Abstract
Epithelial progenitor cells in skin give rise to multiple lineages, comprising the hair follicle, an associated sebaceous gland, and overlying epidermis; however, the signals that regulate sebocyte development are poorly understood. We tested the potential involvement of the Hedgehog pathway in sebaceous gland development using transgenes designed to either block or stimulate Hedgehog signaling in cutaneous keratinocytes in vivo. Whereas inhibition of the Hedgehog pathway selectively suppressed sebocyte development, Hedgehog pathway activation led to a striking increase both in size and number of sebaceous glands. Remarkably, ectopic Hedgehog signaling also triggered the formation of sebaceous glands from footpad epidermis, in regions normally devoid of hair follicles and associated structures. These ectopic sebaceous glands expressed molecular markers of sebocyte differentiation and were functional, secreting their contents directly onto the skin’s surface instead of into a hair canal. The Hedgehog pathway thus plays a key role in sebocyte cell fate decisions and is a potential target for treatment of skin disorders linked to abnormal sebaceous gland function, such as acne.
Epithelial progenitor cells in skin give rise to epidermis as well as the epithelial component of skin appendages, including hair follicles and associated sebaceous glands. 1,2,3 Early-stage follicles consist of an epithelial thickening, called a placode, and an adjacent mesenchymal condensate in the developing dermis. 4 The follicle epithelium grows downward through the dermis and into the subcutaneous fat, where it surrounds the condensate-derived hair follicle papilla to form the hair bulb. Rapidly proliferating matrix cells in the hair bulb give rise to the hair shaft and inner root sheath lineages, which are driven upward toward the skin’s surface during hair maturation. Surrounding these cell layers is the follicle outer root sheath, which is continuous with the interfollicular epidermis and contains epithelial stem cells in a region called the bulge. 5,6 The final differentiated cell type to appear in the developing follicle is the oil-rich sebocyte, which arises from cells within the superficial hair follicle. 7 Over the course of several days, the expanding pool of sebocytes forms a gland located outside of the hair follicle, with sebocytes releasing their contents into the hair canal not far below the skin’s surface.
Proper hair follicle development is dependent on a series of inductive signals traveling between epithelial and mesenchymal follicle progenitors. 8 Recent studies have begun to identify some of the molecules regulating follicle morphogenesis and cell fate, and similar to many other organ systems, hair follicles use both the Wnt and Hedgehog pathways to guide their assembly. Wnt signaling regulates follicle initiation and reactivation of follicle growth during postnatal hair cycling. 9-12 At later stages of follicle maturation, the Wnt pathway also plays an important role in terminal differentiation of hair lineages. 13,14 Hedgehog signaling plays a complementary role, as it is essential for the proliferative expansion of hair follicle epithelium but is not required during follicle initiation or hair lineage differentiation. 15-17
Given the involvement of Hedgehog proteins in regulating cell lineage specification in several other organs, 18 we tested the potential involvement of this pathway in cell fate decisions in skin using both loss-of-function and gain-of-function transgenic mouse models. We find that inhibition of Hedgehog signaling in cutaneous keratinocytes selectively blocks the formation of sebocytes. In contrast, ectopic activation of the Hedgehog pathway promotes sebocyte development, even in regions that normally do not contain hair follicles or associated sebaceous glands. Our findings strongly implicate Hedgehog signaling in sebocyte cell fate decisions, and suggest that modulation of the Hedgehog pathway may provide a novel means of regulating sebaceous gland function.
Materials and Methods
Construction and Breeding of Transgenic Mice
Because Hedgehog signaling modulates gene expression via Gli proteins, 19-21 we generated transgenic mice expressing a skin-targeted dominant-negative Gli mutant designed to block Hedgehog responsiveness in cutaneous epithelium. We produced a Gli2ΔC4 cDNA 22 by digesting pcDNA3.1Flag-Gli2 (kindly provided by Drs. Hiroshi Sasaki and Chi-chung Hui) with ApaI, adding a fragment containing a stop codon flanked by ApaI sites, and re-ligating. Gli2ΔC4 cDNA was released from pcDNA3.1 using PmeI, and subcloned into the SnaBI site of the bovine K5 cassette 23 to generate K5-Gli2ΔC4. All subcloning was verified by sequencing. The insert was purified and injected into C57BL/6 × SJL F2 mouse eggs by the University of Michigan Transgenic Core. Five transgenic founders were produced, and further characterization of these mice and their progeny will be described in detail elsewhere. Studies reported in this manuscript were performed using F1 litters from crosses with C57BL/6J breeders (Jackson Laboratories, Bar Harbor, ME).
