobin and complement 3 in liver tissue in this disease.
Stellate cell activation results in matrix degradation achieved in part by releasing MMPs and their inhibition by TIMPs. 41,42 A number of these molecules were found to be up-regulated in end-stage human ALD cirrhosis in the present study, namely collagen IV (type α1 and α2), collagen VI (type α2 and α3), MMP-2, MMP-7, TIMP-2, and TIMP-3. Of these, increased hepatic collagen IV and laminin production has previously been demonstrated in human ALD 28 and MMP-2 and MMP-9 protein levels are increased in a rat model of ALD. 43
Immune
Several complement components and MHC class II molecules were up-regulated in human ALD whereas MHC class I antigen and β2-microglobulin were up-regulated in the baboon model. HLA class I antigens have been demonstrated on the surface of hepatocytes in patients with alcoholic cirrhosis. 44 In addition, the up-regulation of CCL2, CCL17, CXCL10, and 14-3-3 protein τ and down-regulation of CCR5 in the baboon are novel observations in ALD. The up-regulation of CD44 and the acute T-cell activation marker CD69 in the present study of end-stage ALD is indicative of ongoing immune activation. 17
Neural
Detection of intrahepatic overexpression of several neural genes (see supplementary data for a complete list) in ALD, specifically in the baboon, was interesting. Up-regulation of brain-derived neurotrophic factor and Trk3, among other neurotrophins, occurs in primary biliary cirrhosis and primary sclerosing cholangitis-associated cirrhosis. 4 Several neural genes, for example, synaptosomal-associated protein 25 (SNAP-25), neurofilament triplet M protein, and dopamine receptor D2 were down-regulated in the baboon and human. The role of these genes in liver pathobiology has not been defined, but some have been localized to activated stellate cells suggesting a role in fibrogenesis.
Annexin A2
We report here the first observations that suggest that the annexins may be relevant to the pathogenesis of human liver disease. Annexin A2 and several related molecules were up-regulated in both the baboon model and end-stage human ALD. In the normal liver, moderate expression of different annexins has been reported in the cytoplasm, bile duct, nuclear membrane, hepatocytes, and Kupffer cells. 45 Annexin A2 was immunolocalized in the bile duct but not in hepatocytes in the normal liver. 45 Immunofluorescence showed a marked increase in the expression of annexin A2 protein in human ALD at the hepatocyte cell surface, proliferating bile ducts, and in other small liver cells (Figure 7) ▶ . The Veterans Affairs Cooperative study 46 on alcoholic hepatitis reported proliferation of interlobular and marginal bile ducts with metaplasia and cells resembling oval cells in 122 human biopsy samples. These bile ductal changes correlate strongly with liver fibrosis, cirrhosis, portal inflammation, and overall histological severity scores in patients with ALD. 46 It is intriguing to note that annexin A2 expression was prominent in these proliferating bile ducts (Figure 7) ▶ .
Up-regulation of annexin A2 is consistent with previous observations concerning fibrinolysis in ALD 47 and may contribute to the risk of bleeding. 48 It is relevant to note that alcohol has recently been shown to up-regulate annexin A2 expression and increase fibrinolytic activity in endothelial cells. 49 This may contribute to the cardioprotective effects of moderate alcohol consumption. 50 Based on previous studies, annexin A2 may contribute to ALD via several processes including enhanced fibrinolysis, ECM remodeling, inflammatory cell migration, and inhibition of phospholipase A2 activity.
Annexin A2 has been identified as a surface plasminogen receptor for plasminogen and tissue plasminogen activator (tPA) on various cells. 51 Plasminogen and tPA were also up-regulated in the current study. In view of the fact that endotoxin has been shown to induce a hypercoagulable state in alcohol-fed rats, 52 up-regulation of annexin A2 may reflect a cellular defense to limit procoagulant activity in the liver similar to that which occurs in the vascular endothelium.
The co-expression of annexin A2 and CD14 (Figure 8A) ▶ but not CD68 (supplementary Figure 1A ▶ ) in some small cells in ALD suggests that annexin A2 is selectively expressed in a subpopulation of macrophages. Annexin A2 is also believed to have a role in plasminogen activation for recruitment of macrophages to the site of inflammation and injury, ECM degradation, and migration through ECM and may contribute to the progression of fibrosis and regeneration of liver. Also, suppressing and/or inactivating annexin A2 could be a potential strategy for manipulating its ligand, tissue plasminogen activator, required for the cleavage and activity of MMP-2, and thus controlling the profibrotic action of MMP-2.
