Abstract
Nephrin is a type-1 transmembrane protein and a key component of the podocyte slit diaphragm, the ultimate glomerular plasma filter. Genetic and acquired diseases affecting expression or function of nephrin lead to severe proteinuria and distortion or absence of the slit diaphragm. Here, we showed by using a surface plasmon resonance biosensor that soluble recombinant variants of nephrin, containing the extracellular part of the protein, interact with each other in a specific and concentration-dependent manner. This molecular interaction was increased by twofold in the presence of physiological Ca2+concentration, indicating that the binding is not dependent on, but rather promoted by Ca2+. Furthermore, transfected HEK293 cells and an immortalized mouse podocyte cell line overexpressing full-length human nephrin formed cellular aggregates, with cell-cell contacts staining strongly for nephrin. The distance between plasma membranes at the nephrin-containing contact sites was shown by electron microscopy to be 40 to 50 nm, similar to the width of glomerular slit diaphragm. The cell contacts could be dissociated with antibodies reacting with the first two extracellular Ig-like domains of nephrin. Wild-type HEK293 cells were shown to express slit diaphragm components CD2AP, P-cadherin, FAT, and NEPH1. The results show that nephrin molecules exhibit homophilic interactions that could promote cellular contacts through direct nephrin-nephrin interactions, and that the other slit diaphragm components expressed could contribute to that interaction.
Podocyte foot processes cover the outer aspect of the glomerular capillaries in an interdigitating manner. The slit between adjacent foot processes contains a highly ordered thin structure referred to as slit diaphragm (SD). The SD is thought to act as the ultimate albumin-excluding ultrafilter critical in the formation of primary urine. 1 Based on electron microscopic studies of perfusion-fixed rodent kidneys, the SD has been suggested to have a zipper-like porous structure. 2 The SD is affected in a variety of genetic and acquired diseases with proteinuria and nephrotic syndrome as a result. 1,3 Nephrin is a recently described protein 4 and the first molecule to be localized to the SD. 5-7 In the SD, nephrin molecules of adjacent podocytes could possibly interact with each other and, thus contribute to the filter structure. 8
Congenital nephrotic syndrome of the Finnish type (CNF or NPHS1, MIM 256300) is a rare, but one of the most severe forms of genetic kidney disorders. Development of the clinical syndrome in CNF is closely correlated with major structural and morphological changes in podocyte foot processes and absence of the SD. 3,9 The nephrin gene (NPHS1), mutated in CNF, encodes a type-1 transmembrane glycoprotein belonging to the immunoglobulin superfamily (IgSF). 4,10 The mature polypeptide consists of 1241 amino acid residues with a calculated molecular mass of 132,532. However, because of posttranslational glycosylation, the mature polypeptide migrates as a 180- to 200-kd protein when analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). 11 Nephrin consists of eight extracellular immunoglobulin-like domains followed by a fibronectin type III domain, a short transmembrane part, and a cytoplasmic C-terminal domain. In the kidney, nephrin expression is restricted to the glomerular podocytes, and by immunoelectron microscopy nephrin has been localized to the SD area. 5-7 The central role of nephrin in the formation and function of the SD has been demonstrated in CNF patients 12 and in nephrin-deficient mice, 13 as both cases lead to a lack of the SD and massive proteinuria. Nephrotic syndrome has also been reported in mice lacking CD2AP, an adapter actin-binding protein, 14 and in patients with mutations in the NPHS2 gene-encoding podocin, 15 a new member of the stomatin family with hairpin-like membrane-integrated protein topology. Also, inactivation of the novel SD proteins NEPH1 and FAT results in the development of congenital nephrotic syndrome and neonatal death, respectively. 16,17
It has recently been demonstrated that both CD2AP and podocin interact directly with the intracellular C-terminal part of nephrin and, together with nephrin, can be isolated as a raft-associated component of the podocyte SD. 18-20 Based on its specific location in the SD and the fact that nephrin is a member of the IgSF, a hypothetical model has been proposed that nephrin molecules emerging from adjacent foot processes interact with each other in a homophilic manner and form the backbone of the SD structure; 8 however, evidence for such interactions has, thus far, not been presented.
In the present study, we have shown by using a surface plasmon resonance technology that the extracellular part of soluble nephrin exhibits homophilic interactions. We also demonstrated that cells transfected with full-length nephrin cDNA aggregate to form cell-cell junctions with a spacing of similar width as that of native SD. Furthermore, the cellular aggregates can be dissociated with antibodies against the extracellular domain of nephrin. The results suggest that nephrin molecules could also in vivo, contribute to the SD filter structure through homophilic interactions.
Materials and Methods
Construction of cDNA Clones Encoding Human Nephrin Variants
The construction of the full-length cDNA clone encoding the human nephrin has been reported earlier. 21 For expression of Fc-fused nephrin chimera (NphFc), a cDNA clone encoding the entire extracellular part of human nephrin was cloned into a modified mammalian expression vector pCR-3 (Invitrogen, Carlsbad, CA) with a SalI/NotI cDNA cassette encoding the Fc part (the hinge, CH2, and CH3 domains) of the human IgG1 (the pCR3Fc vector was kindly provided by Dr. Pascal Schneider, Institute of Biochemistry, University of Lausanne, Switzerland). Using polymerase chain reaction (PCR) and 3′-end primer (FNSalI 5′-AGTCAGTCGACCCCCGAGGGTCCT-3′), complementary to the sequence encoding the last four amino acid residues (GPSG) in the fibronectin type III domain of nephrin, followed by a SalI site (underlined), and an upstream primer, UP1 5′-AAGGTTGTGAGTCTGACCCCAC-3′, was used to amplify a short 3′-end fragment. The 3′-end fragment was cleaved with AccI and SalI to generate the AccI/SalI 3′-end fragment. A large EcoRI/AccI fragment corresponding to the middle part of nephrin cDNA together with the HindIII/EcoR1 5′-end fragment (see the full-length construct) and the AccI/SalI 3′-end fragment were ligated into the HindIII/SalI-cleaved pCR3Fc vector to generate a construct (pCR3NphFc) encoding the entire extracellular nephrin followed by the Fc part of human IgG1. Finally, the ligated fragments were checked by sequencing.
The cDNA clone encoding the soluble histidine-tagged nephrin (NphHis) was generated from the pCR3NphFc construct. Two synthetic oligonucleotides, SalIHis 5′-T CGACCATCATCACCATCACCATTGA-3′ with cohesive end SalI site (underlined) and a stop codon (italics and underlined), and NotIHis 5′-GGCCTCAATGGTGATGGTGATGATGG-3′ with a cohesive end NotI site (underlined), were used to construct a SalI/6His/NotI-linker fragment with complementary overlapping 3′-ends encoding six histidine residues and 5′-cohesive ends corresponding to SalI and NotI sites, respectively. After annealing, the linker was inserted into the pCR3NphFc construct cleaved with SalI and NotI. The final construct (pCR3NphHis) encoded the entire extracellular portion of nephrin followed by a 6-His tag and a stop codon.
Cell Lines, Culturing Conditions, and Generation of Stable Transfected Cell Lines
All cell culture media, supplements (GibcoBRL, Grand Island, NY) and plates/flasks (Nunc, Naperville, IL) were supplied by Life Technologies Inc. The conditionally immortalized mouse podocyte cell line (MCP-5, here termed IMP) was kindly provided by Dr. Peter Mundel (Albert Einstein College of Medicine, Bronx, NY). The cells were maintained and propagated at 33°C in RPMI 1640 medium containing 10 U/ml interferon-γ (Sigma, St. Louis, MO) and 10% fetal bovine serum as described. 22 For differentiation the cells were cultured at 37°C in medium without the interferon-γ. When needed, cells were cultured on type I collagen (collagen-A; Biochrom KG, Berlin, Germany) for differentiation. The human embryonic kidney cell line QBI293A (Qbiogene, Carlsbad, CA), derived from the HEK293 cell line, was cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 100 U/ml of penicillin, and 100 mg/ml of streptomycin. Transfections of both cell lines were performed by lipofectin (Roche Molecular Biochemicals, Indianapolis, IN) according to the manufacturer’s protocol. Stable clones were selected for 2 weeks in medium containing 1 mg/ml of geneticin and were then cloned at that stage.
