Abstract
Expression of the c-myc proto-oncogene is deregulated in many human cancers. We examined the role of c-Myc in stimulating angiogenesis and lymphangiogenesis in a highly metastatic murine model of Burkitt’s lymphoma (Eμ-c-myc), where c-Myc is expressed exclusively in B lymphocytes. Immunohistochemical analysis of bone marrow and lymph nodes from young (preneoplastic) Eμ-c-myc transgenic mice revealed increased growth of blood vessels, which are functional by dye flow assay. Lymphatic sinuses also increased in size and number within the lymph nodes, as demonstrated by immunostaining for with a lymphatic endothelial marker 10.1.1. The 10.1.1 antibody recognizes VEGFR-2- and VEGFR-3-positive lymphatic sinuses and vessels within lymph nodes, and also recognizes lymphatic vessels in other tissues. Subcutaneously injected dye traveled more efficiently through draining lymph nodes in Eμ-c-myc mice, indicating that these hypertrophic lymphatic sinuses increase lymph flow. Purified B lymphocytes and lymphoid tissues from Eμ-c-myc mice expressed increased levels of vascular endothelial growth factor (VEGF) by immunohistochemical or immunoblot assays, which could promote blood and lymphatic vessel growth through interaction with VEGFR-2, which is expressed on the endothelium of both vessel types. These results indicate that constitutive c-Myc expression stimulates angiogenesis and lymphangiogenesis, which may promote the rapid growth and metastasis of c-Myc-expressing cancer cells, respectively.
The c-myc proto-oncogene has a central role in regulation of cell growth and differentiation. 1 Overexpression of c-Myc via translocations or other mechanisms is a common feature of many cancers including colon, breast, prostate, and gastrointestinal cancers, as well as leukemias and lymphomas. 2 Experimental c-Myc overexpression in chickens and mice indicates that c-Myc rapidly induces neoplastic transformation of many cell types, including B lymphocytes. In all of these models, increased c-Myc expression impairs cell cycle exit, slows differentiation, 3,4 and induces blood vessel growth. 5-7 Studies using conditional c-Myc expression indicate that c-Myc is continuously required to support c-Myc-induced tumors; loss of c-Myc expression in these tumors results in differentiation, apoptosis, and blood vessel degeneration, followed by tumor resorption. 7,8 These findings indicate that c-Myc is a potent oncogene able to mediate many features of tumor induction, including angiogenesis.
While these studies make plain that c-Myc is important for cell growth and the maintenance of tumors, it is still unclear whether c-Myc may also contribute to tumor metastasis, which is a critical feature in determining the prognosis of cancer patients. Clinical studies indicate that c-Myc expression generally correlates with a less favorable prognosis, 2 and is associated with increased metastasis of breast, 9 colorectal, 10 and cervical cancers. 11 In addition, amplification of L-myc has been closely associated with metastasis of lung cancer to lymph nodes and other organs. 12 Among the potential mechanisms for increasing the metastasis potential of tumor cells, the sprouting of new blood vessels that help feed tumor cells (angiogenesis), and the growth of lymphatic vessels that drain extravasated fluid, proteins, and tumor cells (lymphangiogenesis), 13 are leading candidates for modulation by c-Myc.
Angiogenesis is essential for growth and metastasis of solid tumors, and is involved in hematopoietic malignancies. 14 For example, bone marrow from leukemia and multiple myeloma patients show increased vascularization, 15-17 and non-Hodgkin’s lymphomas also feature increased vessels. 18 Hematopoietic tumors can produce pro-angiogenic factors including vascular endothelial growth factor (VEGF), a potent growth factor for vascular endothelium, and metalloproteinases involved in matrix remodeling. 19,20 Tumor lymphangiogenesis has recently gained attention for its potential involvement in lymphatic dissemination and metastasis of tumor cells. 21 Lymph vessel growth is promoted by VEGF-C or VEGF-D activation of VEGFR-3, whose expression is generally restricted to lymphatic endothelium. For example, VEGF-C- or VEGF-D-expressing tumor cell line implants in immunodeficient mice induced growth of lymph vessels, which promoted metastasis. 22-24 Moreover, inhibition of VEGFR-3 signaling blocked lymphatic vessel growth and tumor metastasis, 25 and it also inhibited corneal lymphangiogenesis. 26 Thus far, few experimental models have been developed to examine the natural role of angiogenesis and lymphangiogenesis in tumor dissemination.
To more fully investigate the ability and mechanism of c-Myc-induced angiogenesis and lymphangiogenesis during hematopoietic cell transformation, we used the well-characterized Eμ-c-myc mouse model of Burkitt’s lymphoma, 27 where the immunoglobulin heavy chain gene enhancer drives c-Myc expression in B-cell progenitors. Overexpression of c-Myc results in accumulation of pre-B cells within the bone marrow, lymph nodes, and spleen beginning before birth. 28 This polyclonal B-cell expansion is followed by the appearance of oligoclonal or monoclonal tumors after 12 to 16 weeks of age, which metastasize to many organs, including the lung, liver, and bone. 29 While Eμ-c-myc mice have been extensively studied to examine the effects of c-Myc on proliferation, differentiation, and apoptosis, the ability of c-Myc to induce angiogenesis and lymphangiogenesis has not yet been examined in this model. We find that young preneoplastic and older tumor-bearing Eμ-c-myc mice show increased growth of functional blood and lymphatic vessels in hematolymphoid tissues relative to otherwise genetically identical normal littermate control mice, which may be promoted at least in part by VEGF production from B lymphocytes.
Materials and Methods
Animals
Eμ-c-myc (C57Bl/6J) transgenic mice 27 obtained from Jackson Laboratories (West Grove, PA) were maintained under specific pathogen-free conditions, and were genotyped by polymerase chain reaction (PCR) according to the supplier’s instructions. Mice were euthanized with CO2, and dissected tissues were snap-frozen in OCT (Sakura Finetek, Torrance, CA) before cryosectioning. Femur marrow was extruded by cutting off the ends of the bone and applying air pressure from a 10-mlsyringe with a 21-gauge needle, before freezing in ornithine carbamyl transferase (OCT). For paraffin sectioning, whole femur bones were formalin- or zinc-fixed 30 and decalcified by 10% nitric acid treatment before embedding. Experimental methods involving animals were approved by the University of Washington and Fred Hutchinson Cancer Research Center Animal Care and Use Committees.
Immunostaining
Serially sectioned organs from 4 to 10 pairs of Eμ-c-myc and wild-type C57Bl/6J littermates were examined in each study. For MECA-32 immunohistochemistry of zinc-fixed (BD Biosciences, San Jose, CA) and decalcified paraffin sections of bone marrow, 2-μm sections were incubated sequentially with MECA-32 antibody (BD Biosciences), biotinylated rabbit anti-rat antibody (Dako, Carpinteria, CA), and Vectastain Universal Elite ABC kit (Vector Laboratories, Burlingame, CA), followed by DAB/NiCl2 detection and Acridine Orange/Safronin O counterstaining. For laminin immunohistochemistry (Sigma, St. Louis, MO), formalin-fixed sections were treated with proteinase K (ProK; Dako) for 5 minutes before immunostaining for laminin.
