Abstract
Keratoacanthoma (KA) is a variant of cutaneous squamous cell carcinoma (SCC) known for rapid growth and potential for involution. Little is known about the basis for the rapid growth because of the dearth of model systems. We hypothesized that amphiregulin (AR), a keratinocyte autocrine growth factor, had a significant role. Using immunohistochemistry, we compared 21 KA, 6 conventional SCC, and 6 basal cell carcinomas (BCC) for AR expression. All KA were positive for AR, the majority with strong immunoreactivity. The SCC were positive (5 of 6), with generally weak staining; no BCC were positive. We developed laboratory model systems to study AR overexpression in keratinocytes and its role in the pathogenesis of KA. A retroviral transduction strategy was used to overexpress AR in the HaCaT keratinocyte-like cell line. The AR overexpressing cells (HaCaT-AR) displayed autonomous proliferation in serum-free media when compared with controls (HaCaT-NIE). To develop an in vivo model, xenografts of HaCaT-AR and HaCaT-NIE were grown on SCID mice. The HaCaT-NIE cells formed thin tumors resembling conventional SCC. The HaCaT-AR cells formed rapidly growing tumors with AR expression similar to KA. HaCaT-AR cells may represent a new system for the further evaluation of KA.
Keratoacanthoma (KA) is a unique cutaneous neoplasm that is classically known for its rapid growth that can be followed by spontaneous regression. Morphologically KA is a distinctive cup-shaped well-differentiated squamous tumor. KA remains controversial as there is still uncertainty regarding its benign versus malignant nature. 1 Traditionally thought to be benign, there is growing evidence that KA is a unique variant of squamous cell carcinoma (SCC). 2-5 Features seen in KA that support this view include lymphatic and perineural invasion and rare instances of metastasis. 2,3,6 Likewise a significant number of KA may undergo so-called “malignant transformation;” 7 this probably represents the evolution of a subclone within KA to a more aggressive phenotype rather than transformation of a benign neoplasm to a malignant tumor. Molecular similarities between KA and SCC include similarities in oncogene expression, 8 in oncostatin M expression, 5 and in mitotic cell cyclin patterns. 4 Although much progress has been made in the elucidation of molecular mechanisms of cutaneous SCC, relatively little effort has been directed toward understanding the KA variant. Hampering the study and understanding of the biology of KA is the dearth of KA-related model systems.
It is well established that epidermal growth factor (EGF) receptor ligands may play an important role in the development of cutaneous SCC and in other hyperproliferative skin diseases. 9 Amphiregulin (AR) is synthesized as a glycosylated 152 amino acid membrane-anchored precursor that can be proteolytically processed into multiple membrane-bound and soluble isoforms. 9-14 Because of differential proteolytic processing and glycoslyation, these AR isoforms may vary widely in molecular weight. 9,13,14 Within its conserved EGF domain, AR shows significant homology to all EGF family members, including EGF, TGFα, and betacellulin. 9 When considering the entire AR protein, it is most structurally similar to HB-EGF. 9 AR is a heparin-inhibited, heparin-binding growth factor and is thought to act as a ligand for the type-1 epidermal growth factor receptor (EGFR-1). 9-13,15 After AR’s initial interaction with cell surface sulfated proteoglycans it may then interact with EGFR-1 and subsequently dimerize with another EGFR subtype (eg, HER-2) to form an active tetrameric receptor-ligand complex. 9 In vitro studies have demonstrated that AR is the predominant autocrine growth factor produced by keratinocytes in cell culture. 9,15,16 AR expression has been documented in several hyperproliferative skin diseases such as psoriasis and in physiological states such as wound healing. 9,17-19 Although qualitatively low levels of AR have been detected in cutaneous SCC, 18 the autocrine growth activity of AR has been suggested to be sufficient for the formation of tumors in v-ras transformed keratinocytes. 20
To our knowledge, no previous study has examined the KA variant of SCC for the expression of AR. We hypothesized that AR may have a central role in the rapid proliferation associated KA in its active growth phase. To test our hypothesis, we examined a series of clinical cases of KA for AR expression in comparison to conventional SCC and basal cell carcinoma (BCC). We also developed potential laboratory model systems to study the effects of AR overexpression on keratinocytes in a cell culture system as well as in vivo xenografts.
Materials and Methods
Clinical Case Selection
Formalin-fixed, paraffin-embedded tissue blocks were retrieved from departmental archives. Twenty-one cases coded as KA were retrieved as well as a small number of representative cases of conventional SCC (n = 6) and BCC (n = 6) for comparative purposes.