We modified the K5 transgenic cassette 23 to enable Cre-mediated expression of M2SMO, which encodes a constitutively activated form of the hedgehog signaling effector SMO, 24 in skin. A fragment containing an EGFP cDNA with bovine growth hormone polyA signal, flanked by loxP sites in reverse orientation, was inserted into the NotI site of the K5 transgenic cassette. M2SMO cDNA 24 was subcloned into the NheI site, yielding a construct with the following elements: bovine K5 promoter, rabbit β-globin intron, loxP, EGFP, bovine growth hormone polyA, loxP, M2SMO, and 2xSV40 polyA, which we designated K5-flxGFP-M2SMO. Eight transgenic founders were produced and several mouse lines established, and these will be characterized in a subsequent publication. To activate M2SMO expression, K5-flxGFP-M2SMO mice were crossed with either K5-Cre or K5-CreERT2 25 mice to generate double-transgenic progeny. Whereas recombinase activity in mice harboring the K5-CreERT2 transgene was previously shown to be dependent on treatment with 4-hydroxytamoxifen, 25 low-level recombination took place in untreated double-transgenic mice described in this report see (Figure 2I ▶ ). This most likely reflects the variable sensitivity of different floxed alleles to Cre-mediated recombination. 26 All mice were housed and maintained according to University of Michigan institutional guidelines.
Figure 2.
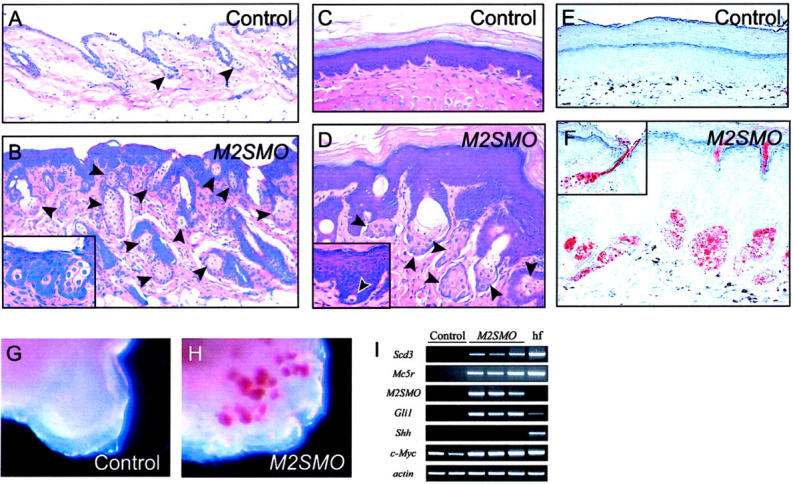
Enhanced activation of Shh signaling in keratinocytes leads to formation of hyperplastic and ectopic sebaceous glands. A and B: H&E staining of control and M2SMO-expressing dorsal mouse skin at 6.5 months of age. Note small size of normal sebaceous glands in control skin (arrowheads in A) compared with increased number and size of sebaceous glands in transgenic skin (arrowheads in B). In addition to glandular structures within the dermis, individual sebocytes or small aggregates are also found ectopically within the epidermis (inset in B). C and D: H&E staining of volar paw skin from control and transgenic mice at 13 months of age. Note thickened epidermis and cornified cell layers typically seen in skin from this region, with a conspicuous absence of hair follicles and sebaceous glands (C). Ectopic sebaceous glands (arrowheads in D) develop from volar epidermis of mice expressing M2SMO. Sebaceous gland development is initiated at the base of epidermal downgrowths (inset in D). E and F: Oil Red O staining for lipids in control and transgenic volar skin. As expected, no staining is detected in control skin in E, whereas multiple sebocytes are stained in transgenic mouse skin in F. Many of the ectopic sebaceous glands secrete Oil Red O-positive material directly onto the skin’s surface (see inset). G and H: Whole-mount analysis of footpads from control and transgenic mice. A large number of Oil Red O-positive sebaceous glands are detected in transgenic (H) but not control (G) footpad. I: Semiquantitative RT-PCR analysis showing expression of sebocyte markers (Scd3, Mc5r) in volar skin from M2SMO-expressing transgenic mice (M2SMO) and control skin containing hair follicles (hf), but not in control volar skin. Transgene-specific primers confirm expression of M2SMO in transgenic samples, which also contain elevated levels of the Shh target gene Gli1.