Eight micrometer cryosections were immunostained with the following antibodies and fixation conditions: for c-Myc (Oncogene Research Products, La Jolla, CA) or Prox-1 antibodies (generously provided by G. Oliver 31 ) sections were fixed in 4% paraformaldehyde for 10 minutes, and permeabilized with 0.2% Triton X-100 in saline for 2 minutes; for MECA-32, laminin, digoxygenin-labeled-10.1.1, 32 and VEGF (sc-507; Santa Cruz Biotechnology, Santa Cruz, CA) sections were fixed in acetone for 10 minutes, dried for 15 minutes, and fixed in 10% formalin for 10 minutes; and for affinity-purified anti-VEGFR-2 (generously provided by R. Brekken 33 ) or VEGFR-3 (R and D Systems Inc., Minneapolis, MN) sections were fixed in acetone for 5 minutes. Endogenous peroxidase or alkaline phosphatase was inhibited by 30 minutes of treatment with 3% hydrogen peroxide, or with levamisole (Vector Laboratories), respectively. Immunohistochemical staining was detected with Vector VIP or Vector Black, followed by Methyl Green counterstaining (Vector Laboratories). For immunofluorescent detection, sections were mounted in Vectashield (Vector Laboratories), and imaged by confocal microscopy, using a Leica SP1 confocal microscope. Stacks of optical sections were collected by optical z sectioning (z step = 0.73 μm). For electron microscopy, BALB/C mice were briefly perfused with 2% paraformaldehyde, 1 mmol/L CaCl2, and 0.1 mol/L cacodylate pH 7.4, before mesenteric lymph nodes were dissected and embedded, and 10.1.1 immunostaining detected with horseradish peroxidase and 3,3′-diaminobenzidine (DAB), as previously described. 32
Dye Injection Analysis of Blood and Lymph Circulation
Eight- to 10-week-old mice were anesthetized by intraperitoneal injection of 87 mg/kg ketamine and 13 mg/kg xylazine. Fluorescein isothiocyanate (FITC)-labeled Lycopersicon esculentum lectin (0.1 ml; Vector Laboratories) was injected into the tail vein to label functional blood vessels. 34 Two minutes later, tissues were rapidly dissected and snap-frozen in OCT. Bone marrow was extruded from the bone before freezing in OCT. In some experiments mice were perfused before harvesting tissues, using an 18-gauge blunt needle placed in the left ventricle to deliver 4% paraformaldehyde at 120 mmHg. The right atrium was incised to allow flow of fixative. Serial sections through each tissue were analyzed by fluorescence or confocal microscopy.
Functional blood vessel density was assessed by fluorescence microscope examination of representative 50-μm cryosections of bone marrow or lymph nodes from FITC-lectin-injected mice. The number of discrete FITC-labeled vessels in three representative and non-overlapping ×400 fields from each section (covering most of the tissue) were counted in a double-blind manner and were averaged together. Vessels in sections from five pairs of mice were counted, and Student’s t-tests of significance were performed.
For lymph flow measurement, the rear footpads of anesthetized mice were injected with 20 μl TRITC-dextran (Sigma), and popliteal and iliac lymph nodes were harvested 5 to 30 minutes later. Cryosections of lymph nodes from TRITC-dextran-injected mice were dried for 1 hour at room temperature, fixed with 4% paraformaldehyde for 20 minutes, and mounted in Vectashield. Representative sections were imaged by confocal microscopy at ×200 magnification. The MetaMorph computer program (Universal Imaging Corp., Downington, PA) was used to measure the spread of dye through each lymph node. The area filled with dye in confocal z stack images covering most of each node was measured in Eμ-c-myc and wild-type lymph nodes. Images from five pairs of mice were detected under the same imaging conditions through each section, and pixel intensity was thresholded relative to uninjected lymph node sections.
B-Cell Purification and Immunoblotting Analysis
Tissues from 4-week-old Eμ-c-myc mice and wild-type littermates were homogenized, and protein was measured by Bradford assay. Splenic B cells were purified by mashing spleens between the frosted ends of microscope slides, followed by erythrocyte lysis, as previously described. 35 T cells were depleted by incubating samples with anti-Thy2 monoclonal antibody, followed by treatment with guinea pig complement (Invitrogen, Carlsbad, CA). The resulting B cells are typically 90% pure, as determined by flow cytometry. B cells were lysed by incubation in 50 mmol/L Tris pH 8.0, 150 mmol/L NaCl, 1% Nonidet P40, 1 mmol/L dithiothreitol, with complete protease inhibitor cocktail (Roche, Indianapolis, IN) on ice for 30 minutes, with occasional mixing, and centrifuged 10 minutes at 14,000 × g to remove debris. Lysate protein was bound to 50 μl heparin-agarose (Sigma) for 1 hour at 4°C, eluted by 0.5% sodium dodecyl sulfate treatment at 95°C for 5 minutes, and protein was measured by Bradford assay. Twenty micrograms of protein from each preparation were immunoblotted and probed with VEGF antibody, and then reprobed with actin antibody (Sigma).
Results
Angiogenesis and Increased Blood Circulation in Bone Marrow of Young Eμ-c-myc Mice
c-Myc-expressing B-cell progenitors proliferate and accumulate within the bone marrow of young Eμ-c-myc mice, 28 suggesting that this model could be useful for analysis of bone marrow angiogenesis. Immunohistochemical staining confirmed that c-Myc-expressing B cells predominate within the marrow of 4-week-old Eμ-c-myc mice (Figure 1A) ▶ , while other hematopoeitic progenitors are reduced, in agreement with previous studies. 28 In contrast, bone marrow from C57Bl6/J wild-type littermates contained few c-Myc-expressing cells. To determine whether c-Myc-expressing B cells induce angiogenesis within the bone marrow, the vasculature of marrow from Eμ-c-myc and wild-type littermates was assessed by immunostaining decalcified and paraffin-embedded femur sections with the MECA-32 antibody, which recognizes most vascular endothelium. 36 Bone marrow from 4- or 8-week-old Eμ-c-myc mice showed numerous small vessels dispersed throughout the marrow, while vessels were sparse in wild-type marrow (Figure 1B ▶ , arrows). Venous sinuses, which are a major feature of bone marrow vasculature, can be detected by their discontinuous laminin coat. 37 Immunostaining for laminin revealed these larger erythrocyte-filled sinuses, which are markedly increased in size and number in Eμ-c-myc bone marrow relative to littermate control bone marrow (Figure 1C ▶ , arrows). These findings indicate that accumulation of c-Myc-expressing progenitors promotes growth of blood vessels and venous sinuses within the marrow. The laminin coating of these sinuses and vessels is much thicker in Eμ-c-myc bone marrow, suggesting that c-Myc also increases laminin deposition as the vessels grow.
Figure 1.

c-Myc-expressing B cells increase the functional vasculature in bone marrow of Eμ-c-myc mice. A: Bone marrow cryosections immunostained for c-Myc show accumulation of c-Myc-expressing B-cell progenitors within Eμ-c-myc marrow from 4-week-old mice, while c-Myc-expressing cells are rare in wild-type littermate marrow. B: Paraffin sections stained for MECA-32 show increased number and size of small vessels (arrows) in 8-week-old Eμ-c-myc mice, relative to littermate controls. C: Paraffin sections stained for laminin reveal increased venous sinuses (arrows) and small blood vessels in Eμ-c-myc mice. D: Cryosections from FITC-lectin-injected mice were immunostained with Texas Red-labeled laminin antibody. The laminin-coated venous sinuses and blood vessels are functional, as the FITC- and Texas Red-laminin immunostaining decorate the same vessels. E: 50-μm bone marrow cryosections from FITC-lectin-injected mice demonstrate increased functional FITC-labeled vessels in Eμ-c-myc mice relative to littermates. F: As a control, 50-μm kidney cryosections from lectin-injected mice show similar blood flow in glomeruli of control and Eμ-c-myc mice. Panels are shown at ×200 magnification; scale bars are 50 μm.