Immunohistochemistry
Immunohistochemical detection of AR was performed according to standard avidin-biotin technique (dilution 1:100) using heat-induced epitope retrieval on the clinical cases of and xenografts (see below). Briefly, slides were deparaffinized, hydrated, and rinsed with Tris-buffered saline with Tween 20 (TBS) (DAKO, Carpenteria, CA). Antigen retrieval was performed using a water bath at 95°C for 20 minutes with DAKO Target Retrieval Buffer. After cooling, the slides were rinsed with TBS, immersed in 3% H2O2 for 10 minutes and incubated with 6R1C anti-AR mAb for 1 hour at room temperature. The slides were rinsed with TBS and incubated with the secondary antibody (DAKO LSAB2) for 20 minutes. After rinsing, the slides were incubated with Strep-Avidin HRP for 20 minutes, rinsed, and stain was developed with DAB for 3 minutes. The slides were rinsed and counter-stained with hematoxylin. Membranous staining was considered positive. Scoring of the immunoreactivity was based on the percentage of staining seen in the basal layer of the tumor. Cases were scored as 3+ (greater than 50% positive cells), 2+ (25 to 50% positive cells, and 1+ (5 to 24% positive cells).
Immunohistochemical stains for the nuclear proliferation marker Ki-67 (DAKO, 1:60) were performed on the xenograft tumors (see below) similarly except for the timing of the incubation steps: 3% H2O2 for 5 minutes, primary antibody for 30 minutes, secondary antibody for 10 minutes and Strep-Avidin HRP for 10 minutes. Only nuclear staining was considered positive. The percentage of basal cell layer immunoreactivity was estimated.
For negative controls on all studies, the primary antibody was omitted, and the slides were otherwise processed with the same procedure as outlined above. A defined positive control for AR in keratinocytes is not available. As our results with conventional SCC and BCC paralleled previously published studies with the same antibody, 18 we considered our methods valid. For Ki-67, sections of skin served as a positive control.
A statistical analysis examining the overall differences in the three distributions was performed with the Kruskal-Wallis Exact Test. As this overall test was significant, pair-wise differences were then tested using Wilcoxon Rank Sum Exact Tests (two-sided).
Generation of the HaCaT Cells Overexpressing Human AR
To create HaCaT cells 21 stably expressing human AR, an 880-bp AR cDNA fragment derived from pTZ-AR-2 13 was cloned into the pGEM-Teasy vector (Promega). The AR cDNA sequence were excised from the pGEM-Teasy vector and subsequently subcloned into the EcoRI site of an MFG-based retroviral DNA vector NIE, which uses EGFP as the selectable marker. 22 The IRES-EGFP portion of pIRES2-EGFP (Clontech, Palo Alto, CA), between the BglII and NotI sites, was subcloned to replace the GFP, between NcoI and BamHI sites, of the MFG. There was a resulting ATG codon at the NcoI site after cloning, and it was destroyed by site-directed mutagenesis (Stratagene, La Jolla, CA). The uptake of the inserts and their orientation were assessed by both restriction endonuclease mapping and sequencing. Infectious amphotropic retroviruses were produced from both NIE-AR and control NIE backbone by transient transfection into the Phoenix amphotropic packaging cell line using Fugene 6 (Roche Molecular Biochemicals, Indianapolis, IN). The supernatants collected 48 hours later containing infectious virions were then used to infect HaCaT cells. Transduced cells were obtained by sorting based on EGFP using FACS.
Northern Blot Analysis
Total RNA was extracted using Tripure (Roche). After the manufacturer’s procedures of purification, an additional phenol (pH 4.2) extraction was performed, followed by ethanol precipitation. The RNA concentration was accessed by UV spectrophotometer. Twenty micrograms of total RNA was separated on a formaldehyde-agarose gel, as previously reported, 23 and transferred to a positively charged nylon membrane (Roche). The blots were then hybridized with 32P-labeled hAR cDNA (cited above) probe using ExpressHyb Hybridization Solution (Clontech) following manufacturer’s protocol. The blots were stripped of the probe using 0.5% sodium SDS at 100°C and later probed with human GAPDH to determine equal loading. Human GAPDH (no. 57091) cDNA clone was purchased from American Type Culture Collection (Rockville, MD).
Western Blot Analysis
Cells were washed with cold phosphate-buffered saline (PBS) (BRL) and lysed with 1 ml of RIPA buffer (150 mmol/L NaCl, 50 mmol/L Tris-HCl, pH 8.0, 0.1% SDS, 0.5% sodium deoxycholate, 1% Nonidet P-40) containing 0.5 nmol/L Pefabloc SC (Roche Molecular Biochemicals). Cell lysate was scraped to collect and sonicated. The protein concentration was determined using the Bio-Rad Protein Assay Kit (Bio-Rad Laboratories, Hercules, CA). Ten μg of protein was heated to 100°C for 5 minutes in Laemmli Sample Buffer without 2-mercaptoethanol, loaded onto a 15% SDS-PAGE gel (Bio-Rad Minigel System). After the gel electrophoresis, the proteins were then transferred to a PVDF membrane (Bio-Rad), using a SD semi-dry electrophoretic cell (Bio-Rad). Detection was performed using ECL-Plus (Amersham, Piscataway, NJ). The mouse-anti hAR antibody clone 6R1C was used at a dilution of 1:300. 15,17,18 The secondary goat anti-mouse IgG-horseradish peroxidase (Bio-Rad) was used at a dilution of 1:3000.