Expression and Purification of Recombinant Proteins
Stable HEK293 cell clones expressing the chimeric NphFc and NphHis recombinant proteins were cultured in triple flasks (Nunc). The cell clone expressing chimeric NphFc was cultured in Dulbecco’s modified Eagle’s medium containing fetal bovine serum to reduce co-purification of serum IgGs with the recombinant nephrin. After 3 days of confluency, the medium was harvested and phenylmethyl sulfonyl fluoride was added to a concentration of 2 mmol/L before filtration through a 0.22-μm membrane. One liter of the harvested medium was loaded circulating onto a 1-ml HiTrap protein A-Sepharose column (Amersham Biosciences, Uppsala, Sweden) equilibrated with phosphate-buffered saline (PBS) in a cold room overnight. The column was connected to a fast performance liquid chromatography (FPLC) system (ΔKTApurifier system; Amersham Biosciences), washed with 10 ml of PBS and the proteins were eluted with 50 mmol/L of citrate-NaOH, pH 3.0. The eluent was neutralized with 1 mol/L Tris-HCl buffer, pH 8.0, and dialyzed against a 20-mmol/L Tris-HCl buffer, pH 7.5. Proteins were then loaded onto an ion-exchange chromatography column (Mono Q HR 5/5, Amersham Biosciences). The NphFc recombinant protein was eluted from the column with a linear 0- to 1-mol/L NaCl-gradient (20-column volume) in 20 mmol/L of Tris-HCl, pH 7.5, and desalted using Sephadex G-50 NICK columns (Amersham Biosciences) equilibrated with 20 mmol/L of Tris-HCl, pH 7.5.
For purification of His-tagged NphHis protein, one liter of the harvested medium was loaded onto a DEAE-Sepharose column using an ΔKTApurifier system and proteins were eluted with 20 mmol/L of Tris-HCl and 1 mol/L of NaCl, pH 7.5. The eluate was loaded onto a Ni-NTA agarose column (Qiagen, Valencia, CA) equilibrated with the washing buffer (20 mmol/L Tris-HCl, 0.5 mol/L NaCl, 10% glycerol, 0.5% Tween 20, pH 8.0). The column was washed with at least 30-column volumes before elution with a stepwise gradient of imidazole 0 to 200 mmol/L in the washing buffer. Collected fractions were analyzed by SDS-PAGE and fractions with the highest amount of NphHis (eluted at 50 to 100 mmol/L of imidazole) were pooled, dialyzed against 20 mmol/L Tris-HCl, pH 7.5, and loaded onto a Mono Q HR 5/5 column. Proteins were eluted with a linear 0- to 1-mol/L NaCl gradient (40-column volume) in 20 mmol/L of Tris-HCl buffer, pH 7.5. The eluate was desalted on Sephadex G-50 NICK columns equilibrated with 20 mmol/L of Tris-HCl, pH 7.5, aliquoted, and frozen at −20°C. The purity of the recombinant proteins was determined by SDS-PAGE and silver staining.
Sample Preparation and Western Blot Analysis
Cell lysates were prepared by washing the cells twice before addition of hot SDS sample buffer (63 mmol/L Tris-HCl, 2% SDS, 10% glycerol, 0.1 mol/L dithiothreitol, pH 6.8). The cell lysates were collected with a rubber scraper and passed through 26-gauge needles and then transferred into Eppendorf tubes, boiled, and centrifuged for 10 minutes before loading. Human glomeruli were isolated by differential sieving through 400-μm and 200-μm mesh brass sieves and washed extensively with ice-cold PBS including protease inhibitors. Isolated glomeruli were collected by centrifugation at 10,000 × g and resuspended in PBS at a concentration of 20,000 glomeruli per ml. Fractions of the isolated glomeruli were lysed in equal volumes of hot 2× SDS sample buffer and centrifuged to remove unsolubilized material. To remove the carbohydrate moieties on nephrin, a N-glycosidase F deglycosylation kit was used according to the manufacturer protocol (catalog no. 1836552, Roche). All samples were subjected to SDS-PAGE and the proteins were transferred to polyvinylidene difluoride membranes. The membranes were blocked and incubated with affinity-purified pAb1 antibodies (0.2 μg/ml), washed, and incubated with horseradish peroxidase-conjugated goat anti-rabbit antibodies. The immunoreactivity was detected by a chemiluminescent kit (Life Science Products) according to the manufacturer’s instructions. The prestained molecular standard used in the SDS-PAGE was from Bio-Rad (catalog number 161-0372; Precision Protein Standards). Protein measurements were performed using a protein assay according to the manufacturer’s protocol (Bio-Rad, Hercules, CA).
Surface Plasmon Resonance
Protein interaction analysis based on surface plasmon resonance technology was performed using a Biacore 2000 optical biosensor (Biacore AB, Uppsala, Sweden). Proteins were immobilized on carboxymethylated dextran surfaces of research-grade (CM5 sensor chips) using amine-coupling chemistry. Flow cells were activated with a 1:1 mixture of 0.1 mol/L N-hydroxysuccinimide and 0.4 mol/L 3-(N,N-dimethylamino)propyl-N-ethylcarbodiimide at a flow rate of 20 μl/min at 25°C. All proteins were chromatographed on a Sephadex G-25 column equilibrated in running buffer, HBS-P [10 mmol/L HEPES, 0.15 mol/L NaCl, 0.005% surfactant P20 (Biacore), pH 7.4], containing 3.4 mmol/L ethylenediaminetetraacetic acid (EDTA) before use. The ligands, NphHis, NphFc, and human IgG1 (DAKO, Glostrup, Denmark), were diluted in immobilization buffer (10 mmol/L malate buffer, pH 6.0) to a final concentration of 10 μg/ml and immobilized in equal molar ratios, resulting in immobilization densities of 5000:5000:2500 response units for NphHis:NphFc:IgG1, respectively. After immobilization, the surfaces were blocked with 1 mol/L of ethanolamine, pH 8.0, followed by extensive wash with regeneration buffer (HBS-P containing 3.4 mmol/L EDTA and 1 mol/L NaCl). Recorded interactions were performed at 25°C with proteins diluted in HBS-P containing either 3.4 mmol/L EDTA or 1 mmol/L Ca2+. Binding of analytes to the immobilized ligands were measured in resonance units, response unit (1000 response units = 1 ng/mm 2 bound protein). Binding data were analyzed as previously described, 23 using an algorithm for calculation of association and dissociation rate constants that corrected for mass transport effects.
Immunofluorescence Staining and Antibody Treatment
Rabbit polyclonal antibodies (pAb1) against the two first Ig-like domains (amino acids 22 to 240) of human nephrin have been described. 5,12 For immunofluorescence staining, cells were cultured on glass coverslips (coated with type I collagen), then washed with PBS and fixed with 2% formaldehyde and 0.1% glutaraldehyde in PBS for 30 minutes. Cells were then washed twice with PBS blocked with 2% bovine serum albumin and 1% casein hydrolysate in PBS for 1 hour at room temperature. The cells were incubated with affinity-purified rabbit anti-nephrin antibodies pAb1 (10 μg/ml) for 1 hour at room temperature. After three washes, the cells were treated for 30 minutes with a secondary antibody (fluorescein isothiocyanate-conjugated swine anti-rabbit IgG, DAKO). Actin cytoskeleton was stained with rhodamine-phalloidin. The cells were finally washed three times, mounted, and examined by a DMRB Leica microscope and photographed with a digital camera (Hamamatsu C4742-95, Bridgewater, NJ).
For experiments with antibody treatment, wild-type HEK293 and IMP cells and cells transfected with full-length nephrin were cultured on glass coverslips coated with type I collagen for 4 to 7 days. Thereafter, the medium was replaced with prewarmed fresh medium containing 1% fetal calf serum and affinity-purified pAb1 in a range of 0 to 40 μg/ml. An equal amount of preimmune rabbit IgG was used in a parallel experiment as negative control. Cells were incubated at 37°C for 1 hour and then either photographed immediately using a phase contrast microscope or fixed and stained for nephrin as described above.