While tumor cells can induce a strong angiogenic response, the newly formed vasculature can be defective. 34,38 We therefore examined whether c-Myc-induced angiogenesis produces functional vessels, by intravenously injecting 8-week-old mice with FITC-labeled L. esculentum lectin, which specifically binds to vascular endothelium. 34 Functional vessels labeled with circulating FITC-labeled lectin were compared with those labeled by Texas Red-laminin immunostaining. Confocal imaging demonstrates that intravenously injected FITC-labeled lectin fills nearly all of the laminin-coated venous sinuses and smaller blood vessels of Eμ-c-myc mice (Figure 1D) ▶ . In wild-type bone marrow, blood vessels are small and sparse, and show faint laminin staining (Figure 1D) ▶ . The co-localization of laminin-coated vessels and injected lectin indicates that the venous sinuses and blood vessels identified by laminin immunostaining in Eμ-c-myc mice are functional. Moreover, these studies indicate that laminin may be a useful marker to reveal the venous sinuses of the bone marrow, which are not detected using standard vascular markers such as MECA-32 (Figure 1B) ▶ .
The lectin injection assay was used to analyze the effect of c-Myc-induced angiogenesis on the bone marrow blood supply, using 50 μm thick cryosections to survey the entire marrow from wild-type and Eμ-c-myc mice. Confocal imaging of FITC-labeled lectin demonstrated that blood flow is greatly increased in marrow from Eμ-c-myc mice, while functional vessels are rare in control marrow (Figure 1E) ▶ . This increase in circulation is specific to Eμ-c-myc bone marrow, as the kidney glomeruli (Figure 1F) ▶ , liver, intestine, and skin (data not shown) from wild-type and Eμ-c-myc mice showed equivalent blood flow. The increased density of functional vessels in Eμ-c-myc marrow was confirmed by counting discrete FITC-lectin-labeled vessels in 50-μm cryosections from five pairs of mice. Functional vessel density increases more than threefold in bone marrow from Eμ-c-myc mice (Figure 2) ▶ . This increase is significant by t-test (P < 0.01%). These results indicate that c-Myc-induced angiogenesis increases the blood supply to the bone marrow, which could support the sustained production of B-cell progenitors observed in bone marrow of Eμ-c-myc mice.
Figure 2.
Functional blood vessel density is increased in bone marrow from Eμ-c-myc mice. The number of discrete FITC-lectin-labeled vessels were counted per 400-μm field, in 50-μm bone marrow cryosections from five pairs of wild-type and Eμ-c-myc FITC lectin-injected mice. Mean functional vessel density and standard errors are shown.
Angiogenesis and Lymphangiogenesis in Eμ-c-myc Lymph Nodes
Lymph nodes of Eμ-c-myc mice grow 2 to 4 times larger than wild-type nodes by 4 weeks of age, due to accumulation of B lymphocytes. 29 We sought to determine whether c-Myc produced by mature B lineage cells in the periphery also induces angiogenesis within lymph nodes. Cryosections of lymph nodes from 4-week-old Eμ-c-myc or control mice were first immunostained with c-Myc antisera, to evaluate the representation of c-Myc-positive cells. c-Myc-expressing cells are distributed throughout the cortex and medulla of lymph nodes from Eμ-c-myc mice (Figure 3A) ▶ and they show high nuclear c-Myc expression, while few cells from wild-type lymph nodes show detectable c-Myc expression (Figure 3B) ▶ . We then evaluated the relative representation of the vasculature within Eμ-c-myc and littermate control-lymph nodes by immunostaining with the MECA-32 antibody. MECA-32-positive blood vessels are increased in number and size throughout the lymph nodes of Eμ-c-myc mice, relative to wild-type nodes (Figure 3C) ▶ . The majority of these vessels were also detected with antisera to the vascular endothelial markers von Willebrand factor or CD31 (data not shown). Hence, lymph nodes from Eμ-c-myc mice contain increased blood vessels relative to lymph nodes from normal littermate control mice.
Figure 3.
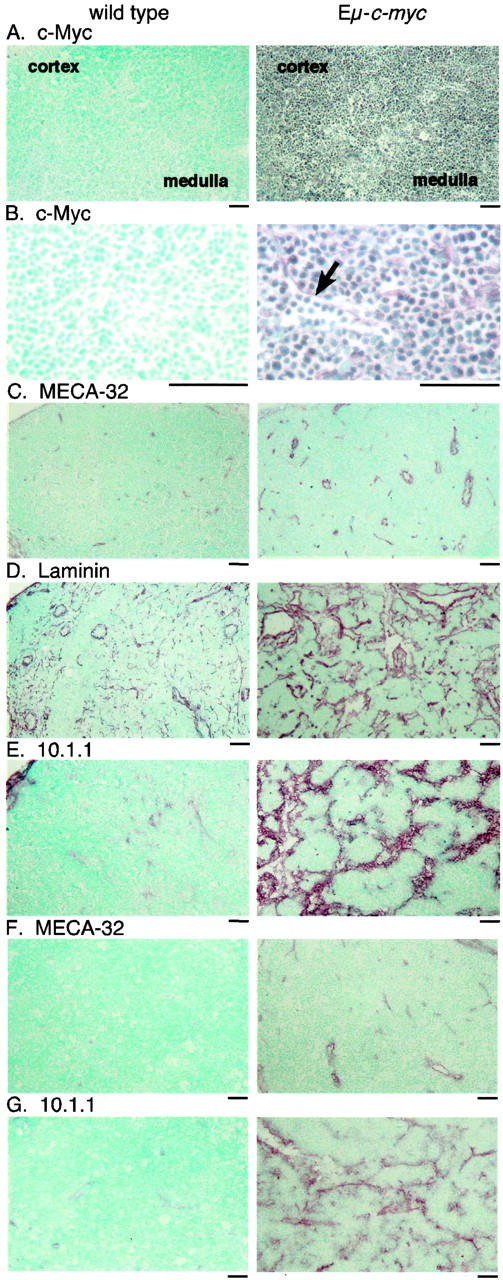
Angiogenesis and lymphangiogenesis in lymph nodes and lymphomas from Eμ-c-myc mice. A: Immunostaining of cryosections from 4-week-old Eμ-c-myc mice demonstrates that the cortex and medulla of lymph nodes are filled with c-Myc-expressing B cells, while few c-Myc-expressing cells are found in wild-type littermate nodes, shown at ×100 magnification. B: At ×400 magnification, sinuses are observed that are filled with c-Myc-expressing B cells in Eμ-c-myc mice (arrow). C: The MECA-32 antibody demonstrates increased blood vessels in Eμ-c-myc lymph nodes, at ×100 magnification. D: Laminin antisera shows pronounced alterations of the architecture of cortical and medullary sinuses in Eμ-c-myc lymph nodes. E: The 10.1.1 antibody identifies increased lymphatic sinuses throughout lymph nodes from 4-week-old Eμ-c-myc mice, and much smaller sinuses mainly in the cortex of wild-type lymph nodes. F: Primary lymphomas from 12-week-old Eμ-c-myc mice show continuing blood vessel growth by MECA-32 immunostaining, while lymph nodes from wild-type littermates show sparse vessels. G: Lymphomas contain abnormal enlarged 10.1.1 sinuses, that are not found in wild-type littermates. Scale bars, 50 μm.
One feature of Eμ-c-myc lymph nodes which was especially striking was the abundance of enlarged sinuses, which are filled with c-Myc-expressing B cells (Figure 3B ▶ , arrow). Immunostaining of lymph nodes from Eμ-c-myc and littermate control mice with laminin antisera revealed extensive alteration of the architecture of the lymph node (Figure 3D) ▶ . Eμ-c-myc lymph nodes are filled with enlarged sinuses relative to wild-type lymph nodes, which could represent lymphatic sinuses. The increased laminin immunostaining observed in Eμ-c-myc lymph nodes (Figure 3D) ▶ indicates that basement membrane deposition on these vessels and sinuses is increased by c-Myc-expressing B cells.
