Abstract
QR-32 tumor cells, a clone derived from a murine fibrosarcoma, are poorly tumorigenic and nonmetastatic when injected into syngeneic C57BL/6 mice. However, they are converted to highly malignant ones once they have grown in vivo after being co-implanted in a subcutaneous site with a foreign body, a gelatin sponge. Early phase of inflammation induced by the gelatin sponge participates in the conversion and histological analysis shows predominant infiltration of neutrophils. The objective of this study was to determine whether the depletion of the infiltrating neutrophils has any effect on the tumor progression. Intraperitoneal administration of a monoclonal anti-granulocyte antibody, RB6-8C5 (RB6), depleted neutrophils from both the peripheral blood circulation and the local inflamed site in mice with co-implantation of QR-32 tumor cells and gelatin sponge. The RB6 administration did not inhibit either tumor development or growth of QR-32 tumor cells. In contrast, tumor cell lines established from RB6-administered mice showed a significant decrease in metastatic incidence as compared with the tumor cell lines obtained from the mice with administration of control rat IgG or saline. Metastatic ability was significantly suppressed when RB6 had been administered in the early phase (from day −2 to day 6 after implantation); however, the administration in the middle (from day 6 to day 14) or late (from day 14 to day 22) phase did not affect the metastatic ability. We confirmed the phenomena by using integrin β2 knockout mice that had impaired neutrophil infiltration into inflamed sites. In the knockout mice, neutrophils hardly infiltrated into the gelatin sponge and the tumors showed dramatically suppressed metastatic phenotype as compared with those in wild-type mice or nude mice. Immunohistochemical analysis demonstrated that expressions of 8-hydroxy-2′-deoxyguanosine and nitrotyrosine were parallel to those in the presence of neutrophils. These results suggested that inflammation, especially when neutrophils infiltrate into tumor tissue, is primarily important for benign tumor cells to acquire metastatic phenotype.
The concept of inflammation-associated carcinogenesis was raised from the recognition of tissue inflammation and chronic infection as risk factors for human cancers. 1,2 Tissue inflammation because of autoimmune diseases such as ulcerative colitis and Crohn’s disease is a well known example of tissue inflammation-associated colon carcinogenesis. 3 Prominent association between persistent infection and cancer is evident in chronic active hepatitis B virus infection, 4 parasite infection, 5,6 and bacterial infection such as Helicobacter pylori. 7 The common feature of inflammation induced by autoimmune diseases and chronic infections is massive recruitment of phagocytes, particularly neutrophils, to target organs, and it is generally believed that the ensuing tissue damage is caused by the inflammatory cell-derived mediators.
It has been postulated that tissue-infiltrating phagocytes not only kill target cells but also damage important biomolecules of the cells through generation of a variety of reactive oxygen species (ROS) and reactive nitrogen species. 8-11 Thereby inflammatory cell-derived reactive nitrogen oxides are considered as one of the major contributors to carcinogenesis. 12,13 It is estimated that ROS derived from chronic inflammatory cells may be a primary factor in the development of up to one-third of all cancers. 13 Despite the apparent epidemiological association of inflammation with cancer, the relationship between inflammation and carcinogenesis/tumor progression is not fully understood because of a lack of suitable animal models.
To better understand the contribution of inflammation to tumor development/progression, we have established an inflammation-mediated murine tumor progression model. A clone (QR-32) derived from a fibrosarcoma cells is poorly tumorigenic and nonmetastatic when injected in normal syngeneic C57BL/6 mice. 14 However, when QR-32 tumor cells are co-implanted with a gelatin sponge, the inflammation induced by the foreign body not only promotes the local growth of the cells but also converts them into more aggressive tumors; ie, they acquire enhanced tumorigenicity and metastatic ability. 15 We showed that an early phase of inflammation contributes to malignant progression of QR-32 tumor cells and that inflammatory cells induced by gelatin sponge implantation convert QR-32 tumor cells into tumorigenic ones after injection of the mixture of both cells. Histological examination revealed that neutrophils mainly infiltrate into the co-implantation site in an early inflammation phase.
In the present study, we herein revealed that the inflammation-promoted tumor progression was inhibited by administration of anti-granulocyte antibody, RB6-8C5, which abrogated the neutrophil infiltration; the inhibition was also seen in β2-integrin knockout mice (CD18-deficient with impaired neutrophil infiltration into inflamed sites). We demonstrated that neutrophils infiltrating into tumor tissues led the tumor cells to acquire metastatic phenotype.
Materials and Methods
Tumor Cell Lines and Culture Conditions
The origin and characteristics of the tumor cell line used in this study have been described previously. 14,16 Briefly, BMT-11, a transplantable fibrosarcoma, was induced in a C57BL/6 mouse by 3-methyl-cholanthrene, and a tumorigenic clone BMT-11 cl-9 was subsequently isolated by limiting dilution. BMT-11 cl-9 cells were exposed in vitro to quercetin, which gave rise to a number of random subclones. 14 They spontaneously regressed when injected into normal syngeneic mice. These variants were named QR clones, representing quercetin-induced regressive tumor. Tumor cells of one of the variant cell clones, QR-32, were used in this study. The QR-32 tumor cells and its derived tumor cell lines were maintained in Eagle’s minimum essential medium (MEM; Nissui Pharm., Japan) supplemented with 8% fetal bovine serum (Filtron), sodium pyruvate, nonessential amino acids, and l-glutamine, at 37°C, in a humidified 5% CO2/95% air mixture.
Mice
C57BL/6J and athymic KSN mice were obtained from Nippon SLC (Hamamatsu, Japan) and integrin β2 knockout mice of the same genetic background (C57BL/6JItgb2tm1Bay, CD18-deficient) were purchased from the Jackson Laboratory (Bar Harbor, ME). We used 6-week-old female mice throughout the experiments. All of the mice were maintained in the complete barrier condition, lit from 7:00 a.m. to 7:00 p.m., at 23 ± 3°C and 50 ± 10% humidity, fed with mouse diet (Nihon Nosan Kogyo, Yokohama, Japan) and UV-irradiated water in the germ-free section of Institute for Animal Experimentation, Hokkaido University Graduate School of Medicine.
Co-Implantation of QR-32 Tumor Cells with Gelatin Sponge in Mice
The animal protocols were approved by the Committee of Institute for Animal Experimentation, Hokkaido University Graduate School of Medicine (no. 10980).
Inflammation was induced by insertion of a foreign body, gelatin sponge, as described previously. 15 The sterile gelatin sponge (Spongel; Yamanouchi Pharm., Japan) was cut into 10 × 5 × 3 mm pieces. A subcutaneous pocket reaching up to the thorax was made from a 10-mm incision on the right flank of the pelvic region in each anesthetized mouse and one piece of gelatin sponge was inserted and the wound was closed with clips. Then QR-32 tumor cells (1 × 105 cells/0.1 ml) were immediately injected into the preinserted gelatin sponge.
To examine the timing of gelatin sponge implantation necessary to accelerate QR-32 tumor cell progression, we had previously inserted the gelatin sponge into a subcutaneous space in the mice on days 0 (simultaneously with co-implantation), 30, 20, 10, 5, and 1 day(s) before QR-32 tumor cell injection.
To determine the potential of gelatin sponge-infiltrating cells to convert QR-32 tumor cells into tumorigenic ones, we first inserted a gelatin sponge into the subcutaneous space of normal mice; removed it 5, 10, 20, and 30 days later; and collected sponge-infiltrated inflammatory cells by squeezing the gelatin sponge in the medium. Gelatin sponge-infiltrated cells (1 × 106) or bone marrow-derived neutrophils were admixed with QR-32 tumor cells (1 × 105) and the mixture was injected subcutaneously into normal mice. Bone marrow-derived neutrophils were purified from bone marrow cells of normal mice by using Mono-Poly resolving medium (Dainippon Pharmaceutical Co. Ltd., Tokyo, Japan). All of the procedures were done under sterile conditions.
Establishment of Culture Cell Lines from the in Vivo Growing Tumors and Estimation of Tumor Progression
To assess whether the arising tumors have acquired malignant phenotype, we removed the subcutaneously growing tumors aseptically 25 days after co-implantation. The tumors were subjected to establishing individual culture cell lines by mechanical disaggregation with scissors. Detailed procedures have been described elsewhere. 15 The tumor lines were allowed at least four passages in culture to eliminate host cell contamination. Each tumor cell line was injected intravenously (1 × 106 cells) in normal C57BL/6J mice. On day 25, the mice were sacrificed and metastatic nodules on the surface of the lungs or other organs were counted macroscopically. If a tumor cell line had a significantly increased incidence of lung colonization, it was defined to have acquired tumor progression. The data indicate representative results of three separate experiments with similar results.