Tissue Harvesting and Oil Red O Staining
For hematoxylin and eosin (H&E) staining, we fixed skin overnight in neutral-buffered formalin, transferred to 70% EtOH, processed, and embedded in paraffin. Skin was also embedded in Optimum Cutting Temperature compound for frozen sections. Oil Red O staining was performed using a modification of a protocol kindly provided by Dr. Karin Müller-Decker (Deutsches Krebsforschungszentrum, Heidelberg, Germany). Frozen sections were fixed in 1% neutral-buffered formalin for 5 minutes, washed in deionized water, and incubated in 60% isopropanol for 5 minutes. Sections were stained with filtered Oil Red O working solution, prepared immediately before use by making a 6:4 mixture of stock (0.5% Oil Red O in 99% isopropanol) and deionized water. Sections were transferred to 60% isopropanol, washed in deionized water, counterstained using hematoxylin, and mounted using 50% glycerol in PBS. A similar protocol was used for Oil Red O staining of whole-mounts, except samples were subsequently washed and stored in deionized water.
Semiquantitative RT-PCR
We isolated RNA from skin lysates prepared using Trizol (Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions. Semiquantitative reverse-transcription-polymerase chain reaction (RT-PCR) was performed using 1-μg samples of total RNA for first-strand DNA synthesis (Superscript II RT kit, Invitrogen). Primers were selected to span an intron whenever possible, or PCR was performed in the absence of reverse transcriptase to rule out the possibility that amplification products were derived from contaminating DNA. PCR conditions are available on request; the following primers were used: actin (421 bp) forward 5′-TACCACAGGCATTGTGATGGA-3′, reverse 5′-CAACGTCACACTTCATGATGG-3′ 27 ; c-Myc (548 bp) forward 5′-AGTGCATTGATCCCTCAGTGGTCTTTCCCTA-3′, reverse 5′-CAGCTCGTTCCTCCTCTGACGTTCCAAGACGTT-3′; Gli1 (364 bp) forward 5′-GTCGGAAGTCCTATTCACGC-3′, reverse 5′-CAGTCTGCTCTCTTCCCTGC-3′; M2SMO (435 bp) forward 5′-AAGCGGATCAAGAAGAGCA-3′, reverse 5′-GAGGCAGTCGAGGAATGGTA-3′; Mc5r (490 bp) forward 5′-AAATCCGATGCCAAGAAGTG-3′, reverse 5′-GGTAGCGCAAGGCATAGAAG-3′; Scd3 (809 bp) forward 5′-CTTGGATAACCACCCTGGGTG-3′, reverse 5′-CTCCTCTGGAACATCACCAGCTTC-3′; 28 Shh (241 bp)forward 5′-TCTGTGATGAACCAGTGGCC-3′, reverse 5′-GCCACGGAGTTCTCTGCTTT-3′. 29
Results
Sonic hedgehog (Shh) is produced and secreted by developing hair follicle keratinocytes and activates signaling both in the follicular epithelium and mesenchyme. 30,31 Gli proteins mediate transcriptional responses to Hedgehog family members, 19,20 and the Gli2ΔC4 transactivation-domain mutant blocks Gli function in a dominant-negative manner. 22 To inhibit Hedgehog signaling selectively in cutaneous epithelium, we used the bovine K5 promoter 23 to generate K5-Gli2ΔC4 transgenic mice. The K5 promoter is active in the epidermal basal layer, embryonic hair follicle progenitor cells, and follicle outer root sheath, including the bulge region which harbors multipotent stem cells. 5,6
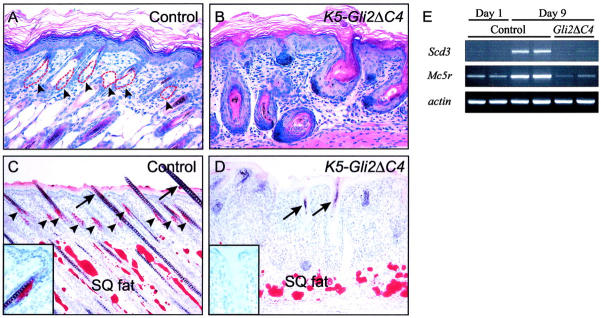
Hair follicles in K5-Gli2ΔC4 mice were shorter than in control littermates but expressed multiple markers for epidermal and hair follicle cell lineages (H. Sheng et al, manuscript in preparation). However, while 7-day-old control dorsal skin contained numerous hair follicle-associated sebaceous glands (Figure 1A) ▶ , these structures were rarely seen in dorsal skin from K5-Gli2ΔC4 littermates (Figure 1B) ▶ . Sebaceous glands were also deficient in transgenic mice at 13 days of age (not shown), but analysis at later times was not possible because of impaired viability of these mice. Staining with Oil Red O to identify lipids revealed subcutaneous adipose tissue in both control and K5-Gli2ΔC4 skin, but sebaceous glands were conspicuously absent in the transgenic skin samples (Figure 1, C and D) ▶ . Extensive sebaceous gland development normally occurs during the perinatal period, reflected by the accumulation of transcripts encoding the sebocyte markers Scd3 28 and Mc5r 32 between day 1 and 9 in control skin (Figure 1E) ▶ . In skin from 9-day-old K5-Gli2ΔC4 mice, however, Scd3 mRNA was nearly undetectable and Mc5r transcripts were present at levels comparable to day 1. In retrospect, there was also a selective deficiency of sebaceous glands in Shh mutant skin allowed to mature on immunodeficient hosts. 15,16 Since multiple markers for epidermal and hair follicle lineages are detected both in Shh mutant 15,16 and K5-Gli2ΔC4 skin, Shh/Gli signaling appears to be specifically required for sebocyte development.