The enlarged sinuses observed in Eμ-c-myc nodes were further characterized by immunostaining with a 10.1.1 monoclonal antibody raised against murine thymic stroma, 32 which also recognized lymphatic vessels. The 10.1.1 antibody decorated the enlarged sinuses in Eμ-c-myc nodes, and the much smaller cortical and medullary lymphatic sinuses of wild-type nodes (Figure 3E) ▶ . These increased vessels and sinuses were consistently observed in lymph nodes from 4-week-old Eμ-c-myc mice during early stage polyclonal pre-B-cell expansion, before oligoclonal B-cell tumors arise at 12 to 16 weeks of age.
MECA-32 immunostaining of primary lymphomas from 12-week-old mice showed that blood vessel growth continues during lymphomagenesis, while vessels are sparse in wild-type littermate lymph nodes at 12 weeks of age (Figure 3F) ▶ . 10.1.1 immunostaining demonstrated enlarged sinuses throughout the tumor, while lymph nodes from 12-week-old wild-type littermates showed few 10.1.1-positive sinuses (Figure 3G) ▶ . The tumor sinuses are filled with c-Myc-expressing lymphocytes (data not shown) as was observed in lymph nodes (Figure 3B) ▶ . These findings indicate that the growth of blood vessels and sinuses is sustained during the formation of lymphomas.
The 10.1.1 Antibody Specifically Recognizes Lymphatic Endothelium
The finding that the 10.1.1 antibody stains lymphocyte-filled sinuses of lymph nodes suggested that this antibody could be a useful marker for lymphatic endothelium. The specificity of the 10.1.1 antibody for lymphatic endothelium was further characterized by immunoelectron microscopy. 10.1.1 immunostaining is restricted to the plasma membrane of lymphatic endothelium in lymph nodes (Figure 4A ▶ , arrows). A trabecula of lymphatic endothelium extending across the lymphatic sinus is also 10.1.1-positive (Figure 4A ▶ , arrowhead). In contrast, adjacent lymphocytes and other cells do not express 10.1.1. Moreover, adjacent capillary endothelium does not express 10.1.1 (Figure 4B ▶ , arrowhead), supporting the idea that 10.1.1. is not expressed on vascular endothelium.
Figure 4.

10.1.1 is expressed on the plasma membrane of lymphatic endothelium. A: Immunoelectron microscopy of 10.1.1 staining of lymph node shows that 10.1.1 localizes to the plasma membrane of lymphatic endothelium (arrows), and does not react with other cell types. A characteristic trabecula of lymphatic endothelium crossing the lymphatic sinus is also 10.1.1-positive (arrowhead) Magnification, ×5400. B: 10.1.1 is expressed on the surface of lymphatic endothelium (arrow), while it does not react with vascular endothelium on an adjacent capillary (arrowhead). Magnification, ×7000.
Immunofluorescence staining was used to further characterize the specificity of the 10.1.1 antibody, in lymph nodes from mice injected with biotinylated L. esculentum lectin to label blood vessels. The resulting Texas Red streptavidin-labeled blood vessels are distinct from the 10.1.1-labeled lymphatic sinuses shown at low magnification (Figure 5A) ▶ and at high magnification (Figure 5B) ▶ , indicating that 10.1.1-positive sinuses are not blood vessels. Further evidence that the 10.1.1 antibody is specific for lymphatic endothelium was obtained by immunostaining with two markers that are expressed by lymphatic endothelium. The Prox-1 transcription factor is specifically expressed in lymphatic endothelium. 31 Immunostaining for 10.1.1 and Prox-1 reveals that Prox-1 nuclear staining is restricted to 10.1.1-positive lymph node sinuses (Figure 5C) ▶ . VEGFR-3 is also generally restricted to lymphatic endothelium, 14 and VEGFR-3 staining decorates the same lymph node sinuses strongly stained with 10.1.1 (Figure 5D ▶ , arrow), supporting the idea that 10.1.1 recognizes lymphatic endothelium. The immunostaining pattern of VEGFR-2 was also analyzed, as this receptor is generally restricted to vascular endothelium. 33,39 We were surprised to find that the VEGFR-2 antisera stained the same 10.1.1-positive sinuses of lymph nodes (Figure 5E ▶ , arrow), in addition to staining 10.1.1-negative small blood vessels (Figure 5E ▶ , arrowhead). The strong expression of VEGFR-2 on the lymphatic sinuses was confirmed by co-staining with the VEGFR-2 and MECA-32 antibodies. The large sinuses express VEGFR-2 only (Figure 5F ▶ , arrow), while smaller blood vessels and high endothelial venules express both VEGFR-2 and MECA-32 (Figure 5F ▶ , arrowhead). The same staining specificity was observed in blood and lymph vessels from wild-type lymph nodes (data not shown). Taken together, these data indicate that 10.1.1 is a specific marker for lymphatic endothelium within lymph nodes. Our finding that VEGFR-2 and VEGFR-3 are both expressed on the lymphatic endothelium of lymph nodes suggests that activation of either or both receptors could promote the expansion of the lymphatic sinuses of Eμ-c-myc mice.
Figure 5.

The 10.1.1 antibody recognizes VEGFR-3 and VEGF-2-positive lymphatic endothelium in lymph nodes, and lymphatic vessels of the dermis. Lymph node cryosections from biotinylated lectin-injected mice were stained with Texas Red streptavidin to detect functional blood vessels, and with 10.1.1 to detect lymphatic endothelium, shown at ×400 magnification (A) and ×1000 magnification (B). C: The Prox-1 transcription factor (Alexa 568-labeled) and 10.1.1 (FITC-labeled) antibodies both stain lymphatic endothelium. D: Sections immunostained for VEGFR-3 (Alexa 568-labeled) and 10.1.1 (FITC-labeled) both identify large lymphatic sinuses (arrow). E: Sections immunostained for VEGFR-2 (Texas Red-labeled) and 10.1.1 (FITC-labeled) both identify large lymphatic sinuses (arrow). VEGFR-2 also stains small blood vessels, while 10.1.1 does not (arrowhead). F: Texas Red-labeled VEGFR-2 identifies larger lymphatic sinuses (arrow), and smaller blood vessels (arrowhead), while FITC-labeled MECA-32 immunostaining identifies blood vessels only. G: Control Texas Red anti-rabbit and FITC anti-rat immunostaining with nonspecific rabbit and rat IgG, respectively. H: Newborn skin immunostained with 10.1.1 (FITC-labeled) and CD31 (Alexa 568-labeled) antibodies show low CD31 expression on 10.1.1-positive lymphatic endothelium (arrow), and high expression on vascular endothelium (arrowhead). I: 10.1.1 (FITC-labeled) and MECA-32 (Alexa 568-labeled) immunostaining of an adjacent skin cryosection demonstrates that MECA-32 stains blood vessels (arrowhead), while 10.1.1 stains lymphatic vessels (arrow). Scale bars, 25 μm.