Anti-Granulocyte Monoclonal Antibody (RB6-8C5) Administration
Monoclonal rat anti-mouse granulocyte antibody, RB6-8C5 (RB6), was a generous gift from Dr. R. Coffman (DNAX Research Institute, Palo Alto, CA). This antibody has extensively been characterized elsewhere and has been shown to bind to and lyse neutrophils. 17
RB6, or rat IgG isotype, or saline as control was systemically administered by intraperitoneal injection at the dose of 10 mg/kg in a volume of 200 μl once daily from preimplantation day −2 through day 10. For determining the period necessary for neutrophil infiltration to accelerate tumor progression, RB6 or control vehicles were intraperitoneally injected at the same dose as indicated above once daily from preimplantation day −2 to day 6, from day 6 to day 14, or from day 14 to day 22, respectively.
White Blood Cell (WBC) Count
Peripheral blood was repeatedly collected from a small cut made at the tip of tail of anesthetized mice. WBCs were counted with a hemacytometer after erythrocyte lysis with Turk’s solution (Kishida Chemical Inc., Osaka, Japan). We obtained differential counts of more than 200 WBCs in blood smear preparations stained with May-Gruenwald’s and Giemsa solutions (Merck, Tokyo, Japan). The total number of peripheral leukocytes was expressed as WBC per mm3 (mean ± SD).
Purification of Neutrophils and Assay of Complement-Dependent Cytotoxicity (CDC)
The CDC assay was performed by using RB6 antibody on QR-32 tumor cells and its derived progressive tumor lines (QRsP-11 and QRsP-12), thymocytes, and neutrophils. Thymocytes were obtained from normal C57BL/6J mice thymus. Neutrophils were obtained by two methods: 1) separating them from bone marrow cells of normal mice; and 2) inserting a gelatin sponge (10 × 5 × 3 mm) into the subcutaneous space of normal mice, removing it 5 days later, and collecting sponge-infiltrated neutrophils. Bone marrow-derived or gelatin sponge-infiltrated neutrophils were purified by using Mono-Poly resolving medium. Purity of the neutrophils was greater than 95%.
The cell suspension was washed and resuspended in RPMI 1640 supplemented with 0.3% bovine serum albumin (no. 735086; Roche, Mannheim, Germany) and 25 mmol/L of Hepes (Sigma-Aldrich Chemie Gmbh H-4034, Steinheim, Germany). Then 6 × 105 cells of each cell type were separately incubated with a serially diluted RB6 antibody at 4°C for 1 hour. After washing and decanting supernatants, the cells were further incubated with rabbit complement (Low-Tox-M; Cedarlane, Ontario, Canada) at 37°C for 1 hour. Then, the plates were placed on the ice and cell cytotoxicity was scored by trypan blue exclusion test. Cytotoxic index (C.I.) was calculated by the following formula, where a represents percentage of cytotoxicity by the complement alone, and b by treatment with RB6 antibody and the complement: C.I. = (a − b)/a.
Immunohistochemistry for Neutrophils, 8-Hydroxy-2′ Deoxyguanosine (8-OHdG), and Nitrotyrosine (NT)
Tumor tissues were excised at the times indicated and trisected. One third was fixed in 10% neutral buffered formalin and 4-μm sections were prepared for hematoxylin and eosin (H&E) staining and neutrophil immunohistochemistry. Another one third was embedded in Tissue Tek OCT compound (no. 4589; Sakura Finetechnical Co. Ltd., Tokyo, Japan), frozen in liquid nitrogen, and stored at −80°C for nitrotyrosine immunostaining. The last one third was fixed in Bouin’s solution overnight, immersed sequentially in 50%, 75%, and 99% ethanol for 24 hours each, which was then embedded in paraffin and sectioned at 4 μm for 8-OHdG immunostaining. After deparaffinization and rehydration, the tissue sections were incubated with 1% hydrogen peroxide in methanol to quench endogenous peroxidase. They were blocked for 30 minutes with the serum adjusted for second antibody or 5% skim milk. Then the sections were incubated with rat monoclonal antibody to mouse granulocyte antigen (RB6-8C5, at a concentration of 1.6 μg/ml), mouse anti-8-OHdG monoclonal antibody (MOG-100 at a concentration of 5.0 μg/ml; Nikken Foods Co. Inc., Shizuoka, Japan) and rabbit anti-nitrotyrosine antibody (06-284 at a concentration of 5.0 μg/ml; Upstate Biotechnology, Lake Placid, NY), respectively, overnight in a humidified chamber at 4°C. After rinsing, the sections were then incubated for 30 minutes at room temperature with corresponding second antibody conjugated with biotin. After another rinse, avidin/biotinylated horseradish peroxidase complex (ABC Elite; Vector, Burlingame, CA) was placed on the tissue for 30 minutes. After a final rinse, specific immunolabeling was respectively examined with the use of 3,3′-diaminobenzidine (Sigma, St. Louis, MO) as the chromogen, which was placed on the tissues for a few minutes. 3,3′-Diaminobenzidine development was stopped by washing the tissues in distilled water. The sections were counterstained with hematoxylin and then dehydrated, mounted, and photographed.
Statistical Analysis
The significance of the differences in tumor and metastatic incidences was calculated by the chi-square test.
Results
Early-Phase Inflammation Is Required for Tumorigenic Conversion of QR-32 Tumor Cells
We have reported that a weakly tumorigenic and nonmetastatic mouse fibrosarcoma cell line, QR-32, is converted into highly metastatic tumor cells once it has grown in vivo after being co-implanted with a foreign body, a gelatin sponge. 15 We have identified that inflammation induced by gelatin sponge implantation participated in this process.
Table 1 ▶ shows the influence of timing of gelatin sponge implantation on the induction of subcutaneous growth of QR-32 tumor cells. QR-32 tumor cells grew progressively at the site where a gelatin sponge had been implanted 0, 1, and 5 days previously. However, tumor incidence was low when QR-32 tumor cells were injected into a gelatin sponge that had been implanted 10 days before or earlier.
Table 1.
Early-Phase Inflammation Is Required for Tumorigenic Conversion of QR-32 Tumor Cells
| Injection of QR-32 tumor cells (1 × 105) into the site where the gelatin sponge had been implanted* (days after gelatin sponge implantation) | Tumorigenicity (no. of mice with tumor take/no. of mice tested) | |||
|---|---|---|---|---|
| Exp. I | Exp. II | Exp. III | Total (%) | |
| Without gelatin sponge | 0/5 | 0/8 | 0/5 | 0/18 (0) |
| 0 (simultaneous co-implantation) | 4/7 | 5/7 | 5/7 | 14/21 (67) |
| 1 | 3/7 | 5/7 | 6/7 | 14/21 (67) |
| 5 | 5/7 | 3/6 | 6/7 | 14/20 (70) |
| 10 | 2/7 | 2/7 | 4/7 | 8/21 (38) |
| 20 | 2/7 | NT | 2/7 | 4/14 (29) |
| 30 | 2/7 | 0/7 | 2/6 | 4/20 (20) |
*Gelatin sponge was implanted into the back of normal mice on the days indicated.
To verify that the infiltrated cells themselves actually had potential to convert QR-32 tumor cells to tumorigenic, we isolated gelatin sponge-infiltrated cells from the preinserted sponge at various intervals and injected them subcutaneously into mice after admixing with QR-32 tumor cells. Table 2 ▶ shows that gelatin sponge-infiltrated cells collected 5 days after implantation converted QR-32 tumor cells to tumorigenic (P < 0.001). However, tumor-forming incidences of infiltrated cells obtained 10 days after implantation or later were low. Moreover, neutrophils obtained from bone marrow cells had low potential to convert QR-32 tumor cells to tumorigenic. These results indicated that an early-phase inflammatory response to gelatin sponge enhanced in vivo growth of QR-32 tumor cells.
Table 2.