Electron Microscopy (EM) and Electron Tomography
For immuno-EM, nephrin-expressing HEK293 cells were fixed with 3.5% paraformaldehyde alone or with different concentrations (0.01 to 0.1%) of glutaraldehyde, processed and immunostained as described. 5,24 In short, LR-White thin sections on gold-nickel grids were incubated with primary antibodies in blocking solution and washed. After incubation with 5- or 10-nm gold-conjugated secondary antibodies, the sections were poststained in 1% uranyl acetate. Controls included use of nonimmune rabbit IgG as primary antibody. A Jeol 1200 EX electron microscope was used for examining the sections.
For electron tomography, the immunoresin sections were prepared as for EM but immuno-marked only with 5-nm-gold and the grids were treated with 10-nm-gold protein A (Amersham Biosciences) for alignment purposes in image reconstruction. The electron tomography was performed essentially as described earlier 24,25 using a Philips CEM 200 FEG transmission electron microscopy. Automatic low-dose tilt series were recorded with a slow-scan camera (2048 × 2048 CCD chip, pixel size 14 μm; TVIPS GmbH, Gauting, Germany) and using the EMMENU software. Images were recorded at 1 or 2° tilt intervals (−65 to + 60°, 26,700×, final pixel size of 5.24 Å). The total dose on the immunoresin sections was below 30 e−/Å. 2 Geometrical image alignment was performed using gold markers (error usually under 1 pixel). Image refinement was done using the COMET technique. 24 The reconstructions were visualized by isodensity contouring as surface rendered or wire-frame representation with the program XTV 25 or by volume rendering with the program BOB (Ken Chin-Purcell, Minnesota Supercomputer Center Inc.).
RNA Isolation, Primer Design, and Reverse Transcriptase (RT)-PCR
Total RNA was isolated from wild-type and nephrin-transfected HEK293 cells using an RNeasy kit according to the manufacturer’s protocol (Qiagen). Forward and reverse primers were designed to examine the expression levels of human genes encoding the SD components, CD2AP, FAT1, NEPH1, nephrin, podocin, and P-cadherin in the cultured cells. For expression levels of human CD2AP gene (GI:11321633), forward primer (5′-ggcatgggaatgtagcaagt-3′, in exon 3) and reverse primer (5′-tctccaaatccaattcctcg-3′, in exon 6) were designed to amplify a 433-bp PCR-fragment. For the gene encoding human FAT1 (GI:1107686), forward primer (5′-ggacccgctacggctttctt-3′, in exon 26) and reverse primer (5′-gctttcccgggcactgtatg-3′, in exon 27) were designed to amplify a 420-bp fragment corresponding to the cytoplasmic part of the molecule. Forward primer (5′-gagaggaccaactcaggcag-3′, in exon 10) and reverse primer (5′-gctctcggttcactgtctt-3′, in exon 12) to human NEPH1 (GI:14572520) were chosen to amplify a 310-bp PCR fragment. For the human nephrin gene, NPHS1 (GI:4758821), forward primer (5′-gtgaacgagggctcccagc-3′, in exon 5) and reverse primer (5′-gcagtccatccatgactgtc-3′, covering the splice junction between exon 9 and 10) were designed to amplify a PCR product of 620 bp. To detect expression of human NPHS2 gene (GI:25137568)-encoding podocin, the forward primer (5′-ggttgaccttcgtctccaaa-3′, exon 4) and reverse primer (5′-gaatctcagctgccatcctc-3′, exon 8) were designed to amplify a 443-bp PCR fragment. For human P-cadherin gene (GI:14589890), forward primer (5′-ggcacgggaacccttctgcta-3′, in exon 13) and reverse primer (5′-cgccatagtagaagacgttgt-3′, in exon 15) were designed to amplify a 520-bp PCR fragment. RT-PCR was applied using a one-step RT-PCR kit, according to the manufacturer’s protocol (Titanium One-Step RT-PCR kit; Clontech, Palo Alto, CA). For each reaction, 600 ng of total RNA was used. To synthesize the first cDNA strand, the thermocycler was set to 50°C for 1 hour followed by a denaturizing step at 94°C for 5 minutes. To amplify gene-specific expression, 35 cycles of 94°C for 5 minutes, 58°C for 30 seconds, and 68°C for 1 minute were repeated followed by an additional extension cycle at 68°C for 2 minutes. The PCR reactions were analyzed by gel electrophoresis on a 1.5% agarose gel, stained with ethidium bromide, and photographed under UV light.
Results
Characterization of Recombinant Nephrin Variants
Three recombinant nephrin variants were produced for studies on the cellular and protein interactions for nephrin (Figure 1A) ▶ . Full-length human nephrin expressed in HEK293 cells was shown to be ∼185 kd by Western blot analysis, using a rabbit polyclonal antibody 5 (pAb1) raised against the first two Ig domains of human nephrin (Figure 1B) ▶ . The same size was obtained for recombinant nephrin expressed in IMP cells (not shown). In contrast, nephrin isolated from normal human glomeruli migrated with an apparent weight of 200 kd. The observed size difference is because of different degrees of glycosylation, as enzymatic removal of the N-linked carbohydrates with N-glycosidase F resulted in proteins with similar migration in SDS-PAGE (Figure 1C) ▶ . When analyzed by gradient SDS-PAGE, the full-length nephrin expressed in IMP-NPH1 and HEK293 cells appeared as a closely migrating double immunoband of 185 kd (Figure 1C) ▶ . However, after removal of all N-linked carbohydrates, a single band was detected (Figure 1C) ▶ , indicating that the minor difference in migration is because of glycosylation. As expected, protein samples derived from glomerular extracts of a CNF patient with a Fin-major mutation 4 and wild-type HEK293 cells were devoid of nephrin (Figure 1B) ▶ .
Figure 1.
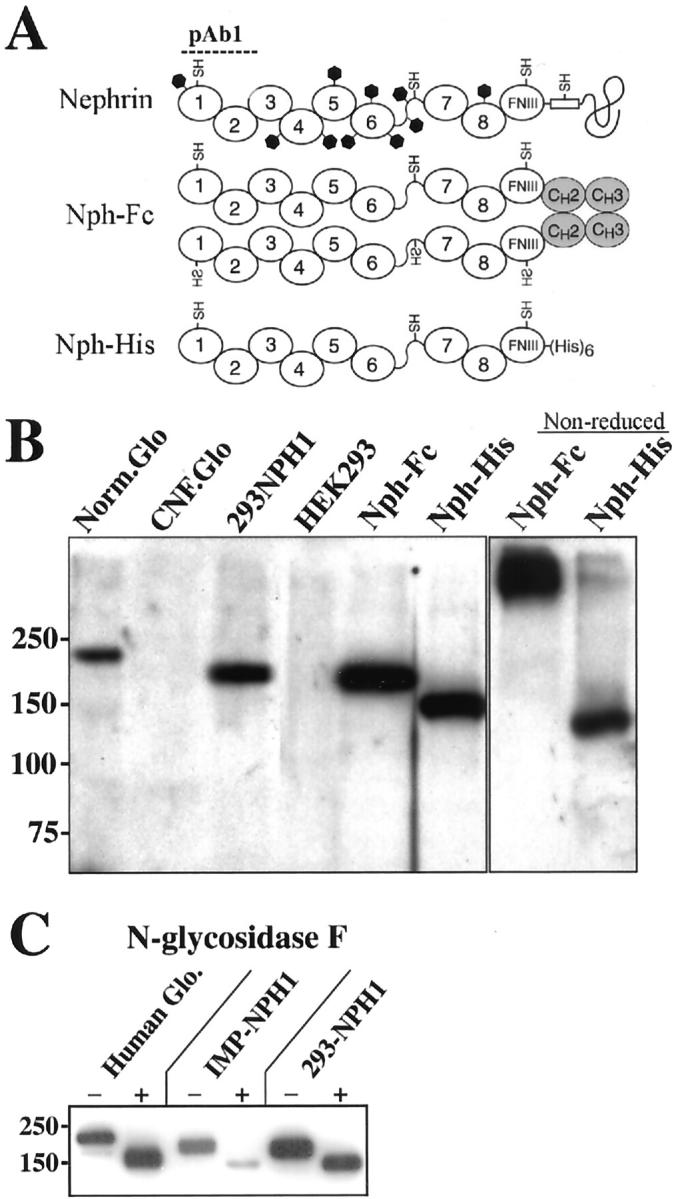
Schematic view of recombinant nephrin variants made in this study and their molecular sizes based on SDS-PAGE. A: Full-length nephrin (Nephrin) with its eight Ig-domains (1–8), a fibronectin type-III module (FNIII), a transmembrane part (boxed), and a cytoplasmic tail. Positions of the four free cysteine residues (−SH) and the 10 potential N-linked glycosylation sites (black hexagonals) are marked. The dimer nephrin construct (NphFc) with its Fc-fusion partner (CH2 and CH3 domains from human IgG1), and the (His)6-tagged monomer nephrin constructs (NphHis) are shown. The binding area of the pAb1 antibody against the first two Ig domains of human nephrin is indicated. B: Immunoblot with polyclonal antibody pAb1 showing the relative molecular migration of nephrin extracted from normal human glomeruli (Norm.Glo) and from the stably transfected HEK293 cell line expressing the full-length nephrin construct, the Fc-fused dimer (NphFc) and the histidine-tagged monomer (NphHis), under reducing conditions. The samples from a CNF patient with a Fin-major mutation (CNF.Glo) and the nontransfected parental HEK293 cell line (HEK293) show no detectable nephrin. Migration of the soluble recombinant nephrin constructs (NphFc and NphHis) under nonreducing condition is also shown. C: Immunoblot of nephrin samples from normal human glomeruli (Human Glo), a stable transfected immortalized mouse podocyte cell line (IMP-NPH1), and the stable transfected HEK293 cell line (293-NPH1) before (−) and after (+) treatment with N-glycosidase F. Nephrin molecules from all samples migrate as a 150-kd immunoband after removal of the N-glycosylations, indicating that the small difference in migration is because of somewhat different degrees of glycosylation. The positions of the molecular marker proteins are shown in kd.