The expression of 10.1.1 in other tissues was examined by immunohistochemical staining, to determine whether this antibody recognizes lymphatic vessels in organs other than the lymph node. Confocal microscopy of newborn mouse skin immunostained with 10.1.1 (FITC-labeled) and CD31 (Alexa 568-labeled) demonstrates that 10.1.1-positive lymphatic vessels show low CD31 staining (Figure 5H ▶ , arrow), relative to adjacent blood vessels which show high CD31 staining (Figure 5H ▶ , arrowhead). Moreover, the same 10.1.1-positive lymphatics of an adjacent section are negative for MECA-32 (Figure 5I ▶ , arrow), while adjacent blood vessels stain with MECA-32 (Figure 5I ▶ , arrowhead). 10.1.1 also recognizes lymphatic vessels in the colon submucosa and in lacteal lymphatics of the small intestine (data not shown). Moreover, the size and distribution of lymphatics in these tissues, which do not contain c-Myc-expressing lymphocytes, 29 is similar in wild-type and Eμ-c-myc mice (data not shown). Taken together, these studies demonstrate that 10.1.1 recognizes lymphatic endothelium within lymph nodes and other organs of wild-type and Eμ-c-myc mice. 10.1.1 immunostaining identifies a strong lymphangiogenic phenotype in Eμ-c-myc lymph nodes populated with c-Myc-expressing B lymphocytes, while the lymphatic vessels remain normal in tissues that do not contain significant numbers of B lymphocytes. The 10.1.1 antibody should be useful for studies of lymphangiogenesis, as few reagents specific for murine lymphatic endothelium are available thus far. 21
Dye Injection Reveals Increased Blood and Lymph Circulation in Eμ-c-myc Lymph Nodes
To determine whether c-Myc-induced angiogenesis results in functional increases in lymph node blood supply, the circulation within popliteal and mesenteric lymph nodes from FITC lectin-injected mice was assessed by fluorescence microscopy of 50-μm cryosections through mesenteric lymph nodes. The number and diameter of large and small FITC-lectin-labeled blood vessels was uniformly increased throughout the nodes from 8-week-old Eμ-c-myc mice relative to wild-type littermates (Figure 6A) ▶ . This observation was confirmed by counting vessels in representative cryosections from five pairs of mice, which demonstrated a threefold increase in functional blood vessel density in Eμ-c-myc nodes (Figure 7A) ▶ that is significant by t-test (P < 0.001%). The increased circulation of FITC-lectin correlates with the increased blood vessels observed by MECA-32 immunohistochemistry of Eμ-c-myc lymph nodes (Figure 3C) ▶ , indicating that these new blood vessels are indeed functional.
Figure 6.
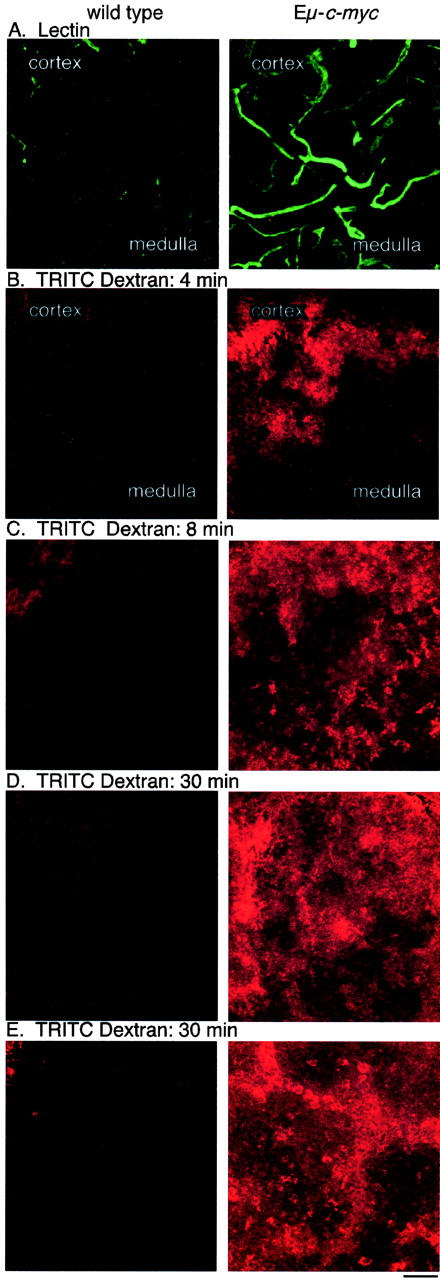
Functional increase in blood and lymph circulation in lymph nodes from Eμ-c-myc mice. A: FITC lectin-labeled functional blood vessels are increased in 50-μm cryosections of nodes from lectin-injected Eμ-c-myc mice relative to wild-type littermates. B: TRITC-dextran-labeled lymph flow is increased throughout the cortex and medulla of Eμ-c-myc popliteal nodes from mice receiving TRITC-dextran footpad injections, while small amounts of dye are limited to the cortical sinuses of nodes from wild-type littermates, at 4 minutes after injection. After 8 minutes (C) or 30 minutes (D), lymph flow through the popliteal node continues at higher rates in Eμ-c-myc mice. E: Lymph flow into the draining iliac lymph nodes is much higher in Eμ-c-myc mice at 30 minutes after dye injection. Magnification, ×200; scale bars, 50 μm.
Figure 7.

Functional blood vessel density and lymph flow is increased in lymph nodes from Eμ-c-myc mice. A: The number of FITC-lectin-labeled vessels per 400 μm were counted field in 50-μm popliteal lymph node cryosections from five pairs of wild-type and Eμ-c-myc FITC lectin-injected mice. Mean functional vessel density and standard errors are shown. B: Area measurement using the MetaMorph computer program was used to assess the spread of TRITC-dextran through the cortex and medulla of popliteal lymph nodes, at 8 minutes after footpad injection of Eμ-c-myc and littermate controls. The area of 50-μm lymph node cryosections filled with dye was measured in five pairs of mice, and lymph flow was expressed in arbitrary area units. Standard errors are shown.
We next examined whether the lymphangiogenesis observed in Eμ-c-myc lymph nodes results in an increase in lymph circulation. Anesthetized Eμ-c-myc and littermate control mice were injected with TRITC-dextran subcutaneously in the footpads, and the draining popliteal nodes were removed after 4 to 30 minutes. Confocal imaging of 50-μm cryosections demonstrated that TRITC-dextran uptake into lymph nodes from Eμ-c-myc mice was consistently higher and was distributed throughout the cortex and medulla, while in wild-type nodes only a small amount of dye reached the cortical sinuses by 4 (Figure 6B) ▶ or 8 minutes (Figure 6C) ▶ after injection into the footpad. The spread of TRITC-dextran through popliteal nodes at the 8-minute timepoint was compared in confocal images from five pairs of Eμ-c-myc and wild-type mice, using the MetaMorph computer program to measure the area of each node which was filled with dye. The TRITC-dextran spread through Eμ-c-myc lymph nodes 23-fold more than through wild-type nodes during this interval (Figure 7B) ▶ , and this increase was statistically significant (P < 0.05%).
The popliteal nodes were examined at 20 and 30 minutes after injection of TRITC-dextran into the footpad, to determine whether nodes from wild-type mice show a delayed increase in lymph flow. At 20 minutes (data not shown) or 30 minutes (Figure 6D) ▶ after injection, lymph flow into the popliteal node is much higher in Eμ-c-myc mice, with perfusion of dye throughout the cortex and medullar of Eμ-c-myc nodes, while dye remains restricted to the cortex of wild-type nodes. Lymph flow through the iliac lymph node located on the dorsal peritoneum, which drains lymph from the popliteal node, was also assessed, to determine whether lymph flow to more central lymph nodes is increased in Eμ-c-myc mice. At 30 minutes after TRITC-dextran injection, iliac lymph nodes from Eμ-c-myc mice consistently showed much greater TRITC-dextran uptake and spread, relative to those of wild-type littermates (Figure 6E) ▶ . These findings indicate that the c-Myc-expressing B lymphocytes from Eμ-c-myc mice increase lymph flow both into and out of lymph nodes, which could facilitate the dissemination of tumor cells.