Figure 1.
Dominant-negative inhibition of Gli activity blocks sebocyte development. A and B: H&E staining of skin from 7-day-old control and K5-Gli2ΔC4 transgenic mice. Clusters of sebocytes are seen associated within the superficial portion of control hair follicles (dashed red lines and arrowheads in A, but not in aberrant hair follicles arising in transgenic skin in B). C and D: Oil Red O staining for lipids. Note the presence of large, amorphous masses of adipocytes staining in subcutaneous fat layer (SQ fat) in both the control (C) and transgenic (D) skin sections. Staining of sebaceous glands is limited to control skin (inset and arrowheads in C). Despite the reduced length of follicles in K5-Gli2ΔC4 mice (compare B and D with A and C), the transgenic hair follicles express multiple markers for hair follicle lineages (in preparation) and assemble abortive, pigmented hair shafts (arrows in D). E: Semiquantitative RT-PCR for sebocyte markers reveals up-regulation of Scd3 and Mc5r in control skin between postnatal day 1 and day 9, reflecting expansion of the sebocyte lineage during this time. Expression of transcripts encoding sebocyte markers in skin from day 9 K5-Gli2ΔC4 samples (Gli2ΔC4) was similar to that of day 1 control samples.
Shh acts on target cells by inhibiting the function of its receptor Ptch, which normally represses the signal transducer Smo. 33 To expand the pool of keratinocytes in which the Shh pathway is active, we generated K5 promoter-driven mice using a gain-of-function SMO mutant designated M2SMO, which is largely resistant to inhibition by Ptch. 34 We bypassed the perinatal lethality previously reported in K5-M2SMO mice 24 by modifying the K5 transgenic cassette to enable Cre-dependent induction of M2SMO, as described in the Materials and Methods. K5 promoter-driven M2SMO expression resulted in an increase in both the number and size of sebaceous glands in haired skin (Figure 2, A and B) ▶ . The appearance of ectopic sebocytes within the epidermis (Figure 2B ▶ , inset) suggested that Hedgehog signaling could act as a switch directing competent keratinocytes to enter the sebocyte lineage. To further explore this possibility, we examined regions of volar skin that are normally devoid of hair follicles or sebaceous glands. In striking contrast to controls, volar skin from M2SMO-expressing mice was thicker and contained numerous, well-formed ectopic sebocytes (Figure 2, C and D) ▶ . Much of the adjacent epidermis contained typical keratinocytes, suggesting that only a subset of epidermal cells was capable of forming sebocytes in response to M2SMO. Intriguingly, during early stages of development, ectopic sebocytes arose from cells at the base of epidermal invaginations (Figure 2D ▶ , inset), one of the sites where epidermal stem cells have been proposed to reside in non-hairy skin. 35
The ectopic sebocytes in M2SMO-expressing volar skin stained with Oil Red O and were frequently organized into glands (Figure 2, E and F) ▶ . In the absence of hair follicles, many of these glands released their contents into ducts leading directly to the skin’s surface (Figure 2F ▶ , inset), instead of into hair canals. Thus, the ectopic sebocytes exhibited a remarkable degree of autonomy with regard to assembly into glandular structures with rudimentary ducts. To better assess the extent of ectopic sebaceous gland development, footpads were removed from control and M2SMO transgenic mice, stained with Oil Red O, and examined as whole mounts. Large numbers of ectopic, Oil Red O-positive sebaceous glands were detected in footpads from M2SMO transgenic mice, but not controls (Figure 2, G and H) ▶ . Semiquantitative RT-PCR analysis confirmed expression of sebocyte markers in volar skin samples from M2SMO-expressing mice but not controls (Figure 2I) ▶ . As expected, the transgenic samples contained M2SMO mRNA and elevated levels of the Shh target gene Gli1, confirming enhanced Hedgehog pathway activity. M2SMO samples did not contain detectable levels of Shh mRNA, arguing that the appearance of sebocytes in transgenic skin was not the result of up-regulated Shh expression. In light of recent studies documenting enhanced sebocyte development in mice expressing MYC in skin, 36,37 it is noteworthy that c-Myc expression appeared to be up-regulated in volar skin of M2SMO mice, compared to controls.