Annexin A1 inhibits lipopolysaccharide-stimulated release of tumor necrosis factor-α 53 and may down-regulate the inflammatory response in several animal models 54,55 and potentially also in ALD. The present observations concerning annexin A1 expression suggest that annexin A1 may also play a significant role in the pathogenesis of ALD and warrants further investigation.
Conclusion
The study has defined the global gene expression profile in ALD and has confirmed the role of several molecules and pathways previously implicated in ALD and/or liver fibrosis. The study has also identified many novel genes and pathways unrelated to any other form of liver pathophysiology. The differential expression of a number of molecules belonging to and related to the annexin family and its many ligands, in particular the up-regulation of annexin A2 at the RNA and protein level in ALD was unexpected. This raises the possibility of its involvement in the progression of fibrosis and its potential as a therapeutic target.
Acknowledgments
We thank Drs. Nicholas Shackel and G. Alex Bishop for advice, Dr. Qihan Dong of Royal Prince Alfred Hospital for assistance with phosphorimaging, and Dr. Wolfgang Rettig of Boehringer Ingelheim Pharma KG for providing the antibody to fibroblast activation protein.
Footnotes
Address reprint requests to Devanshi Seth, Royal Prince Alfred Hospital, Drug Health Services, Missenden Rd., Camperdown, NSW 2050, Australia. E-mail: phaber@mail.usyd.edu.au.
Supported by the Department of Veteran’s Affairs, Australia; National Institutes of Health grant DK56402 to the University of Sydney HCV Pathogenesis Group, the Australian National Health and Medical Research Council, the Clive and Vera Ramaciotti Foundation and the Australian Brewer’s Foundation and by Wellcome Trust in supporting the purchase of a Sequence Detector. We also acknowledge NIH grant AA11115, The US Department of Veteran’s Affairs, and the Kingsbridge Research Foundation for support to Drs. C.S. Lieber and M.A. Leo.
References
- 1.Hill DB, Deaciuc IV, Nanji AA, McClain CJ: Mechanisms of hepatic injury in alcoholic liver disease. Gitlin N McCullough AJ eds. Clinics in Liver Disease. 1998:pp 703-721 Saunders, Philadelphia
- 2.Thurman RG, II: Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol 1998, 275:G605-G611 [DOI] [PubMed] [Google Scholar]
- 3.Fernandez-Checa JC, Kaplowitz N, Garcia-Ruiz C, Colell A: Mitochondrial glutathione: importance and transport. Semin Liver Dis 1998, 18:389-401 [DOI] [PubMed] [Google Scholar]
- 4.Shackel NA, McGuinness PH, Abbott CA, Gorrell MD, McCaughan GW: Identification of novel molecules and pathogenic pathways in primary biliary cirrhosis: cDNA array analysis of intrahepatic differential gene expression. Gut 2001, 49:565-576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shackel NA, McGuinness PH, Abbott CA, Gorrell MD, McCaughan GW: Insights into the pathobiology of hepatitis C virus-associated cirrhosis: analysis of intrahepatic differential gene expression. Am J Pathol 2002, 160:641-654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lieber CS, DeCarli L, Rubin E: Sequential production of fatty liver, hepatitis, and cirrhosis in sub-human primates fed ethanol with adequate diets. Proc Natl Acad Sci USA 1975, 72:437-441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seth D, Gorrell MD, McGuinness PH, Leo MA, Lieber CS, McCaughan GW, Haber PS: SMART amplification maintains representation of relative gene expression: quantitative validation by real time PCR and application to studies of alcoholic liver disease in primates. J Biochem Biophys Methods 2003, 55:53-66 [DOI] [PubMed] [Google Scholar]