Increased VEGF Production by c-Myc-Expressing B Lymphocytes
VEGF is a potent vascular endothelial growth factor commonly expressed by tumors. 14 We used immunohistochemical staining to assess the relative amounts of VEGF in hematopoeitic tissues from Eμ-c-myc and littermate control mice at 4 weeks of age, to determine whether c-Myc expression in B lineage cells may stimulate VEGF production as a mechanism to induce vessel growth,. We found that VEGF is highly expressed in bone marrow and lymph nodes from young Eμ-c-myc mice (Figure 8, B and E) ▶ , relative to wild-type mice (Figure 8, A and D) ▶ . Immunostaining is observed over most but not all of the cells within these tissues. Lymphomas from older mice also express increased VEGF levels (data not shown). Since c-Myc-expressing B cells predominate within these tissues, B lymphocytes may be the source of this VEGF.
Figure 8.
VEGF accumulates in bone marrow and lymph nodes from Eμ-c-myc mice. Immunostaining of cryosections from bone marrow (A to C) or mesenteric lymph nodes (D to F) from 4-week-old wild-type or Eμ-c-myc littermates demonstrates VEGF accumulation in Eμ-c-myc tissues. A and D: Immunostaining of wild-type tissues with VEGF antibody demonstrates low levels of VEGF. B and E: Eμ-c-myc tissues immunostained with VEGF antibody show increased VEGF over most but not all lymphocytes. C and F: Eμ-c-myc tissues immunostained with nonspecific rabbit IgG. Magnification, ×400; scale bars, 50 μm.
Immunoblots of lymph nodes, spleen, and bone marrow from Eμ-c-myc and littermate control mice further revealed that the 23-kd 164 aa VEGF protein 40 is the major VEGF isoform expressed in hematolymphoid tissues, and expression of this isoform is increased in these c-Myc-expressing tissues (Figure 9A) ▶ . Reprobing of the blots with actin confirmed equal loading of protein from each pair of tissues (Figure 8B) ▶ . By contrast, VEGF abundance in kidney, a non-lymphoid organ, was the same in Eμ-c-myc and control mice, indicating that the increase in VEGF is specific to tissues containing large numbers of c-Myc-expressing B lymphocytes.
Figure 9.
The 164 aa VEGF isoform is increased in lymphoid tissues and in purified B cells from Eμ-c-myc mice. A: Western blot analysis under reducing conditions of VEGF, using 20 μg of protein homogenate from tissues of 4-week-old wild-type (wt) and Eμ-c-myc (c-Myc) mice, shown at left. B: The blot was re-probed for actin, as a loading control. The panels on the right show immunoblot analysis of VEGF and actin using heparin-binding protein fractions isolated from B lymphocytes, which were purified from 4-week-old wild-type and Eμ-c-myc spleens.
The increased VEGF production in Eμ-c-myc lymphoid tissues suggested that that this growth factor could mainly originate from the c-Myc-expressing B cells in these tissues. Lymphoid organs, however, contain other cell types such as macrophages and stromal cells, which could also produce VEGF. To determine whether c-Myc-expressing B cells are a source of VEGF, B lymphocytes were purified from the spleen. Heparin-agarose enrichment of lysates from purified B-cell lymphocytes was used to concentrate the heparin-binding 164 aa VEGF isoform, 41 as this factor is too rare to detect by immunoblotting of whole cell lysates. Immunoblot analysis consistently showed higher VEGF levels in the heparin-binding protein fraction from Eμ-c-myc B cells, relative to levels in wild-type B cells (Figure 9B) ▶ . Taken together, the immunohistochemistry and immunoblot results demonstrate that c-Myc-expressing B cells within these tissues are a major source of VEGF, which could promote the growth of vessels observed in Eμ-c-myc mice.
Discussion
Increased blood vessel density is a feature of most human leukemias 15,16 and is associated with the progression of multiple myeloma. 17 However, an understanding of the relationship between cellular transformation and angiogenesis in hematopoeitic cancers has been limited due in part to the lack of an animal model showing a similar angiogenic phenotype. In this study, we determined that B-lymphocyte-specific expression of c-Myc in bone marrow and lymph nodes derived from Eμ-c-myc mice induces early and persistent growth of blood vessels. In addition, analysis of blood flow in bone marrow and lymph nodes from Eμ-c-myc mice indicates that the majority of the new vessels formed are functional. 34,38 The ability of c-Myc to effectively increase blood supply during the initial polyclonal expansion phase, and through lymphomagenesis, indicates that c-Myc stimulates angiogenesis both before and after transformation, and that blood vessel growth during lymphomagenesis is not simply a consequence of the transformation process itself. The increase in blood vessel growth early during lymphomagenesis may support the c-Myc-induced polyclonal expansion of B-cell progenitors. c-Myc-induced angiogenesis following transformation may also support the rapid growth of tumors in this model, which can reach more than 1 cm in diameter by 12 weeks of age. c-Myc-induced blood vessel growth has also been observed when c-Myc is ectopically expressed in keratinocytes and pancreatic islet cells in transgenic mice, suggesting that this phenomenon is not restricted to B lymphocytes. 7,42 In humans, c-Myc is commonly overexpressed in solid tumors as well as in hematopoietic malignancies, 2 suggesting that this oncogene could promote growth of a variety of tumors at least in part through its ability to promote angiogenesis. A recent report that c-Myc is required for vascularization of embryos 43 suggests that c-Myc also plays a significant role in normal vascular physiology during development.
While numerous studies have revealed the importance of angiogenesis in normal cellular growth, development, and the pathogenesis of numerous diseases, much less is known about lymphangiogenesis. However, it is clear that insufficient lymphangiogenesis leads to incapacitating lymphedema, while excess lymphangiogenesis can promote metastatic spread of tumor cells to distant sites. 44 Hence, understanding the molecular mechanisms that control lymphangiogenesis are critically important for comprehending normal physiology and perhaps the pathophysiology of cancer. In this study, we find that lymph nodes from Eμ-c-myc mice show intensive growth of lymphatic sinuses and vessels, beginning during early stage polyclonal B-cell expansion, and continuing during lymphomagenesis. The enlarged lymphatic sinuses of Eμ-c-myc lymph nodes show increased lymph flow after TRITC-dextran injection into the footpad. Both the popliteal lymph node, which immediately drains the foot, and also the more central iliac lymph node, which drains from the popliteal lymph node, show greatly increased lymph flow in Eμ-c-myc mice, indicating that lymph flow both into and out of lymph nodes is amplified in Eμ-c-myc mice.
Our finding that accumulation of c-Myc-expressing lymphocytes stimulates lymph node lymphangiogenesis suggests an active mechanism by which tumor cells could metastasize to draining lymph nodes, and then to other organs. Tumor cells producing lymphangiogenic factors could induce the growth of lymphatic vessels, to facilitate spread of tumors to the lymph node. Once seeded into the draining lymph node, stimulation of lymphatic sinus growth, and the resulting increased lymph flow would then promote dissemination of tumor cells to distant sites. Consistent with this hypothesis, ectopic expression of VEGF-C or VEGF-D in tumor cells in various murine tissues induces lymphangiogenesis, metastasis to lymph nodes, 22,24 and tumor dissemination to other organs. 23 In addition, the enlarged lymphatic sinuses in Eμ-c-myc nodes and lymphomas are filled with c-Myc-expressing B cells, and lymphomas from Eμ-c-myc mice rapidly metastasize to many organs including the lung, which features c-Myc-expressing lymphocytes within lymphatic vessels. 29 In several human cancers, increased c-Myc expression correlates with poor prognosis, 2 which could at least in part reflect increased lymphatic metastasis. Further characterization of the Eμ-c-myc model should provide insight to the role of c-Myc in lymph node lymphangiogenesis and tumor metastasis.