Discussion
The results of our studies strongly implicate Hedgehog signaling in the development of sebocytes from competent progenitor cells. Although sebaceous glands are normally derived from hair follicles, ectopic Hedgehog signaling leads to sebocyte formation within the epidermis of hairy skin, as well as hairless volar skin. Previous work using hairless skin 38,39 or even corneal epithelium 40 has demonstrated the presence of multipotent progenitor cells capable of giving rise to hair follicle and sebocyte cell lineages. However, these studies entailed the use of tissue or cell-suspension recombinants, and did not identify molecular signals that determine the fates of progenitor cells. The appearance of sebaceous glands in response to ectopic M2SMO expression in keratinocytes points to the Hedgehog pathway as a critical determinant of sebocyte cell fate. This concept is further strengthened by the finding that formation of sebocytes, but not other cutaneous epithelial cell types, is inhibited by blocking Gli protein function in K5-Gli2ΔC4 mice. Normal sebaceous gland development is thus likely to be controlled, at least in part, by expression of Shh and downstream targets in developing hair germs but not epidermis. 30,31 M2SMO can bypass this requirement, even in hairless skin, by activating Hedgehog signaling in a ligand-independent manner. 41
Sebaceous gland hyperplasia is also occasionally seen in other mouse models with deregulated Hedgehog signaling in skin, including K5-Gli1 42 and K5-Gli2 mice 43 (data not shown). However, additional studies are needed to define the precise conditions required for Hedgehog-pathway-driven formation of this specialized cell type. Interestingly, ectopic expression of Shh in embryonic mouse skin is sufficient to drive the formation of basal cell carcinoma-like proliferations but not sebaceous glands, 44 in keeping with studies suggesting that both the timing 45 and level 46 of Hedgehog signaling activity are important determinants of skin phenotype. In addition to regulating normal sebaceous gland development, it will be interesting to ascertain whether Hedgehog signaling is involved in the formation of sebaceous gland tumors and hyperplasias.
In contrast to its role in sebocyte development, Shh is not required for terminal differentiation of hair lineages, 15,16 which is controlled by members of the Wnt signaling pathway. 1,2 Moreover, inhibition of Wnt target genes using a dominant-negative Lef-1 promotes sebocyte development while inhibiting differentiation of hair lineages. 13,14 Taken together with these findings, our data raise the interesting possibility that sebocyte cell fate is governed by the relative levels of stimulatory (Hedgehog) and inhibitory (Wnt) signals acting on multipotent progenitors. Further studies will be required to test this hypothesis, and to ascertain whether the effects of mesenchymal cell types on sebaceous gland development 47,48 can be attributed to modulation of either of these developmental signaling pathways. The potential influence of cross-talk involving Hedgehog and Wnt 49 or BMP 50 pathways will also need to be examined. In addition, it will be important to explore the relationship between the Hedgehog pathway and other molecules implicated in sebocyte development, including c-Myc, 36,37 PPARγ, 51,52 and COX-2. 53
Hair follicles and sebaceous glands both undergo cyclic changes after birth, 54 and Shh plays a major role in regulating proliferation of follicle epithelium during times of active growth. 17 Thus, in addition to its involvement in the initiation of sebaceous gland formation, the Hedgehog pathway is also likely to play a role in postnatal function of sebaceous glands. Acne is the most common skin disease in humans, and in its most severe forms it can lead to significant disfigurement and emotional distress. 55 Although a number of factors contribute to the development of acne, there is compelling evidence that oil-producing sebaceous glands play a central role in the pathogenesis of this disease. 56 Agents aimed at inhibiting Hedgehog signaling may thus provide a novel means of reducing sebaceous gland activity and thereby improving acne. In keeping with this possibility, retinoids used in the treatment of acne can inhibit sebocyte differentiation, 57 and have also been shown to reduce Gli transcriptional activity in cultured keratinocytes. 58
Note Added in Proof
While this manuscript was under review, a report was published implicating Indian hedgehog in the proliferation of sebocyte progenitors (Niemann C, Unden AB, Lyle S, Zouboulis CC, Toftgard R, Watt FM: Indian hedgehog and b-catenin signaling: role in the sebaceous lineage of normal and neoplastic mammalian epidermis. Proc Natl Acad Sci USA 2003, 100(Suppl 1):11873–11880).
Acknowledgments
We thank Drs. Hiroshi Sasaki, Chi-chung Hui, and Fred de Sauvage for reagents; Drs. Bruce Morgan, Sarah Millar, Deb Gumucio, Sean Morrison, Steve Prouty, and Ulrike Lichti for helpful discussions. Transgenic mice were generated by the Transgenic Animal Model Core (Mark Berard and Thomas Saunders) of the University of Michigan’s Biomedical Research Core Facilities.