Two soluble nephrin variants containing the entire extracellular domain were also produced in HEK293 cells. One contained a six histidine residue tag (NphHis), and the other the Fc domain of human IgG1 (NphFc). The NphHis protein migrated as a monomer with expected size under both reducing and nonreducing conditions (Figure 1B) ▶ . However, without reduction, a minor fraction of NphHis also migrated as protein complexes with high molecular weights (Figure 1B) ▶ , indicating that intermolecular disulfide bridges might be formed in vitro by the free cysteine residues present in the extracellular part. As predicted, the NphFc chimera migrated without reduction with a size corresponding to a dimer, while it migrated with the size of a monomer after reduction (Figure 1B) ▶ .
Soluble Nephrin Molecules Exhibit Homophilic Interactions
To examine the molecular interactions of nephrin molecules in solution, we used a surface plasmon resonance biosensor, which allows for monitoring of association and dissociation between biomolecules in real time, provided that one of the binding partners is covalently immobilized or captured onto a sensor surface. 26 Both NphFc and NphHis were covalently immobilized on separate lanes of a CM5 sensor chip using amine-coupling chemistry (see Materials and Methods). As a background control, human IgG1, which shares an identical Fc portion with the NphFc chimera, was immobilized on a separate lane. The association and dissociation of NphHis to a surface of NphHis is shown in Figure 2, A and C ▶ . Increased binding was detected with higher concentrations of NphHis in the fluid phase. A similar pattern of increasing interaction was seen when NphHis was flushed over immobilized NphFc (data not shown). Human IgG1 did not show any binding to either immobilized NphHis or immobilized IgG1 (Figure 2B) ▶ , demonstrating that the observed binding of NphHis to immobilized nephrin represents a specific homophilic binding between nephrin molecules. A small, nonspecific binding was observed when NphHis was run over immobilized IgG1 (Figure 2A) ▶ ; subtraction of this binding from the nephrin-nephrin binding curve gave smooth association-dissociation binding curves (Figure 2A) ▶ . A series of such binding curves, obtained by running different concentrations of NphHis (Figure 2C) ▶ , were used in a global curve-fitting analysis to calculate association and dissociation rate constants for the nephrin-nephrin binding interaction. This resulted in an association rate constant ka = 2.2 × 105 mol/L−1 s−1, a dissociation rate constant kd = 0.0011 seconds−1, and an equilibrium binding constant KD = 5.0 × 10−9 mol/L, indicating that the primary nephrin-nephrin binding is of high affinity. We further studied the nature of the nephrin-nephrin homophilic binding in the presence of Ca2+. When Ca2+ at the physiological concentration of 1 mmol/L was included in the buffer, a more than twofold increase of the binding was observed, indicating that the homophilic binding of nephrin is promoted by Ca2+ ions (Figure 2D) ▶ . Because of the complex nature of the nephrin-nephrin binding in the presence of Ca2+, we did not attempt to calculate the association-dissociation rate constants under these conditions.
Figure 2.
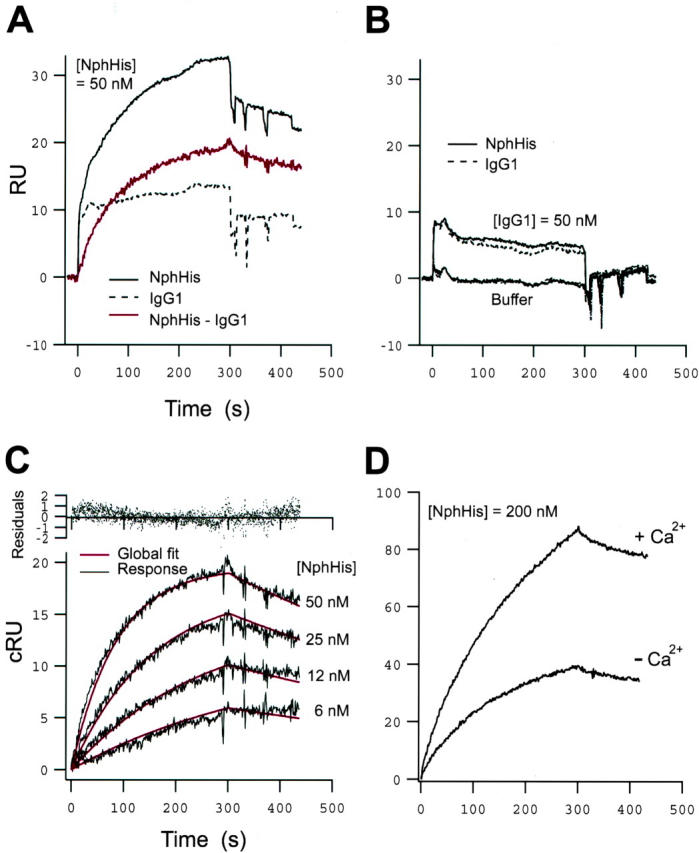
Sensorgrams showing binding responses of nephrin. A: Sensorgrams obtained from injection of NphHis (50 nm) over surfaces of immobilized NphHis (solid curve) and IgG1 (stippled curve), respectively. The corrected response of NphHis binding to the NphHis-surface, obtained after subtraction of the nonspecific binding to the IgG1 surface, is shown in red. B: Sensorgrams obtained from injection of IgG1 (50 nmol/L) over surfaces of immobilized NphHis (solid curve) and IgG1 (stippled curve), respectively. C: Corrected response sensorgrams obtained from injections of NphHis (6 to 50 nmol/L) over a surface of immobilized NphHis, showing the increase in binding response with higher concentrations. Global curve fitting analysis (red curves) yielded the following binding parameters: ka = 2.2 × 105 mol/L−1s−1, kd = 0.0011 seconds−1, KD = 5.0 × 10−9 mol/L. D: Corrected response sensorgrams obtained from injections of NphHis samples (200 nmol/L) in buffer with or without 1 mmol/L of Ca2+, showing a more than twofold increase in binding response in the presence of Ca2+. In all of the sensorgrams the sample injection phase lasted for 300 seconds, and was followed by injection of buffer without sample. Corrected response units represent the observed response after subtraction of the nonspecific background interactions on the background control ligand, IgG1.