Cell Culture Studies
HaCaT cells with empty vector (HaCaT-NIE) and AR overexpressing HaCaT cells (HaCaT-AR) were grown in Dulbecco’s modified Eagle’s medium (Life Technologies, Inc.) with 10% Fetal Clone III (Hyclone, Logan, UT). For the proliferation studies, cells were plated at a density of 200,000cells/10-cm2 dish. On the following day the cells were washed twice with PBS, and grown in the presence of serum (Dulbecco’s modified Eagle’s medium, 0.5% bovine serum albumin), without serum and in some plates with exogenous AR (R and D Systems, Minneapolis, MN) added (final concentration 20 ng/ml). Cells were harvested at various times by trypsinization and counted to assess proliferation over time.
Establishment of Xenografts
Dispersed HaCaT cells were grafted onto NODLtSz/scid/scid (SCID) mice as previously described. 22 Before use, a sample of peripheral blood was screened by flow cytometry for the presence of T cells. Animals used in these studies had <1% T cells. Briefly, silicone-grafting domes (Renner GMBH, Germany) were placed on the dorsum of 2-to-3-month-old mice using 1% avertin as anesthesia. A suspension of 5 × 106 HaCaT-NIE (five mice) or HaCaT-AR cells (four mice) mixed with 2.5 × 106 human neonatal foreskin fibroblasts were placed into the grafting domes. The domes were removed after 2 weeks, and white petrolatum placed on the xenografts on a daily basis. At 60 days post-transplantation, the mice were injected with BrdU as described. 24 Two hours after intraperitoneal BrdU injection, the mice were sacrificed, and the xenograft excised, and sections were either placed in formalin for histology and immunohistochemistry, or snap-frozen in liquid nitrogen in OCT media for BrdU-labeling studies.
Results
Immunohistochemistry Studies from Clinical Cases
Examination of archival specimens revealed AR expression only in the tumor cells of KA and SCC; no AR immunoreactivity was seen in the cases of BCC (Table 1) ▶ . The immunoreactivity had a granular, membranous pattern. In the SCC, 5 of 6 cases were positive; all 21 KA were positive for AR. There were significant quantitative differences in the immunoreactivity of KA and SCC. In SCC the immunoreactivity was seen primarily in the basal layer with a patchy distribution (1+ staining) (Figure 1A) ▶ . Only one case showed intense staining of the basal layer with the majority of the basal layer positive. In KA 12 of 21 (57%) had more intense (2+ to 3+) staining. Immunoreactivity was seen in some of the suprabasilar cells as well. In two cases of KA with a clinical history of developing rapidly over the span of only a few weeks, there was confluent staining of the basal layer with the majority of the suprabasilar keratinocytes showing strong AR expression (Figure 1B) ▶ . Overall, there were significant differences in the % basal layer positivity (P value <0.0001) using the Kruskal-Wallis Exact Test. In addition, all pair-wise differences were significant (P value = 0.0494 for KA versus SCC, <0.0001 for KA versus BCC, and 0.0152 for SCC versus BCC) using Wilcoxon Rank Sum Exact Tests (two-sided). The KA group tended to have higher scores than both SCC and BCC, and SCC tended to have higher scores than BCC.
Table 1.
Immunohistochemistry for AR: Clinical Cases
| Diagnosis | Score | |||
|---|---|---|---|---|
| 0+ | 1+ | 2+ | 3+ | |
| Keratoacanthoma (n = 21) | 0 | 9 | 7 | 5 |
| Conventional SCC (n = 6) | 1 | 4 | 1 | 0 |
| BCC (n = 6) | 6 | 0 | 0 | 0 |
Figure 1.
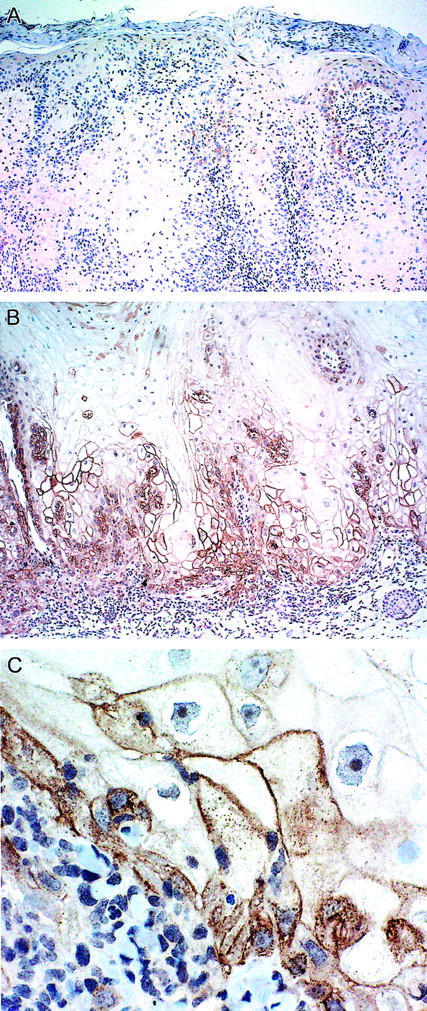
Comparison of amphiregulin expression in conventional squamous cell carcinoma and keratoacanthoma. A: Conventional squamous cell carcinoma with relatively weak and patchy staining for amphiregulin in the basal layer. Original magnification, ×100. B: Keratoacanthoma with strong, diffuse staining for amphiregulin. Original magnification, ×100. C: High power view of amphiregulin staining in a keratoacanthoma demonstrating the membranous pattern of staining. Original magnification, ×400.