- 8.Ihaka R, Gentleman RR: “R”: a language for data analysis and graphics. J Comput Graph Statist 1996, 5:299-314 [Google Scholar]
- 9.Yang YH, Dudoit S, Luu P, Speed TP: Normalization for cDNA microarray data. Bittner ML Chen Y Dorsal AN Dougherty ER eds. Microarrays: Optical Technologies and Informatics. 2001:pp 141-152 Proc SPIE (The International Society for Optical Engineering)
- 10.Yang YH, Dudoit S, Luu P, Lin DM, Peng V, Ngai J, Speed TP: Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res 2002, 30:1-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouton CM, Pevsner J: DRAGON View: information visualization for annotated microarray data. Bioinformatics 2002, 18:323-324 [DOI] [PubMed] [Google Scholar]
- 12.Yin JL, Shackel NA, Zekry A, McGuinness PH, Richards C, Putten KV, McCaughan GW, Eris JM, Bishop GA: Real-time reverse transcriptase-polymerase chain reaction (RT-PCR) for measurement of cytokine and growth factor mRNA expression with fluorogenic probes or SYBR Green I. Immunol Cell Biol 2001, 79:213-221 [DOI] [PubMed] [Google Scholar]
- 13.Shackel NA, Gorrell MD, McCaughan GW: Gene array analysis and the liver. Hepatology 2002, 36:1313-1325 [DOI] [PubMed] [Google Scholar]
- 14.Chetcuti A, Margan SH, Russell P, Mann S, Millar DS, Clark SJ, Rogers J, Handelsman DJ, Dong Q: Loss of annexin II heavy and light chains in prostate cancer and its precursors. Cancer Res 2001, 61:6331-6334 [PubMed] [Google Scholar]
- 15.Levy MT, McCaughan GW, Abbott CA, Park JE, Cunningham AM, Mueller E, Retting WJ, Gorrell MD: Fibroblast activation protein: a cell surface dipeptidyl peptidase and gelatinase expressed by stellate cells at the tissue remodelling interface in human cirrhosis. Hepatology 1999, 29:1768-1778 [DOI] [PubMed] [Google Scholar]
- 16.Ajami K, Abbott CA, Obradovic M, Gysbers V, Kähne T, McCaughan GW, Gorrell MD: Structural requirements for catalysis, expression, and dimerization in the CD26/DPIV gene family. Biochemistry 2003, 42:694-701 [DOI] [PubMed] [Google Scholar]
- 17.Honda M, Kaneko S, Kawai H, Shirota Y, Kobayashi K: Differential gene expression between chronic hepatitis B and C hepatic lesion. Gastroenterology 2001, 120:955-966 [DOI] [PubMed] [Google Scholar]
- 18.McCaughan GW, Shackel NA, Gorrell MD: Differential gene expression between chronic hepatitis B and C hepatic lesions (letter). Gastroenterology 2001, 121:1263-1264 [DOI] [PubMed] [Google Scholar]
- 19.Deaciuc IV, D’Souza NB, de Villiers WJ, Burikhanov R, Sarphie TG, Hill DB, McClain CJ: Inhibition of caspases in vivo protects the rat liver against alcohol-induced sensitization to bacterial lipopolysaccharide. Alcohol Clin Exp Res 2001, 25:935-943 [PubMed] [Google Scholar]
- 20.Zuo GQ, Gong JP, Liu CA, Li SW, Wu XC, Yang K, Li Y: Expression of lipopolysaccharide binding protein and its receptor CD14 in experimental alcoholic liver disease. World J Gastroenterol 2001, 7:836-840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bautista AP: Acute alcohol intoxication and endotoxemia desensitize HIV-1 gp120-induced CC-chemokine production by Kupffer cells. Life Sci 2001, 68:1939-1949 [DOI] [PubMed] [Google Scholar]
- 22.Okazaki I, Watanabe T, Hozawa S, Arai M, Maruyama K: Molecular mechanism of the reversibility of hepatic fibrosis: with special reference to the role of matrix metalloproteinases. J Gastroenterol Hepatol 2000, 15:D26-D32 [DOI] [PubMed] [Google Scholar]