Expression of Nephrin Induces Cell-Cell Contacts
Wild-type HEK cells and stable nephrin-expressing transfectants were used to study the effects of nephrin expression on cellular behavior. Wild-type cells not expressing nephrin did not form clusters (Figure 3A) ▶ . In phase contrast microscopy, cell clones with low levels of expression did not significantly differ from parental nontransfected cells with respect to morphology (not shown). In contrast, cell clones with high nephrin expression formed clusters of cells with aggregated morphology (Figure 3B) ▶ . Higher magnification of such aggregated colonies showed localization of nephrin at the cell membrane, especially at the intercellular plasma membranes between nephrin-expressing cells (Figure 3C) ▶ . Immortalized mouse podocytes transfected with a human nephrin construct also revealed an aggregated cell morphology (Figure 3E) ▶ , while the nontransfected counterparts did not cluster (Figure 3D) ▶ .
Figure 3.
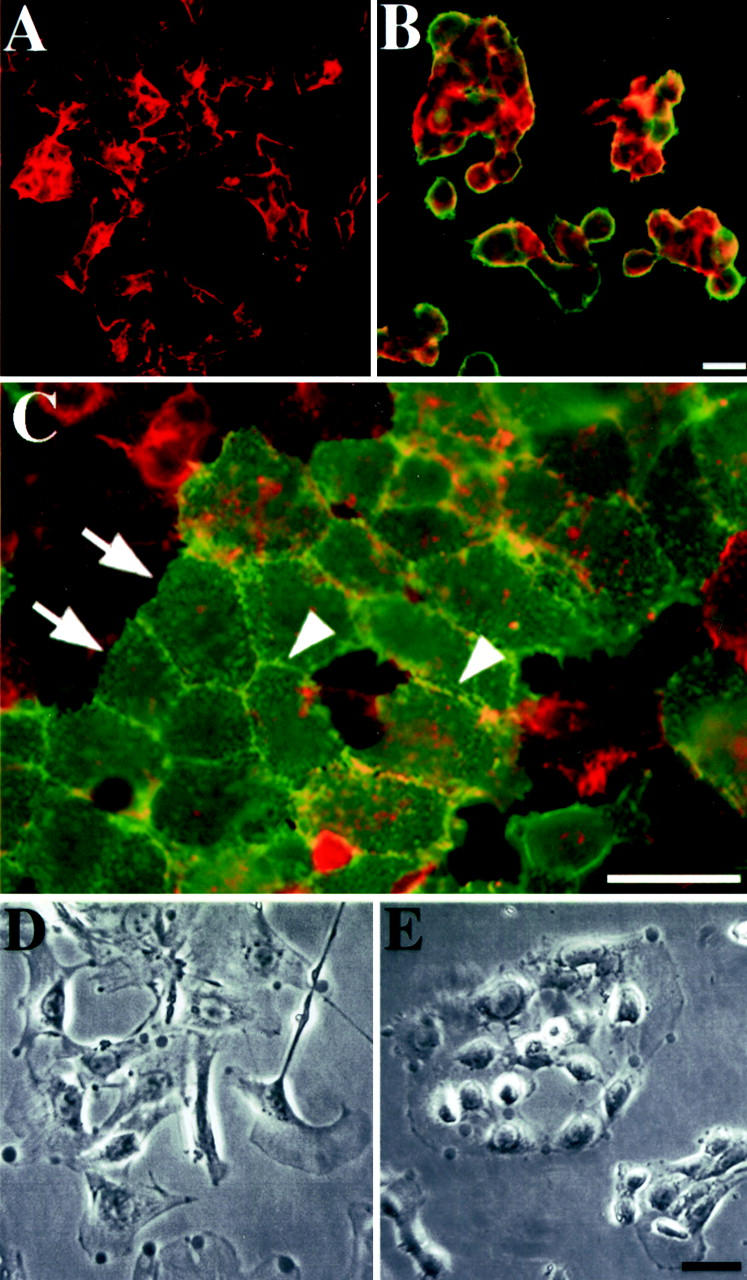
Immunofluorescence microscopy of wild-type HEK293 cells and a stably transfected cell line expressing a full-length human nephrin cDNA construct. Transfected and nontransfected cells were cultured separately (A, B) or together (C) on glass coverslips overnight, fixed, and incubated with rabbit polyclonal anti-nephrin antibodies (pAb1). Cells were double-stained with fluorescein isothiocyanate-conjugated anti-rabbit antibodies for nephrin (green) and TRITC-phalloidin for F-actin (red). A: Wild-type cells staining positive for actin are negative for nephrin. B: Transfected HEK cells with high nephrin expression exhibit aggregated morphology. C: In higher magnification of co-cultured wild-type and transfected cells, the transfectants are distinguished from nontransfected cells by their tight adherence to form flat cell aggregates. Nephrin expression was observed predominantly at intercellular plasma membranes (arrowheads), but hardly at free borders (arrows) or between transfected cells and loosely associated wild-type cells only expressing F-actin. D: Phase contrast microscopy of differentiated wild-type immortalized mouse podocytes. E: When transfected with full-length nephrin cDNA construct, the transfected cell line IMP-NPH1 shows aggregated cell morphology. Scale bars, 10 μm.
We wished to investigate the nephrin-containing contact sites formed between transfected HEK293 cells in more detail. In thin sections cut parallel to the growth substratum, tightly growing cells were seen in EM to have long intercellular contact areas. At numerous places, the nonspecialized normal contact was widened to 40 to 50 nm, containing visible extracellular material that in immuno-EM was associated with immunogold label against N-terminal nephrin (Figure 4A) ▶ . Electron tomography of such areas (Figure 4B) ▶ provided three-dimensional visualization possibilities of the contact sites (Figure 4, C and D) ▶ . In face view, a layer of extracellular strands was typically seen at the site of nephrin label, usually consisting of several immunogold particles (Figure 4C) ▶ . Irregular winding pores of varying size (5 to 10 nm) penetrated the layer. Visualized in cross-section (Figure 4D) ▶ , the layer characteristically formed a horizontal 40- to 50-nm-wide and 20- to 30-nm-thick shelf between the cell membranes. Outside the 40- to 50-nm-wide contact sites, solitary gold particles were seen on the free cell surfaces (Figure 4B) ▶ . Gold label was not found along areas where cells formed usual 10- to 20-nm contact faces lacking visible extracellular material.
Figure 4.
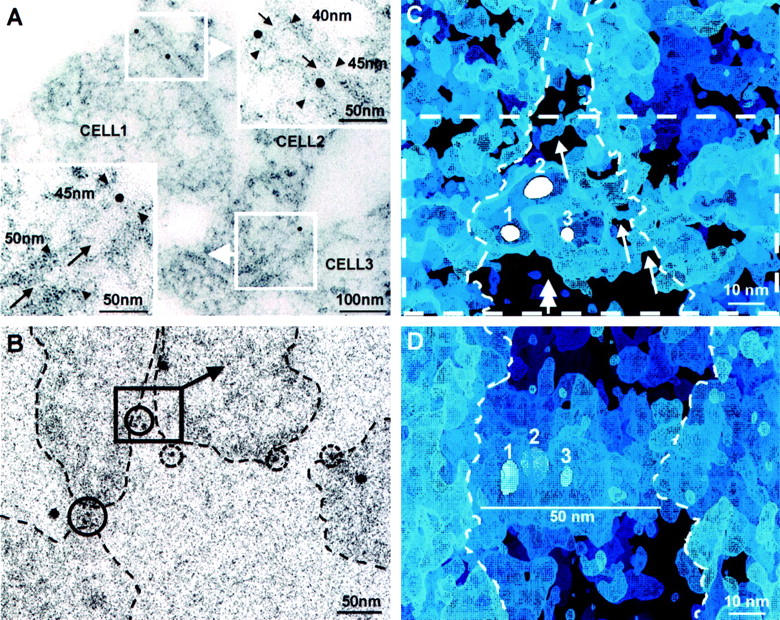
Nephrin-transfected HEK293 cells immunogold-labeled for extracellular nephrin. A: In horizontal EM thin section of a cell monolayer, nephrin immunolabel (10 nm gold) is seen at contacts between three cells (cells 1, 2, and 3). In blow-ups, cell membranes are ∼40 to 50 nm apart at sites of gold label, where also extracellular material is visible (arrows). B: Single low-dose image at 0° tilt from an electron tomography tilt series of sectioned cells as in A. Nephrin label (5-nm immunogold within solid circles) is seen concentrated at cell contact areas and solitarily (broken circles) on free cell surfaces. Cell boundaries marked by dashed lines. Volume chosen for three-dimensional visualization, shown in C, is marked by square. Large 10-nm unmarked gold particles are protein A-gold laid on grids for alignment purposes. C: A digital section of the electron tomography volume marked in B. Three immunogold particles (white spheres 1, 2, and 3, digital sections of gold particles located at different levels) mark sites of immunolabeled nephrin amino-ends in extracellular material between contacting cells. Thin ∼5-nm strands emanating from cell membrane are discernible in the labeled area (arrows). Strands border irregular pores in intercellular material. Dashed rectangle marks volume seen in D, viewed from indicated direction (double-headed arrow). Cell boundaries marked by dashed line as seen in stereo viewing. D: Slanted side-view of volume marked by dashed rectangle in C. A distinct layer of extracellular material bridges the cell boundaries (dashed lines). Immunogold particles (1 to 3) lie within the up to 50-nm wide extracellular layer. Cells cross-cut, growth substratum downward.