Footnotes
Address reprint requests to Andrzej A. Dlugosz, M.D., U-M Cancer Center/Dermatology, 3310 CCGC, Box 0932, 1500E Medical Center Drive, Ann Arbor, MI 48109-0932. E-mail: dlugosza@umich.edu.
Supported by the National Institutes of Health through the University of Michigan Comprehensive Cancer Center (CA46592) and the Center for Organogenesis; and grants AR45973 and CA87837 (to A.A.D.).
References
- 1.Fuchs E, Merrill BJ, Jamora C, DasGupta R: At the roots of a never-ending cycle. Dev Cell 2001, 1:13-25 [DOI] [PubMed] [Google Scholar]
- 2.Niemann C, Watt FM: Designer skin: lineage commitment in postnatal epidermis. Trends Cell Biol 2002, 12:185-192 [DOI] [PubMed] [Google Scholar]
- 3.Byrne C, Hardman M, Nield K: Covering the limb: formation of the integument. J Anat 2003, 202:113-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardy MH: The secret life of the hair follicle. Trends Genet 1992, 8:55-61 [DOI] [PubMed] [Google Scholar]
- 5.Cotsarelis G, Sun TT, Lavker RM: Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 1990, 61:1329-1337 [DOI] [PubMed] [Google Scholar]
- 6.Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y: Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell 2001, 104:233-245 [DOI] [PubMed] [Google Scholar]
- 7.Paus R, Muller-Rover S, van der Veen C, Maurer M, Eichmuller S, Ling G, Hofmann U, Foitzik K, Mecklenburg L, Handjiski B: A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol 1999, 113:523-532 [DOI] [PubMed] [Google Scholar]
- 8.Millar SE: Molecular mechanisms regulating hair follicle development. J Invest Dermatol 2002, 118:216-225 [DOI] [PubMed] [Google Scholar]
- 9.Gat U, DasGupta R, Degenstein L, Fuchs E: De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated β-catenin in skin. Cell 1998, 95:605-614 [DOI] [PubMed] [Google Scholar]
- 10.Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W: β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 2001, 105:533-545 [DOI] [PubMed] [Google Scholar]
- 11.Andl T, Reddy ST, Gaddapara T, Millar SE: WNT signals are required for the initiation of hair follicle development. Dev Cell 2002, 2:643-653 [DOI] [PubMed] [Google Scholar]
- 12.Van Mater D, Kolligs FT, Dlugosz AA, Fearon ER: Transient activation of β-catenin signaling in cutaneous keratinocytes is sufficient to trigger the active growth phase of the hair cycle in mice. Genes Dev 2003, 17:1219-1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merrill BJ, Gat U, DasGupta R, Fuchs E: Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev 2001, 15:1688-1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niemann C, Owens DM, Hulsken J, Birchmeier W, Watt FM: Expression of DeltaNLef1 in mouse epidermis results in differentiation of hair follicles into squamous epidermal cysts and formation of skin tumours. Development 2002, 129:95-109 [DOI] [PubMed] [Google Scholar]
- 15.St Jacques B, Dassule HR, Karavanova I, Botchkarev VA, Li J, Danielian PS, McMahon JA, Lewis PM, Paus R, McMahon AP: Sonic hedgehog signaling is essential for hair development. Curr Biol 1998, 8:1058-1068 [DOI] [PubMed] [Google Scholar]
- 16.Chiang C, Swan RZ, Grachtchouk M, Bolinger M, Litingtung Y, Robertson EK, Cooper MK, Gaffield W, Westphal H, Beachy PA, Dlugosz AA: Essential role for sonic hedgehog during hair follicle morphogenesis. Dev Biol 1999, 205:1-9 [DOI] [PubMed] [Google Scholar]
- 17.Wang LC, Liu ZY, Gambardella L, Delacour A, Shapiro R, Yang J, Sizing I, Rayhorn P, Garber EA, Benjamin CD, Williams KP, Taylor FR, Barrandon Y, Ling L, Burkly LC: Regular articles: conditional disruption of hedgehog signaling pathway defines its critical role in hair development and regeneration. J Invest Dermatol 2000, 114:901-908 [DOI] [PubMed] [Google Scholar]
- 18.Ingham PW, McMahon AP: Hedgehog signaling in animal development: paradigms and principles. Genes Dev 2001, 15:3059-3087 [DOI] [PubMed] [Google Scholar]