Cell Culture Studies
Given our findings of increased levels of AR immunoreactivity associated with KA, we next developed a model system to assess the effects of overexpression of this growth factor in epithelial function. HaCaT cells are a human immortalized keratinocyte-derived cell line. 21 HaCaT cells were transduced with a retroviral vector encoding the human AR cDNA or empty vector as control. As shown in Figure 2A ▶ , Northern blots detected AR mRNA only in HaCaT-AR cells.
Figure 2.
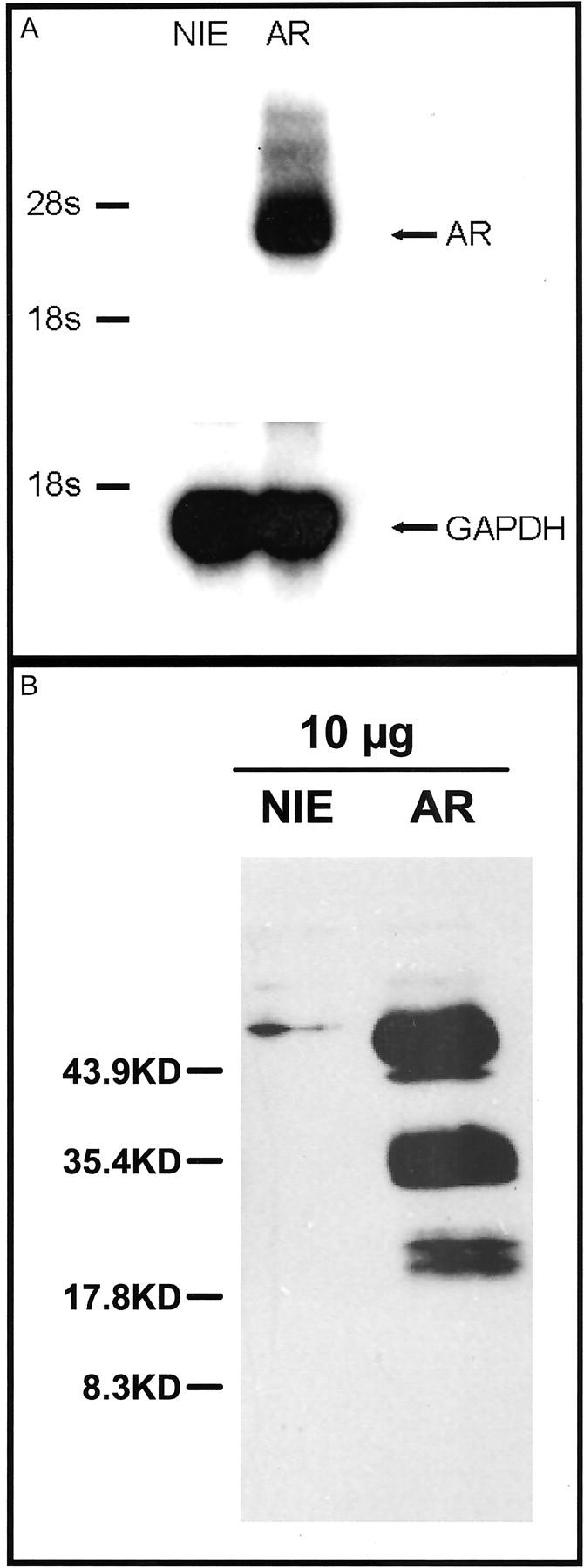
Molecular analysis of HaCaT-AR cells. A: Northern blot detected AR mRNA only in the HaCaT-AR cells (right). B: Western blots detected a small amount of AR in the control cells (left). The AR overexpressing cells had much higher amounts of AR with different forms ranging from approximately 18 to 70 kd.