Antibodies to the Extracellular Domain of Nephrin Disrupt Cell-Cell Contacts
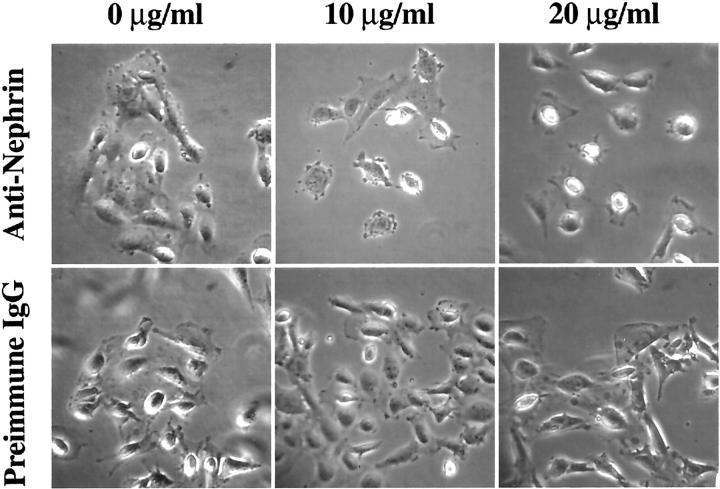
To examine whether nephrin was functionally involved in cell-cell interaction and aggregation, we challenged transfected cells with medium containing increasing concentrations of affinity-purified rabbit IgG directed against the two first extracellular Ig-like motifs of human nephrin. The cells were cultured at 37°C on glass coverslips coated with type I collagen for 4 to 7 days. The cell culture medium was then replaced by prewarmed medium containing different concentrations of pAb1 and incubated for 1 hour. Equal concentrations of preimmune rabbit IgG were also used in a parallel experiment as negative control. The cells were then fixed and studied in immunofluorescence microscopy. Aggregated nephrin-expressing HEK293 cells (Figure 3C) ▶ retracted from each other after addition of the antibodies (data not shown). This phenomenon was also evident in comparison of wild-type and nephrin-expressing IMP cells (Figure 5) ▶ . Addition of preimmune serum to the nephrin-expressing IMP cells did not affect the cell clustering, while addition of the anti-nephrin antibodies resulted in dissociation of the aggregated cells. This effect was also shown to be dependent on the antibody concentration (Figure 5) ▶ , so that with increasing antibody concentration, the transfected IMP cells retracted from each other completely and rounded up.
Figure 5.
Disruption of cell-cell contacts in nephrin-expressing cells by anti-nephrin antibodies. Cultured transfected IMP-Nph1 cells were challenged with medium containing different concentrations of affinity-purified polyclonal antibodies (pAb1) against the first two Ig domains of human nephrin. The cells were incubated for 1 hour and then photographed under a phase contrast microscope. In cells treated with pAb1, intercellular adhesion was disrupted and the cell morphology changed in a concentration-dependent manner. The morphological change was very clear at concentrations 10 and 20 μg/ml of the pAb1 antibody, which resulted in total rounding up of the cells. No change in morphology was seen in cells treated with preimmune rabbit IgG as negative control.
Expression of Podocyte SD Complex Proteins in HEK293 Cells
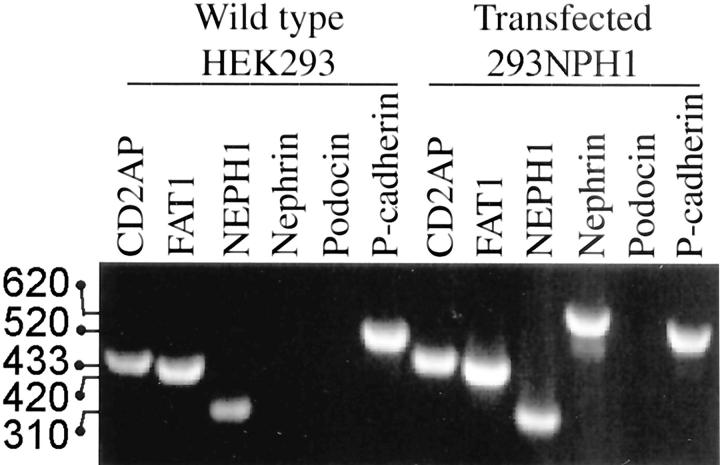
In addition to nephrin, the podocyte SD region contains podocin, CD2AP, P-cadherin, FAT, and NEPH1, that are essential for maintaining a functional SD. To examine if any of those are expressed in the HEK293 cells used in this study, we examined the expression of those by RT-PCR. Gene-specific primers were designed from two exon sequences flanked by at least one intron to ensure that each amplified PCR fragment is corresponding to the size of targeted mRNA and not contaminating genomic DNA. As shown in Figure 6 ▶ , the wild-type HEK293 cells exhibit expression of CD2AP, FAT1, NEPH1, and P-cadherin, but not nephrin or podocin. In the nephrin-transfected HEK293 cells no alterations were observed in the expression of the other genes examined.
Figure 6.
Agarose gel electrophoresis showing expression analysis of genes encoding podocyte SD proteins using one-step RT-PCR. Equal amounts (600 ng) of total RNA isolated from wild-type and nephrin-transfected HEK293 cells were used in each reaction to amplify targeted sequences in each corresponding cDNA. Gene-specific forward and reverse primers were designed from exon sequences separated by at least one intron to ensure that each amplified PCR fragment is corresponding to the size of targeted sequence on each corresponding cDNA. Wild-type HEK293 cells expressed CD2AP, FAT, NEPH1, and P-cadherin, but not podocin or nephrin. In addition to nephrin, the transfected HEK293 cells expressed CD2AP, FAT, NEPH1, and P-cadherin, while no podocin expression was detected. Molecular sizes of PCR fragments corresponding to genes for CD2AP (433 bp), FAT1 (420 bp), NEPH1 (310 bp), nephrin (620 bp), and P-cadherin (520 bp) are indicated.
Discussion
Nephrin is homologous to cell adhesion molecules (CAMs) of the immunoglobulin super family. Several CAMs have been attributed to have diverse functions in cell-cell or cell-matrix interactions. 27 The nature of these interactions can be homophilic, heterophilic, or both. On the basis of its restricted localization at the podocyte SD and its structural similarities to CAMs, nephrin molecules have been proposed to interact with each other in trans configuration and form the backbone of the SD. 8,28 The results of this study provide support for this model and show that such trans interactions occur between the extracellular part of nephrin molecules. First, homophilic interactions were shown to occur between the extracellular parts of nephrin molecules as demonstrated by surface plasmon resonance. Second, mouse podocytes and HEK293 cells, that normally do not express nephrin, were shown to aggregate and form tight contact sites on transfection with full-length nephrin cDNA. The nephrin-containing intercellular structures at the contact sites resembled podocyte SDs seen in vivo. Third, cell-cell contacts between the transfected cells could be dissociated by antibodies reacting with the extracellular domain of nephrin.