Acquisition of Subcutaneous Growth Properties of QR-32 Tumor Cells Injected after Admix with Early-Phase of Gelatin Sponge-Infiltrated Inflammatory Cells
| No. of QR-32 cells | Inflammatory cells obtained from | No. of mice with tumor/no. of mice injected* | Mean survival time | ||
|---|---|---|---|---|---|
| Exp. I | Exp. II | Total | |||
| 1 × 105 | - | 0/10 | 0/10 | 0/20 (0%) | - |
| 1 × 105 | Day 5 gelatin sponge | 6/12 | 5/10 | 11/22† (50%) | 51.8 ± 5.3 |
| 1 × 105 | Day 10 gelatin sponge | 2/12 | 3/10 | 5/22‡ (23%) | 53.4 ± 6.8 |
| 1 × 105 | Day 20 gelatin sponge | 1/12 | 0/10 | 1/22§ (5%) | 53 |
| 1 × 105 | Day 30 gelatin sponge | NA | NA | NA | NA |
| 1 × 105 | Neutrophils purified from bone marrow | 1/10 | 1/19 | 2/19§ (11%) | 58.5 ± 7.8 |
*Gelatin sponge was inserted into the subcutaneous space of mice. The sponge was removed as indicated days and infiltrated cells were collected. QR-32 cells (1 × 105 cells) were admixed with or without 1 × 106 cells of gelatin sponge-infiltrated cells or neutrophils obtained from bone marrow, and then injected subcutaneously into mice.
†P < 0.001;
‡P < 0.05;
§not significant versus QR-32 cells alone.
Depletion of Circulating Peripheral Blood and Extravasating Neutrophils by Administration of Monoclonal Antibody RB6-8C5
Histological examination after H&E staining 5 days after co-implantation revealed massive neutrophil infiltration (Figure 1A) ▶ . To examine the relationship between infiltration of neutrophils and tumor progression, we depleted neutrophils by administrating an anti-granulocyte antibody, RB6-8C5 (RB6). 17 Previous studies have indicated that intraperitoneal injection of mice with monoclonal anti-mouse granulocyte antibody, RB6 dramatically decreased the number of neutrophils in the blood circulation and spleen for up to 3 days after inoculation. 17 To verify whether the administration of RB6 resulted in neutrophil deletion, RB6 (10 mg/kg) was administered to the co-implanted mice for 13 consecutive days from day −2 to day 10. Control mice received rat IgG or saline at the same protein concentration (or volume) and period.
Figure 1.
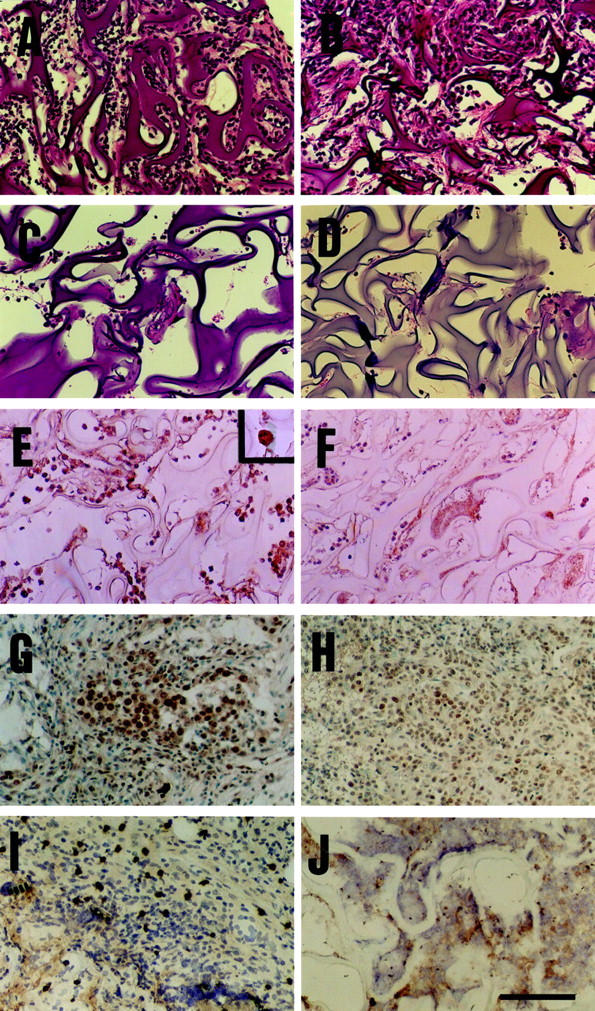
Histopathological findings and immunohistochemistry with antibodies against granulocytes, 8-OHdG, and nitrotyrosine of the arising tumors after administration of RB6-8C5 antibody or control vehicles. Histological sections were obtained from the arising tumors after subcutaneous co-implantation of QR-32 tumor cells with gelatin sponge. Tissues were obtained from co-implantation sites on day 5 (A–F) or on day 12 (G–J). They were from C57BL/6J mice with RB6 antibody (C, F, H, J), rat IgG (B, E, G, I), or saline (A) administration, and from integrin β2 knockout mice (C57BL/6JItgb2tm1Bay) (D). H&E staining (A–D) and immunohistochemistry were performed with monoclonal antibodies against granulocytes (E, F), 8-OHdG (G, H), or nitrotyrosine (I, J), respectively. High magnification (×400) of RB6 antibody-positive neutrophils is indicated (E, top right). Scale bar, 100 μm.
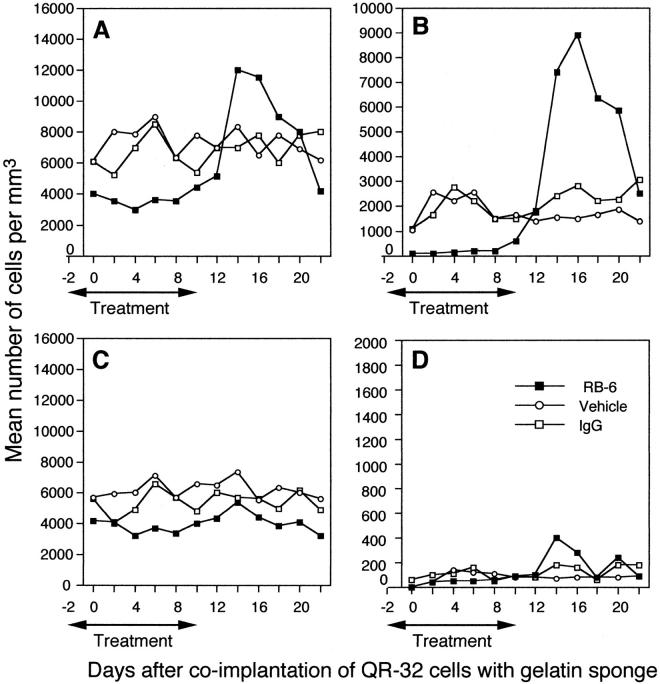
Total leukocyte counts gradually increased in the co-implanted mice in response to QR-32 tumor cells, and the increase was not affected by IgG administration (Figure 2A) ▶ . However, RB6 administration reduced the number of WBCs in circulation to 40% as compared to control IgG-administered mice. The mean differential leukocyte counts are shown in Figure 2 ▶ ; B to D. In IgG-treated mice, the majority of circulating cells on day 4 was lymphocytes (5.4 × 103 cells) and neutrophils (2.5 × 103 cells), whereas the monocyte content was poor. In contrast, only 1.6 × 102 neutrophils (both segmented and band type) were detected in the peripheral blood of RB6-treated mice. RB6 administration also reduced the number of lymphocytes because they share the common antigen to neutrophils with CD8+ cells, 17,18 although circulating platelets were not significantly altered quantitatively compared to those in the controls (data not shown).
Figure 2.
Depletion of neutrophils by administration of RB6-8C5 monoclonal antibody from peripheral blood of the mice in which QR-32 tumor cells had been co-implanted with gelatin sponge. Ten mg/kg of RB6-8C5 antibody was injected intraperitoneally into mice in which QR-32 tumor cells had been co-implanted with gelatin sponge from day −2 to day 10. At various intervals, the numbers of peripheral blood leukocytes (A, all leukocytes; B, neutrophils; C, lymphocytes; D, monocytes) were counted, and the hemograms of more than 200 leukocytes were examined by May-Gruenwald’s and Giemsa staining. ▪, RB6-8C5-treated mice; ○, saline-treated mice; □, rat IgG-treated mice. Mean values are presented as the number of cells per ml from more than 17 mice with each treatment and the data indicates representative results of at least two separate experiments with similar results.