Western blots demonstrated high levels of AR protein in the HaCaT-AR cells (Figure 2B) ▶ . At least five distinct bands ranging from approximately 18 to 70 kd were detected corresponding to previously published results. 9,13,14 The range of molecular sizes suggests that AR produced by HaCaT-AR cells was both differentially processed and glycosylated. 9,13,14 Supernatants from HaCaT-AR cells also contained low levels of AR protein, indicating that the cells were releasing only small amounts of proteolytic processed AR. In contradistinction, no AR was detected in the supernatant from the control HaCaT-NIE cells (data not shown). These studies confirmed that HaCaT AR cells expressed significant levels of variably processed and glycosylated AR, and that most of the AR was apparently cell associated, implying that AR-dependent growth-promoting activity may be mediated by a cell-cell dependent juxtacrine mechanism, as has been previously suggested for human keratinocytes. 9
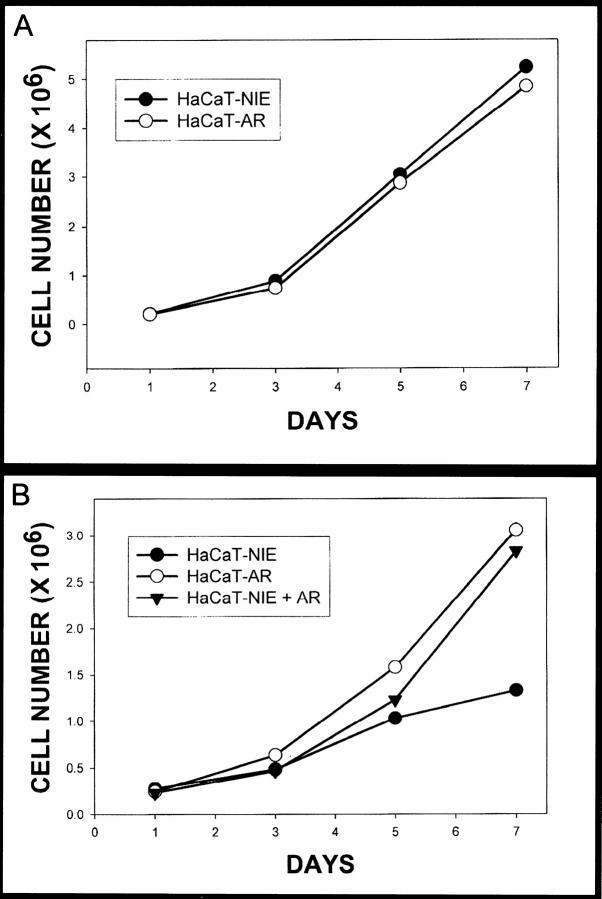
Our next studies examined the functional significance of AR overexpression in HaCaT cells. Both the HaCaT-AR and HaCaT-NIE cell lines had essentially the same growth curves when grown in the presence of serum (Figure 3) ▶ . In serum-free conditions however, the growth rate of HaCaT-AR cells was only slightly decreased in contrast to the markedly lower growth rate seen in control empty vector HaCaT-NIE cells. When exogenous AR was added to the serum-free medium, the HaCaT-NIE control cells had equivalent growth as compared to the HaCaT-AR cells grown in serum-free medium. These studies confirm that AR overexpression can affect HaCaT proliferation.
Figure 3.
Cell culture studies of HaCaT-AR cells and HaCaT-NIE controls. A: In the cell culture studies, the control cells and the AR overexpressing cells had equivalent growth rates in the presence of serum. B: In the absence of serum, the AR overexpressing cells had significantly higher rates of growth in serum-free media as compared with controls. This growth advantage was abolished with the addition of exogenous AR to the media.
Xenograft Studies
To assess the functional significance of AR overexpression in HaCaT cells in vivo, HaCaT-AR or control HaCaT-NIE cells were xenografted onto SCID mice. Establishment of xenografts of both cell types resulted in tumors. While the predicted tumors derived from the HaCaT-NIE cells were thin (mean volume ± SEM: 301 ± 53 mm3) and resembled minimally invasive SCC (Figure 4,A and C) ▶ , tumors from HaCaT-AR cells were rapidly growing, much larger, and formed cup-shaped nodules reminiscent of KA (mean volume ± SEM: 10,740 ± 1850 mm3) (Figure 4, A, B, and D) ▶ . The control HaCaT-NIE tumors showed no significant staining for AR in contrast to the HaCaT-AR tumors which demonstrated diffuse, strong membranous for AR (Figure 4, E and F) ▶ . Although the pattern of staining was overall more diffuse than the majority of the clinical cases of KA, the pattern of staining was essentially indistinguishable from the cases of KA with a clinical history of being present for only 2 to 4 weeks (Figures 1B and 4F) ▶ .
Figure 4.
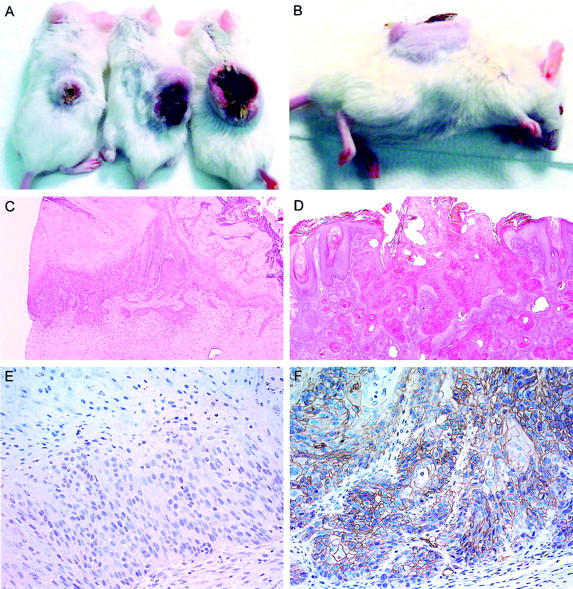
Comparison of control and amphiregulin overexpressing xenograft tumors. A: The far left mouse had a xenograft with the control HaCaT-NIE cells. The right two mice had HaCaT-AR xenografts and formed rapidly growing cup-shaped tumors. B: Side view of HaCaT-AR xenograft tumor showing the cup-shaped morphology similar to keratoacanthoma. C: Histologically the HaCaT-NIE xenografts resembled thin squamous cell carcinomas. H&E; original magnification, ×40. D: The HaCaT-AR xenografts formed cup-shaped tumors that resembled keratoacanthomas. H&E; original magnification, ×20. E: Immunohistochemistry for amphiregulin detected no significant expression in the HaCaT-NIE control xenograft tumors. Original magnification, ×200. F: Strong staining for amphiregulin was seen in the HaCaT-AR xenografts. Original magnification, ×200.