The study of lymphangiogenesis in murine models is hampered by the lack of readily available markers. In this study, we used antibodies against a variety of antigens to characterize the lymphatic endothelium of lymph nodes from Eμ-c-myc and wild-type littermate mice. We find that the 10.1.1 antibody shows good specificity for lymphatic endothelium of lymph nodes and other tissues, as its staining coincides with that of VEGFR-3 and Prox-1, two other markers of lymphatic endothelium. The 10.1.1 antibody may be especially useful since it does not recognize vascular endothelium in normal tissues or in lymph nodes, unlike VEGFR-3, which can also be expressed on lymph node high endothelial venules 45 and tumor blood vessels. 46
The molecular mechanisms that regulate lymphangiogenesis in health or in disease states remain poorly characterized. However, the VEGF-C and VEGF-D members of the VEGF family of growth factors can induce lymphangiogenesis via interaction with VEGFR-3 expressed on lymphatic endothelium (reviewed by 13 ). A recent study has found that ectopic VEGF expression from subcutaneously delivered adenovirus vectors or from tumor implants also stimulates a strong local lymphangiogenic response, perhaps via interaction with VEGFR-2 expressed on adjacent lymphatic vessels. 47 In this study, we find that the lymphatic sinuses of wild-type or Eμ-c-myc lymph nodes express both VEGFR-2 and VEGFR-3. In addition, we find that purified c-Myc-expressing B lymphocytes from Eμ-c-myc mice show increased VEGF production. This VEGF could promote the growth of VEGFR-2-expressing blood vessels in the lymph nodes and bone marrow, and could also potentially drive the growth of VEGFR-2-expressing lymphatic sinuses within the lymph nodes. The blood and lymphatic vessels induced by treatment with purified VEGF are structurally and functionally abnormal. 39,47 However, the blood and lymphatic vessels of Eμ-c-myc mice are highly functional, suggesting that c-Myc expression induces additional endothelial growth factors. We find that low levels of VEGF-C and VEGF-D mRNA are expressed in c-Myc-expressing B lymphocytes by RT-PCR (data not shown), which could also contribute to the growth of lymphatic endothelium in Eμ-c-myc lymph nodes. Further studies will be required to distinguish the contributions of VEGF and other VEGF family ligands, and of VEGFR-2 and VEGFR-3 signaling, to the robust blood and lymph vessel growth observed in Eμ-c-myc mice.
Acknowledgments
We thank Rolf Brekken for generously providing the VEGFR-2 antisera, and Guillermo Oliver for a gift of PROX-1 antisera. We thank Sean Parghi, Meredith Klacking, and Andrea Nicks for excellent technical assistance. We thank Patricia Parsons-Wingerter, Maureen Ryan, Denny Liggitt, the FHCRC Pathology and Scientific Imaging Facilities for their helpful advice.
Footnotes
Address reprint requests to Alanna Ruddell, Fred Hutchinson Cancer Research Center, P.O. Box 19024, 1100 Fairview Avenue N. MS-C2–023, Seattle, WA 98109-1024. E-mail aruddell@fred.fhcrc.org.
Supported by NIH NCI grant R01 CA68328 to A. Ruddell, NIH NIAID grant R01 AI 24137 to A. Farr, and NIH NIAID grant K08 AJ01445 to B. Iritani.
References
- 1.Schmidt EV: The role of c-myc in cellular growth control. Oncogene 1999, 18:2988-2996 [DOI] [PubMed] [Google Scholar]
- 2.Nesbit CE, Tersak JM, Prochownik EV: MYC oncogenes and human neoplastic disease. Oncogene 1999, 18:3004-3016 [DOI] [PubMed] [Google Scholar]
- 3.Brandvold KA, Ewert DL, Kent SC, Neiman P, Ruddell A: Blocked B cell differentiation and emigration support the early growth of Myc-induced lymphomas. Oncogene 2001, 20:3226-3234 [DOI] [PubMed] [Google Scholar]
- 4.Iritani BM, Eisenman RN: c-Myc enhances protein synthesis and cell size during B lymphocyte development. Proc Natl Acad Sci USA 1999, 96:13180-13185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandvold KA, Neiman P, Ruddell A: Angiogenesis is an early event in the generation of myc-induced lymphomas. Oncogene 2000, 19:2780-2785 [DOI] [PubMed] [Google Scholar]
- 6.Ngo CV, Gee M, Akhtar N, Yu D, Volpert O, Auerbach R, Thomas-Tikhonenko A: An in vivo function for the transforming Myc protein: elicitation of the angiogenic phenotype. Cell Growth Differ 2000, 11:201-210 [PMC free article] [PubMed] [Google Scholar]
- 7.Pelengaris S, Littlewood T, Khan M, Elia G, Evan G: Reversible activation of c-Myc in skin: induction of a complex neoplastic phenotype by a single oncogenic lesion. Mol Cell 1999, 3:565-577 [DOI] [PubMed] [Google Scholar]
- 8.Felsher DW, Bishop JM: Transient excess of MYC activity can elicit genomic instability and tumorigenesis. Proc Natl Acad Sci USA 1999, 96:3940-3944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watson PH, Safneck JR, Le K, Dubik D, Shiu RP: Relationship of c-myc amplification to progression of breast cancer from in situ to invasive tumor and lymph node metastasis. J Natl Cancer Inst 1993, 85:902-907 [DOI] [PubMed] [Google Scholar]
- 10.Sato K, Miyahara M, Saito T, Kobayashi M: c-myc mRNA overexpression is associated with lymph node metastasis in colorectal cancer. Eur J Cancer 1994, 30A:1113-1117 [DOI] [PubMed] [Google Scholar]
- 11.Riou G, Le MG, Favre M, Jeannel D, Bourhis J, Orth G: Human papillomavirus-negative status and c-myc gene overexpression: independent prognostic indicators of distant metastasis for early-stage invasive cervical cancers. J Natl Cancer Inst 1992, 84:1525-1526 [DOI] [PubMed] [Google Scholar]
- 12.Kawashima K, Shikama H, Imoto K, Izawa M, Naruke T, Okabayashi K, Nishimura S: Close correlation between restriction fragment length polymorphism of the L-MYC gene and metastasis of human lung cancer to the lymph nodes and other organs. Proc Natl Acad Sci USA 1988, 85:2353-2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alitalo K, Carmeliet P: Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell 2002, 1:219-227 [DOI] [PubMed] [Google Scholar]
- 14.Saaristo A, Karpanen T, Alitalo K: Mechanisms of angiogenesis and their use in the inhibition of tumor growth and metastasis. Oncogene 2000, 19:6122-6129 [DOI] [PubMed] [Google Scholar]
- 15.Aguayo A, Kantarjian H, Manshouri T, Gidel C, Estey E, Thomas D, Koller C, Estrov Z, O’Brien S, Keating M, Freireich E, Albitar M: Angiogenesis in acute and chronic leukemias and myelodysplastic syndromes. Blood 2000, 96:2240-2245 [PubMed] [Google Scholar]
- 16.Perez-Atayde AR, Sallan SE, Tedrow U, Connors S, Allred E, Folkman J: Spectrum of tumor angiogenesis in the bone marrow of children with acute lymphoblastic leukemia. Am J Pathol 1997, 150:815-821 [PMC free article] [PubMed] [Google Scholar]
- 17.Vacca A, Ribatti D, Presta M, Minischetti M, Iurlaro M, Ria R, Albini A, Bussolino F, Dammacco F: Bone marrow neovascularization, plasma cell angiogenic potential, and matrix metalloproteinase-2 secretion parallel progression of human multiple myeloma. Blood 1999, 93:3064-3073 [PubMed] [Google Scholar]