Specificity of RB6 to neutrophil depletion was apparent when RB6 administration was interrupted (Figure 2) ▶ ; namely, the number of circulating neutrophils substantially increased in RB6-treated mice (8.9 × 103 cells) compared with the IgG- and saline-treated mice on day 16 (2.8 × 103 and 1.5 × 103, respectively). Lymphocytes and monocytes also slightly increased by the interruption of the antibody administration. The mice with antibody interruption showed obvious splenomegaly coincident with neutrophil proliferation. Thus we consider that the phenomenon may be a type of physiological rebound after the sudden loss and degradation of circulating neutrophils.
We then examined the effect of RB6 administration on extravasation of neutrophils into gelatin sponge. As shown in Figure 1, A and B ▶ , the majority of emigrated cells in saline- or IgG-treated mice was neutrophils. In contrast, neutrophil infiltration into the gelatin sponge in RB6-treated mice was completely inhibited (Figure 1C) ▶ . To confirm the efficacy of RB6 to inhibit neutrophil infiltration, we stained the extravasated leukocytes with RB6 antibody, which is a marker of mature murine neutrophils, identical to Gr-1 antibody. 17 In control mice, most of the infiltrated cells showed high RB6 expression (Figure 1E) ▶ . In contrast, hardly any of extravasated cells was positive for RB6 in RB6 antibody-treated mice (Figure 1F) ▶ , demonstrating that RB6 abrogated neutrophil extravasation into the gelatin sponge.
Requirement of Neutrophils for Inflammation-Based Tumor Progression
QR-32 tumor cell growth after co-implantation with a gelatin sponge was observed in 17 of 25 (68%) saline-treated mice, 18 of 26 (69%) RB6-treated mice, and 22 of 27 (81%) rat IgG-treated mice (Figure 3) ▶ . The mean tumor diameters on day 25 were 9.5 ± 0.7 mm, 9.4 ± 0.6 mm, and 7.8 ± 0.9 mm and the mean body weights were 17.4 ± 0.6 g, 17.7 ± 0.6 g, and 18.6 ± 0.7 g, respectively. Namely, RB6 administration had no inhibitory effect on either tumor growth or body weight.
Figure 3.
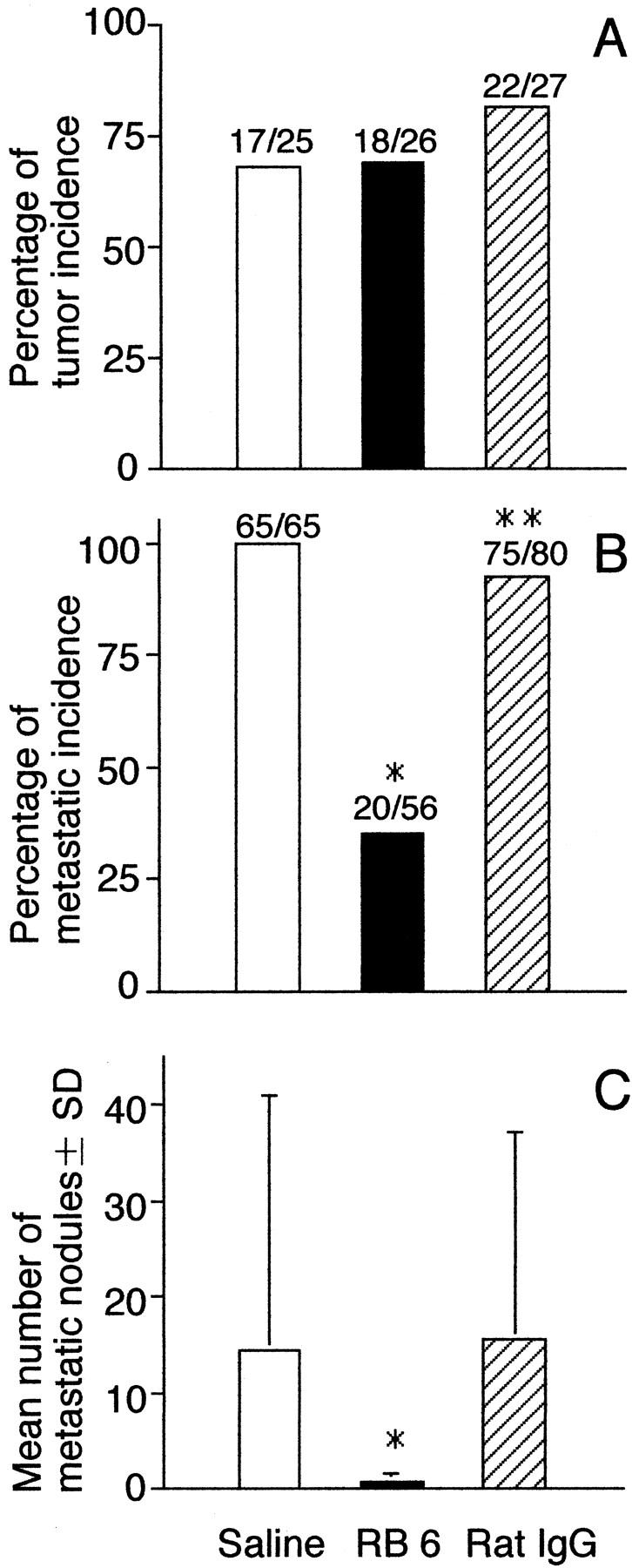
Inhibition of acquisition of metastatic ability of QR-32 tumor cells by administration of RB6-8C5 anti-granulocyte monoclonal antibody. A: QR-32 tumor cells (1 × 105) were co-implanted with gelatin sponge in mice. They were given the anti-granulocyte monoclonal antibody RB6-8C5, or control vehicles, rat IgG, or saline. B: Metastatic incidence of the arising tumors. Mice were injected intravenously with 1 × 106 of each cell line. *, P < 0.001; **, P < 0.05 versus saline group. C: Mean numbers of metastatic nodules in lung per mouse. *, P < 0.001 versus saline group. The actual number of incidences is indicated above each bar (number of mice with tumor or metastasis/number of mice tested).
We next established in vitro culture cell lines from the arising tumors in individual mice with RB6, rat IgG, or saline administration and examined their metastatic ability in mice. In contrast to the groups with rat IgG or saline administration, in the group with RB6 the grown tumor cell lines acquired significantly low metastatic phenotype, both in the incidence of lung metastasis (Figure 3B) ▶ and the number of colonies per lung (Figure 3C ▶ ; P < 0.001). Among the tumor cell lines we used for the analysis of metastasis, significant differences were not observed in their in vitro growth properties such as doubling time, plating efficiency, or colony formation in soft agar (data not shown).
Complement-Dependent Cytotoxicity Induced by RB6 Antibody Is Specific to Neutrophils
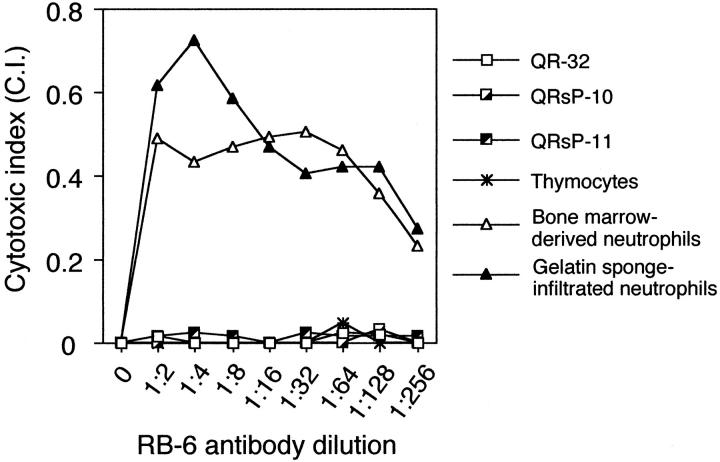
To reduce the possibility that these results were obtained not by depletion of neutrophils but by activating complement-dependent cytotoxic ability against progressive tumor cells converted from QR-32 tumor cells co-implanted with a gelatin sponge, we examined the effect of CDC in using RB6 antibody. As a result, RB6-mediated CDC was not observed in the typical QRsP tumor cell lines (QRsP-10 and QRsP-11), QR-32 tumor cells, or thymocytes; however, specific CDC-mediated neutrophil lysis was observed (Figure 4) ▶ . These results suggested that the prevention of acquisition of metastatic phenotype of tumor cells by RB6 administration is not because of selective cytotoxic activity of the antibody against progressive tumor cells.
Figure 4.
Complement-dependent cytotoxicity induced by RB6-8C5 antibody is specific to neutrophils. Tumor cells (QR-32, QRsP-10, and QRsP-11), thymocytes, and neutrophils were separately incubated with a serially diluted RB6-8C5 antibody with rabbit complement. The cytotoxic indices are indicated as representative results of at least two separate experiments with similar results.