The immunohistochemical results from the clinical cases and the xenografts strongly suggested a role for AR-related proliferation in the growth of KA. Since the rapid tumor growth could be due to either proliferation or lack of cell death, we evaluated epithelial proliferation of HaCaT-NIE and HaCaT-AR tumors. We stained the tumors with the nuclear proliferation marker Ki-67. Both the HaCaT-AR and HaCaT-NIE xenograft tumors expressed Ki-67, but the AR overexpressing tumors exhibited higher levels of basal layer staining compared with the vector controls (Table 2) ▶ . The HaCaT-AR tumors also had more suprabasilar staining of keratinocytes as compared with the vector controls. Paralleling the Ki-67 results, there was also much higher BrdU uptake in HaCaT-AR xenografts compared with the control xenografts (data not shown).
Table 2.
Immunohistochemistry for Ki-67
| Xenografts | Percentage of Basal Cells Positive |
|---|---|
| HaCaT-AR | 50–70% |
| HaCaT-NIE | 20–30% |
Discussion
The results of our immunohistochemical studies indicate a role for AR in the molecular pathogenesis of KA. All 19 cases of KA were immunoreactive for AR with over half of the cases (12 of 21) showing strong staining. The cases of SCC were likewise frequently immunoreactive for AR (5 of 6 cases), but AR expression levels appeared to be less than in KA. The relatively low levels of AR seen in some KA could potentially be explained by the growth stage of the KA. As mentioned previously, KA classically has a phase of rapid growth followed by spontaneous involution. The cases of KA with weaker staining may have already passed their zenith of growth activity. Although there was insufficient clinical history in terms of the growth of the tumors in the majority of cases to determine the age of the tumor, two cases with 3+ staining had the clinical history of being present for only 2 to 4 weeks. This supports the theory that the tumors with stronger staining were in the more active phase of growth. Unlike the positive AR expression in KA and some SCC, no appreciable AR staining was seen in the cases of BCC (0 of 6 cases). The results of the immunohistochemical studies of SCC and BCC are consistent with previous reports of AR expression in these tumors. 18
Our results suggest a greater role for AR in KA than in conventional SCC. Since the clinical cases relied on archival material, however, the role of AR in the pathogenesis of KA remained speculative. Theoretically, the AR detected in the cases of KA may not have been functional or could represent an epiphenomenon. Therefore we developed a potential laboratory model system using a retroviral transduction method to overexpress the AR gene product in HaCaT cells for use in a xenograft system. The results of the in vitro studies on the HaCaT cells confirm the activity of the overexpressed AR. The ability of the HaCaT-AR cells to grow in the absence of serum and exogenous growth factors is not surprising, as AR has been characterized as an autocrine/juxtacrine growth factor that drives the autonomous proliferation of human keratinocytes in serum- and growth factor-free media. 9,15,16 The ability of exogenously added AR to enable the empty vector control HaCaT-NIE cells to proliferate in a fashion similar to that observed for HaCaT-AR in the absence of exogenous AR, further supports the role of AR in the proliferation seen in our current cell culture studies. AR expressed in HaCaT cells exists in a range of isoforms through post-translational glycosylation and proteolytic cleavage. 9,13,14 The significance of the different forms of AR as it relates to the growth-promoting activity in epidermal neoplasms such as KA is unknown. This could provide an avenue of further investigation.
The xenograft model provides supporting evidence for a potential role for AR in the pathogenesis of KA. The HaCaT-AR overexpressing tumors formed large cup-shaped masses that grew very quickly over the course of a few weeks. The gross morphology and the rapid growth were very reminiscent of KA. Histologically, the AR overexpressing tumor also resembled classic KA. The strong expression of AR seen in the resulting xenograft tumors was essentially indistinguishable from the clinical cases of KA in which the rapidly growing tumor had been present for only a few weeks. The rapid proliferation was confirmed by two separate measures: immunoreactivity for Ki-67 and BrdU-labeling studies. The HaCaT-AR xenograft tumors exhibited high levels of Ki-67 expression as well as high levels of BrdU uptake. By contrast, the HaCaT-NIE-control xenografts formed thin tumors that grossly and histologically resembled conventional cutaneous SCC. No significant AR expression was seen by immunohistochemistry, and there was less Ki-67 expression and BrdU uptake in tumors derived from the HaCaT-NIE cells in comparison to the HaCaT-AR tumors.