- 19.Matise MP, Joyner AL: Gli genes in development and cancer. Oncogene 1999, 18:7852-7859 [DOI] [PubMed] [Google Scholar]
- 20.Ruiz I, Altaba A, Sanchez P, Dahmane N: Gli and hedgehog in cancer: tumours, embryos and stem cells. Nat Rev Cancer 2002, 2:361-372 [DOI] [PubMed] [Google Scholar]
- 21.Mill P, Mo R, Fu H, Grachtchouk M, Kim PC, Dlugosz AA, Hui CC: Sonic hedgehog-dependent activation of Gli2 is essential for embryonic hair follicle development. Genes Dev 2003, 17:282-294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sasaki H, Nishizaki Y, Hui C, Nakafuku M, Kondoh H: Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development 1999, 126:3915-3924 [DOI] [PubMed] [Google Scholar]
- 23.Ramirez A, Bravo A, Jorcano JL, Vidal M: Sequences 5′ of the bovine keratin 5 gene direct tissue- and cell-type-specific expression of a lacZ gene in the adult and during development. Differentiation 1994, 58:53-64 [DOI] [PubMed] [Google Scholar]
- 24.Xie J, Murone M, Luoh SM, Ryan A, Gu Q, Zhang C, Bonifas JM, Lam CW, Hynes M, Goddard A, Rosenthal A, Epstein EH, Jr, de Sauvage F: Activating smoothened mutations in sporadic basal-cell carcinoma. Nature 1998, 391:90-92 [DOI] [PubMed] [Google Scholar]
- 25.Indra AK, Warot X, Brocard J, Bornert JM, Xiao JH, Chambon P, Metzger D: Temporally controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic Acids Res 1999, 27:4324-4327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vooijs M, Jonkers J, Berns A: A highly efficient ligand-regulated Cre recombinase mouse line shows that LoxP recombination is position dependent. EMBO Rep 2001, 2:292-297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walterhouse D, Ahmed M, Slusarski D, Kalamaras J, Boucher D, Holmgren R, Iannaccone P: Gli, a zinc finger transcription factor and oncogene, is expressed during normal mouse development. Dev Dyn 1993, 196:91-102 [DOI] [PubMed] [Google Scholar]
- 28.Zheng Y, Prouty SM, Harmon A, Sundberg JP, Stenn KS, Parimoo S: Scd3: a novel gene of the stearoyl-CoA desaturase family with restricted expression in skin. Genomics 2001, 71:182-191 [DOI] [PubMed] [Google Scholar]
- 29.Takabatake T, Ogawa M, Takahashi TC, Mizuno M, Okamoto M, Takeshima K: Hedgehog and patched gene expression in adult ocular tissues. FEBS Lett 1997, 410:485-489 [DOI] [PubMed] [Google Scholar]
- 30.Bitgood MJ, McMahon AP: Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol 1995, 172:126-138 [DOI] [PubMed] [Google Scholar]
- 31.Iseki S, Araga A, Ohuchi H, Nohno T, Yoshioka H, Hayashi F, Noji S: Sonic hedgehog is expressed in epithelial cells during development of whisker, hair, and tooth. Biochem Biophys Res Commun 1996, 218:688-693 [DOI] [PubMed] [Google Scholar]
- 32.Chen W, Kelly MA, Opitz-Araya X, Thomas RE, Low MJ, Cone RD: Exocrine gland dysfunction in MC5-R-deficient mice: evidence for coordinated regulation of exocrine gland function by melanocortin peptides. Cell 1997, 91:789-798 [DOI] [PubMed] [Google Scholar]
- 33.Kalderon D: Transducing the hedgehog signal. Cell 2000, 103:371-374 [DOI] [PubMed] [Google Scholar]
- 34.Murone M, Rosenthal A, de Sauvage FJ: Sonic hedgehog signaling by the patched-smoothened receptor complex. Curr Biol 1999, 9:76-84 [DOI] [PubMed] [Google Scholar]
- 35.Potten CS, Booth C: Keratinocyte stem cells: a commentary. J Invest Dermatol 2002, 119:888-899 [DOI] [PubMed] [Google Scholar]
- 36.Waikel RL, Kawachi Y, Waikel PA, Wang XJ, Roop DR: Deregulated expression of c-Myc depletes epidermal stem cells. Nat Genet 2001, 28:165-168 [DOI] [PubMed] [Google Scholar]
- 37.Arnold I, Watt FM: c-Myc activation in transgenic mouse epidermis results in mobilization of stem cells and differentiation of their progeny. Curr Biol 2001, 11:558-568 [DOI] [PubMed] [Google Scholar]
- 38.Reynolds AJ, Jahoda CA: Cultured dermal papilla cells induce follicle formation and hair growth by transdifferentiation of an adult epidermis. Development 1992, 115:587-593 [DOI] [PubMed] [Google Scholar]