The present surface plasmon resonance biosensor studies demonstrated that soluble nephrin molecules can participate in high-affinity homophilic interactions. The fact that this interaction is increased by Ca2+ indicates that nephrin is a Ca2+-binding protein and that the binding of Ca2+ promotes and regulates the homophilic binding properties of nephrin. The binding scenario is likely to be more complex in vivo, where other molecular components of the SD may also interact with nephrin. Beside nephrin, several other proteins have been reported to be located in the SD region. 28-30 Such molecules include ZO-1, CD2AP, podocin, P-cadherin, and FAT, of which the two latter belong to the cadherin superfamily. ZO-1 has long been known for its location in the cytoplasmic vicinity of the SD, and it is most likely involved in signaling events during the modification of cell junctions between podocyte foot processes. 31 CD2AP and podocin have been shown to be intracellular binding partners for nephrin. 18-20,32 Recently, NEPH1, a novel homologue of nephrin, has been reported to be essential for the glomerular filtration barrier, as interruption of its gene in mice leads to foot process effacement and nephrotic syndrome. 33 Like nephrin, NEPH1 is a type-1 transmembrane protein with somewhat shorter extracellular part (five Ig-like domains) and an intracellular part that interacts with podocin. 17 While this article was being revised, two separate groups reported on evidence for homophilic and heterophilic interactions of nephrin and NEPH1, 34,35 thus supporting the results of the present study.
The results of this study clearly demonstrated that nephrin molecules can interact with each other through their extracellular part and that this molecular interaction is not dependent on but rather promoted by Ca2+ ions. The reversibility of this interaction also indicates that the proposed disulfide bonds between the free cysteine residues of the extracellular part of nephrin are not involved in this molecular interaction.
The cell culture results provided further support for nephrin-nephrin interactions, as transfected cell lines with high expression of nephrin formed aggregated cell colonies. The observation that transfected cells with low expression of nephrin did not show aggregated cell morphology indicates that high local levels of nephrin at the plasma membrane are needed to promote tight cell-cell interaction. Importantly, it was shown that wild-type HEK293 cells express key components of the podocyte SD, ie, CD2AP, P-cadherin, FAT, and NEPH1. Yet, clustering and formation of cell-cell junctions first occurred on transfection and expression of nephrin. This implies that nephrin is crucial for junction formation. Considering that isolated nephrin molecules self-associate, such junction formation is likely to reflect homophilic binding between nephrin molecules on adjacent cells, although heterophilic binding with endogenous NEPH1 cannot be excluded. Our electron microscopic analysis revealed that the spacing between cells at the nephrin-containing contact sites had similar dimensions as podocyte slits in vivo. 2 This further suggests that nephrin molecules determine the width of the SD in vivo. Electron tomography, is a relatively new imaging method that enables detailed three-dimensional structural analysis of individual cellular structures and macromolecular complexes in situ. 36,37 When applied here to the nephrin-containing cell-cell junctions between transfected cells, this method revealed that these contact sites include an anti-nephrin-labeled layer of strands forming a porous structure similar to that seen in SD in vivo by conventional EM 2,5-7 and by electron tomography (our unpublished results).
The results of this work also provided additional support for a direct role of nephrin in cell-cell interactions, as the cell-cell junctions observed in nephrin-expressing cells could be dissociated by anti-nephrin antibodies. Such outside-in signaling could be mediated through tyrosine phosphorylation, resulting in a rapid disruption of intercellular junctions. In fact, such nephrin-mediated outside-in signaling may have a major role in some acquired kidney diseases, especially in patients having anti-nephrin antibodies in their blood circulation, 38 or in experimental nephrosis in rat models. 31,39,40 Hence, the localization of nephrin at the SD is crucial for maintaining proper foot process morphology and both width and structure of the SD. The intimate relationship between the actin cytoskeleton, nephrin, CD2AP, and podocin that has recently been shown, 18-20 suggests that any interference with nephrin adhesion could lead to reorganization of the cortical F-actin and withdrawal of foot processes with consequent proteinuria. The present results indicate that nephrin-expressing cells can provide a useful in vitro model for studies on the podocyte SD.
Acknowledgments
We thank Dr. Peter Mundel (Albert Einstein College of Medicine, Bronx, NY) for kindly providing us with the immortalized mouse podocyte cell line; the staff of the Electron Microscopy Unit, Institute of Biotechnology, University of Helsinki, for skillful technical help; and Eetu Mäkelä for contribution in image processing.
Footnotes
Address reprint requests to Karl Tryggvason, M.D., Ph.D., Division of Matrix Biology, Department of Medical Biochemistry and Biophysics, Karolinska Institutet, SE-171 77 Stockholm, Sweden. E-mail: karl.tryggvason@mbb.ki.se.
Supported by the Swedish Research Council (projects 05200 and 11545), the Swedish Cancer Foundation (project 4720), Polysackaridforskning AB, the Novo Nordisk Foundation, the Sigrid Jusélius Foundation, the National Institutes of Health (grant DK-54724), and the European Union (grants BIO4-CT97-2364 and BIO4-CT96-0099).
Present address for J. K.: Department of Medicine, Division of Nephrology and Hypertension, Vanderbilt University Medical Center, S-3223 Medical Center North, 1161 21st Avenue South, Nashville, TN 37232-2372.
References
- 1.Tisher CC, Madsen KM: Anatomy of the kidney. Brenner BM eds. Brenner and Rector’s The Kidney ed 6 2000:pp 3-67 WB Saunders Company Philadelphia
- 2.Rodewald R, Karnovsky MJ: Porous substructure of the glomerular slit diaphragm in the rat and mouse. J Cell Biol 1974, 60:423-433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khoshnoodi J, Tryggvason K: Congenital nephrotic syndromes. Curr Opin Genet Dev 2001, 11:322-327 [DOI] [PubMed] [Google Scholar]
- 4.Kestilä M, Lenkkeri U, Männikkö M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K: Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell 1998, 1:575-582 [DOI] [PubMed] [Google Scholar]
- 5.Ruotsalainen V, Ljungberg P, Wartiovaara J, Lenkkeri U, Kestilä M, Jalanko H, Holmberg C, Tryggvason K: Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci USA 1999, 96:7962-7967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holzman LB, St. John PL, Kovari IA, Verma R, Holthöfer H, Abrahamson DR: Nephrin localizes to the slit pore of the glomerular epithelial cell. Kidney Int 1999, 56:1481-1491 [DOI] [PubMed] [Google Scholar]
- 7.Holthöfer H, Ahola H, Solin M-L, Wang S, Palmen T, Luimula P, Miettinen A, Kerjaschki D: Nephrin localizes at the podocyte filtration slit area and is characteristically spliced in the human kidney. Am J Pathol 1999, 155:1681-1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tryggvason K: Unraveling the mechanisms of glomerular ultrafiltration: nephrin, a key component of the slit diaphragm. J Am Soc Nephrol 1999, 10:2440-2445 [DOI] [PubMed] [Google Scholar]