The results from the clinical cases and the murine xenograft model support a role for AR in the molecular pathogenesis of KA. Increased expression of AR likely contributes to the rapid growth of KA. It remains to be seen what other EGFR ligands may participate in the rapid growth of KA. In a mouse model, large exophytic squamous papillomas developed in skin grafts of mouse epidermal cells overexpressing transforming growth factor α. 25 The determination of the role of other EGFR ligands in the pathogenesis of KA will require further study. It should be noted that targeted overexpression of AR to the epidermis in mice resulted in an inflammatory hyperproliferative dermatitis that strongly resembles the human disease psoriasis. 19 That AR may have a prominent role in KA and psoriasis may seem somewhat counterintuitive given the resistance of psoriatic plaques to malignant transformation. 26 Presumably KA has other genetic damage acting as an initiating event and AR overexpression occurs later in the molecular pathogenesis.
Interestingly, the potential role of AR in psoriasis invites speculation on the role of AR for the involution of KA. In psoriasis it has been proposed that AR may induce proinflammatory cytokine circuits in the proliferating epidermis. 19 In the setting of a neoplastic proliferation, activation of a proinflammatory cytokine circuit may enable the immune system to recognize the tumor as foreign and allow immune destruction of the neoplasm. It has been postulated that immune surveillance may contribute to the destruction of KA. Thus, it is possible that the AR expressed by aggressively growing KA may stimulate both keratinocyte hyperprolferation, and proinflammatory events leading to KA regression. An alternate and less likely possibility is the potential inhibitory role for AR on tumor growth reported in some in vitro studies. 12
In summary, the present studies provide evidence suggesting a role for AR in the biology of KA. In addition, these studies describe a potential laboratory model system for the study of KA that might allow further exploration regarding the role of AR in the molecular pathogenesis of KA. Future studies are needed to define the exact role of AR in both the rapid growth as well as a potential role for AR in the spontaneous involution of KA.
Footnotes
Address reprint requests to Steven D. Billings M.D., Indiana University Hospital, Department of Pathology and Laboratory Medicine, UH3274, 550 N. University Blvd, Indianapolis, IN 46202. E-mail: sdbillin@iupui.edu.
Supported in part by grants from the Riley Memorial Association, and National Institutes of Health grants AR01993 and HL62996.
References
- 1.LeBoit PE: Can we understand keratoacanthoma? Am J Dermatopathol 2002, 24:166-168 [DOI] [PubMed] [Google Scholar]
- 2.Beham A, Regauer S, Soyer HP, Beham-Schmid C: Keratoacanthoma: a clinically distinct variant of well-differentiated squamous cell carcinoma. Adv Anat Pathol 1998, 5:269-280 [PubMed] [Google Scholar]
- 3.Cribier B, Asch P, Grosshans E: Differentiating squamous cell carcinoma from keratoacanthoma using histopathological criteria: is it possible? A study of 296 cases. Dermatology 1999, 199:208-212 [DOI] [PubMed] [Google Scholar]
- 4.Tran TA, Ross JS, Boehm JR, Carlson JA: Comparison of mitotic cyclins and cyclin-dependent kinase expression in keratoacanthoma and squamous cell carcinoma. J Cutan Pathol 1999, 26:391-397 [DOI] [PubMed] [Google Scholar]
- 5.Tran TA, Ross JS, Sheehan CE, Carlson JA: Comparison of oncostatin M expression in keratoacanthoma and squamous cell carcinoma. Mod Pathol 2000, 13:427-432 [DOI] [PubMed] [Google Scholar]
- 6.Hodak E, Jones RE, Ackerman AB: Solitary keratoacanthoma is a squamous-cell carcinoma: three examples with metastases. Am J Dermatopathol 1993, 15:332-342; 343352 [DOI] [PubMed] [Google Scholar]
- 7.Sanchez Yus E, Simon P, Requena L, Ambrojo P, de Eusebio E: Solitary keratoacanthoma: a self-healing proliferation that frequently becomes malignant. Am J Dermatopathol 2000, 22:305-310 [DOI] [PubMed] [Google Scholar]
- 8.Peng X, Griffith JW, Han R, Lang CM, Kreider JW: Development of keratoacanthomas and squamous cell carcinomas in transgenic rabbits with targeted expression of EJras oncogene in epidermis. Am J Pathol 1999, 155:315-324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piepkorn M, Pittelkow MR, Cook PW: Autocrine regulation of keratinocytes: the emerging role of heparin-binding, epidermal growth factor-related growth factors. J Invest Dermatol 1998, 111:715-721 [DOI] [PubMed] [Google Scholar]