Influence of Timing of RB6 Administration
The mice in which QR-32 tumor cells had been co-implanted with gelatin sponge were divided into six groups, and RB6 or control IgG was administered to them for different periods. The administration was in the early (from day −2 to day 6), middle (from day 6 to day 14), and late (from day 14 to day 22) phases after co-implantation. As shown in Figure 5 ▶ , acquisition of metastatic phenotype was prevented by the administration of RB6 especially in the early phase of inflammation.
Figure 5.
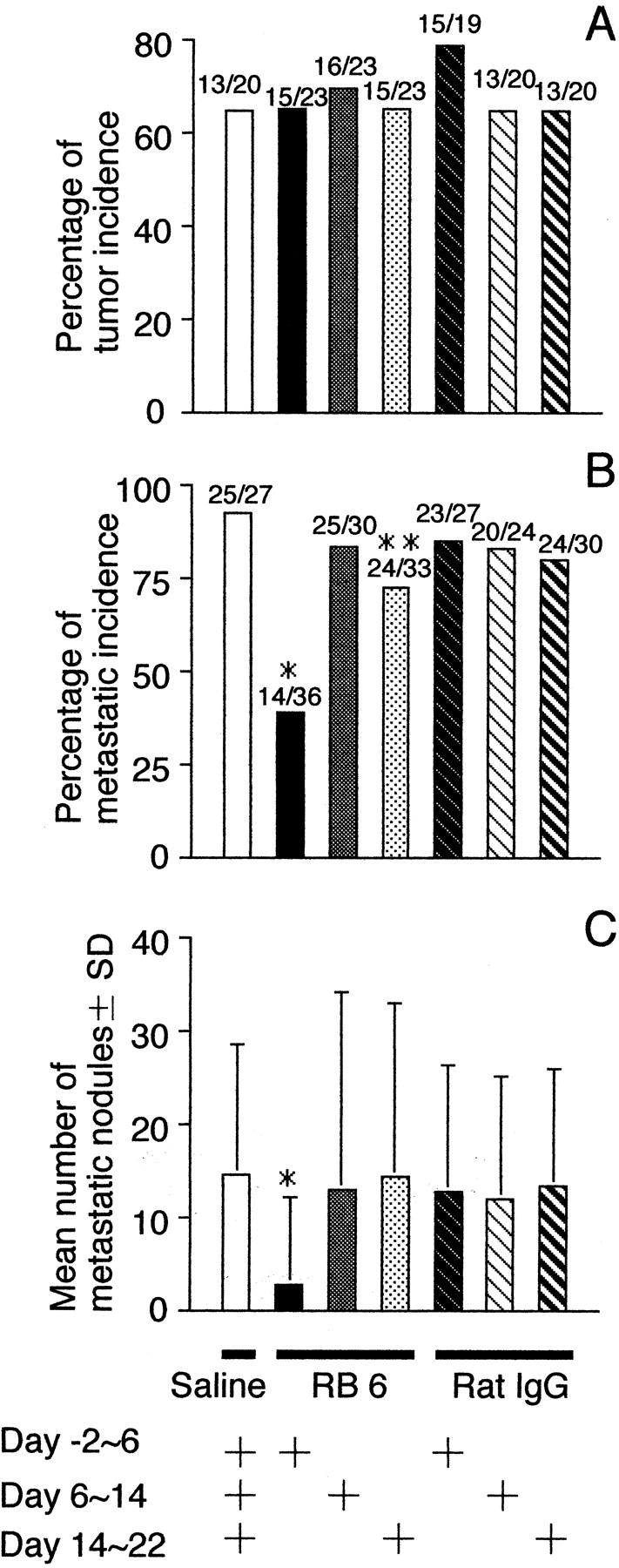
Early-phase RB6 administration inhibits acquisition of metastatic ability of QR-32 tumor cells. QR-32 tumor cells (1 × 105) were co-implanted with gelatin sponge in normal mice to which RB6-8C5 monoclonal antibody, rat IgG, or saline had been administered for different periods. A: Metastatic incidence of the arising tumors. Mice were injected intravenously with 1 × 106 of each cell line. *, P < 0.001; **, P < 0.05 versus saline group. B: Mean numbers of metastatic nodules in lung per mouse. *, P < 0.001 versus saline group. The actual number of incidences is indicated above each bar (number of mice with tumor or metastasis/number of mice tested).
Major inflammatory responses to the inserted gelatin sponge around days −2 to 6 were those of neutrophils; however, from days 14 to 22 they were mainly those of lymphocytes, which has been confirmed by histological examination (data not shown).
Immunohistochemical Analysis for 8-OHdG and Nitrotyrosine in Tumor Tissues
Because neutrophils have been shown to produce a number of ROS and reactive nitrogen species, 9,8-11 we examined the by-products of those reactive species formed in the tumor tissue by immunohistochemical analysis and by evaluating the formation of 8-hydroxy-2′-deoxyguanosine (8-OHdG) adducts as a marker of oxidative DNA damage in response to ROS. Twelve days after implantation, a small tumor mass was formed within the gelatin sponge and intense staining for 8-OHdG in tumor cells was observed in control IgG-treated group (Figure 1G) ▶ . However, tumors grown in RB6-treated mice showed weak staining compared to controls (Figure 1H) ▶ . Tyrosine nitration, an index of the nitrosylation of proteins by peroxynitrate (formed from NO reacting with the superoxide anion), was then detected. Tumors obtained from the mice treated with control IgG revealed positive staining for nitrotyrosine (Figure 1I) ▶ , which was mainly localized in the stromal tissue. In contrast, a few spots of positive staining for nitrotyrosine was found in the tumor tissues of the mice treated with RB6 (Figure 1J) ▶ . The specificity of the antibody to 8-OHdG or nitrotyrosine was determined by disappearance of the positive staining by incubating with 10 ng of 8-OHdG polynucleotide or 1 mmol/L of nitrotyrosine (data not shown).
Inhibition of Acquisition of Metastatic Ability of QR-32 Tumor Cells Grown in Integrin β2-Knockout Mice
The β2-integrins are known to play a pivotal role in the extravasation of neutrophils to the sites of inflammation. 19,20 To further investigate the role of neutrophils in inflammation-promoted tumor progression, we used integrin β2-knockout mice that do not express β2 integrin and consequently lack neutrophil extravasation from blood circulation to the inflamed sites. 19-21
Knockout mice have significantly elevated levels of circulating WBCs and neutrophil counts as compared to wild-type mice: 8025 ± 695 mm3 versus 4873 ± 1042 mm3 and 2536 ± 219 mm3 versus 1540 ± 329 mm3, respectively. Tumorigenic incidences of QR-32 tumor cells co-implanted with the gelatin sponge were in 10 of 12 (83%) wild-type mice, 7 of 10 (70%) integrin β2-knockout mice, and all of the 10 KSN nude mice (Figure 6A) ▶ . Dramatical suppression of neutrophil emigration into the gelatin sponge was observed histologically in the knockout mice as compared to wild-type C57BL/6J mice (Figure 1, A and D) ▶ . Metastatic ability of the tumor cell lines established from individually arising tumors was then analyzed in normal C57BL/6J mice. Tumor cell lines obtained from the knockout mice were significantly less metastatic to lungs (Figure 6B) ▶ , with no macroscopical metastatic nodules (Figure 6C) ▶ , whereas those from wild-type mice or nude mice aggressively metastasized to the lungs.
Figure 6.