- 18.Vacca A, Ribatti D, Ruco L, Giacchetta F, Nico B, Quondamatteo F, Ria R, Iurlaro M, Dammacco F: Angiogenesis extent and macrophage density increase simultaneously with pathological progression in B-cell non-Hodgkin’s lymphomas. Br J Cancer 1999, 79:965-970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kossakowska AE, Hinek A, Edwards DR, Lim MS, Zhang C-L, Breitman DR: Proteolytic activity of human non-Hodgkins lymphomas. Am J Pathol 1998, 152:565-576 [PMC free article] [PubMed] [Google Scholar]
- 20.Vacca A, Ribatti D, Iurlaro M, Albini A, Minischetti M, Bussolino F, Pellegrino A, Ria R, Rusnati M, Presta M, Vincenti V, Persico MG, Dammacco F: Human lymphoblastoid cells produce extracellular matrix-degrading enzymes and induce endothelial cell proliferation, migration, morphogenesis, and angiogenesis. Int J Clin Lab Res 1998, 28:55-68 [DOI] [PubMed] [Google Scholar]
- 21.Stacker SA, Baldwin ME, Achen MG: The role of tumor lymphangiogenesis in metastatic spread. EMBO J 2002, 16:922-934 [DOI] [PubMed] [Google Scholar]
- 22.Mandriota SJ, Jussila L, Jeltsch M, Compagni A, Baetens D, Prevo R, Banerji S, Huarte J, Montesano R, Jackson DG, Orci L, Alitalo K, Christofori G, Pepper MS: Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J 2001, 20:672-682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M: Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med 2001, 7:192-198 [DOI] [PubMed] [Google Scholar]
- 24.Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H, Achen MG: VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med 2001, 7:186-191 [DOI] [PubMed] [Google Scholar]
- 25.He Y, Kozaki K, Karpanen T, Koshikawa K, Yla-Herttuala S, Takahashi T, Alitalo K: Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J Natl Cancer Inst 2002, 94:819-825 [DOI] [PubMed] [Google Scholar]
- 26.Kubo H, Cao R, Brakenhielm E, Makinen T, Cao Y, Alitalo K: Blockade of vascular endothelial growth factor receptor-3 signaling inhibits fibroblast growth factor-2-induced lymphangiogenesis in mouse cornea. Proc Natl Acad Sci USA 2002, 99:8868-8873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams JM, Harris AW, Pinkert CA, Corcoran LM, Alexander WS, Cory S, Palmiter RD, Brinster RL: The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature 1985, 318:533-538 [DOI] [PubMed] [Google Scholar]
- 28.Langdon WY, Harris AW, Cory S, Adams JM: The c-myc oncogene perturbs B lymphocyte development in Eu-myc transgenic mice. Cell 1986, 47:11-18 [DOI] [PubMed] [Google Scholar]
- 29.Harris AW, Pinkert CA, Crawford M, Langdon WY, Brinster RL, Adams JM: The E μ-c-myc transgenic mouse: a model for high-incidence spontaneous lymphoma and leukemia of early B cells. J Exp Med 1988, 167:353-371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beckstead J: A simple technique for preservation of fixation-sensitive antigens in paraffin-embedded tissues. J Histochem Cytochem 1994, 42:8-13 [DOI] [PubMed] [Google Scholar]
- 31.Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G: An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J 2002, 21:1505-1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farr A, Nelson A, Hosier S, Kim A: A novel cytokine-responsive cell surface glycoprotein defines a subset of medullary thymic epithelium in situ. J Immunol 1993, 150:116-1171 [PubMed] [Google Scholar]
- 33.Brekken RA, Huang X, King SW, Thorpe PE: Vascular endothelial growth factor as a marker of tumor endothelium. Cancer Res 1998, 58:1952-1959 [PubMed] [Google Scholar]
- 34.Hashizume H, Baluk P, Morikawa S, McLean JW, Thurston G, Roberge S, Jain RK, McDonald DM: Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol 2000, 156:1363-1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iritani BM, Forbush KA, Farrar MA, Perlmutter RM: Control of B cell development by Ras-mediated activation of Raf. EMBO J 1997, 16:7019-7031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leppink DM, Bishop DK, Sedmak DD, Henry ML, Ferguson RM, Streeter PR, Butcher EC, Orosz CG: Inducible expression of an endothelial cell antigen on murine myocardial vasculature in association with interstitial cellular infiltration. Transplantation 1989, 48:874-877 [DOI] [PubMed] [Google Scholar]
- 37.Nilsson SK, Debatis ME, Dooner MS, Madri JA, Quesenberry PJ, Becker PS: Immunofluorescence characterization of key extracellular matrix proteins in murine bone marrow in situ. J Histochem Cytochem 1998, 46:371-377 [DOI] [PubMed] [Google Scholar]
- 38.Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Wiegand SJ: Vessel cooption, regression, and growth in tumors mediated by angiopoeitins and VEGF. Science 1999, 284:1994-1997 [DOI] [PubMed] [Google Scholar]
- 39.Feng D, Nagy JA, Brekken RA, Pettersson A, Manseau EJ, Pyne K, Mulligan R, Thorpe PE, Dvorak HF, Dvorak AM: Ultrastructural localization of the vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) receptor-2 (FLK-1, KDR) in normal mouse kidney and in the hyperpermeable vessels induced by VPF/VEGF-expressing tumors and adenoviral vectors. J Histochem Cytochem 2000, 48:545-556 [DOI] [PubMed] [Google Scholar]
- 40.Rosenthal RA, Megyesi JF, Henzel WJ, Ferrara N, Folkman J: Conditioned medium from mouse sarcoma 180 cells contains vascular endothelial growth factor. Growth Factors 1990, 4:53-59 [DOI] [PubMed] [Google Scholar]
- 41.Park JE, Keller GA, Ferrara N: Vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell 1993, 4:1317-1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pelengaris S, Khan M, Evan GI: Suppression of Myc-induced apoptosis in beta cells exposes multiple oncogenic properties of Myc and triggers carcinogenic progression. Cell 2002, 109:321-334 [DOI] [PubMed] [Google Scholar]
- 43.Baudino TA, McKay C, Pendeville-Samain H, Nilsson JA, Maclean KH, White EL, Davis AC, Ihle JN, Cleveland JL: c-Myc is essential for vasculogenesis and angiogenesis during development and tumor progression. Genes Dev 2002, 16:2530-2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K: Lymphangiogenesis and cancer metastasis: nature reviews. Cancer 2002, 2:573-583 [DOI] [PubMed] [Google Scholar]
- 45.Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K: Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci USA 1995, 92:3566-3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valtola R, Salven P, Heikkila P, Taipale J, Joensuu H, Rehn M, Pihlajaniemi T, Weich H, deWaal R, Alitalo K: VEGFR-3 and its ligand VEGF-C are associated with angiogenesis in breast cancer. Am J Pathol 1999, 154:1381-1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagy JA, Vasile E, Feng D, Sundberg C, Brown LF, Detmar MJ, Lawitts JA, Benjamin L, Tan X, Manseau EJ, Dvorak AM, Dvorak HF: Vascular permeability factor/vascular endothelial growth factor induces lymphangiogenesis as well as angiogenesis. J Exp Med 2002, 196:1497-1506 [DOI] [PMC free article] [PubMed] [Google Scholar]