- 23.Okazaki I, Watanabe T, Hozawa S, Niioka M, Arai M, Maruyama K: Reversibility of hepatic fibrosis: from the first report of collagenase in the liver to the possibility of gene therapy for recovery. Keio J Med 2001, 50:58-65 [DOI] [PubMed] [Google Scholar]
- 24.Potter JJ, Rennie-Tankersley L, Mezey E: Endotoxin enhances liver alcohol dehydrogenase by action through upstream stimulatory factor but not by nuclear factor-kappa B. J Biol Chem 2003, 278:4353-4357 [DOI] [PubMed] [Google Scholar]
- 25.Dodd PR, Lewohl JM: Cell death mediated by amino acid transmitter receptors in human alcoholic brain damage: conflicts in the evidence. Ann NY Acad Sci 1998, 844:50-58 [PubMed] [Google Scholar]
- 26.Thomas GJ, Harper CG, Dodd PR: Expression of GABA(A) receptor isoform genes in the cerebral cortex of cirrhotic and alcoholic cases assessed by S1 nuclease protection assays. Neurochem Int 1998, 32:375-385 [DOI] [PubMed] [Google Scholar]
- 27.Booyse FM, Aikens ML, Grenett HE: Endothelial cell fibrinolysis: transcriptional regulation of fibrinolytic protein gene expression (t-PA, u-PA, and PAI-1) by low alcohol. Alcohol Clin Exp Res 1999, 23:1119-1124 [DOI] [PubMed] [Google Scholar]
- 28.Tsutsumi M, Urashima S, Nakase K, Takase S, Takada A: Type IV collagen and laminin contents of livers from patients with alcoholic liver disease. Alcohol Alcohol Suppl 1993, 1A:45-52 [DOI] [PubMed] [Google Scholar]
- 29.Yamada H, Aida T, Taguchi K, Asano G: Expression of type III and IV procollagen, prolyl 4-hydroxylase mRNAs in fibrotic human liver. Nippon Rinsho-Jpn J Clin Med 1993, 51:423-427 [PubMed] [Google Scholar]
- 30.Lieber CS: Cytochrome P-4502E1: its physiological and pathological role. Physiol Rev 1997, 77:517-544 [DOI] [PubMed] [Google Scholar]
- 31.Oneta CM, Lieber CS, Li J, Ruttimann S, Schmid B, Lattmann J, Rosman AS, Seitz HK: Dynamics of cytochrome P4502E1 activity in man: induction by ethanol and disappearance during withdrawal phase. J Hepatol 2002, 36:47-52 [DOI] [PubMed] [Google Scholar]
- 32.Lasker JM, Tsutsumi M, Bloswick BP, Lieber CS: Characterization of benzoflavone (BF)-inducible hamster liver cytochrome P-450 isozyme catalytically similar to cytochrome P-450-ALC. Hepatology 1987, 7:432(Abstract) [Google Scholar]
- 33.Salmela KS, Kessova IG, Tsyrlov IB, Lieber CS: Respective roles of human cytochrome P-4502E1, 1A2, and 3A4 in the hepatic microsomal ethanol oxidizing system. Alcohol Clin Exp Res 1998, 22:2125-2132 [PubMed] [Google Scholar]
- 34.Kono H, Bradford BU, Yin M, Sulik KK, Koop DR, Peters JM, Gonzalez FJ, McDonald T, Dikalova A, Kadiiska MB, Mason RP, Thurman RG: CYP2E1 is not involved in early alcohol-induced liver injury. Am J Physiol 1999, 277:G1259-G1267 [DOI] [PubMed] [Google Scholar]
- 35.Lieber CS: Metabolism of alcohol. McCullough A eds. Clinics in Liver Disease: Alcoholic Liver Disease. 1998:pp 673-702 WB Saunders Company, Philadelphia
- 36.Zhao M, Matter K, Laissue JA, Zimmermann A: Copper/zinc and manganese superoxide dismutases in alcoholic liver disease: immunohistochemical quantitation. Histol Histopathol 1996, 11:899-907 [PubMed] [Google Scholar]
- 37.Zhou Z, Sun X, James KY: Metallothionein protection against alcoholic liver injury through inhibition of oxidative stress. Exp Biol Med 2002, 227:214-222 [DOI] [PubMed] [Google Scholar]
- 38.Friedman SL: Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem 2000, 275:2247-2250 [DOI] [PubMed] [Google Scholar]