- 10.Shoyab M, McDonald VL, Bradley JG, Todaro GJ: Amphiregulin: a bifunctional growth-modulating glycoprotein produced by the phorbol 12-myristate 13-acetate-treated human breast adenocarcinoma cell line MCF-7. Proc Natl Acad Sci USA 1988, 85:6528-6532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shoyab M, Plowman GD, McDonald VL, Bradley JG, Todaro GJ: Structure and function of human amphiregulin: a member of the epidermal growth factor family. Science 1989, 243:1074-1076 [DOI] [PubMed] [Google Scholar]
- 12.Plowman GD, Green JM, McDonald VL, Neubauer MG, Disteche CM, Todaro GJ, Shoyab M: The amphiregulin gene encodes a novel epidermal growth factor-related protein with tumor-inhibitory activity. Mol Cell Biol 1990, 10:1969-1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown CL, Meise KS, Plowman GD, Coffey RJ, Dempsey PJ: Cell surface ectodomain cleavage of human amphiregulin precursor is sensitive to a metalloprotease inhibitor: release of a predominant N-glycosylated 43-kDa soluble form. J Biol Chem 1998, 273:17258-17268 [DOI] [PubMed] [Google Scholar]
- 14.Garach-Jehoshua O, Ravid A, Liberman UA, Koren R: 1, 25-Dihydroxyvitamin D3 increases the growth-promoting activity of autocrine epidermal growth factor receptor ligands in keratinocytes. Endocrinology 1999, 140:713-721 [DOI] [PubMed] [Google Scholar]
- 15.Cook PW, Mattox PA, Keeble WW, Pittelkow MR, Plowman GD, Shoyab M, Adelman JP, Shipley GD: A heparin sulfate-regulated human keratinocyte autocrine factor is similar or identical to amphiregulin. Mol Cell Biol 1991, 11:2547-2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piepkorn M, Lo C, Plowman G: Amphiregulin-dependent proliferation of cultured human keratinocytes: autocrine growth, the effects of exogenous recombinant cytokine, and apparent requirement for heparin-like glycosaminoglycans. J Cell Physiol 1994, 159:114-120 [DOI] [PubMed] [Google Scholar]
- 17.Cook PW, Pittelkow MR, Keeble WW, Graves-Deal R, Coffey RJ, Jr, Shipley GD: Amphiregulin messenger RNA is elevated in psoriatic epidermis and gastrointestinal carcinomas. Cancer Res 1992, 52:3224-3227 [PubMed] [Google Scholar]
- 18.Piepkorn M: Overexpression of amphiregulin, a major autocrine growth factor for cultured human keratinocytes, in hyperproliferative skin diseases. Am J Dermatopathol 1996, 18:165-171 [DOI] [PubMed] [Google Scholar]
- 19.Cook PW, Piepkorn M, Clegg CH, Plowman GD, DeMay JM, Brown JR, Pittelkow MR: Transgenic expression of the human amphiregulin gene induces a psoriasis-like phenotype. J Clin Invest 1997, 100:2286-2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dlugosz AA, Cheng C, Williams EK, Darwiche N, Dempsey PJ, Mann B, Dunn AR, Coffey RJ, Jr, Yuspa SH: Autocrine transforming growth factor α is dispensible for v-rasHa-induced epidermal neoplasia: potential involvement of alternate epidermal growth factor receptor ligands. Cancer Res 1995, 55:1883-1893 [PubMed] [Google Scholar]
- 21.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE: Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol 1988, 106:761-771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spandau DF, Marques M, Bierhuizen M, Wagemaker G, Hurwitz S, Pei Y, Breese R, Travers JB: Use of enhanced green fluorescent protein to monitor retroviral-mediated gene therapy in human keratinocytes. Exp Dermatol 2000, 9:252-257 [DOI] [PubMed] [Google Scholar]
- 23.Pei Y, Barber LA, Murphy RC, Johnson CA, Kelley SW, Dy LC, Fertel RH, Nguyen TM, Williams DA, Travers JB: Activation of the epidermal platelet-activating factor receptor results in cytokine and cyclooxygenase-2 biosynthesis. J Immunol 1998, 161:1954-1961 [PubMed] [Google Scholar]
- 24.Sato S, Kume K, Ito C, Ishii S, Shimizu T: Accelerated proliferation of epidermal keratinocytes by the transgenic expression of the platelet-activating factor receptor. Arch Dermatol Res 1999, 291:614-621 [DOI] [PubMed] [Google Scholar]
- 25.Finzi E, Kilkenny A, Strickland JE, Balaschak M, Bringman T, Derynck R, Aaronson S, Yuspa SH: TGF α stimulates growth of skin papillomas by autocrine and paracrine mechanisms but does not cause neoplastic progression. Mol Carcinog 1988, 1:7-12 [DOI] [PubMed] [Google Scholar]
- 26.Nickoloff BJ: Creation of psoriatic plaques: the ultimate tumor suppressor pathway. A new model for an ancient T-cell-mediated skin disease. Viewpoint J Cutan Pathol 2001, 28:57-64 [DOI] [PubMed] [Google Scholar]