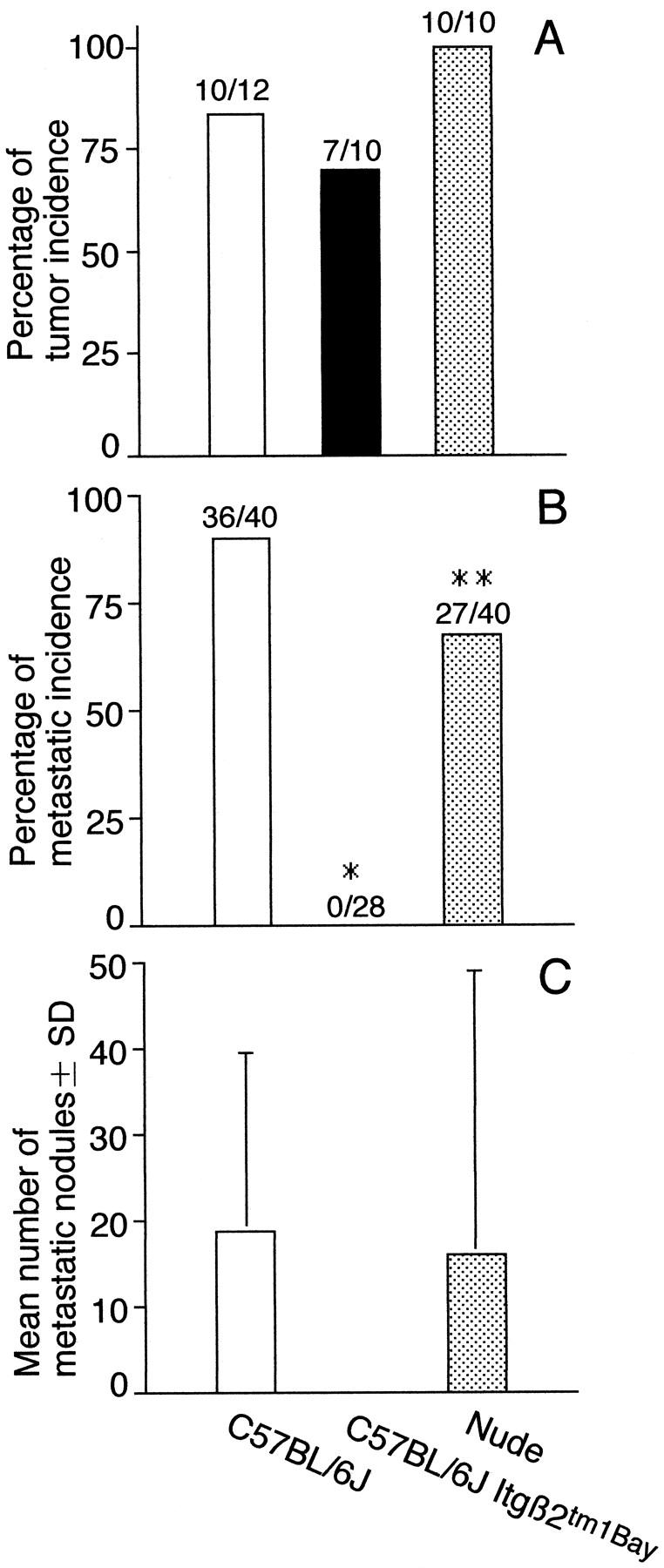
Inhibition of acquisition of metastatic ability of QR-32 tumor cells grown in integrin β2-knockout mice. A: QR-32 tumor cells (1 × 105) were co-implanted with gelatin sponge in normal syngeneic C57BL/6J or C57BL/6J Itgβ2tm1Bay mice or KSN nude mice. B: Metastatic incidence of the arising tumors. One × 106 of each cell line was injected intravenously into normal C57BL/6J mice. *, P < 0.001; **, P < 0.05 versus saline group. C: Mean numbers of metastatic nodules in lung per mouse. The actual number of incidence is indicated above each bar (number of mice with tumor or metastasis/number of mice tested).
Discussion
We demonstrated that the acquisition of metastatic phenotype of QR-32 tumor cells was suppressed by depletion of neutrophil infiltration into the tumor mass by administration of anti-granulocyte antibody, RB6-8C5, in the inflammation-promoted tumor progression model, and we further confirmed it in integrin β2 knockout mice with impaired neutrophil extravasation.
The role of neutrophils in the process of tumor metastasis has been controversial. It has been shown in vivo and in vitro that neutrophils are involved in inhibition of experimental metastasis 22 because of anti-tumor immunity. 23,24 In contrast, intravenous injection of neutrophils, regardless whether they have been primed with tumor cells or not, tends to increase experimental metastases. 25,26 Ishikawa and colleagues 27 have shown that increased lung metastasis was observed in experimentally induced granulocytosis-positive mice. Moreover, they showed that either simultaneous injection or preinjection of neutrophils with tumor cells enhanced the metastatic capacity of tumor cells. 27 In all those experiments, the authors investigated whether tumor cells metastasized to distant organs after they were admixed with neutrophils or injected into mice with neutrophilia. The major cause of the augmented metastasis is aggregate (emboli) formation between tumor cells and neutrophils or among tumor cells themselves that are most likely to be arrested in microvasculature. 27 Apart from or conjunction with the mechanical and rheological circumstance, 28 or enzymatic activity of neutrophils in the blood circulation, 29 the unresolved question is whether neutrophils actually have the potential to alter metastatic phenotype of tumor cells qualitatively. To answer this question, we established an inflammatory cell-promoting tumor progression model that demonstrates conversion of low-tumorigenic and nonmetastatic tumor cells to highly tumorigenic and metastatic ones after benign tumor cells contacted with foreign body-induced early-phase inflammatory cells. 15 In this model, it is possible to determine whether the tumor cell itself, without infiltrated inflammatory cells, acquires metastatic ability because the tumor lines have been established by culturing the cells from the arising growing tumors in mice. During several subcultures, contaminated host cells including inflammatory cells have been eliminated. We demonstrated in this study that neutrophils are likely to have the potential to endow benign tumor cells with metastatic phenotype.
The gelatin sponge is known to induce a classic inflammatory response; initially, neutrophils infiltrate into the gelatin sponge, which is followed by an accumulation of monocytes/macrophages and other inflammatory cells. 30-32 Similar immunological responses are thought to occur also in tumor cell transplantation. Neutrophils primarily play an initial role in the anti-tumor immunity because they act in the priming phase of tumor-associated transplantation antigens, 33 and thereafter produce chemotactic factors for accumulating macrophages, 34 lymphocytes, 35,36 and tumor-infiltrating lymphocytes. 37 Considering their immune responses to either a foreign body or tumor cells, infiltration of neutrophils is necessary for subsequent extravasation and infiltration of immune effector cells. Thus a lack of neutrophil infiltration into tumor tissues because of RB6 antibody treatment, or a lack of integrin β2 expression may inhibit the arrival of subsequential immune-effector cells to tumor tissues. In our previous work, we had postulated that antigen nonspecific immune effector cells (including neutrophils, natural killer cells, lymphokine-activated killer cells, and resident/activated macrophages) have progression-enhancing activity against QR-32 tumor cells whereas antigen-specific immune effector cells, ie, cytotoxic T lymphocytes, have not. 38 Therefore inhibition of accumulating antigen-nonspecific immune effector cells to tumor masses may prevent tumor cells from acquiring metastatic phenotype. From these, we suggest that infiltrating neutrophils are primarily important in the acquisition of metastatic phenotype of tumor cells.
The RB6 antibody used in this study shares a common antigen to neutrophils with CD8+ lymphocytes. 17,18 At present time, we could not rule out the contribution of other lymphocytes in the acquisition of metastatic phenotype of tumor cells. As shown in Figure 5 ▶ , late-phase RB6 administration (from day 14 to day 22) slightly but significantly reduced the metastasis incidence (Figure 5B) ▶ . We also found that the major inflammatory responses to the inserted gelatin sponge around day 14 to 22 are mainly of lymphocytes but not neutrophils. In our model, neutrophils are primarily and mainly important in the conversion of benign tumors although we do note that other types of inflammatory cells also contribute to the process.
We noticed that the potential of neutrophils to give tumor cells metastatic ability is dependent on their activation status. As shown in Table 2 ▶ , neutrophils that were obtained from bone marrow have a low potential to convert QR-32 tumor cells to tumorigenic ones; however, early phase of gelatin sponge-infiltrated neutrophils convert QR-32 tumor cells more efficiently. We believe that neutrophils are activated while they invade and migrate into the inflammatory lesion; and those neutrophils infiltrated into inflammation are supposed to be different from the resident or circulating neutrophils as far as the potential to convert benign tumor cells to more malignant ones is concerned.
The infiltration process of circulating neutrophils into the inflamed region consists of at least three steps. First the circulating neutrophils attach to the vascular endothelium near the inflammatory site. Secondly, the attached neutrophils penetrate through the endothelium. Thirdly, extravasated neutrophils move to the inflamed site, oriented to the chemotactic factors released from the inflamed region. In our experiments, RB6 antibody inhibited neutrophil infiltration into the inflamed site because the antibody exhibited specific cytolysis against neutrophils. Therefore, we believe that neutrophils were lysed in the peripheral circulation; however, we could not rule out the possibility that the RB6 antibody reacted with some adhesion molecule(s) on the neutrophils and thus inhibited neutrophil attachment to the endothelium. In integrin β2 knockout mice (lacking adhesion molecule), circulating neutrophils are hard to adhere to the endothelium; therefore neutrophils stay in the circulation, being unable to migrate into the inflamed site. Actually, in the knockout mice, neutrophil counts in the circulation are higher than in the wild-type mice. We intend to conduct further study to compare the ability of neutrophil adherence to endothelium after treated with or without RB6 antibody.