- 9.Holmberg C, Jalanko H, Tryggvason K, Rapola J: Congenital nephrotic syndrome. Barratt TM Avner ED Harmon WE eds. Pediatric Nephrology, ed 4 1999:pp 765-777 Lippincott Williams & Wilkins Baltimore
- 10.Lenkkeri U, Männikkö M, McCready P, Lamerdin J, Gribouval O, Niaudet PM, Antignac CK, Kashtan E, Holmberg C, Olsen A, Kestilä M, Tryggvason K: Structure of the gene for congenital nephrotic syndrome of the Finnish type (NPHS1) and characterization of mutations. Am J Hum Genet 1999, 64:51-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan K, Khoshnoodi J, Ruotsalainen V, Tryggvason K: N-linked glycosylation is critical for the plasma membrane localization of nephrin. J Am Soc Nephrol 2002, 13:1385-1389 [DOI] [PubMed] [Google Scholar]
- 12.Ruotsalainen V, Patrakka J, Tissari P, Reponen P, Hess M, Kestilä M, Holmberg C, Salonen R, Heikinheimo M, Wartiovaara J, Tryggvason K, Jalanko H: Role of nephrin in cell junction formation in human nephrogenesis. Am J Pathol 2000, 157:1905-1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Putaala H, Soininen R, Kilpeläinen P, Wartiovaara J, Tryggvason K: The murine nephrin gene is specifically expressed in kidney, brain and pancreas: inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet 2001, 10:1-8 [DOI] [PubMed] [Google Scholar]
- 14.Shih N-Y, Li J, Karpitskii V, Nguyen A, Dustin ML, Kanagawa O, Miner JH, Shaw AS: Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 1999, 286:312-315 [DOI] [PubMed] [Google Scholar]
- 15.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler M-C, Niaudet P, Antignac C: NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 2000, 24:349-354 [DOI] [PubMed] [Google Scholar]
- 16.Mitchell KJ, Pinson KI, Kelly OG, Brennan J, Zupicich J, Scherz P, Leighton PA, Goodrich LV, Lu X, Avery BJ, Tate P, Dill K, Pangilinan E, Wakenight P, Tessier-Lavigne M, Skarnes WC: Functional analysis of secreted and transmembrane proteins critical to mouse development. Nat Genet 2001, 28:241-249 [DOI] [PubMed] [Google Scholar]
- 17.Sellin L, Huber TB, Gerke P, Quack I, Pavenstädt H, Walz G: NEPH1 defines a novel family of podocin-interacting proteins. EMBO J 2003, 17:115-117 [DOI] [PubMed] [Google Scholar]
- 18.Shih NY, Li J, Cotran R, Mundel P, Miner JH, Shaw AS: CD2AP localizes to the slit diaphragm and binds to nephrin via a novel C-terminal domain. Am J Pathol 2001, 159:2303-2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saleem MA, Ni L, Witherden I, Tryggvason K, Ruotsalainen V, Mundel P, Mathieson PW: Co-localisation of nephrin, podocin and the actin cytoskeleton. Evidence for a role in podocyte foot process formation. Am J Pathol 2002, 161:1459-1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwarz K, Simons M, Reiser J, Saleem MA, Faul C, Kriz W, Shaw AS, Holzman LB, Mundel P: Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J Clin Invest 2001, 108:1621-1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu L, Cotta Doné S, Khoshnoodi J, Bertorello A, Wartiovaara J, Berggren P-O, Tryggvason K: Defective nephrin trafficking caused by missense mutations in the NPHS1 gene: insight into the mechanisms of congenital nephrotic syndrome. Hum Mol Genet 2001, 10:2637-2644 [DOI] [PubMed] [Google Scholar]
- 22.Mundel P, Reiser J, Zuniga Mejia Borja A, Pavenstadt H, Davidson GR, Kriz W, Zeller R: Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 1997, 236:248-258 [DOI] [PubMed] [Google Scholar]
- 23.Sigmundsson K, Másson G, Rice R, Beauchemin N, Öbrink B: Determination of active concentrations and association/dissociation rate constants of interacting biomolecules: an analytical solution to the theory for kinetic and mass transport limitations in biosensor technology and its experimental verification. Biochemistry 2002, 41:8263-8276 [DOI] [PubMed] [Google Scholar]
- 24.Skoglund U, Öfverstedt L-G, Burnet RM, Bricogne G: Maximum-entropy three-dimensional reconstruction with deconvolution of the contrast transfer function: a test application with adenovirus. J Struct Biol 1996, 117:173-188 [DOI] [PubMed] [Google Scholar]
- 25.Miralles F, Öfverstedt L-G, Sabri N, Aissouni Y, Hellman U, Skoglund U, Visa N: Electron tomography reveals posttranscriptional binding of pre-mRNPs to specific fibers in the nucleoplasm. J Cell Biol 2000, 148:271-282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karlsson R, Michaelsson A, Mattsson L: Kinetic analysis of monoclonal antibody-antigen interactions with a new biosensor based analytical system. J Immunol Methods 1991, 145:229-240 [DOI] [PubMed] [Google Scholar]
- 27.Brummendorf T, Rathjen FG: Cell adhesion molecules 1: immunoglobulin superfamily. Protein Profile 1995, 2:963-1108 [PubMed] [Google Scholar]
- 28.Khoshnoodi J, Tryggvason K: Unraveling the molecular make-up of the glomerular podocyte slit diaphragm. Exp Nephrol 2001, 9:355-359 [DOI] [PubMed] [Google Scholar]
- 29.Reiser J, Von Gersdorff G, Simons M, Schwarz K, Faul C, Giardino L, Heider T, Loos M, Mudel P: Novel concepts in understanding and management of glomerular proteinuria. Nephrol Dial Transplant 2002, 17:951-955 [DOI] [PubMed] [Google Scholar]
- 30.Mundel P, Shankland SJ: Podocyte biology and response to injury. J Am Soc Nephrol 2002, 13:3005-3015 [DOI] [PubMed] [Google Scholar]
- 31.Kurihara H, Anderson JM, Farquhar MG: Increased Tyr phosphorylation of ZO-1 during modification of tight junctions between glomerular foot processes. Am J Physiol 1995, 268:F514-F524 [DOI] [PubMed] [Google Scholar]
- 32.Roselli S, Boute N, Sich M, Benessy F, Attie T, Gubler MC, Antignac C: Podocin localizes in the kidney to the slit diaphragm area. Am J Pathol 2002, 160:131-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donoviel DB, Freed DD, Vogel H, Potter DG, Hawkins E, Barrish JP, Mathur BN, Turner CA, Geske R, Montgomery CA, Starbuck M, Brandt M, Gupta A, Ramirez-Solis R, Zambrowicz BP, Powell DR: Proteinuria and prenatal lethality in mice lacking NEPH1, a novel protein with homology to nephrin. Mol Cell Biol 2001, 21:4829-4836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerke P, Huber TB, Sellin L, Benzing T, Walz G: Homodimerization and heterodimerization of the glomerular podocyte proteins nephrin and NEPH1. J Am Soc Nephrol 2003, 14:918-926 [DOI] [PubMed] [Google Scholar]
- 35.Barletta GM, Kovari IA, Verma RK, Kerjaschki D, Holzman LB: Nephrin and Neph1 co-localize at the podocyte foot process intercellular junction and form cis hetero-oligomers. J Biol Chem 2003, 278:19266-19271 [DOI] [PubMed] [Google Scholar]
- 36.McEwen BF, Marko M: The emergence of electron tomography as an important tool for investigating cellular ultrastructure. J Histochem Cytochem 2001, 49:553-563 [DOI] [PubMed] [Google Scholar]
- 37.Medalia O, Weber I, Frangakis AS, Nicastro D, Gerisch G, Baumeister W: Macromolecular architecture in eukaryotic cells visualized by cryoelectron tomography. Science 2002, 298:1209-1213 [DOI] [PubMed] [Google Scholar]
- 38.Patrakka J, Ruotsalainen V, Reponen P, Qvist E, Laine J, Holmberg C, Tryggvason K, Jalanko H: Recurrence of nephrotic syndrome in kidney grafts of patients with congenital nephrotic syndrome of the Finnish type: role of nephrin. Transplantation 2002, 73:394-403 [DOI] [PubMed] [Google Scholar]
- 39.Orikasa M, Matsui K, Oite T, Shimizu F: Massive proteinuria induced in rats by a single intravenous injection of a monoclonal antibody. J Immunol 1988, 141:807-814 [PubMed] [Google Scholar]
- 40.Topham PS, Kawachi H, Haydar SA, Chugh S, Addona TA, Charron KB, Holzman LB, Shia M, Shimizu F, Salant DJ: Nephritogenic mAb 5-1-6 is directed at the extracellular domain of rat nephrin. J Clin Invest 1999, 104:1559-1566 [DOI] [PMC free article] [PubMed] [Google Scholar]