- 39.Ferraris C, Bernard BA, Dhouailly D: Adult epidermal keratinocytes are endowed with pilosebaceous forming abilities. Int J Dev Biol 1997, 41:491-498 [PubMed] [Google Scholar]
- 40.Ferraris C, Chevalier G, Favier B, Jahoda CA, Dhouailly D: Adult corneal epithelium basal cells possess the capacity to activate epidermal, pilosebaceous and sweat gland genetic programs in response to embryonic dermal stimuli. Development 2000, 127:5487-5495 [DOI] [PubMed] [Google Scholar]
- 41.Hynes M, Ye W, Wang K, Stone D, Murone M, Sauvage F, Rosenthal A: The seven-transmembrane receptor smoothened cell autonomously induces multiple ventral cell types. Nat Neurosci 2000, 3:41-46 [DOI] [PubMed] [Google Scholar]
- 42.Oro AE, Higgins K: Hair cycle regulation of Hedgehog signal reception. Dev Biol 2003, 255:238-248 [DOI] [PubMed] [Google Scholar]
- 43.Grachtchouk M, Mo R, Yu S, Zhang X, Sasaki H, Hui CC, Dlugosz AA: Basal cell carcinomas in mice overexpressing Gli2 in skin. Nat Genet 2000, 24:216-217 [DOI] [PubMed] [Google Scholar]
- 44.Oro AE, Higgins KM, Hu ZL, Bonifas JM, Epstein EH, Jr, Scott MP: Basal cell carcinomas in mice overexpressing sonic hedgehog. Science 1997, 276:817-821 [DOI] [PubMed] [Google Scholar]
- 45.Morgan BA, Orkin RW, Noramly S, Perez A: Stage-specific effects of sonic hedgehog expression in the epidermis. Dev Biol 1998, 201:1-12 [DOI] [PubMed] [Google Scholar]
- 46.Grachtchouk V, Grachtchouk M, Lowe L, Johnson T, Wei L, Wang A, de Sauvage F, Dlugosz AA: The magnitude of hedgehog signaling activity defines skin tumor phenotype. EMBO J 2003, 22:2741-2751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kartasova T, Scandurro AB, Denning MF, Wirth PJ, Yuspa SH, Lichti U: Factors mediating the interactions between epidermal and dermal cells in skin grafts that might be important for hair follicle development. J Invest Dermatol 1995, 104:21S-22S [DOI] [PubMed] [Google Scholar]
- 48.Prouty SM, Lawrence L, Stenn KS: Fibroblast-dependent induction of a murine skin lesion similar to human nevus sebaceus of Jadassohn. Lab Invest 1997, 76:179-189 [PubMed] [Google Scholar]
- 49.Reddy S, Andl T, Bagasra A, Lu MM, Epstein DJ, Morrisey EE, Millar SE: Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech Dev 2001, 107:69-82 [DOI] [PubMed] [Google Scholar]
- 50.Botchkarev VA, Botchkareva NV, Nakamura M, Huber O, Funa K, Lauster R, Paus R, Gilchrest BA: Noggin is required for induction of the hair follicle growth phase in postnatal skin. EMBO J 2001, 15:2205-2214 [DOI] [PubMed] [Google Scholar]
- 51.Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM: PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell 1999, 4:611-617 [DOI] [PubMed] [Google Scholar]
- 52.Rosenfield RL, Deplewski D, Greene ME: Peroxisome proliferator-activated receptors and skin development. Horm Res 2000, 54:269-274 [DOI] [PubMed] [Google Scholar]
- 53.Neufang G, Furstenberger G, Heidt M, Marks F, Muller-Decker K: Abnormal differentiation of epidermis in transgenic mice constitutively expressing cyclooxygenase-2 in skin. Proc Natl Acad Sci USA 2001, 98:7629-7634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stenn KS, Paus R: Controls of hair follicle cycling. Physiol Rev 2001, 81:449-494 [DOI] [PubMed] [Google Scholar]
- 55.Webster GF: Acne vulgaris. BMJ 2002, 325:475-479 [PMC free article] [PubMed] [Google Scholar]
- 56.Leyden JJ: New understandings of the pathogenesis of acne. J Am Acad Dermatol 1995, 32:S15-S25 [DOI] [PubMed] [Google Scholar]
- 57.Deplewski D, Rosenfield RL: Role of hormones in pilosebaceous unit development. Endocr Rev 2000, 21:363-392 [DOI] [PubMed] [Google Scholar]
- 58.Goyette P, Allan D, Peschard P, Chen CF, Wang W, Lohnes D: Regulation of gli activity by all-trans retinoic acid in mouse keratinocytes. Cancer Res 2000, 60:5386-5389 [PubMed] [Google Scholar]