Sugawara and colleagues 39 have reported species-dependent differences of neutrophil functions. They found that the neutrophil migration induced by known chemotactic factors is different among animal species. Therefore, we may not be able to extrapolate the condition in human diseases related with neutrophil infiltration from the results of our studies; however, as far as the mouse system is concerned, neutrophils infiltrated into the inflammatory site do have the potential to convert benign tumors to more malignant ones.
Carcinogenesis induced by neutrophils was first reported by Weitzman and colleagues, 40 in which they showed malignant transformation of 10T1/2 cells; namely, C3H mouse fibroblast cell line was converted to form tumors in nude mice after co-culture with neutrophils. They proved that neutrophil-derived ROS were involved in this conversion. Yamamoto and colleagues 41 and Kawai and colleagues 42 demonstrated that migration of neutrophils converted rat normal urothelium into bladder cancer by means of repeated instillation of killed Escherichia coli. ROS derived from E. coli-elicited neutrophils was thought responsible for the tumorigenic conversion. 40,43 Sandhu and colleagues 44 found in the Mutatect mouse tumor model, by measuring mutations occurring at the specific gene locus, that tumor-infiltrating neutrophils had the ability to induce mutations in tumor cells. They showed that the number of gene mutations in individually growing tumors was associated with the number of infiltrating neutrophils and the amount of nitric oxide synthase. 44 The results of those studies, as well as ours, suggest the role of mutagenic factors such as reactive nitrogen oxides in inflammatory neutrophils. 8,45-49 The mechanism responsible for the neutrophil-mediated acceleration of tumor progression is suspected to involve, at least in part, reactive nitrogen oxides produced by neutrophils. We have revealed that gelatin sponge-reactive inflammatory cell-derived ROS are involved in the QR-32 tumor progression. The gelatin sponge-reactive inflammatory cell-induced somatic mutation or the content of 8-OHdG in QR tumor cells with low levels of anti-oxidative enzymes were associated with the frequencies of inflammation-promoted tumor progression. 50 Reversely, induction of an anti-oxidative enzyme at the co-implantation site prevented tumor progression. 51 Considering those results, inflammatory cell-derived reactive nitrogen oxides are suspected to be involved. Finally, the present results indicate that neutrophil infiltration into tumor cells may be primarily important to accelerate malignancy of tumor cells, especially acquiring metastatic phenotype.
Acknowledgments
We thank Dr. Hiroyasu Esumi (National Cancer Center Research Institute East) for helpful advice, Miss Masako Yanome for help in the English revision of this manuscript, and Mrs. Yumiko Shinohe for immunohistochemical assistance.
Footnotes
Address reprint requests to Futoshi Okada, Ph.D., Division of Cancer Pathobiology, Research Section of Pathophysiology, Institute for Genetic Medicine, Hokkaido University, Kita-15, Nishi-7, Kita-ku, Sapporo, 060-0815, Japan. E-mail: fuokada@med.hokudai.ac.jp.
Supported in part by a grant-in-aid for cancer research (10-36) from the Japanese Ministry of Health, Labor, and Welfare; a grant-in-aid from the Japanese Ministry of Education, Culture, Sports, Science, and Technology; and grants from the Japan Society for the Promotion of Science (grant 15390367).
References
- 1.Ohshima H, Bartsch H: Chronic infections and inflammatory processes as cancer risk factors: possible role of nitric oxide in carcinogenesis. Mutat Res 1994, 305:253-264 [DOI] [PubMed] [Google Scholar]
- 2.Wiseman H, Halliwell B: Damage to DNA by reactive oxygen and nitrogen species: role of inflammatory disease and progression to cancer. Biochem J 1996, 313:17-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levin B: Ulcerative colitis and colon cancer: biology and surveillance. J Cell Biochem Suppl 1992, 16G:47-50 [DOI] [PubMed] [Google Scholar]
- 4.Tiollais P, Pourcel C, Dejean A: The hepatitis B virus. Nature 1985, 317:489-495 [DOI] [PubMed] [Google Scholar]
- 5.Rosin MP, Anwar WA, Ward AJ: Inflammation, chromosomal instability, and cancer: the schistosomiasis model. Cancer Res 1994, 54:1929s-1933s [PubMed] [Google Scholar]
- 6.Haswell-Elkins MR, Satarug S, Tsuda M, Mairiang E, Esumi H, Sithithaworn P, Mairiang P, Saitoh M, Yongvanit P, Elkins DB: Liver fluke infection and cholangiocarcinoma: model of endogenous nitric oxide and extragastric nitrosation in human carcinogenesis. Mutat Res 1994, 305:241-252 [DOI] [PubMed] [Google Scholar]
- 7.Houben GM, Stockbrugger RW: Bacteria in the aetio-pathogenesis of gastric cancer: a review. Scand J Gastroenterol 1995, 212:13-18 [DOI] [PubMed] [Google Scholar]
- 8.Weitzman SA, Gordon LI: Inflammation and cancer: role of phagocyte-generated oxidants in carcinogenesis. Blood 1990, 76:655-663 [PubMed] [Google Scholar]
- 9.Shacter E, Beecham EJ, Covey JM, Kohn KW, Potter M: Activated neutrophils induce prolonged DNA damage in neighboring cells. Carcinogenesis 1988, 9:2297-2304 [DOI] [PubMed] [Google Scholar]
- 10.Birnboim HC: DNA strand breakage in human leukocytes exposed to a tumor promoter, phorbol myristate acetate. Science 1982, 215:1247-1249 [DOI] [PubMed] [Google Scholar]
- 11.Troll W, Wiesner R: The role of oxygen radicals as a possible mechanism of tumor promotion. Annu Rev Pharmacol Toxicol 1985, 25:509-528 [DOI] [PubMed] [Google Scholar]
- 12.Lewis JG, Adams DO: Inflammation, oxidative DNA damage, and carcinogenesis. Environ Health Perspect 1987, 76:19-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ames BN, Shigenaga M, Hagen TM: Oxidants, antioxidants, and the degenerative disease of aging. Proc Natl Acad Sci USA 1993, 90:7915-7922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishikawa M, Okada F, Hamada J, Hosokawa M, Kobayashi H: Changes in the tumorigenic and metastatic properties of tumor cells treated with quercetin or 5-azacitidine. Int J Cancer 1987, 39:338-342 [DOI] [PubMed] [Google Scholar]
- 15.Okada F, Hosokawa M, Hamada J, Hasegawa J, Kato M, Mizutani M, Ren J, Takeichi N, Kobayashi H: Malignant progression of a mouse fibrosarcoma by host cells reactive to a foreign body (gelatin sponge). Br J Cancer 1992, 66:635-639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishikawa M, Hosokawa M, Oh-hara N, Niho Y, Kobayashi H: Marked granulocytosis in C57BL/6 mice bearing a transplantable BMT-11 fibrosarcoma. J Natl Cancer Inst 1987, 78:567-571 [PubMed] [Google Scholar]
- 17.Czuprynski CJ, Brown JF, Maroushek N, Wagner RD, Steinberg H: Administration of anti-granulocyte mAB-RB6-8C5 impairs the resistance of mice to Listeria monocytogenes infections. J Immunol 1994, 152:1836-1842 [PubMed] [Google Scholar]
- 18.Smelt SC, Cotterell SEJ, Engwerda CR, Kaye PM: B cell-deficient mice are highly resistant to Leishmania donovani infection, but develop neutrophil-mediated tissue pathology. J Immunol 2000, 164:3681-3688 [DOI] [PubMed] [Google Scholar]
- 19.Walzog B, Scharffetter-Kochanek K, Gaehtgens P: Impairment of neutrophil emigration in CD18-null mice. Am J Physiol 1999, 276:G1125-G1130 [DOI] [PubMed] [Google Scholar]
- 20.Arnaout MA: Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood 1990, 75:1037-1050 [PubMed] [Google Scholar]