- 39.Spitsyn VA, Nafikova A, Spitsyna N, Afanas’eva IS: Genetic predisposition to development of toxic liver cirrhosis caused by alcohol. Genetika 2001, 37:698-707 [PubMed] [Google Scholar]
- 40.Skrede S, Blomhoff JP, Elgjo K, Gjone E: Serum proteins in diseases of the liver. Scand J Clin Lab Invest 1975, 35:399-406 [PubMed] [Google Scholar]
- 41.Rahkonen OP, Koskivirta IM, Oksjoki SM, Jokinen E, Vuorio EI: Characterization of the murine Timp4 gene, localization within intron 5 of the synapsin 2 gene and tissue distribution of the mRNA. Biochim Biophys Acta 2002, 1577:45-52 [DOI] [PubMed] [Google Scholar]
- 42.Arthur MJ, Iredale JP, Mann DA: Tissue inhibitors of metalloproteinases: role in liver fibrosis and alcoholic liver disease. Alcohol Clin Exp Res 1999, 23:940-943 [DOI] [PubMed] [Google Scholar]
- 43.Lu X, Wang B, Xie Y, Liu C, Fu B: Dynamic change and expression of matrix metalloproteinase-2, -9 in alcoholic liver disease in rats. Chung Hua Kan Tsang Ping Tsa Chih 2001, 9:268-270 [PubMed] [Google Scholar]
- 44.Fukusato T, Gerber MA, Thung SN, Ferrone S, Schaffner F: Expression of HLA class I antigens on hepatocytes in liver disease. Am J Pathol 1986, 123:264-270 [PMC free article] [PubMed] [Google Scholar]
- 45.Dreier R, Schmid KW, Gerke V, Riehemann K: Differential expression of annexins I, II and IV in human tissues: an immunohistochemical study. Histochem Cell Biol 1998, 110:137-148 [DOI] [PubMed] [Google Scholar]
- 46.Ray MB, Mendenhall CL, French SW, Gartside PS: Bile duct changesin alcoholic liver disease. The Veterans Administration Cooperative Study Group. Liver 1993, 13:36-45 [DOI] [PubMed] [Google Scholar]
- 47.Gram J, Duscha H, Zurborn KH, Bruhn HD: Increased levels of fibrinolysis reaction products (D-dimer) in patients with decompensated alcoholic liver cirrhosis. Scand J Gastroenterol 1991, 26:1173-1178 [DOI] [PubMed] [Google Scholar]
- 48.Hu KQ, Yu AS, Tiyyagura L, Redeker AG, Reynolds TB: Hyperfibrinolytic activity in hospitalized cirrhotic patients in a referral liver unit. Am J Gastroenterol 2001, 96:1581-1586 [DOI] [PubMed] [Google Scholar]
- 49.Tabengwa EM, Abou-Agag LH, Benza RL, Torres JA, Aikens ML, Booyse FM: Ethanol-induced up-regulation of candidate plasminogen receptor annexin II in cultured human endothelial cells. Alcohol Clin Exp Res 2000, 24:754-761 [PubMed] [Google Scholar]
- 50.Abou-Agag LH, Tabengwa EM, Tresnak JA, Wheeler CG, Taylor KB, Booyse FM: Ethanol-induced increased surface-localised fibrinolytic activity in cultured human endothelial cells: kinetic analysis. Alcohol Clin Exp Res 2001, 25:351-361 [PubMed] [Google Scholar]
- 51.Falcone DJ, Borth W, Khan KMF, Hajjar KA: Plasminogen-mediated matrix invasion and degradation by macrophages is dependent on surface expression of annexin II. Blood 2001, 97:777-784 [DOI] [PubMed] [Google Scholar]
- 52.Arai M, Nakano S, Okuno F, Hirano Y, Sujita K, Kobayashi T, Ishii H, Tsuchiya M: Endotoxin-induced hypercoagulability: a possible aggravating factor of alcoholic liver disease. Hepatology 1989, 9:846-851 [DOI] [PubMed] [Google Scholar]
- 53.de Coupade C, Ajuebor MN, Russo-Marie F, Perretti M, Solito E: Cytokine modulation of liver annexin 1 expression during experimental endotoxemia. Am J Pathol 2001, 159:1435-1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Flower RJ: Lipocortin. Prog Clin Biol Res 1990, 349:11-25 [PubMed] [Google Scholar]
- 55.Rothwell NJ, Flower R: Lipocortin-1 exhibits novel actions, providing clinical opportunities. Trends Pharmacol Sci 1992, 13:45-46 [DOI] [PubMed] [Google Scholar]