- 21.Coxon A, Rieu P, Barkalow FJ, Askari S, Sharpe AH, von Adrian U, Arnaout MA, Mayadas TN: A novel role for β2 integrin CD11b/CD18 in neutrophil apoptosis. A homeostatic mechanism in inflammation. Immunity 1996, 5:653-666 [DOI] [PubMed] [Google Scholar]
- 22.Glaves D: Role of polymorphonuclear leukocytes in the pulmonary clearance of arrested cancer cells. Invasion Metastasis 1983, 3:160-173 [PubMed] [Google Scholar]
- 23.van Spriel AB, Leusen JH, van Egmond M, Dijkman HB, Assmann KJ, Mayadas TN, van de Winkel JG: Mac-1 (CD11b/CD18) is essential for Fc receptor-mediated neutrophil cytotoxicity and immunologic synapse formation. Blood 2001, 97:2478-2486 [DOI] [PubMed] [Google Scholar]
- 24.Inoue T, Sendo F: In vitro induction of cytotoxic polymorphonuclear leukocytes by supernatant from a concanavalin-A-stimulated spleen cell culture. J Immunol 1983, 131:2508-2514 [PubMed] [Google Scholar]
- 25.Starkey JR, Liggitt HD, Jones W, Hosick HL: Influence of migratory blood cells on the attachment of tumor cells to vascular endothelium. Int J Cancer 1984, 34:535-543 [DOI] [PubMed] [Google Scholar]
- 26.Welch DR, Schissel DJ, Howrey RP, Aeed PA: Tumor-elicited polymorphonuclear cells, in contrast to “normal” circulating polymorphonuclear cells, stimulate invasive and metastatic potentials of rat mammary adenocarcinoma cells. Proc Natl Acad Sci USA 1989, 86:5859-5863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishikawa M, Koga Y, Hosokawa M, Kobayashi H: Augmentation of B16 melanoma lung colony formation in C57BL/6 mice having marked granulocytosis. Int J Cancer 1986, 37:919-924 [DOI] [PubMed] [Google Scholar]
- 28.Schmid-Schonbein GW, Fung YC, Zweifach BW: Vascular endothelium-leukocyte interaction; stickling shear force in venules. Circ Res 1975, 36:173-184 [DOI] [PubMed] [Google Scholar]
- 29.Aeed PA, Nakajima M, Welch DR: The role of polymorphonuclear leukocytes (PMN) on the growth and metastatic potential of 13762NF mammary adenocarcinoma cells. Int J Cancer 1988, 42:748-759 [DOI] [PubMed] [Google Scholar]
- 30.Ascher NA, Eerguson RM, Hoffman R, Simmons RL: Partial characterization of cytotoxic cells infiltrating sponge matrix allografts. Transplantation 1979, 27:254-259 [DOI] [PubMed] [Google Scholar]
- 31.Akporiaye ET, Kudalore MK: Implantation of a gelatin sponge as a model for effector recruitment: tumor growth inhibition by T-lymphocytes recovered from a site of tumor rejection. Cancer Immunol Immunother 1989, 29:199-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Middleton MM, Campbell PA: Functions of purified mouse neutrophils isolated from gelatin sponges. J Leukoc Biol 1989, 46:416-466 [DOI] [PubMed] [Google Scholar]
- 33.Midorikawa Y, Yamashita T, Sendo F: Modulation of immune response to transplanted tumors in rats by selective depletion of neutrophils in vivo using a monoclonal antibody: abrogation of specific transplantation resistance to chemical carcinogen-induced syngeneic tumors by selective depletion of neutrophils in vivo. Cancer Res 1990, 50:6243-6247 [PubMed] [Google Scholar]
- 34.Ishida M, Honda M, Hayashi H: In vitro macrophage chemotactic generation from serum immunoglobulin G by neutrophil neutral seryl protease. Immunology 1978, 35:167-176 [PMC free article] [PubMed] [Google Scholar]
- 35.Higuchi Y, Honda M, Hayashi H: Production of chemotactic factor for lymphocytes by neutral SH-dependent protease of rabbit PMN leukocytes from immunoglobulins, especially IgM. Cell Immunol 1975, 15:100-108 [DOI] [PubMed] [Google Scholar]
- 36.Kudo C, Araki A, Matsushima K, Sendo F: Inhibition of IL-8-induced W3/25+ (CD4+) T lymphocyte recruitment into subcutaneous tissues of rats by selective depletion of in vivo neutrophils with a monoclonal antibody. J Immunol 1991, 147:2196-2201 [PubMed] [Google Scholar]
- 37.Yamaki T, Uede T, Shijubo N, Kikuchi K: Functional analysis of mononuclear cells infiltrating into tumors. III. Soluble factors involved in the regulation of T lymphocyte infiltration into tumors. J Immunol 1988, 140:4388-4396 [PubMed] [Google Scholar]
- 38.Okada F, Hosokawa M, Hasegawa J, Kuramitsu Y, Nakai K, Yuan L, Lao H, Kobayashi H, Takeichi N: Enhancement of in vitro prostaglandin E2 production by mouse fibrosarcoma cells after co-culture with various anti-tumour effector cells. Br J Cancer 1994, 70:233-238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sugawara T, Miyamoto M, Takayama S, Kato M: Separation of neutrophils from blood in human and laboratory animals and comparison of the chemotaxis. J Pharmacol Toxicol Methods 1995, 33:91-100 [DOI] [PubMed] [Google Scholar]
- 40.Weitzman SA, Weitberg AB, Clark EP, Stossel TP: Phagocytes as carcinogens: malignant transformation produced by human neutrophils. Science 1985, 227:1231-1233 [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto M, Wu HH, Momose H, Rademaker A, Oyasu R: Marked enhancement of rat urinary bladder carcinogenesis by heat-killed Escherichia coli. Cancer Res 1992, 52:5329-5333 [PubMed] [Google Scholar]
- 42.Kawai K, Yamamoto M, Kameyama S, Kawamata H, Rademaker A, Oyasu R: Enhancement of rat urinary bladder tumorigenesis by lipopolysaccharide-induced inflammation. Cancer Res 1993, 53:5172-5175 [PubMed] [Google Scholar]
- 43.Tamatani T, Turk P, Weitzman S, Oyasu R: Tumorigenic conversion of a rat urothelial cell line by human polymorphonuclear leukocytes activated by lipopolysaccharide. Jpn J Cancer Res 1999, 90:829-836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sandhu JK, Privora HF, Wenckebach G, Birnboim HC: Neutrophils, nitric oxide synthase, and mutations in the mutatect murine tumor model. Am J Pathol 2000, 156:509-518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gal A, Wogan GN: Mutagenesis associated with nitric oxide production in transgenic SJL mice. Proc Natl Acad Sci USA 1996, 93:15102-15107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilkinson D, Sandhu JK, Breneman JW, Tucker JD, Birnboim HC: Hprt mutants in a transplantable murine tumour arise more frequency in vivo than in vitro. Br J Cancer 1995, 72:1234-1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weitzman SA, Stossel TP: Mutation caused by human phagocytes. Science 1981, 212:546-547 [DOI] [PubMed] [Google Scholar]
- 48.Tamir S, Tannenbaum SR: The role of nitric oxide (NO.) in the carcinogenic process. Biochim Biophys Acta 1996, 1288:F31-F36 [DOI] [PubMed] [Google Scholar]
- 49.Felley-Bosco E: Role of nitric oxide in genotoxicity: implication for carcinogenesis. Cancer Metastasis Rev 1998, 17:25-37 [DOI] [PubMed] [Google Scholar]
- 50.Okada F, Nakai K, Kobayashi T, Shibata T, Tagami S, Kawakami Y, Kitazawa T, Kominami R, Yoshimura S, Suzuki K, Taniguchi N, Inanami O, Kuwabara M, Kishida H, Nakae D, Konishi Y, Moriuchi T, Hosokawa M: Inflammatory cell-mediated tumour progression and minisatellite mutation correlate with the decrease of antioxidative enzymes in murine fibrosarcoma cells. Br J Cancer 1999, 79:377-385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Habelhah H, Okada F, Nakai K, Choi SK, Hamada J, Kobayashi M, Hosokawa M: Polysaccharide K induces Mn superoxide dismutase (Mn-SOD) in tumor tissues and inhibits malignant progression of QR-32 tumor cells: possible roles of interferon α, tumor necrosis factor α and transforming growth factor β in Mn-SOD induction by polysaccharide K. Cancer Immunol Immunother 1998, 46:338-344 [DOI] [PMC free article] [PubMed] [Google Scholar]